Abstract
The biofilm is a microbial community characterised by sessile bacterial cells strongly adherent to a substrate and/or an interface and incorporated in a polymeric matrix of microbial origin. In this condition, microbial cells exhibit an altered phenotype in comparison to corresponding free or planktonic forms. Life in a biofilm represents probably the prevailing mode of growth for microbes in several environments. After the development of new observation methods and the modification of different procedures, biofilms may be identified on known substrates and on new sites. The concept of biofilm has acquired the importance that still plays in the health sector, especially in areas where the use of invasive instruments and the continuous temporal localisation is expected. An important role is also played in the food industry because of the direct presence on food surfaces.
….There are several steps that we must take to optimize our lives in a city. The first is to choose the city in which we will live, then we must select the neighborhood in the city that best suits our needs, and finally we must make our home amongst the homes of many others. Occasionally, when life in the city sours, we leave….
P. Watnick, R. Kolter (2000) Biofilm, City of Microbes
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alginate
- Biofilm
- Cellulose
- Exopolysaccharide
- Extracellular polymeric substance
- N-acetylglucosamine
- Succinoglycan
3.1 Biofilm: An Introduction
The biofilm is a microbial community characterised by sessile bacterial cells strongly adherent to a substrate and/or an interface and incorporated in a polymeric matrix of microbial origin. In this condition, microbial cells exhibit an altered phenotype with respect to growth rates and the gene transcription in comparison to the corresponding free or planktonic forms.
It has been reported that the bacterial adhesion triggers gene control mechanisms for the production of required molecules in the formation of biofilms (Donlan and Costerton 2002). The definition of biofilm involves not only a description of the morphostructural type but also biomolecular factors (Donlan 2002).
After the development of new observation methods such as scanning electron microscopy and the modification of different procedures, biofilms may be identified on known substrates and on new sites. Consequently, persistent and antibiotic-resistant infections have been explained in this way. In recent years, the ‘biofilm phenomenon’ has been analysed in different ways and situations. Many microorganisms have been the object of study, including:
-
Candida albicans (Hawser and Douglas 1994; Sherry et al. 2014)
-
Escherichia coli (Pratt and Kolter 1998)
-
Klebsiella pneumoniae (di Martino et al. 2003)
-
Legionella pneumophila (Atlas 1999; Declerck 2010; Declerck et al. 2007; Hindré et al. 2008; Murga et al. 2001; Storey et al. 2004)
-
Proteus mirabilis (Holling et al. 2014; Jones et al. 2007; Moryl et al. 2014)
-
Pseudomonas aeruginosa (Klausen et al. 2003; O’Toole and Kolter 1998; Ramsey and Whiteley 2004; Savoia 2014)
-
Staphylococcus aureus (Kiedrowski et al. 2014; Islam et al. 2014; Wojtyczka et al. 2014).
3.1.1 Extracellular Polymeric Substances
As mentioned above, biofilm is a complex structure which is made of aggregates of microbial cells within a matrix of extracellular polymeric substances (EPS). EPS are high-molecular-weight compounds secreted by microorganisms into their environment.
The matrix structure constitutes the elastic part of the biofilm. Interstitial voids and channels separating micro-colonies contain a liquid phase, mainly constituted by water. The liquid phase is the viscous part of biofilms. The EPS matrix provides the biofilm with mechanical stability through these viscoelastic properties (Shaw et al. 2004) and the consequent functional and structural integrity. Physiochemical properties of biofilms are mainly determined by the qualitative and quantitative composition of EPS matrices (Flemming and Wingender 2010)
All major classes of macromolecules—polysaccharides, proteins, nucleic acids, peptidoglycan and lipids—can be present in a biofilm. Although extracellular polysaccharides are considered as the major structural components of the biofilm matrix, extracellular deoxyribonucleic acid (DNA) plays an important role in the establishment of biofilm structures (Whitchurch et al. 2002)
EPS are the construction material of bacterial settlements and either remain attached to outer cell surfaces or are secreted into its growth medium. These compounds are important in biofilm formation and cells’ attachment to surfaces. EPS constitutes 50–90 % of the total organic matter in biofilms (Donlan 2002; Donlan and Costerton 2002; Flemming et al. 2000).
The development of microbial biofilms is observed virtually on all submerged surfaces in natural and industrial environments. Biofilms are also observed at interfaces as pellicles, or in the bulk of aquatic environments as flocs or granules (Marti et al. 2011).
Microorganisms synthesise a wide spectrum of multifunctional polysaccharides including intracellular, structural and extracellular polysaccharides or exopolysaccharides (ES). ES are high-molecular-weight polymers, the composition includes sugar residues which are secreted by a microorganism into the surrounding environment.
ES generally consist of monosaccharides and some non-carbohydrate substituents such as acetate, pyruvate, succinate and phosphate groups. Owing to the wide diversity in composition, ES have found multifarious applications in medical, pharmaceutical and food industries.
ES of some strains of lactic acid bacteria, including Lactococcus lactis subsp. cremoris, contribute a gelatinous texture to fermented milk products such as Viili (a yoghurt-like mesophilic fermented milk); these polysaccharides are also digestible (Ljungh and Wadstrom 2009; Welman 2009). An example for the industrial use of ES is the application of dextran in the Italian panettone and other breads in the bakery industry (Ullrich 2009).
Lembre and coworkers have reported various examples of ES and carbohydrates in bacterial biofilm (Lembre et al. 2012). The composition of ES can vary according to producing bacterial species (Sects. 3.1.1.1–3.1.1.3).
3.1.1.1 Alginate and N-Acetylglucosamine
Alginate, a polysaccharide extracted by brown algae and different bacteria such as Azotobacter vinelandii (Gorin and Spencer 1966) and P. aeruginosa (Davies et al. 1993; Davies and Geesey 1995; Linker and Jones 1964), has been extensively studied (Fig. 3.1). In detail, alginate is an ES with a relatively high molecular mass (104–106 g/ml). This compound consists (Gacesa 1998) of two uronic acid residues: β-D-mannuronate, also named M, and its C-5 epimer, α-L-guluronate, also named G (Fig. 3.1).
Exopolysaccharides in biofilm matrices: structure of G- and M-alginates. G-structures mean ‘poly-α-L-guluronate’ while M-blocks are ‘poly-β-D-mannuronate’. BKchem version 0.13.0, 2009 (http://bkchem.zirael.org/index.html) has been used for drawing this structure
Another interesting ES, N-acetylglucosamine (Fig. 3.2), is produced by E. coli, S. aureus and S. epidermidis (Cerca and Jefferson 2008; Kaplan et al. 2004; Izano et al. 2008).
Structure of N-acetylglucosamine. BKchem version 0.13.0, 2009 (http://bkchem.zirael.org/index.html) has been used for drawing this structure
3.1.1.2 Succinoglycan
Succinoglycan is produced by Alcaligenes faecalis var. myxogenes 10C3, a microorganism our research group has isolated from soils. This microorganism produces a water-soluble and an insoluble extracellular polysaccharide. The former compound, succinoglycan, is composed of glucose, galactose, pyruvic acid and succinic acid (molar proportions are 7:1:1:1), with (β1-3)-, (β1-4)- and (β1-6)-glucosidic linkages. These polymers or oligomers are also produced by many Agrobacterium and Rhizobium strains (Harada 1983; Harada and Amemura 1979; Hisamatsu et al. 1982; Tomlinson et al. 2010).
3.1.1.3 Cellulose
Cellulose (Fig. 3.3) is the most abundant sugar polymer found on the surface of the planet Earth. It is found throughout the living world: in plants, animals, fungi and in bacteria such as Salmonella spp, E. coli, Acetobacter, Agrobacterium and Rhizobium (Matthysse et al. 2005; Solomon et al. 2005; Spiers and Rainey 2005).
Structure of cellulose. BKchem version 0.13.0, 2009 (http://bkchem.zirael.org/index.html) has been used for drawing this structure
3.2 The Biofilm and Its Creation
As mentioned earlier, biofilms are an aggregation of microorganisms attached to and growing on a surface (Costerton and Stewart 2001). The formation and the subsequent development of biofilms are affected by many factors, including the specific bacteria strain (Borucki et al. 2003; Chae and Schraft 2000), material surface properties and environmental parameters such as pH, available nutrient levels and temperature (Donlan 2002).
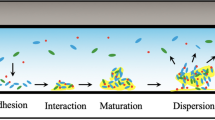
The biofilm formation is a dynamical process consisting of five steps: (1) initial attachment, (2) irreversible attachment, (3) early development of biofilm architecture, (4) maturation and (5) final dispersion (Fig. 3.4).
3.2.1 Initial Attachment
The initial attachment of microorganisms can be active or passive, depending on their motility, the gravitational transportation of their planktonic species (free floating) or the diffusion of the surrounding fluid phase (Kumar and Anand 1998). Physical properties of the environment are essential for microorganism’s attachment to the substratum, biofilm formation and microbial processes. The cell adhesion during this process strongly depends on the physiochemical properties of the bacterial cell surface (Begoude et al. 2007). At first, adherent cells possess only a small quantity of EPS; many of these life forms are capable of independent movement (O’Toole and Kolter 1998) by pilus-mediated twitching or gliding motility. The adhesion is reversible in this stage (Fig. 3.4).
3.2.2 Irreversible Attachment
The switch of the biofilm from the reversible to the irreversible state is related to the interaction between the presence of bacteria, EPS production and the surface (Fig. 3.4). In this step, cells lose their flagella-driven motility (Stoodley et al. 2002). After this irreversible attachment, a very strong force is required to remove the biofilm. For this reason, there is the need of chemicals such as enzymes, detergents or surfactants. McCoy and coworkers have shown that microbial adhesion strongly depends on hydrophobic–hydrophilic properties of interacting surfaces (McCoy and Brown 1998).
3.2.3 Micro-Colony Formation
Micro-colony formation results from the simultaneous aggregation and the increase of microorganisms (Fig. 3.4). As a result, the formation of biofilms is associated with the production of EPS, which helps to reinforce links between bacteria and the substratum. In addition, the colony is protected from any environmental stress (Donlan 2002).
Micro-colonies are discrete matrix-enclosed communities that may include cells of one or many species. Depending on the involved species, the micro-colony may be composed of 10–29 % cells and 75–90 % EPS- matrix (Costerton et al. 1987). Bacterial cells within the matrix are characterised by their lack of Brownian motion; the structural analysis of many micro-colonies often reveals a mushroom-like shape (Costerton et al. 1995).
Studies of bacterial species in natural systems have shown (McLean et al. 1997; Swift et al. 2001) that planktonic cells from the neighbouring medium can be included in this stage into the biofilm using the cell–cell communication (also named quorum sensing).
3.2.4 Maturation
After attachment to a surface, bacteria undergo further adaption to life in a biofilm. Two significant properties are often associated with this step: the increased EPS synthesis and the development of antibiotic resistance. In this step, bacteria develop other properties such as ultraviolet light—resistance, increased rates of genetic exchange, altered biodegradative capabilities and the increased production of secondary metabolites (O’Toole et al. 2000). For example, the presence of protein from milk or meat can alter physicochemical properties of surfaces (charge, free energy, hydrophobicity, etc.), which further results in a greater bacterial attachment (Kumar and Anand 1998).
The biofilm maturation (Fig. 3.4) is the step where the living matrix develops into an organised structure which can be flat or mushroom-shaped (Fig. 3.4), depending on available nutrient sources (Klausen et al. 2003).
In order to reach structural maturity, periods of 10 days or more are required (Stoodley et al. 2002). Bacteria grow under sessile form in motley complex-enclosed micro-colonies scattered with open water channels (Davey and O’ Toole 2000). Mature biofilms have also been evaluated and compared with chemostat cultures of P. aeruginosa by the DNA microarray technology. Results have shown that over 70 genes were altered, including genes encoding proteins involved in translation, metabolism, membrane transport and/or secretion, and gene regulation.
3.2.5 Dispersion
After the fourth stage, several attacked bacteria can leave the ‘aged’ biofilm individually or in groups and disperse in the environment. Results are the survival of these life forms and the colonisation of new niches. This detachment is a discontinuous and occasional process; it depends on various factors such as:
-
(a)
Flow variations of the surrounding liquid
-
(b)
The presence of chemicals or the modification of surface properties of cells or the colonised substrate
-
(c)
Internal biofilm processes, such as endogenous enzymatic degradation
-
(d)
The release of EPS or surface-binding protein.
These causes can occur at the same time; in addition, starvation is considered as a reason for the detachment and forces bacteria to search for a nutrient-rich environment (Srey et al. 2013). Every microorganism that breaks off can be transported in the environment and start again the process of biofilm formation (Fig. 3.4).
3.3 Some Environmental Factors Affecting Biofilm Formation
3.3.1 pH and Temperature
Bacteria respond to internal and external pH and temperature modifications with the variation of the activity and synthesis of proteins associated with many different cellular processes (Olsen 1993). A gradual increase in acidity determines good chances of cell survival if compared to a sudden increase by the rapid addition of hydrochloric acid (Li 2001). This result suggests that bacteria contain mechanisms in place which allow the microbial population to adapt to small pH changes (Garrett et al. 2008). Bacteria have membrane-bound proton pumps which extrude protons from the cytoplasm to generate a trans-membrane electrochemical gradient, i.e. the proton motor force. The passive influx of protons in response to the proton motive force can be a problem for cells attempting to regulate their cytoplasmic pH (Booth 1985). Large variations of external pH values can overwhelm such mechanisms and have a biocidal effect on microorganisms. The optimum pH for polysaccharide production depends on the individual species; however, it has been reported that approximate pH values of around 7 are good for most bacteria (Oliveira et al. 1994).
3.3.2 Surface Topography
The roughness of substrates is known to be one of the key factors in determining the extent of bacterial colonisation (Crawford et al. 2012; Oh et al. 2009). Also, it plays a significant role in the attachment process, particularly when superficial irregularities are comparable to microbial sizes and can provide shelter from unfavourable environmental factors (Mitik-Dineva et al. 2008). Moreover, levels of bacterial adhesion are determined by the surface topography. The relationship between surface roughness and the attachment (and growth) of bacteria may vary depending on involved microbial species. Furthermore, once bacteria have colonised a surface, the resistance of biofilms to the removal by simple friction increases in proportion to the superficial roughness (Preedy et al. 2014).
3.3.3 Hydrophobicity and Hydrophilicity
Hydrophobicity plays an important role in the microbial attachment on surfaces: this factor has an impact on the temporal length of cells association with the substratum. The hydrophobicity of cell surfaces is also important in adhesion because the hydrophobic interaction tends to augment when non-polar properties of each surface increase. (Alsteens et al. 2007; Faille et al. 2002; van Oss 1995, 1997)
3.4 Food and Food Industry
The role of biofilms in the food sector is relevant. Many authors have demonstrated the presence of microorganisms organised in biofilms directly on the food as well as on different materials that will be in contact with it. Germs can adhere strongly to glass or stainless steel tools and ceramic and polypropylene surfaces, with the resulting difficult eradication (Adetunji et al. 2014; Balsanelli et al. 2014; Regina et al. 2014).
In environments of food processing bacteria with other organic and inorganic molecules, such as milk—or meat—proteins, a conditioning film can be formed. This film, composed of many complexes, can be displaced by diffusion or transported by liquid flows. The accumulation of molecules at the solid–liquid interface on the contact surface of the food can lead to a high concentration of nutrients with respect to the fluid phase. The increased level of nutrients deposited on contact surfaces can favour the formation of biofilm, acting as a conditioning film.
The adhesion of bacteria to food or food- contact surfaces represents a serious hygiene problem; it can determine economic losses due to possible food spoilage. In addition, some studies have demonstrated the persistence of some foodborne pathogens on surfaces in contact with foods and some germs has been recently added to the existing list: Salmonella (de Oliveira et al. 2014; Lianou and Koutsoumanis 2012, 2013; Patel et al. 2013; Schonewille et al. 2012; Wang et al. 2013); Listeria monocytogenes (Barbosa et al. 2013; Borucki et al. 2003; Ferreira et al. 2014; Valderrama and Cutter 2013); Yersinia enterocolitica (Zhou and Yang 2011; Campylobacter jejuni (Joshua et al. 2006; Reeser et al. 2007; Teh et al. 2010, 2014); Aeromonas hydrophila (Elhariry 2011; Jahid et al. 2013); E. coli and E. coli 0157: H7 (Dourou et al. 2011; Gomes et al. 2014; Silagyi et al. 2009; Simpson Beauchamp et al. 2012); S. aureus (Gutiérrez et al. 2012). The persistence and the accumulation of germs in biofilms could be a source of subsequent contamination, leading to a reduced shelf life of the product. The transmission of pathogens may also occur by produced aerosols during surface-cleaning processes in food plants (Italian Institute of Packaging 2009; Parisi 2011, 2012; Steinka 2015).
One of the main objectives of the food industry is the production of safe and healthy products with good organoleptic quality. Consequently, the growth of microorganisms has to be monitored in order to minimise the risk of food contamination by germs into food plants (environmental contamination).
Moreover, monitoring activities have to consider methods of production and handling of food products, involving frequent contact of raw materials, semi-finished and finished products with work surfaces and utensils. It can be assumed that the transfer of microbial flora with pathogenic or degrading features to foods may be easily observed when considering work surfaces, utensils, packaging materials and correlated machinery equipment (Parisi 2013).
The presence of nutrient residuals on surfaces can promote the formation of biofilms; on the other hand, the simple presence of organic substance in drinking water may be sufficient, when surfaces are washed.
From the industrial viewpoint, biofilms represent a problem that mainly concerns food industries: breweries, dairy companies, meat industries, etc. (Chen et al. 2007; Frank et al. 2003; Jessen and Lammert 2003; Simões and Vieira 2009; Simões et al. 2010; Somers and Wong 2004).
With reference to food production environments, bacteria and chemical compounds (organic and inorganic molecules such as milk or meat proteins) can adhere to non-food surfaces with the consequent formation of conditioning films. Organic and inorganic molecules are transported together with microorganisms on surfaces by diffusion or by the turbulent flow of liquids in some cases (Poulsen 1999; Sharma and Anand 2002). In these systems, the amount of residual nutrients with strong adherence to surfaces is notable and can condition the surface itself (Hood and Zottola 1997). Basically, the increase of nutrients promotes biofilm formation (Jeong and Frank 1994).
The creation of biofilm on food-contact materials leads to a number of hygienic and economic problems at the same time: in fact, biologic contamination can cause food deterioration before of the labelled expiration date (Parisi 2002a, b, c).
On the other hand, it is known that surfaces and tooling in contact with foods must be made with easily cleanable and disinfectable materials (Gurnari 2015). The use of steel and hardness plastics has been promoted for this reason, excluding porous materials such as wood.
For example, dirty or unclean equipment are considered as the main sources of contamination (with airborne microflora) of milk and milk products. The accumulation and the persistence of biofilms can cause post-process contamination, shortening shelf-life values of food products (Jin and Zhang 2008; Zottola and Sasahara 1994).
The contamination may occur between packaging and consumption steps at any time. According to recent literature on polyethylene terephthalate (common plastic bottles for drinks are made with this plastic matter), bacteria can adhere tenaciously and constitute biofilms in less than 24 h; on the other hand, polypropylene (containers and plates) is not easily contaminated by moulds and bacteria (Byun et al. 2007).
The modern equipment used in food production processes are made of different materials such as stainless steel, glass, plastic, rubber and polytetrafluoroethylene (Teflon®); the degree of hydrophobicity of these surfaces can influence microbial adhesion (Sinde and Carballo 2000; Teixeira et al. 2005).
Food contamination may appear at different stages of the production chain; in particular, certain perishable products such as milk have to be considered. Milk can be contaminated during the milking stage by faccal contamination or the simple use of contaminated water. With specific reference to milk and its derivatives, saprophytic or pathogens bacteria may creep under the seals of transformation or packaging machinery and contaminate food products and/or the final container (or single parts), thus leading to the formation of biofilm. Should the contamination concern packaging materials, this phenomenon and the consequent biofilm development would occur after pasteurisation. Surfaces such as shelves and walls can be indirectly source of contamination: germs can be transported from the air, as well as by operators’ hands.
The design of equipment and the careful selection of materials of work surfaces are of crucial importance in preventing the formation of biofilms (Gurnari 2015). The most suitable material is stainless steel (it can be easily cleaned using common detergents).
The prerequisite for an efficient sanitation programme is correlated with the so-called hygienic design (Gurnari 2015). Corners, cracks, joints, gaskets and crevices are vulnerable points for the accumulation of biofilm. Should the design of equipment and work surfaces be improper, any sanitation programme would represent possible contamination problems. Therefore, best strategies for the control of food-contact surfaces can be expressed as follows:
-
(a)
The design of equipment should aim towards an efficient hygiene program, perform a proper cleansing and implement a good sanitation
-
(b)
An effective cleaning programme removes undesired materials from food-contact surfaces, including microorganisms, foreign matter and any residues of detergent.
3.5 Strategies of Biofilm Control
3.5.1 Physical Methods
With exclusive reference to the removal of biofilms in food industries, the most innovative methods of physical nature include:
-
(a)
High-frequency ultrasound
-
(b)
High-frequency pulsed electric fields (the concomitant use of organic acids can be considered)
-
(c)
Low-frequency electric fields (with the aim of increasing the effect of biocides).
Recently, the use of electric fields associated with antibiotics has been defined effective when speaking of Pseudomonas biofilm control.
3.5.2 Chemical Methods
The effective elimination of microorganisms on surfaces is important before disinfection procedures. Germs become much more sensitive to disinfectants when the biofilm is removed from surfaces. Even the mechanical or chemical break of polysaccharide matrices is important for the control of biofilms. Among the detergents with effective power against biofilms, chelating substances should be mentioned: these compounds include ethylenediaminetetraacetic and aminoethylene glycolethyl tetracetic acids. Some oxidising disinfectants are able to depolymerise ES, including acid peracetic acid, chlorine, iodine and hydrogen peroxide. These substances allow the detachment of biofilms formed by certain types of bacteria from surfaces.
3.5.3 Biological Methods
An innovative strategy for the control of biofilms consists in the adsorption of bioactive molecules such as bacteriocins and enzymes on food-contact surfaces. For example, nisin has been adjudged effective when speaking of adherence reduction of L. monocytogenes to surfaces (Saà Ibusquiza et al. 2011). The use of peculiar lactic bacteria cultures and their extracts allows the inhibition of degrading microorganisms and pathogens on food-contact (poultry products). As regards enzymes, they have been defined very efficient as detergents because of the inactivation of biofilm exocellular polymers and the enhancement of biofilm removal.
3.6 Biofilm in Hospital Setting and Antibiotic Resistance
It has to be noted that the chronicity of persistent bacterial infections is due to bacterial biofilm formation, in contrast with planktonic bacteria found in acute infections (Bjarnsholt 2013).
The epidemiologic evidence has clearly shown that biofilms have a role in infectious diseases (cystic fibrosis, periodontitis, bloodstream and urinary tract infections) as a result of indwelling medical devices. The process may be particularly relevant for immunocompromised patients who lack the ability to contrast invading organisms. Beyond the evidence, however, the exact processes by which biofilm-associated organisms elicit disease in the human host are poorly understood. Suggested mechanisms include the following steps:
-
(1)
Detachment of cells or cell aggregates from indwelling medical device biofilms, resulting in bloodstream or urinary tract infections
-
(2)
Production of endotoxins
-
(3)
Resistance to the host immune system
-
(4)
Provision of a niche for the generation of resistant organisms (through resistance plasmid exchange).
One of the most fascinating defence mechanisms of a biofilm is based on a peculiar type of intercellular signalling, the so-called quorum sensing. Some bacteria release a molecular signal, also called inductor. These molecules are acylated homoserine lactone for Gram-negative bacteria and peptic substances (peptides, amines, amino acids) for Gram-positive microorganisms.
As the cell density rises, the concentration of these molecules increases. Inductors interact with specific receptors in each cell to activate quorum sensing genes and start a cascade of events, thus causing the expression or repression of numerous other genes on the bacterial chromosome.
The structure and physiological attributes of biofilm-producing organisms confirm the inherent resistance to antimicrobial agents such as antibiotics, disinfectants and germicides. The mechanisms responsible for resistance may be described approximately as follows:
-
1.
Loss of penetration power of antibiotics through the biofilm matrix
-
2.
Percentage of altered growth of germs which constitute the biofilm
-
3.
Other physiological changes due to the mode of growth of the biofilm.
Antibiotic molecules must diffuse into the biofilm matrix to inactivate included cells. Extracellular polymeric substances that constitute the matrix represent a barrier to the diffusion of these molecules so as to hold both the percentage of the transport of molecules to the inside of biofilm and the interaction of the antimicrobial matter with the material from the matrix. A loss of power of penetration of ciprofloxacin against P. aeruginosa has been demonstrated (Suci et al. 1994); in other words, the normal sterilisation of surfaces (needed time: 40 s) would require 21 min when surfaces are covered by biofilms. Bacterial cells have been reported to be 15 times more sensitive to tobramycin if compared to cells of an intact biofilm (Hoyle et al. 1992).
Another mechanism has been proposed to explain the resistance to antimicrobial agents. In brief, cells associated with the biofilm may grow significantly more slowly than planktonic cells with the delayed absorption of antimicrobial. Anwar and coworkers have demonstrated that old cells (life: ten days) of P. aeruginosa biofilms were much more resistant to tobramycin and piperacillin biofilm cells (Anwar et al. 1989) if compared to younger cells (life: two days). A dosage of 500 mg of piperacillin + tobramycin (5 mg/ml) can inactivate completely planktonic cells and biofilms of young cells. On the other side, older cells have been reduced only by approximately 20 % in the same condition.
3.6.1 Measurement and Observation of Biofilms
Biofilm growth and structure has been measured using diversified methods: fluorescence, transmission electron, scanning electron, atomic force and confocal laser scanning microscopy (CLSM). In relation to the CLSM system, our research group has used a TCS SP2- laser scanning confocal microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany), equipped with argon-krypton laser and coupled to a Leica DMIRB-inverted fluorescence microscope. The use of CLSM requires the use of fluorescent stains as propidium iodide, a nucleic acid intercalating and fluorescent agent (Sigma). A biofilm produced by S. aureus, grown on glass and analysed by CLSM, is shown in Fig. 3.5.
Numerous methods are used with the aim of quantifying biofilm production (Sternberg et al. 2014). Microtiter plates are among the most frequently used biofilm model systems (Djordjevic et al. 2002; Elkhatib et al. 2014; Kwasny and Opperman 2010; Pierce et al. 2010). In these systems, biofilms are either grown on the bottom and the walls of the microtiter plate (most commonly a 96-well plate); results are usually read using a spectrometer (Fig. 3.6) (Table 3.1).
Abbreviations
- CLSM:
-
Confocal laser scanning microscopy
- DNA:
-
Deoxyribonucleic acid
- ES:
-
Exopolysaccharide
- EPS:
-
Extracellular polymeric substance
References
Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib NE (2014a) Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol 196(7):453–472. doi:10.1007/s00203-014-0983-1
Abdallah M, Chataigne G, Ferreira-Theret P, Benoliel C, Drider D, Dhulster P, Chihib NE (2014b) Effect of growth temperature, surface type and incubation time on the resistance of Staphylococcus aureus biofilms to disinfectants. Appl Microbiol Biotechnol 98(6):2597–2607. doi:10.1007/s00253-013-5479-4
Adetunji VO, Adedeji AO, Kwaga J (2014) Assessment of the contamination potentials of some foodborne bacteria in biofilms for food products. Asian Pac J Trop Med 7(1):S232–S237. doi:10.1016/S1995-7645(14)60238-8
Alsteens D, Dague E, Rouxhet PG, Baulard AR, Dufrêne YF (2007) Direct measurement of hydrophobic forces on cell surfaces using AFM. Langmuir 23(24):11977–11979. doi:10.1021/la702765c
Anwar H, van Biesen T, Dasgupta M, Lam K, Costerton JW (1989) Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother 33(10):1824–1826
Atlas RM (1999) Legionella: from environmental habitats to disease pathology, detection and control. Environ Microbiol 1:283–293. doi:10.1046/j.1462-2920.1999.00046.x
Balsanelli E, de Baura VA, Pedrosa Fde O, de Souza EM, Monteiro RA (2014) Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae. PLoS ONE 9(10):e110392. doi:10.1371/journal.pone.0110392
Barbosa J, Borges S, Camilo R, Magalhães R, Ferreira V, Santos I, Silva J, Almeida G, Teixeira P (2013) Biofilm formation among clinical and food isolates of Listeria monocytogenes. Int J Microbiol 2013:524975. doi:10.1155/2013/52497
Begoude BA, Lahlali R, Friel D, Tondje PR, Jijakli MH (2007) Response surface methodology study of the combined effects of temperature, pH, and aw on the growth rate of Trichoderma asperellum. J Appl Microbiol 103(4):845–854. doi:10.1111/j.1365-2672.2007.03305.x
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS 121(Suppl 136):1–54. doi: 10.1111/apm.12099
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49(4):359–378
Borucki MK, Peppin JD, White D, Loge F, Call DR (2003) Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol 69(12):7336–7342. doi:10.1128/AEM.69.12.7336-7342.2003
Byun MW, Kim JH, Kim DH, Kim HJ, Jo C (2007) Effects of irradiation and sodium hypochlorite on the micro-organisms attached to a commercial food container. Food Microbiol 24(5):544–548. doi:10.1016/j.fm.2006.08.005
Cerca N, Jefferson KK (2008) Effect of growth conditions on poly-N-acetylglucosamine expression and biofilm formation in Escherichia coli. FEMS Microbiol Lett 283(1):36–41. doi:10.1111/j.1574-6968.2008.01142.x
Chae MS, Schraft H (2000) Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int J Food Microbiol 62(1–2):103–111. doi:10.1016/S0168-1605(00)00406-2
Chen J, Rossman ML, Pawar DM (2007) Attachment of enterohemorragic Escherichia coli to the surface of beef and a culture medium. LWT Food Sci Technol 40(2):249–254. doi:10.1016/j.lwt.2005.10.011
Costerton JW, Stewart PS (2001) Battling biofilms. Sci Am 285(1):74–81
Costerton JW, Cheng K-J, Geesey GG, Ladd T, Nickel JC, Dasgupta M, Marrie JT (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464. doi:10.1146/annurev.mi.41.100187.002251
Costerton JW, Lewandowski Z, Caldwell D, Korber D, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745. doi:10.1146/annurev.mi.49.100195.003431
Crawford RJ, Webb HK, Truong VK, Hasan J, Ivanova EP (2012) Surface topographical factors influencing bacterial attachment. Adv Colloid Interface Sci 179–182:142–149. doi:10.1016/j.cis.2012.06.015
Davey ME, O’ Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847–867. doi:10.1128/MMBR.64.4.847-867.2000
Davies DG, Geesey GG (1995) Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol 61(3):860–867
Davies DG, Chakrabarty AM, Geesey GG (1993) Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol 59(4):1181–1186
De Oliveira DC, Fernandes Júnior A, Kaneno R, Silva MG, Araújo Júnior JP, Silva NC, Rall VL (2014) Ability of Salmonella spp. to produce biofilm is dependent on temperature and surface material. Foodborne Pathog Dis 11(6):478–483. doi:10.1089/fpd.2013.1710
Declerck P (2010) Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol 12(3):557–566. doi:10.1111/j.1462-2920.2009.02025.x
Declerck P, Behets J, van Hoef V, Bouckaert V, Ollevier F (2007) Replication of Legionella pneumophila in floating biofilms. Curr Microbiol 55(5):435–440. doi:10.1007/s00284-007-9006-7
Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A (2003) Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res Microbiol 154(1):9–16. doi:10.1016/S0923-2508(02)00004-9
Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 68(6):2950–2958. doi:10.1128/AEM.68.6.2950-2958.2002
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881–890. doi:10.3201/eid0809.020063
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193. doi:10.1128/CMR.15.2.167-193.2002
Dourou D, Beauchamp CS, Yoon Y, Geornaras I, Belk KE, Smith GC, Nychas GJ, Sofos JN (2011) Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int J Food Microbiol 149(3):262–268. doi:10.1016/j.ijfoodmicro.2011.07.004
Elhariry HM (2011) Biofilm formation by Aeromonas hydrophila on green-leafy vegetables: cabbage and lettuce. Foodborne Pathog Dis 8(1):125–131. doi:10.1089/fpd.2010.0642
Elkhatib WF, Khairalla AS, Ashour HM (2014) Evaluation of different microtiter plate-based methods for the quantitative assessment of Staphylococcus aureus biofilms. Future Microbiol 9(6):725–735. doi:10.2217/fmb.14.33
Faille C, Jullien C, Fontaine F, Bellon-Fontaine MN, Slomianny C, Benezech T (2002) Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can J Microbiol 48(8):728–738. doi:10.1139/w02-063
Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ (2014) Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot 77(1):150–170. doi:10.4315/0362-028X
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. doi:10.1038/nrmicro2415
Flemming HC, Wingender J, Griebe T, Mayer C (2000) Physico-chemical properties of biofilms. In: Evans LV (ed) Biofilms: recent advances in their study and control. Harwood Academic Publishers, Amsterdam
Frank JF, Ehlers J, Wicher L (2003) Removal of Listeria monocytogenes and poultry soil-containing biofilms using chemical cleaning and sanitizing agents under static conditions. Food Prot Trends 23:654–663
Gacesa P (1998) Bacterial alginate biosynthesis-recent progress and future prospects. Microbiol 144(5):1133–1143. doi:10.1099/00221287-144-5-1133
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1046. doi:10.1016/j.pnsc.2008.04.001
Gomes LC, Silva LN, Simões M, Melo LF, Mergulhão FJ (2014) Escherichia coli adhesion biofilm development and antibiotic susceptibility on biomedical materials. J Biomed Mater Res A 103(4):1414–1423. doi:10.1002/jbm.a.35277
Gorin JPA, Spencer TJF (1966) Exocellular alginic acid from Azotobacter vinelandii. Can J Chem 44:993–998. doi:10.1139/v66-147
Gurnari G (2015) Hygiene of food-contact approved materials for machinery. In: Gurnari G (ed) Safety protocols in the food industry and emerging concerns. SpringerBriefs in Chemistry of Foods, Springer International Publishing, Heidelberg. doi: 10.1007/978-3-319-16492-2_3
Gutiérrez D, Delgado S, Vázquez-Sánchez D, Martínez B, Cabo ML, Rodríguez A, Herrera JJ, García P (2012) Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl Environ Microbiol 78(24):8547–8554. doi:10.1128/AEM.02045-12
Harada T (1983) Special bacterial polysaccharides and polysaccharases. Biochem Soc Symp 48:97–116
Harada T, Amemura A (1979) Comparative studies of polysaccharides elaborated by Rhizobium, Alcaligenes, and Agrobacterium. Carbohydr Res 77(1):285–288. doi:10.1016/S0008-6215(00)83821-5
Hawser SP, Douglas LJ (1994) Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun 62(3):915–921
Hindré T, Brüggemann H, Buchrieser C, Héchard Y (2008) Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiol 154(1):30–41. doi:10.1099/mic.0.2007/008698-0
Hisamatsu M, Amemura A, Matsuo T, Matsuda H, Harada T (1982) Cyclic (1 → 2)-β-D-Glucan and the Octasaccharide Repeating-unit of Succinoglycan Produced by Agrobacterium. Microbiol 128(8):1873–1879. doi:10.1099/00221287-128-8-1873
Holling N, Lednor D, Tsang S, Bissell A, Campbell L, Nzakizwanayo J, Dedi C, Hawthorne JA, Hanlon G, Ogilvie LA, Salvage JP, Patel BA, Barnes LM, Jones BV (2014) Elucidating the genetic basis of crystalline biofilm formation in Proteus mirabilis. Infect Immun 82(4):1616–1626. doi:10.1128/IAI.01652-13
Hood SK, Zottola EA (1997) Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int J Food Microbiol 37(2–3):145–153
Hoyle BD, Wong CK, Costerton JW (1992) Disparate efficacy of tobramycin on Ca(2 +)-, Mg(2 +)-, and HEPES-treated Pseudomonas aeruginosa biofilms. Can J Microbiol 38(11):1214–1218. doi:10.1139/m92-201
Islam N, Kim Y, Ross JM, Marten MR (2014) Proteomic analysis of Staphylococcus aureus biofilm cells grown under physiologically relevant fluid shear stress conditions. Proteome Sci 30(12):21. doi:10.1186/1477-5956-12-21
Italian Institute of Packaging (2009) Aspetti analitici a dimostrazione della conformità del food packaging: linee guida. Prove, Calcoli, Modellazione e altre argomentazioni. The Italian Institute of Packaging, Milan
Izano EA, Amarante MA, Kher WB, Kaplan JB (2008) Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 74(2):470–476. doi:10.1128/AEM.02073-07
Jahid IK, Lee NY, Kim A, Ha SD (2013) Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J Food Prot 76(2):239–247. doi:10.4315/0362-028X.JFP-12-321
Jeong DK, Frank JF (1994) Growth of listeria monocytogenes at 21 °C in biofilms with micro-organisms isolated from meat and dairy processing environments. LWT Food Sci Technol 27(5):415–424
Jessen B, Lammert L (2003) Biofilm and disinfection in meat processing plants. Int Biodeterior Biodegrad 51(4):265–269
Jin T, Zhang H (2008) Biodegradable polylactic acid Polymer with nisin for use in antimicrobial food packaging. J Food Sci 2008 73(3):M127–M134. doi: 10.1111/j.1750-3841.2008.00681.x
Jones SM, Yerly J, Hu Y, Ceri H, Martinuzzi R (2007) Structure of P. mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiol Lett 268(1):16–21. doi:10.1111/j.1574-6968.2006.00587.x
Joshua GW, Guthrie-Irons C, Karlyshev AV, Wren BW (2006) Biofilm formation in Campylobacter jejuni. Microbiol 152(2):387–396. doi:10.1099/mic.0.28358-0
Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JK, Ramasubbu N (2004) Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol 186(24):8213–8220. doi:10.1128/JB.186.24.8213-8220.2004
Kiedrowski MR, Crosby HA, Hernandez FJ, Malone CL, McNamara JO 2nd, Horswill AR (2014) Staphylococcus aureus Nuc2 is a functional, surface-attached extracellular nuclease. PLoS ONE 9(4):e95574. doi:10.1371/journal.pone.0095574
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T (2003) Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48(6):1511–1524. doi:10.1046/j.1365-2958.2003.03525.x
Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42(1–2):9–27
Kwasny SM, Opperman TJ (2010) Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr Protoc Pharmacol chapter 13:unit 13A.8. doi: 10.1002/0471141755.ph13a08s50
Lembre P, Lorentz C, di Martino P (2012) Exopolysaccharides of the biofilm matrix: a complex biophysical world, chapter 13. In: Karunaratne DN (ed) The complex world of polysaccharides InTech Open Access Publisher. http://dx.doi.org/10.5772/51213. Accessed 31 March 2015
Li Y (2001) Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J Bacteriol 183(23):6875–6884. doi:10.1128/JB.183.23.6875-6884.2001
Lianou A, Koutsoumanis KP (2012) Strain variability of the biofilm-forming ability of Salmonella enterica under various environmental conditions. Int J Food Microbiol 160(2):171–178. doi:10.1016/j.ijfoodmicro.2012.10.002
Lianou A, Koutsoumanis KP (2013) Strain variability of the behavior of foodborne bacterial pathogens: a review. Int J Food Microbiol 167(3):310–321. doi:10.1016/j.ijfoodmicro.2013.09.016
Linker A, Jones RS (1964) A Polysaccharide resembling Alginic Acid from a Pseudomonas Micro-organism. Nature 204:187–188. doi:10.1038/204187a0
Ljungh A, Wadstrom T (eds) (2009) Lactobacillus molecular biology: from genomics to probiotics. Caister Academic Press, Norfolk
Marti S, Nait Chabane Y, Alexandre S, Coquet L, Vila J, Jouenne T, Dé E (2011) Growth of A. baumannii in pellicle enhanced the expression of potential virulence factors. PLoS ONE 6(10):e26030. doi:10.1371/journal.pone.002603
Matthysse AG, Marry M, Krall L, Kaye M, Ramey BE, Fuqua C, White AR (2005) The effect of cellulose overproduction on binding and biofilm formation on roots by Agrobacterium tumefaciens. Mol Plant Microbe Interact 18(9):1002–1010. doi:10.1094/MPMI-18-1002
McCoy DI, Brown KM (1998) Hydrocarbon pollutants alter short-term recruitment in the barnacle Balanus eburneus. Mar Environ Res 45(3):209–224
McLean RJ, Whiteley M, Stickler DJ, Fuqua WC (1997) Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett 154(2):259–263. doi:10.1111/j.1574-6968.1997.tb12653.x
Mitik-Dineva N, Wang J, Mocanasu RC, Stoddart PR, Crawford RJ, Ivanova EP (2008) Impact of nano-topography on bacterial attachment. Biotechnol J 3(4):536–544. doi:10.1002/biot.200700244
Moryl M, Kaleta A, Strzelecki K, Różalska S, Różalski A (2014) Effect of nutrient and stress factors on polysaccharides synthesis in P. mirabilis biofilm. Acta Biochim Pol 61(1):133–139
Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM (2001) Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiol 147(11):3121–3126
Oh YJ, Lee NR, Jo W, Jung WK, Lim JS (2009) Effects of substrates on biofilm formation observed by atomic force microscopy. Ultramicroscopy 109(8):874–880. doi:10.1016/j.ultramic.2009.03.042
Oliveira R, Melo L, Oliveira A, Salgueiro R (1994) Polysaccharide production and biofilm formation by Pseudomonas fluorescens: effects of pH and surface material. Colloids Surf B Biointerfaces 2:41–46
Olsen ER (1993) Influence of pH on bacterial gene expression. Mol Microbiol 8(1):5–14. doi:10.1111/j.1365-2958.1993.tb01198.x
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30(2):295–304. doi: 10.1046/j.1365-2958.1998.01062.x
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. doi:10.1146/annurev.micro.54.1.49
Parisi S (2002a) Profili evolutivi dei contenuti batterici e chimico-fisici in prodotti lattiero-caseari. Ind Aliment 412:295–306
Parisi S (2002b) I fondamenti del calcolo della data di scadenza degli alimenti: principi ed applicazioni. Ind Aliment 417:905–919
Parisi S (2002c) Previsione della crescita della flora microbica non lattica in formaggi. Ind Aliment 419:1195–1203
Parisi S (2011) Food packaging and technological compliance. The importance of correct storage procedures. Food Packag Bull 20(9 & 10):14–18
Parisi S (2012) Food packaging and food alterations: the user-oriented approach. Smithers Rapra Technology, Shawbury
Parisi S (2013) Food Industry and packaging materials: performance-oriented guidelines for users. Smithers Rapra Technology, Shawbury
Patel J, Singh M, Macarisin D, Sharma M, Shelton D (2013) Differences in biofilm formation of produce and poultry Salmonella enterica isolates and their persistence on spinach plants. Food Microbiol 36(2):388–394. doi:10.1016/j.fm.2013.06.019
Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL (2010) A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J Vis Exp 21(44):2287. doi:10.3791/2287
Poulsen LV (1999) Microbial biofilm in food processing. LWT Food Sci Technol 32(6):321–326
Pratt LA, Kolter R (1998) Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30:285–293
Preedy E, Perni S, Nipiĉ D, Bohinc K, Prokopovich P (2014) Surface roughness mediated adhesion forces between borosilicate glass and Gram-Positive bacteria. Langmuir 30(31):9466–9476. doi:10.1021/la501711t
Ramsey MM, Whiteley M (2004) Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol Microbiol 53(4):1075–1087. doi:10.1111/j.1365-2958.2004.04181.x
Reeser RJ, Medler RT, Billington SJ, Jost BH, Joens LA (2007) Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl Environ Microbiol 73(6):1908–1913. doi:10.1128/AEM.00740-06
Regina VR, Lokanathan AR, Modrzyński JJ, Sutherland DS, Meyer RL (2014) Surface physicochemistry and ionic strength affects eDNA’s role in bacterial adhesion to abiotic surfaces. PLoS ONE 9(8):e105033. doi:10.1371/journal.pone.0105033
Saá Ibusquiza P, Herrera JJ, Cabo ML (2011) Resistance to benzalkonium chloride, peracetic acid and nisin during formation of mature biofilmsby Listeria monocytogenes. Food Microbiol 28(3):418–425. doi:10.1016/j.fm.2010.09.014
Savoia D (2014) New perspectives in the management of Pseudomonas aeruginosa infections. Future Microbiol 9:917–928. doi:10.2217/fmb.14.42
Schonewille E, Nesse LL, Hauck R, Windhorst D, Hafez HM, Vestby LK (2012) Biofilm building capacity of Salmonella enterica strains from the poultry farm environment. FEMS Immunol Med Microbiol 65(2):360–365. doi:10.1111/j.1574-695X.2012.00966.x
Sharma M, Anand SK (2002) Bacterial biofilm on food contact surfaces. A Rev J Food Sci Technol 39(6):573–593
Shaw T, Winston M, Rupp CJ, Klapper I, Stoodley P (2004) Commonality of elastic relaxation times in biofilms. Phys Rev Lett 93(9):098102. doi:10.1103/PhysRevLett.93.098102
Sherry L, Rajendran R, Lappin DF, Borghi E, Perdoni F, Falleni M, Tosi D, Smith K, Williams C, Jones B, Nile CJ, Ramage G (2014) Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol 14:182. doi:10.1186/1471-2180-14-182
Silagyi K, Kim SH, Lo YM, Wei CI (2009) Production of biofilm and quorum sensing by Escherichia coli O157:H7 and its transfer from contact surfaces to meat, poultry, ready-to-eat deli, and produce products. Food Microbiol 26(5):514–519
Simões M, Vieira MJ (2009) Persister cells in Pseudomonas fluorescens biofilms treated with a biocide. Proceedings of the Specialized Conference: Processes in Biofilms: Fundamentals to Applications, 13–16 September 2009. Davis, California, pp 58–62
Simões LC, Simões M, Vieira MJ (2010) A review of current and emergent biofilm control strategies. LWT Food Sci Technol 43(4):573–583
Simpson Beauchamp C, Dourou D, Geornaras I, Yoon Y, Scanga JA, Belk KE, Smith GC, Nychas GJ, Sofos JN (2012) Transfer, attachment, and formation of biofilms by Escherichia coli O157:H7 on meat-contact surface materials. J Food Sci 77(6):M343–M347. doi:10.1111/j.1750-3841.2012.02695.x
Sinde E, Carballo J (2000) Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17(4):439–447
Solomon EB, Niemira BA, Sapers GM, Annous BA (2005) Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J Food Prot 68(5):906–912
Somers EB, Wong ACL (2004) Efficacy of two cleaning and sanitizing combinations on Listeria monocytogenes biofilms formed at low temperature on a variety of materials in the presence of ready-to-eat meat residue. J Food Prot 67:2218–2229
Spiers AJ, Rainey PB (2005) The Pseudomonas fluorescens SBW25 wrinkly spreader biofilm requires attachment factor, cellulose fibre and LPS interactions to maintain strength and integrity. Microbiol 151(9):2829–2839. doi:10.1099/mic.0.27984-0
Srey S, Jahid IK, Ha SD (2013) Biofilm formation in food industries: a food safety concern. Food Control 31(2):572–585
Steinka I (2015) Chemical and microbiological aspects of the interaction between food and food packages. In: Barone C, Bolzoni L, Caruso G, Montanari A, Parisi S, Steinka I (eds) Food packaging hygiene. SpringerBriefs in Chemistry of Foods, Springer, Heidelberg. doi: 10.1007/978-3-319-14827-4
Sternberg C, Bjarnsholt T, Shirtliff M (2014) Methods for dynamic investigations of surface-attached in vitro bacterial and fungal biofilms. Methods Mol Biol 1147:3–22. doi:10.1007/978-1-4939-0467-9_1
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi:10.1146/annurev.micro.56.012302.160705
Storey MV, Ashbolt NJ, Stenström TA (2004) Biofilms, thermophilic amoebae and Legionella pneumophila, a quantitative risk assessment for distributed water. Water Sci Technol 50:77–82
Suci PA, Mittelman MW, Yu FP, Geesey GG (1994) Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 38(9):2125–2133. doi:10.1128/AAC.38.9.2125
Swift S, Downie JA, Whitehead NA, Barnard AM, Salmond GP, Williams P (2001) Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv Microb Physiol 45:199–270
Teh KH, Flint S, French N (2010) Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int J Food Microbiol 143(3):118–124
Teh AH, Lee SM, Dykes GA (2014) Does Campylobacter jejuni Form Biofilms in Food-Related Environments? Appl Environ Microbiol 80(17):5154–5160. doi:10.1128/AEM.01493-14
Teixeira P, Lopes Z, Azeredo J, Oliveira R, Vieira MJ (2005) Physico-chemical surface characterization of a bacterial population isolated from a milking machine. Food Microbiol 22(2–3):247–251
Tomlinson AD, Ramey-Hartung B, Day TW, Merritt PM, Fuqua C (2010) Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiol 156(9):2670–2681. doi:10.1099/mic.0.039032-0
Ullrich M (ed) (2009) Bacterial polysaccharides: current innovations and future trends. Caister Academic Press, Norfolk
Valderrama WB, Cutter CN (2013) An ecological perspective of Listeria monocytogenes biofilms in food processing facilities. Crit Rev Food Sci Nutr 53(8):801–817. doi:10.1080/10408398.2011.561378
van Oss CJ (1995) Hydrophobicity of biosurfaces: origin, quantitative determination and interaction energies. Colloids Surf B 5(3&4):91–110
van Oss CJ (1997) Hydrophobicity and hydrophilicity of biosurfaces. Curr Opin Colloid Interface Sci 2(5):503–512
Wang H, Ding S, Dong Y, Ye K, Xu X, Zhou G (2013) Biofilm formation of Salmonella serotypes in simulated meat processing environments and its relationship to cell characteristics. J Food Prot 76(10):1784–1789. doi:10.4315/0362-028X
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182(10):2675–2679. doi:10.1128/JB.182.10.2675-2679.2000
Welman AD (2009) Exploitation of Exopolysaccharides from lactic acid bacteria. Bacterial polysaccharides: current innovations and future trends. Caister Academic Press
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295(5559):1487. doi:10.1126/science.295.5559.1487
Wojtyczka RD, Orlewska K, Kępa M, Idzik D, Dziedzic A, Mularz T, Krawczyk M, Miklasińska M, Wąsik TJ (2014) Biofilm formation and antimicrobial susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int J Environ Res Public Health 11(5):4619–4633. doi:10.3390/ijerph110504619
Zhou D, Yang R (2011) Formation and regulation of Yersinia biofilms. Protein Cell 2(3):173–179. doi:10.1007/s13238-011-1024-3
Zottola EA, Sasahara KC (1994) Microbial biofilms in the food-processing industry – Should they be a concern. Int J of Food Microbiol 23(2):125–148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Laganà, P., Caruso, G., Mazzù, F., Caruso, G., Parisi, S., Santi Delia, A. (2015). Brief Notes About Biofilms. In: Microbial Toxins and Related Contamination in the Food Industry. SpringerBriefs in Molecular Science(). Springer, Cham. https://doi.org/10.1007/978-3-319-20559-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-20559-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20558-8
Online ISBN: 978-3-319-20559-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)