Abstract
Daily periodic plant leaf movements, known since antiquity, are dramatic manifestations of “osmotic motors” regulated by the endogenous biological clock and by light, perceived by phytochrome and, possibly, by phototropins . Both the reversible movements and their regulation usually occur in specialized motor leaf organs, pulvini. The movements result from opposing volume changes in two oppositely positioned parts of the pulvinus . Water fluxes into the motor cells in the swelling part and out of the motor cells in the concomitantly shrinking part are powered by ion fluxes into and out of these cells, and all of these fluxes occur through tightly regulated membranal proteins: pumps, carriers, and ion and water channels. This chapter attempts to piece together those findings and insights about this mechanism which have accumulated during the past two and a half decades.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Historical Perspective
Almost every text on chronobiology tells us that the ancients were already aware of the rhythmic movements of plants and even relied on them in scheduling their prayers. The first documented experiment, attempting to resolve if this rhythm originated inside the plant and not in the light from the Sun, was that of the French astronomer, De Mairan. His sensitive plant (probably Mimosa pudica) continued moving its leaves even when kept in darkness (De Mairan 1729). Since De Mairan’s days, and for over 2 centuries, leaf movements served as the sole indicators of the internal workings, and increasingly intricate designs were invested in the movement-monitoring devices. During the eighteenth and the nineteenth centuries, experiments with the “sleep movements” of leaves (a name coined by Linnaeus) led to the gradual emergence of the concept of the osmotic motor on the one hand (Pfeffer 1877) and the concept of an internal oscillator—an endogenous biological clock—for which the leaf movements serve as the “clock hands” . In the twentieth century, biological clock began to be studied also in animals. Beatrice Sweeney presented a detailed and vivid account of this thought evolution (Sweeney 1987).
Among the best studied leaf rhythmic movements are those of the pulvini of the compound leaves of the legumes Albizzia, Mimosa, Samanea, Robinia and Phaseolus. While observing the “hands of the clock”, investigators probed the internal mechanism, in an attempt to map the susceptibility of the oscillator and thus to deduce its chemical nature, as well as to map out the signalling paths. They altered the illumination regimes, varied the light intensity and quality and applied various pharmacological agents to the pulvinus (e.g. see the reviews by Satter and Galston 1981; Mayer et al. 1997; Gomez et al. 1999). During the past few decades, an increasing arsenal of technological developments enabled more sophisticated measurements and monitoring of variables other than just the leaf displacement. The forces involved in the movement have been measured (Gorton 1990; Irving et al. 1997; Koller 2001), immunohistochemistry has been applied (e.g. in the cellular immunogold localization of phytochrome, the photoreceptor affecting leaf movement; Moysset et al. 2001), the related distribution of various ions and other elements was studied using ion-selective microelectrodes (e.g. Lee and Satter 1989; Lowen and Satter 1989) and X-ray microanalysis (e.g. Satter et al. 1982; Fromm and Eschrich 1988c; Moysset et al. 1991), and patch clamp and molecular biology analyses of pulvinar channels have begun (Moran et al. 1988; Stoeckel and Takeda 1993; Jaensch and Findlay 1998; Moshelion et al. 2002a, b).
Initial answers to the intriguing questions about how the movement is executed and how the endogenous rhythm—and external signals, mainly light—affects the pulvinar “motor”, have been collected in a small but a thorough compendium on the pulvinus (Satter et al. 1990). During the 25 years that followed, these questions have been addressed with an increasing resolution, sometimes “borrowing” from the molecular insights developed in the much more numerous and extensive studies of stomatal guard cells (as in Fan et al. 2004). These later findings and insights into the leaf movements, revised since the former edition (Moran 2007b), are the main focus of this chapter.
1.2 The Types of Leaf Movements
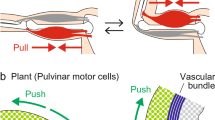
Leaf movements can be repetitious and rhythmic (Fig. 4.1a), or provoked (Fig. 4.1b). Stimulated movements can be classified according to their directionality: tropic movements are related to the direction of the stimulus that caused them, while nastic movements are unrelated. Thus, leaf unfolding in response to the turning on of diffuse light is photonastic and leaf folding with the onset of darkness—scotonastic; the turning of leaves towards directed light is termed phototropic and towards the sun—heliotropic (Fig. 4.1c). Movement in response to touch–like the clasping of the Venus fly trap (Dionaea muscipula) leaf lobes when irritated by an insect, or the curling of a gently stroked pea tendril—is termed thigmonastic; the folding down of the M. pudica leaf upon shaking the plant is seismonastic and upon exposure to the heat of a flame—thermonastic; the turning of leaves upwardly after the shoot is placed horizontally is negatively gravitropic. Frequently, leaves perform more than one type of movements, and different parts of a leaf can perform different types of movements. For example, Mimosa primary pulvinus exhibits also nyctinasty, seismonasty and thigmonasty, while the secondary pulvinus does not respond to seismonastic stimuli (Fig. 4.1b, Fromm and Eschrich 1988a). Samanea leaf movements are largely nyctinastic and insensitive to touch and shaking.
Types of leaf movements. a Nyctinastic movement of the terminal pinnae of the compound leaf of Samanea saman (Jacq.) Merrill, between open during the DAY and folded during the NIGHT. Inset: a schematic drawing of a pulvinus : E extensor, F flexor, vb vascular bundle, P II–P III secondary and tertiary pulvini, ra rachilla, rs rachis (with permission, Moshelion et al. 2002b). b Seismonastic and nyctinastic leaf movement of Mimosa pudica L., p pinnae. P I primary pulvinus; other abbreviations as in A (with permission, Fromm and Eschrich 1988a). c Primary (laminar) leaves of Phaseolus vulgaris L., showing paraheliotropism in the field (with permission, Berg 1986). Note the movement of the leaf blades (arrows) adjusting the angle of the incident light (dashed arrows) at the indicated hours. A “purely” nyctinastic movement in the laboratory would occur between a horizontal- and a vertical-down position of both leaves (not shown)
Rhythmic leaf movements can be related to growth and be non-reversible, like those of cotyledons of the Arabidopsis seedlings or the leaves of the growing tobacco plants. The epinastic leaf movement of tobacco, for example, is based on alternating spurts of growth of the upper and lower leaf surface, and this uneven growth reveals a control by light and the circadian clock (Siefritz et al. 2004). Other examples can be found in a review by Wetherell (1990). While the tissue expansion likely occurs via a mechanism similar to that of pulvinar tissues (see below), the irreversibility of these growth processes is thought to be related to the interstitial deposits in cell wall material and decrease in wall extensibility (Wetherell 1990, and references therein).
Rhythmic nyctinastic leaf movements occur in mature, non-growing leaves and are completely reversible, like those found in many of the legumes (Samanea saman, Accacia lophanta, Albizzia julibrissin, Phaseolus vulgaris, Desmodium gyrans and the previously mentioned M. pudica) and also in some plants of a few other families, such as wood sorrels (Oxalidaceae) and mallows (Malvaceae).
It is of interest to mention in this context the diurnal movements of flower petals, which underlie the repetitive opening and closing of some flowers and which may persist for several days. This petal movement also involves an “osmotic motor”, like in leaves, either in differentially elongating cells in growing petals, or in non-growing petals, in cells which change their size reversibly, by loss of water during the day and refilling during the night (reviewed in great detail by van Doorn and van Meeteren 2003; van Doorn and Kamdee 2014).
The reversible leaf movements originate in the pulvinus (Fig. 4.1a), a mature, specialized motor organ at the leaf base. The identity of the pulvinus as a motor organ is genetically determined, as discovered through pulvini-devoid, non-moving mutants of three orthologs of the same gene: elongated petiolule1 (elp1) of Medicago truncatula, apulvinic (apu) of Pisum sativum and sleepless (slp) of Lotus japonicus (Chen et al. 2012; Cortizo and Laufs 2012; Zhou et al. 2012).
The daily persistence of the leaf movements is a manifestation of regulation by light and the circadian clock. In the dark or under constant low-level illumination, the circadian rhythm displays its “free-running”, genetically dictated periodicity which can range from roughly 20 to 29 h. Period length and its manifestation depend also on other factors. For example, in Phaseolus coccineus, the circadian laminar leaf movement started 9 days after sowing in soil. The period length decreased progressively with pulvinus maturation (from 31.3 to 28.6 h under constant illumination), and these periods became shorter by more than one hour when the leaves were cut-off and watered via petioles (Mayer et al. 1999).
Normally, however, daily light resets the phase of the rhythm and adjusts it to a 24-h period. Rhythmic movements can comprise additionally one or more ultradian rhythms (with a period significantly shorter—between tens of minutes to several hours Millet et al. 1988; Engelmann and Antkowiak 1998).
Light has a profound effect on the rhythmic leaf movement, and it is also easily quantifiable. Therefore, it is a most widely used stimulus to perturb (and “entrain”) the leaf movement rhythms , to change their phase and to alter their period. Changing these two rhythm properties is a criterion for having affected the internal “oscillator”. Red, far-red and blue light (BL) have different effects on the rhythm (reviewed by Satter and Galston 1981; Sweeney 1987; Moran 2007a, b). Curiously, petals of Calendula arvensis flowers with diurnal rhythm of opening and closure could be entrained by light during the bud stage, but the rhythm phase became fixed when they matured (van Doorn and van Meeteren 2003, and references therein).
Acute versus circadian. It is important to note that the same light stimuli evoke also short-lived, or acute, responses lasting for only one to a few periods following the stimulus. These transient responses are superimposed on (“masking”) the responses attributable to changes in the clock (shifting the phase and changing the period length), which persist during many cycles. In the very schematic general portrayal of the system (Fig. 4.2), the clock-resetting stimulus acts along an input pathway to the clock, altering the way the clock directs the osmotic motor of the leaf movement, while the acute stimulus bypasses the clock and acts directly on the osmotic motor. Employing “acute” stimuli in the study of the clock’s role in regulating leaf movement is justified by the underlying assumptions: (a) that the mechanism of the execution of the movements, i.e. of the volume and turgor changes, is identical for both types of movements, the stimulated and the rhythmic, and (b) that the photoreceptors in both pathways are identical (which, in plants, has not yet been disproved). Thus, both pathways are assumed to differ wholly, or partially, “only” in the transduction cascades, i.e. in the chemical reactions between light perception and the regulation of the transporters.
Light stimulates cell volume changes. A model of clock-mediated (circadian) and clock-independent (acute) pathways. a Light, perceived by one or more light receptor(s), R, affects the clock. b The clock governs volume changes, imparting fluctuations (~) in activity or abundance to the pathway intermediates. c Light affects directly the volume changes (a bidirectional arrow)
2 The Mechanism of Leaf Movement: The Osmotic Motor
2.1 Volume Changes
2.1.1 The Mechanics of Movement
Since the movement of a leaf or leaflet results from the changes in the shape of its subtending pulvinus , volume changes must occur anisotropically in the pulvinar tissues. Indeed, the pulvinar motor consists of two distinct positionally and functionally opposed regions: an “extensor” —which extends longitudinally during leaf opening, and “flexor” , which appears contracting (“flexing”) longitudinally at the same time. During leaf closure, the opposite changes occur. Radial inflexibility of the epidermis constrains these changes to the longitudinal axis, but the flexibility of the vascular core, along with its inextendability, causes the curvature of the pulvinus without affecting its length (Koller and Zamski 2002). It appears that extensors and flexors differ also in the extent of the movement-driving pressures they generate. For example, in P. vulgaris laminar pulvinus, the excision of flexor did not seem to alter any of the properties of the circadian leaf oscillation: period, phase and amplitude, whereas when the major part of the extensor was cut away, the amplitude was greatly reduced (although the period and the phase of the leaf movements were unchanged, Millet et al. 1989).
2.1.2 Volume Changes of Isolated Protoplasts
The turgor changes in the pulvinar motor tissues reflect the turgor changes of the individual motor cells, and these, in turn, reflect the elastic properties of the cell walls, together with the volume changes. Confounding effects of the cell wall may be avoided if experiments are performed on protoplasts. Indeed, protoplasts appear to be an appropriate physiological system for studying the circadian rhythm of volume changes. Flexor protoplasts isolated from the bean (P. coccineus) laminar pulvini swelled and shrank under continuous light for over 200 h with a 28 h period , resembling the period of the original pulvinar cells in situ in similar conditions (Mayer and Fischer 1994). Extensor protoplasts seemed to exhibit the same rhythm, but, curiously, they cycled with the same phase as the flexors, at least during the first 70 h, as if their internal clock shifted by 180o relative to their original in situ rhythm. Notwithstanding, the extensors could be entrained to a 24 h rhythm by cycles of 14 h light/10 h dark, this time, shrinking “appropriately” in darkness (Mayer and Fischer 1994).
Protoplasts isolated from Samanea flexors also swelled and shrank rhythmically, in continuous dim light, in phase with the in situ intact cells, and both flexor and extensor Samanea protoplasts reacted to white light (WL) illumination during the dark period as did the in situ intact cells in the pulvinus (extensors swelled and flexors shrank, Moran et al. 1996).
Thus, the isolated pulvinar protoplasts “remember” their origin and retain the physiological properties of their source tissues. Moreover, the motor cells of the pulvinus are themselves the site of the rhythm generator, containing both, the “oscillator” and the “motor”, as evident from the rhythmic volume changes of the isolated pulvinar protoplasts. No less importantly, they also contain the light signal receptors, for both circadian entraining and acute signalling (see also Sect. 4.3.2.1 below).
2.2 The Ionic Basis for the Osmotic Motor
2.2.1 The Current Model
The currently accepted model for the volume changes of pulvinar cells does not differ in principle from that accepted for the stomata guard cells, with the exception that in contrast to guard cells , in the intact pulvinus solute and water fluxes may occur to some extent also via plasmodesmata interconnecting the pulvinar motor cells (Morse and Satter 1979; Satter et al. 1982; noting that plasmodesmata permeability, even to ions, may be regulated, Wigoda et al. 2014).
In the swelling phase, an activated proton pump (a P-type H+-ATPase) hyperpolarizes the cell, which creates the electrochemical gradient for the influx of K+ via K channels (Kim et al. 1992, 1993; Suh et al. 2000) and the proton-motive force for the uphill uptake of Cl−, possibly via a proton–anion symporter (Satter et al. 1987). The hyperpolarization also opens the gates of K+-influx channels. Eventually, K+ and Cl− accumulate in the cell vacuole. In the absence of external Cl−, malate content of the swelling tissues increases (Mayer et al. 1987; Satter et al. 1987). Water, driven by the changing water potential difference across the cell membrane , increases the cell volume and turgor, entering the cells via the membrane matrix and via aquaporins .
In the shrinking phase, the proton pump halts and the motor cell depolarizes. Depolarization may be aided by passive influx of Ca2+ via Ca channels and efflux of Cl− via anion channels. K+-influx channels close, while K+-release channels open. The electrochemical gradient now drives also K+ efflux. Loss of solutes (KCl) drives water efflux via the membrane matrix and aquaporins . The volume and turgor of the motor cells decrease.
Unlike in leaf pulvini, during rhythmic flower opening, rather than ions, the osmoticum driving water movements into the flower petals consists mainly of sugars mobilized from stored polysaccharides (starch and/or fructan) and/or imported sucrose. In some cases, the petal movements occur without proton pump involvement (van Doorn and van Meeteren 2003, and references therein), which may be explained by the internal source of the produced osmoticum.
2.2.2 Membrane Potential
Changes in membrane potential provided early clues about the ionic basis of leaf movement. Racusen and Satter measured the membrane potential in Samanea flexors and extensors in whole, continuously darkened secondary terminal pulvini impaled with microelectrodes and found it to oscillate with about 24-h rhythm between −85 and −40 mV (extensor) and between −100 and −35 mV (flexors) , with the extensors “sinusoid” preceding that of flexors by about 8 h (Racusen and Satter 1975). Membrane potential varied also in response to light signals which caused leaf movement (see Sect. 4.3.2.1 below, and Racusen and Satter 1975, and also Sect. 4.3.2.2). Later measurements of membrane potential, using a membrane-soluble fluorescent dye (3,3′-dipropylthiadicarbocyanine iodide, DiS-C3(5)), provided additional details about the translocation of ions (Kim et al. 1992, and see Sect. 4.2.3.5 below). Membrane potential was also used to learn about the early effects of the hormone salicylic acid (SA) (Saeedi et al. 2013, and Sect. 4.3.4.1 below).
2.2.3 Mechanisms Underlying Volume Changes
Ions involved in leaf movements. Results of X-ray microanalysis in pulvini suggested that the solute concentration changes are primarily those of potassium and chloride , consistent with the occurrence of their massive fluxes across the plasma membrane into the swelling cells and out of the shrinking cells (Satter and Galston 1974; Kiyosawa 1979; Satter et al. 1982; Gorton and Satter 1984; Moysset et al. 1991). At the same time, measurements with ion-sensitive electrodes allowed dynamic, real-time observations of changes in the apoplastic activity of protons (Lee and Satter 1989) and potassium ions (Lowen and Satter 1989; Starrach and Meyer 1989). Generally, proton and K+ activities varied in opposite directions (see also Starrach and Meyer 1989, and references therein; Lee 1990).
Non - ionic regulation. Osmotically driven shrinking based on the efflux of ions normally suffices to explain volume changes on the scale of minutes. The puzzling rate of the seismonastic response of M. pudica (leaflet folding on the scale of seconds) invited additional investigations. Thus, seismonastic stimulation of the leaf caused sudden unloading of 14C-labelled sucrose from the phloem into the pulvinar apoplast in the primary pulvinus , lowering the water potential beneath that of the extensors and probably enhancing their shrinkage, leading to leaf closure within a few seconds. This was accompanied by a brief membrane depolarization of the sieve element, recorded via an aphid stylet serving as an intracellular microelectrode (Fromm and Eschrich 1988b). During reswelling, the extensors accumulated the labelled material (Fromm and Eschrich 1988a).
Could cytoskeletal elements—actin filaments, microtubuli—perform actively the fast shrinking (as suggested already by Toriyama and Jaffe 1972)? While both types of proteins were localized to the Mimosa primary pulvinus (using antibodies against muscular actin and a protozoan tubulin, Fleurat Lessard et al. 1993), a combination of pharmacological and immunocytochemical approaches implicated only actin in the seismonastic responses, indicating, additionally, the involvement of its phosphorylation by a tyrosine kinase (distinct from a serine/threonine kinase, Kanzawa et al. 2006). Interestingly, depolymerized actin in guard cells was required for stomatal opening and for the activity of K+-influx channels, and this was independent of the activity of the H+-ATPase (Hwang et al. 1997; Eun and Lee 2000), suggesting that actin may perhaps be involved not only in the “dramatic” movements of the pulvinus, but also in the regulation of its “mundane”, nastic movement.
2.3 Plasma Membrane Transporters
What transporters are involved in the ion fluxes across the pulvinar cell membrane? Although it is obvious that the fluxes of K+, Cl− and water occur between the vacuole and the apoplast, i.e. across two membranes, there is little information about the tonoplast transporters of the pulvinar motor cells. Somewhat more detailed are the observations about the function in situ of a few plasma membrane transporters in the pulvini. Their partial characterization is described below.
2.3.1 H+-Pump Activity
The activity of the proton pump in the plasma membrane in the Samanea pulvini was assayed indirectly via changes in the light-stimulated acidification of the medium bathing extensor and flexor tissues (Iglesias and Satter 1983; Lee and Satter 1989). BL acidified the extensor apoplast, consistent with pump activation, and alkalinized the flexor apoplast, consistent with cessation of pump activity (Lee and Satter 1989). In accord with this, in patch-clamp experiments with intact Samanea flexor protoplasts, BL depolarized the flexor cells, probably by halting the action of the H+ pump (Suh et al. 2000). Red light or dark, following BL, activated the H+ pump in flexors (acidifying the flexor apoplast) and inactivated the pump in extensors (alkalinizing the extensor apoplast, Lee and Satter 1989).
The motor cells of the Phaseolus laminar pulvinus (both extensors and flexors) reacted to BL like the Samanea flexors: shrinking (Koller et al. 1996), depolarizing (Nishizaki 1990, 1994) and alkalinizing their external milieu (as a suspension of protoplasts Okazaki 2002). Vanadate, which blocks P-type proton ATPases, inhibited the BL-induced depolarization (Nishizaki 1994). Additionally, the inhibitory effect of BL was demonstrated directly on the vanadate-sensitive H+-ATPase activity of membranes from disrupted Phaseolus pulvinar protoplasts (Okazaki 2002).
Extensor protoplasts isolated from the P. coccineus pulvinus reacted to WL and dark (D) similarly to extensors of Samanea: they swelled in WL and shrank in D (Mayer et al. 1997). This too may be taken as an indirect evidence of the activation/deactivation of the proton pump , respectively, by WL and D.
2.3.2 H+/Cl− Symporter
The presence of an H+/anion symporter has been suggested based on the experiments in which the net H+ efflux from excised Samanea flexor tissue pieces, bathed in a weakly buffered medium, was greater with the impermeant iminodiacetate anions than with the permeant Cl− in the external solution (Satter et al. 1987).
2.3.3 K+-Release Channels
These channels are presumed to mediate K+ efflux from pulvinar motor cell during their shrinking. Patch-clamp studies revealed depolarization-dependent, K+-release (depolarization-dependent, KD) channels in the plasma membrane of pulvinar cell protoplasts (Moran et al. 1988; Stoeckel and Takeda 1993; Jaensch and Findlay 1998).
Ion selectivity. The selectivity for K+ of the Samanea KD channel was somewhat higher than that for Rb+ and much higher than that for Na+ and Li+, and the channel was blocked by Cs+, Ba2+, Cd2+ and Gd3+ (Moran et al. 1990) and also by TEA (Moran et al. 1988). KD channels in extensors were slightly less K+ selective than in flexors (Moshelion and Moran 2000). Extensors and flexors differed also in the details of the cytosolic Ca2+ sensitivity of the KD channel gating, but the overall effect of cytosolic Ca2+ on these channels was rather minor (Moshelion and Moran 2000). In contrast, the Mimosa KD channel currents, although generally similar in their voltage dependence and similarly blockable by external Ba2+ and TEA (Stoeckel and Takeda 1993), were severely attenuated (they “ran down”) by treatments interpreted as increasing cytosolic Ca2+ (cytosolic Ca2+ concentration was not measured in these experiments, Stoeckel and Takeda 1995). Also, in difference to Samanea KD channels, they were not blocked by external La3+ and Gd3+ at a concentration comparable to the blocking Gd3+ concentration in Samanea. In fact, both lanthanide ions prevented the “rundown” of the Mimosa KD channels.
Regulation by light. Using patch clamp, Suh et al. (2000) demonstrated an increase in the activity of KD channels in cell-attached membrane patches of intact Samanea flexor protoplasts within a few min illumination with BL and a decrease in their activity within a few minutes of darkness, preceded by a brief red-light pulse (Fig. 4.3, Suh et al. 2000). No circadian control, however, was evident in the responsiveness of the flexor KD channels to BL. The authors resolved the blue-light effect into two: (a) membrane depolarization-dependent KD channel activation (a consequence of a blue-light-induced arrest of the proton pump ) and (b) a voltage-independent increase of KD channel availability.
Blue light enhances the activity of the KD Samanea channels in flexor protoplasts. a Light-induced shift of the membrane potential, manifested as shifts of the reversal potential, V rev of KD-channel currents in single cell-attached membrane patches during alternating between blue light (BL) and dark (DK). A negative shift of V rev indicates membrane depolarization (mean ± SE). The asterisks indicate the significance level of difference from zero; *P, 0.05; **P, 0.01; ***P, 0.005. n is the number of membrane patches. b BL-induced, membrane potential-independent changes of KD-channel activity, manifested as changes in G@40, the mean patch conductance at a 40 mV depolarization relative to the V rev of the patch (mean ± SE). The asterisks and n, as in (a) (with permission, Suh et al. 2000)
Molecular identity. Among the four putative K channel genes cloned from the Samanea saman pulvinar cDNA library, which possess the universal K-channel-specific pore signature, TXXTT/VGYG, the Samanea predicted protein sequence of SPORK1 is similar to SKOR and GORK, the only Arabidopsis outward-rectifying Shaker-like K channels. SPORK1 was expressed in all parts of the pulvinus and in the leaf blades (mainly mesophyll; Fig. 4.1), as demonstrated in Northern blots of total mRNA. SPORK1 expression was regulated diurnally and also in a circadian manner in extensor and flexor , but not in the vascular bundle (rachis) or in the leaflet blades (Moshelion et al. 2002b). While the functional expression of SPORK1 has yet to be achieved, these findings strongly indicate that SPORK1 is the molecular entity underlying the pulvinar KD channels.
2.3.4 K+-Influx Channels
Using patch clamp in the whole-cell configuration, Yu et al. described hyperpolarization-gated K+-influx (KH) channels in the plasma membrane of Samanea extensor and flexor protoplasts (Yu et al. 2001). Paradoxically, these channels were blocked by external protons, contrary to what would be expected of channels presumed to mediate K+ influx during cell swelling which is concurrent with external acidification. This was particularly surprising in view of the external acidification-promoted K+-influx channels in guard cells (Blatt 1992; Ilan et al. 1996). Yu et al. (2001) were able to resolve this paradox by quantitative comparisons of the actual versus the required K+ influx, in particular when they “recruited” into their calculations also the relatively large voltage-independent and acidification-insensitive, leak-like currents recorded along with currents activated by hyperpolarization (Yu et al. 2001). No diurnal variation in the activity of the K+-influx channel was noted in the patch-clamp experiments.
K+-selective channels were reportedly observed during membrane hyperpolarization also in extensor protoplasts from pulvini of Phaseolus (Jaensch and Findlay 1998). However, hyperpolarizing pulses failed to activate such channels in protoplasts from the primary pulvini of Mimosa (Stoeckel and Takeda 1993).
Regulation by light. Kim et al. (1992) monitored membrane potential in isolated Samanea extensor and flexor protoplasts using the fluorescent dye DiS-C3(5) and pulses of elevated external K+ concentration to detect specifically states of high potassium permeability of the cell membrane (manifested as depolarization). They interpreted this high permeability as a high level of activity of K+-influx channels (KH channels). They were thus able to demonstrate an almost full (21-h-long) cycle of K+-influx channel activity (in continuous darkness), which was out of phase in extensors and flexors , paralleling the periods of expected swelling in these protoplasts: the activity of the channels was high in extensors anticipating a “lights-on” signal during early morning hours and in flexors anticipating a “lights-off” signal in the evening (Kim et al. 1993). In addition, these authors demonstrated circadian-enabled (gated) responsiveness of extensors and flexors to light stimuli: during the 2nd half of the night of a normal day cycle, BL opened K+-influx channels in extensors and closed them in flexors, and red light had no effect at all at this time. Then, during the last third of the day (of a normal day cycle), BL opened these channels in extensors, but had no effect on flexors, and darkness closed these channels in extensors (without red light) and opened them in flexors (when preceded by red light; ibid.).
Molecular identity. Two of the Shaker K-channel-like genes cloned from the Samanea cDNA pulvinar library were SPICK1 and SPICK2, and their predicted protein sequences were homologous to AKT2, a weakly inward-rectifying Shaker-like Arabidopsis K channel. KAT1 and KAT2, genes of the chief K+-influx channels of the Arabidopsis guard cells, were not detected in the pulvinar cDNA library in several repeated trials. Based on Northern blot analysis, the SPICK1 and SPICK2 transcript level was regulated diurnally (SPICK2 in extensor and flexor, SPICK1 in extensor and rachis) and their expression in the extensor and flexor was also under a circadian control (Moshelion et al. 2002b). Because circadian rhythm governs also the resting membrane K+ permeability in extensor and flexor protoplasts and the susceptibility of this permeability to light stimulation (Kim et al. 1993), SPICK1 and SPICK2 are very likely the molecular entities underlying the activity of the in situ KH channels. Samanea pulvinar motor cells are thus the first described system combining light and circadian regulation of K channels at the level of transcript and membrane transport .
2.3.5 Ca2+ Channels
A KD channel rundown (gradual loss of activity) by increased hyperpolarization was used as an indicator—as an indirect evidence—for the influx of Ca2+ and thus for the existence and function of hyperpolarization-activated Ca channels in the plasma membrane of protoplasts from pulvini of Mimosa (Stoeckel and Takeda 1995, but see a comment in Sect. 4.2.3.3 above).
2.3.6 Anion Channels
There is practically no information about anion channels in the pulvinar plasma membrane. Pharmacological evidence that Cl channels mediate ABA -induced shrinking of protoplasts isolated from a laminar pulvinus of P. vulgaris (Iino et al. 2001) are not conclusive, as NPPB (an inhibitor used in the above study) has been shown also to inhibit plant K+-release channels with an even higher affinity (Garrill et al. 1996).
2.3.7 Mechanical Stretch-Activated Channels
Stretch-activated channels (SACs) were detected by patch clamp in cell membranes in virtually all cell types assayed, including prokaryotes (see, for example, references mentioned in the review by Kung 2005). In Samanea flexors and extensors, these channels were observed quite frequently upon application of pressure to the patch pipette, during and after the formation of a giga-seal between the patch-pipette and the protoplast membrane. Channels of undefined selectivity (cation non-selective or anion selective, but not specifically K+ selective) were activated reversibly in outside-out patches by outwardly directed (i.e. membrane-extending) pressure pulses under 30 mm Hg. These stimuli were well within the physiological range of estimated turgor values occurring in the Samaea pulvini (Moran et al. 1996). The possible physiological role of these channels might be in volume regulation of motor cells, thus constituting a part of the rhythm-regulating process.
2.3.8 Water Channels (Aquaporins)
Water permeability (Pf) of the plasma membrane was determined in motor cell protoplasts of Samanea by monitoring their swelling upon exposure to a hypotonic solution. The Pf of the protoplasts was regulated diurnally, being the highest in the morning (extensor and flexor) and in the evening (extensor), corresponding to the periods of most pronounced volume changes, i.e. the periods of highest water fluxes. Pf increases were inhibited down to the lowest, noon level, by 50 μM HgCl2 and by 250 μM phloretin, both non-specific transport inhibitors, shown to inhibit aquaporins in some systems (e.g. Dordas et al. 2000), and by 2 mM cycloheximide, an inhibitor of protein synthesis. The susceptibility of Pf to fast modification by pharmacological agents has been interpreted as evidence for the function of plasma membrane aquaporins (Moshelion et al. 2002a).
Molecular identity. Two plasma membrane intrinsic protein homolog genes, SsAQP1 and SsAQP2, representing two separate subfamilies of aquaporins, PIP1 and PIP2, were cloned from the Samanea pulvinar cDNA library and characterized as aquaporins in Xenopus laevis oocytes. Pf was 10 times higher in SsAQP2-expressing oocytes than in SsAQP1-expressing oocytes, and SsAQP1 was found to be glycerol permeable. In the oocytes, SsAQP2 was inhibited by 0.5 mM HgCl2 and by 1 mM phloretin. In the leaf, the aquaporin mRNA levels differed in their spatial distribution, with the most prominent expression of SsAQP2 found in pulvini. The transcript levels of both aquaporins were regulated diurnally in phase with leaflet movements. Additionally, SsAQP2 transcription was under circadian control. These results linked SsAQP2 to the physiological function of rhythmic cell volume changes (Moshelion et al. 2002a).
Two plasma membrane aquaporins PIP1;1 and PIP2;1, representing PIP1 and PIP2, like in Samanea, were isolated from a Mimosa pudica (Mp) cDNA library and characterized in heterologous expression systems, the frog oocytes and mammalian Cos cells. MpPIP1;1 alone exhibited no water channel activity, but it facilitated the water channel activity of MpPIP2;1 and immunoprecipitation analysis revealed that MpPIP1;1 binds directly to MpPIP2;1 (Temmei et al. 2005). However, the relation of the Mimosa MpPIP1 and MpPIP2 to the rhythmic movement of the pulvinus (localization and function in the pulvinus) has yet to be demonstrated.
2.4 Tonoplast Transporters
The solutes and water traversing the plasma membrane cross also the tonoplast. Vacuoles appear to fragment and coalesce during leaf movements (Setty and Jaffe 1972; Campbell and Garber 1980). However, very little is known about vacuolar transporters in pulvini.
2.4.1 H+-ATPase
The only evidence so far for a proton transporter across a pulvinar tonoplast comes from immunolocalization studies in the primary pulvinus of Mimosa (Fleurat-Lessard et al. 1997). A catalytic α-subunit of an H+-ATPase was detected abundantly and almost exclusively in the tonoplast of the aqueous (colloidal) vacuoles. The maturation of the pulvinus and the acquisition of the very rapid responsiveness to external stimuli was accompanied by a more than threefold increase in the abundance of the H+-ATPase per length unit of membrane (Fleurat-Lessard et al. 1997).
2.4.2 Ion Channels
SPOCK1, a homologue of the Arabidopsis KCO1 (two-pore-in-tandem K-signature channel) cloned from the Samanea cDNA pulvinar library (Moshelion et al. 2002b), likely represents, like the Arabidopsis TPK1 (formerly KCO1, Czempinski et al. 2002), a K+ selective voltage-independent VK vacuolar channel (Bihler et al. 2005). SPOCK1 mRNA level in the Samanea pulvini fluctuated under diurnal control (with the highest level in the morning), but not in constant darkness, and only in extensor and flexor (and not in the rachis or the leaflet blades, Moshelion et al. 2002b). While the vacuole reservoir of ions most likely participates in the pulvinar cell volume changes, SPOCK1 is a likely K+-release pathway across the tonoplast during the diurnal leaf movements of Samanea.
2.4.3 Aquaporins
γ-tonoplast intrinsic protein (TIP) was detected in the membrane of aqueous (colloidal) vacuoles of Mimosa primary pulvinus using immunocytochemical approaches. Development of the pulvinus into a motor organ was accompanied by a more than threefold increase in the abundance of the aquaporin (per length unit of membrane measured in electron microscopy micrographs), paralleling the development of the ability to respond rapidly to an external stimulus (Fleurat-Lessard et al. 1997). A single TIP aquaporins gene, TIP1;1, was cloned from Mimosa cDNA library, and its product, expressed in a frog oocytes, conducted water (Temmei et al. 2005). Its identity with the γ-TIP of the pulvinus and its involvement in the pulvinar function have yet to be determined.
3 Mechanisms of Regulation
Membrane transporters are the end point in the signalling cascades regulating pulvinus movement. This regulation is rather complex (Fig. 4.4) and includes a large number of factors, such as light, circadian clock, hormones, and temperature. Such regulation occurs at both transcriptional and post-translational levels (Fig. 4.4).
Regulation of membrane transporters in the pulvinus at the levels of transcription, translation and protein modification (a schematic model). bn are clock output signalling pathways, and cn are signalling pathways from the light-activated receptor, R. T is the transporter protein in the membrane. The processes affected are indicated. The other signs are as in Fig. 4.2. Feedback loops are not indicated
3.1 Regulation by Protein Modification—Phosphorylation
As yet, evidence for rhythmic phosphorylation of pulvinar proteins in situ is lacking. The accumulating information pertains to in vitro assays, or, at best, to acute stimuli. Notwithstanding, this may be also one of the ways the clock affects transporters, for example gating their responsiveness to acute stimuli (see 4.2.3.4 above).
3.1.1 Phosphorylation of the Proton Pump
The recently discovered immunologically undistinguishable three iso-phototropins of the P. vulgaris pulvinus (see 4.3.2.2 below, and Inoue et al. 2005) were identified as the first element in the phototransduction cascade in a shrinking pulvinar motor cell (Fig. 4.5). In the dark, they existed in a dephosphorylated state and the plasma membrane H+-ATPase existed in a phosphorylated state. A 30 s pulse of BL induced the phosphorylation of the phototropins and the dephosphorylation of the H+-ATPase. Three results indicated that these phototropins may function upstream of the H+-ATPase and decrease the activity of H+-ATPase by dephosphorylation: the phototropin phosphorylation peaked the earliest (Fig. 4.5a); the phosphorylation and dephosphorylation exhibited similar fluence rate dependencies on BL (Fig. 4.5b); and inhibitors of the phototropin phosphorylation (the specific flavoprotein inhibitor diphenyleneiodonium and the protein kinase inhibitors K-252a and staurosporine) inhibited not only the phototropin phosphorylation, but also the H+-ATPase dephosphorylation (Fig. 4.5c–f). This indicated that H+-ATPase dephosphorylation is depended on phototropin phosphorylation (Inoue et al. 2005).
Pulvinar phototropins mediate the dephosphorylation of the plasmalemmal H+ATPase by blue light. a Time courses of recombinant 14-3-3 protein binding to phototropin and to H+-ATPase (as a measure of their phosphorylation status) in pulvinar microsomal membranes in response to a blue-light pulse (30 s at 100 μmol m−2 s−1; mean ± SE, n = 3). b The dependencies of 14-3-3 protein binding to the H+-ATPase and to phototropin on blue-light fluence rate (a representative of 3 similar experiments). c–d The effect of 1-h preincubation of excised pulvini in flavoprotein inhibitor, DPI (100 μM) in the dark. e–f The effect of similar pretreatment with Ser/Thr protein kinase inhibitors, K-252a (10 μM) (with permission, Inoue et al. 2005)
Very interestingly, the dephosphorylation of the H+-ATPase upon BL stimulation in the Phaseolus pulvinus was just the opposite from what occurred in the guard cell , where BL stimulated H+-ATPase phosphorylation (Kinoshita and Shimazaki 1999) and activated the H+-ATPase. Such contrast was manifested also in the opposite reactions of the H+-ATPase activity to BL illumination in flexors and extensors of Samanea (Lee and Satter 1989)—a decrease of H+ secretion in Samanea flexor (albeit, after a brief, transient increase in activity, Okazaki et al. 1995) and activation of H+ secretion in Samanea extensors (like in guard cells, Shimazaki et al. 1985). Thus, it may be concluded that the whole pulvinus of Phaseolus reacts to BL like the Samanea flexor.
3.1.2 Phosphorylation of Samanea K Channels
In situ phosphorylation of the K D channel. The enhancement of the activity of KD channels in flexor protoplasts by BL implicated a voltage-independent component, which could be a phosphorylation (see 4.2.3.3 above and Suh et al. 2000). Indeed, the activity of KD channels in Samanea extensor protoplasts, assayed using patch clamp in a whole-cell configuration and in inside-out patches (Moran 1996), required the presence of Mg2+ and ATP (or its hydrolyzable analog, ATP-γ-S) at the cytoplasmic surface of the plasma membrane. In their absence, channel activity decayed completely within 15 min, but could be restored by adding ATP and Mg2+. A non-hydrolyzable ATP analogue, AMP-PNP (5′-Adenylylimidodiphosphate), did not substitute for ATP. H7 (1-(5-IsoquinolinesulphonyI)-2-methylpiperazine), a broad-range kinase inhibitor, blocked reversibly the activity of KD channels in the presence of MgATP (ibid.).
In another series of experiments, several proteins in isolated plasma membrane-enriched vesicles of Samanea extensors and flexors underwent phosphorylation without an added kinase in solutions similar to patch clamp. The pattern of phosphorylation in the two cell types was not identical (Yu et al. 2006). These results strongly suggested that the activation of the outward-rectifying K channels by depolarization depended critically on phosphorylation by a kinase tightly associated with the membrane. However, it still remains unclear whether the KD channel itself needs to be phosphorylated to function, or an accessory protein or even a lipid need to be phosphorylated. A support for the latter notion originated in a study, where the addition of PtdInsP2 (phosphatidylinositol(4,5)bisphosphate) replaced MgATP in restoring the “rundown” activity of SKOR channels (the presumed Arabidopsis molecular equivalent of the Samanea KD channels), in inside-out patches of a frog oocyte (Liu et al. 2005).
In situ phosphorylation of the K H channel. The voltage-dependent K+-selective fraction of the inward current in extensor and flexor cells protoplasts (i.e. the activity of their KH channels) was assayed in whole-cell patch-clamp assays (see 4.2.3.4 above). The promotion of phosphorylation was achieved using okadaic acid, OA, an inhibitor of protein phosphatase type 1 and 2A. High levels of phosphorylation (300 nM of OA) inhibited KH channel activity, while low levels of phosphorylation (5 nM of OA) promoted channel activity in flexors, but did not affect them in extensors (Yu et al. 2006). This difference between flexor and extensor in the susceptibility of their KH channels activity to phosphorylation may be related to their time-shifted contribution to the pulvinar movement.
In vitro phosphorylation of SPICK 2. The putative SPICK2-channel protein, the molecular candidate for the KH channel (see 4.2.3.4), raised in cultured insect cells (Sf9), was phosphorylated in vitro by the catalytic subunit of the broad-range cyclic-AMP (cAMP)-dependent protein kinase (PKA, Yu et al. 2006). While this finding does not necessarily imply that PKA regulation of KH channels is physiologically relevant, it is consistent with the notion that the SPICK2 channel (assuming it is a pulvinar K+-influx channel) may be regulated in vivo by direct phosphorylation.
3.1.3 Phosphorylation of Water Channels
The water permeability of frog oocytes expressing solely MpPIP2;1, one of the two Mimosa plasma membrane aquaporins (see 4.2.3.8), was independent of phosphorylation. Its interaction (demonstrated by immunoprecipitation) with the water-impermeable MpPIP1;1 was also phosphorylation independent. Yet, the water permeability of this complex increased in parallel to its phosphorylation, curiously, localized to Ser-131 of MpPIP1;1 (Temmei et al. 2005).
3.2 The Perception of Light
Plant photoreception has been reviewed recently (e.g. Wang et al. 2014; Christie et al. 2015; Wang and Wang 2015). Our focus here is on photoreception related to leaf movement. How are the different light stimuli perceived in the pulvinus ? Are the acute and clock signals (Fig. 4.2) perceived via different receptors? What are they? Physiological experiments delineated broad classes of receptors and biochemical–molecular tools are just beginning to be applied in this area.
3.2.1 Phytochrome
Phytochrome - mediated responses. A hallmark for a phytochrome-perceived red-light (and sometimes, blue-light) signal is its reversal by far-red light. Light triggers phytochrome holoproteins to interconvert between the R-absorbing form (Pr) and the FR-absorbing form (Pfr), which represents the biologically inactive and active form, respectively (Wang and Wang 2015, and references therein).
Phytochrome mediates phase shifting of leaf movement rhythms in various plants, e.g. in Samanea and Albizzia (Simon et al. 1976; Satter et al. 1981). It is also a receptor for acute signals: in Samanea, when red light preceded darkness, it enhanced leaf closure, transmitting a swelling signal to the pulvinar flexor cells (reviewed by Satter and Galston 1981). This signalling was replicated in isolated flexor protoplast (Kim et al. 1992, 1993). At the same time, phytochrome -perceived red light, followed by darkness, was thought to signal shrinking to pulvinar extensors (Satter and Galston 1981), but in isolated extensor protoplast, red illumination (i.e. Pfr) appeared to be unnecessary for darkness to initiate shrinking (Kim et al. 1992, 1993).
In the pulvinar protoplasts of P. vulgaris, the Pfr form of the phytochrome had to be present for the shrinking response to be induced by the BL. Far-red light abolished the BL responsiveness, but red light (preceding the blue) restored it (Wang et al. 2001).
In Samanea, in whole darkened pulvinar flexors illuminated with red light, phytochrome-mediated hyperpolarization (measured directly) and subsequently—upon illumination with far-red light—depolarization (Racusen and Satter 1975, see also 4.2.2.2).
Molecular identity. Phytochrome is a multi-gene protein (in Arabidopsis, it is denoted PHYA through PHYE) with a linear tetrapyrrole cofactor, changing its conformation between a red-light-absorbing form (Pr) and a far-red-light-absorbing form (Pfr). A putative Robinia phytochrome A (PHYA) was detected by immunoblotting pulvinar sections using an antibody to mustard (Sinapis alba L.) PHYA (CP2/9). In contrast, an antibody against the cucumber (Cucumis sativus L.) phytochrome B (PHYB) (mAT1) did not produce any signal in these blots (Moysset et al. 2001). Thus, immunochemistry suggests it could be PHYA-like. In further support of this notion, in tobacco (Nicotiana plumbaginifolia), the absence of PHYB in the hlg mutant did not prevent the normal entraining of the endogenous rhythm of growth movements of rosette leaves (although it did affect the sensitivity of bolting to photoperiod, i.e. to short vs long-day regimes, Hudson and Smith 1998).
On the other hand, a suggestion that the pulvinar phytochrome could be related to PHYB is based on an Arabidopsis nonsense oop1 (out of phase 1) mutation in the PHYB apoprotein. This mutation caused defective photoreception and defective circadian phase setting in light–dark cycles (although it did not prevent normal entrainment by temperature cycles, Salome et al. 2002). A physiological hint in support of this latter notion is the low-fluence irradiance, at the range of 1–1000 μmol m−2 s of light, characterized by red/far-red reversibility (Wang 2005), effective in stimulating the known phytochrome responses of pulvinar cells (as, for example, in Moysset and Simon 1989; Kim et al. 1993).
Localization. The phytochrome was localized to the pulvinar cells by examining pulvinar responses during selective illumination of different leaf parts, but even more convincingly—by demonstrating red/far-red-responsiveness in isolated protoplasts (e.g. in Samanea, by Kim et al. 1992, 1993). Immunological evidence for a motor cell-specific localization was provided in Robinia. The labelling with anti-PHYA antibody (see above) was restricted to cortical cells, and there was no evidence of labelling either in the vascular system or in the epidermis. The pattern of labelling was the same in both extensor and flexor cells irrespective of whether phytochrome was in the Pfr or in the Pr form (Moysset et al. 2001).
3.2.2 Blue Light Photoreceptor
Blue light - mediated responses. BL, perceived by an unknown photoreceptor in pulvini, can also shift the rhythm of leaf movement, although this requires hours-long illumination. Acting “acutely”, it is a “shrinking signal” to flexor cells and a “swelling signal” to extensor cells, causing leaf unfolding in Samanea and Albizzia (Satter et al. 1981).
Unlike in Samanea, in P. vulgaris BL caused motor cell shrinking in the laminar pulvinus on the irradiated side, wherever it occurred, irrespective of the stereotyped division of the pulvinus into extensor (abaxial) and flexor (adaxial) , causing the phototropic bending of the pulvinus towards the light source. Such bending orients the leaves maximizing their light-receptive area and probably accounts for movements of solar tracking described in Phaseolus (e.g. Berg 1986, Fig. 4.1c). In accordance with this, in protoplasts isolated from the Phaseolus laminar pulvinus, BL evoked shrinking, without distinction between the extensor and flexor cells, but it required the presence of pfr of phytochrome (i.e. red-light preillumination, Wang et al. 2001).
The action spectrum of the depolarization recorded in the Phaseolus laminar pulvinus (concomitant with initiation of the shrinking signalling ) peaked at 460 nm with lower peaks at 380–420 nm. Almost no sensitivity was observed at wavelengths shorter than 360 nm and longer than 520 nm (Nishizaki et al. 1997). This earlier study failed to notice any red- and far-red light effects on the depolarization of the motor cell, thus excluding phytochrome participation in this movement .
A similar action spectrum was found for both diaheliotropic and paraheliotropic movements of greenhouse-grown soya bean (Glycine max) seedlings. The action spectrum of the movements of the pulvini of the unifoliolate leaves—performed with interference filters—peaked between 410 and 440 nm and between 470 and 490 nm (Donahue and Berg 1990). Indeed, BL was found necessary for these movements. Thus, spectroscopic studies suggested that the pulvinar BL receptor is similar to the receptor involved in the general phototropic responses (reviewed by Briggs and Christie 2002; Christie et al. 2015).
Molecular identity. Three genes of phototropins , PvPHOT1a, PvPHOT1b and PvPHOT2, have been cloned from the bean pulvinus , and their protein products were demonstrated to be the pulvinar BL receptor(s) for the acute responses (Inoue et al. 2005). Their Arabidopsis homologs, PHOT1 and PHOT2, have been localized to the plasma membrane (Harada et al. 2003), suggesting the bean phototropins may be localized similarly. The pulvinar phototropins appear to participate in what appears to be the first step of phototransduction, causing—through unknown step(s)—the dephosphorylation of the plasma membrane H+-ATPase .
The intriguing question is as follows: are the photoreceptors which feed into the clock the same as those mediating the acute responses? With respect to phytochrome , an affirmative answer appears to receive support from findings in Arabidopsis. There, a physical interaction was demonstrated between the C terminal fragments of phytochrome B (PHYB) and the “clock oscillator proteins”, Zeitlupe (ZTL) and cryptochrome 1 (CRY1, Jarillo et al. 2001).
Also phototropins may mediate BL signals to the clock. This is suggested by the finding that in Arabidopsis, the double mutant lacking the cryptochromes cry1 and cry2, and even a quadruple mutant lacking the phytochromes phyA and phyB as well as cry1 and cry2, retained robust circadian rhythmicity, as reflected in the growth movements of the cotyledons. Moreover, this movement could be still phase shifted by (unspecified, but apparently white) light; i.e., while nearly “blind” for developmental responses, the quadruple mutant perceived a light cue for entraining the circadian clock (Yanovsky et al. 2000).
3.3 Intermediate Steps
The most established second messenger in plant signalling is cytosolic Ca2+ (Hetherington and Brownlee 2004), which has also been the central focus in the in most of the studies of signalling in the pulvinus , with attempts to confirm it as a part of the phosphatidylinositol (PtdIns) signalling pathway. The possible target effectors of Ca2+ may be calmodulin (CAM), actin and annexins ; these have just begun to be examined in pulvini.
3.3.1 The Involvement of Calcium
Pharmacological alteration of rhythm. Applying effectors of Ca2+ to pulvini interfered with their rhythms as well as with their acute responses to illumination. EGTA, a Ca2+ chelator, applied to P. vulgaris primary pulvinus suppressed its circadian movements (strongly depending on the phase of application, Kayali et al. 1997). Various CAM antagonists (chlorpromazine (CPZ), trifluoperazine (TFP), calmidazolium and N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7), but not W5, the inactive analog of W7), and also 8-(diethylamino)octyl 3,4,5-trimethoxybenzoate hypochloride (TMB-8, an inhibitor of intracellular IP3-mediated cytosolic calcium mobilization, Schumaker and Sze 1987), all shifted the circadian phase of the Robinia pseudoacacia leaflet movement in continuous darkness, characterized by phase response curves (PRCs). The amplitudes of the advances were proportional to the concentrations of the agents. All these antagonists produced PRCs somewhat similar in shape to the PRC produced by two-hour pulses of BL, but only TMB-8 produced a PRC almost identical to the BL PRC, with advances during the subjective day and delays during the subjective night (Gomez et al. 1999). Interestingly, applying agents presumed to increase the internal Ca2+ concentration, such as calcium ionophore A23187 and, separately, two-hour pulses of 10 mM CaCl2, created PRCs almost identical to the PRC of 15 min of red light, with delays during the subjective day and advances during the subjective night, i.e. opposite to that of BL (Gomez and Simon 1995). Ca2+ signalling is also implicated in temperature sensing (see 4.3.4.4 below), and it could provide the means of convergence of various signals perceived via different receptors.
Pharmacological alteration of acute responses. Acute effects of red light and BL were also altered by applying Ca2+ effectors to whole pulvini of Albizzia and Cassia (Moysset and Simon 1989; Roblin et al. 1989). Most instructive, however, was a pharmacological study conducted on isolated extensor protoplasts of P. coccineus during their swelling and shrinking in a regime of 9 h light to 15 h dark (which paralleled their expected behaviour in the intact pulvinus, Mayer et al. 1997). Light-induced swelling required Ca2+ influx from the surrounding medium. Promoting Ca2+ influx from outside elicited swelling in the D, mimicking the “light on” signal. Dark-induced shrinking occurred in Ca2+-free medium, but was sensitive to manipulations of Ca2+ release from internal stores via the activation or inhibition of the PtdIns pathway, suggesting that the shrinking signal “light off” is—but the swelling signal of “light on” is not—transduced through PtdIns hydrolysis and Ca2+ release from internal stores. However, increasing internal Ca2+ in the light could not substitute for the “light off” signal, although it could nullify the inhibition (by TMB-8) of mobilization of cytosolic Ca2+ in the presence of the “light off” signal. Thus, while Ca2+ itself is necessary, it is not sufficient for shrinking, and “light off” signal provides this additional required element (Mayer et al. 1997).
Phytochrome has been shown directly to increase cytosolic Ca2+ in other systems. For example, in etiolated wheat leaf protoplast, red-light evoked Ca2+ increase mediated by phytochrome, associated with protoplast swelling (Shacklock et al. 1992). However, no such evidence has been obtained for pulvinar cells.
Phototropins . In protoplasts isolated from motor cells of M. pudica pulvini, UV(A) light (360 nm; possibly perceived by phototropins) increased transiently the cytosolic-free Ca2+ concentration. This Ca2+ increase was not significantly modified when protoplasts were incubated in a nominally calcium-free medium and was not inhibited by calcium influx blockers (LaCl3 and nifedipine), arguing thus for a mobilization from intracellular stores (Moyen et al. 1995). The BL-induced movement of the primary pulvinus of Mimosa is similar to the seismonastic response in its direction and in the underlying loss of osmoticum (Stoeckel and Takeda 1993).
This distinct response to UV(A) resembles the PtdIns pathway-related response mediated by phot2 in deetiolated Arabidopsis seedlings. There, while both phot1 and phot2 could induce Ca2+ influx from the apoplast through a Ca2+ channel in the plasma membrane in response to BL (phot1, at lower fluence rates: 0.1–50 mmol m−2 s−1, and phot2, at higher fluence rates: 1–250 mmol m−2 s−11), phot2 alone induced phospholipase C (PLC)-mediated phosphoinositide signalling (Harada et al. 2003).
Circadian Ca 2+ oscillations seem almost inevitable in the mature pulvinar cells in view of the strong evidence for the involvement of Ca2+ in the rhythmic movements (see above). However, in plants, they have been documented so far only in tobacco (N. plumbaginifolia) and in Arabidopsis seedlings (Fig. 4.6, and Johnson et al. 1995; see also the review by Hetherington and Brownlee 2004; Love et al. 2004), most likely in synchrony with the growth movements of the cotyledons. Circadian oscillations in free Ca2+ were not detected in nuclei (Wood et al. 2001); thus, this is not obvious how the cytosolic oscillations communicate—as an input to and/or as an output from the clock, the core elements of which (LHY, CCA1 and TOC1) reside only in the nucleus (Dodd et al. 2005, and references therein).
Circadian oscillations of [Ca2+]cyt in Arabidopsis seedlings entrained to different photoperiods. Aequorin luminescence emitted by seedlings kept under 110 µmol m−2 s−1 constant light (LL). Shown are measurements from seedlings entrained in 8L/16D (a) and 16L/8D (b) for 11 d before LL. During the entrainment light period, the photon flux density was 60 µmol m−2 s−1. Points represent the mean bioluminescence of 12 seedling clusters ± SE. Open areas indicate the subjective day, and shaded areas indicate the subjective night (with permission, Love et al. 2004)
3.3.2 PhosphatidylInositides (PIs)
Although the animal paradigm cannot be applied uncritically to plants, the general scheme for signal propagation via the PI pathway has received considerable support in plants (reviewed in Cote et al. 1996; Drobak et al. 1999; Stevenson et al. 2000; Hetherington and Brownlee 2004). Plants possess most of the enzymes producing the different phosphoinositides (only phosphatidylinositol trisphosphate is not produced in plants). The changes in free cytosolic Ca2+ concentration, when attributed to mobilization from internal stores, suggested the activation of the PtdIns pathway, in particular the hydrolysis of PtdInsP2 by PLC into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (InsP3). This was confirmed in some cases by pharmacological agents, such as PLC inhibitors, and also in direct lipid assays (see Stevenson et al. 2000). Indeed, light, when it served as a cell-shrinking signal, increased the level of InsP3 in motor cells of leaf-moving organs (Morse et al. 1987; Kim et al. 1996; Mayer et al. 1997).
In addition to affecting the activity of PLC, light could affect other enzymes. For example, in etiolated sunflower hypocotyls , light transiently down-regulated the activity of PIP 5-kinase consequently down-regulating the level of its product, PtdInsP2 (Memon and Boss 1990). In protoplasts isolated from BY2 cultured tobacco cells with genetically and pharmacologically manipulated PtdInsP2 and InsP3 levels, the activity of the K+-release channel NtORK1 was inversely correlated with the PtdInsP2 level (Ma et al. 2009). Remarkably, in those protoplasts, in which the expression of the human type I PIP 5-kinase increased the levels of PtdInsP2 and InsP3 PIP , the osmotic water permeability of the plasma membrane was a few fold higher than that of protoplasts from control cells (wild type, or transformed with the plasmid without the kinase gene). The increased water permeability of the membranes appears to have been caused by an increased activity of aquaporins , due to the elevated levels of inositol phospholipids (Ma et al. 2014).
PIs in the leaf - moving motor cells. 15 s of WL illumination to the Samanea pulvini was sufficient to accelerate the turnover of phosphoinositides in the motor tissues (Morse et al. 1987). Furthermore, in Samanea pulvinar protoplasts, cell-shrinking stimuli applied at the appropriate circadian time (darkness, to the pulvinar extensors during the last third of the day period, or BL, to the pulvinar flexors, during the 2nd part of the night) increased InsP3. This “shrinking light” effect was inhibited by neomycin, at a concentration of 10 μM which inhibits PtdInsP2 hydrolysis, and mimicked by mastoparan, a G protein activator (Fig. 4.7 and Kim et al. 1996; Moran et al. 1996). In parallel, the K+-influx channels were shown to close in response to the same leaf-closing stimuli, i.e. in the protoplasts with increased InsP3 levels (Kim et al. 1993, 1996). The authors concluded from these results that a PLC-catalysed hydrolysis of phosphoinositides , possibly activated by a G protein, was an early step in the signal transduction pathway by which BL and darkness closed K+-influx channels in (the appropriate) Samanea pulvinar cells (Kim et al. 1996).
lnositol 4,5 trisphosphate (InsP3) and K+ permeability of protoplasts in response to shrinking signals. Protoplasts were isolated during light period and transferred to growth chamber at time of normal “light off” (means ± SD of 3 or 4 separate experiments, each done in duplicate). a flexor responses to blue light (BL) at hours 4–8 of dark period. Cells were treated with indicated agent, and change in the fluorescence of membrane potential indicator dye 3,3′-dipropylthiodicarboxycyanide iodide was measured as a function of time after addition of 200 mM K+. ΔF/F 0 is the values of fluorescence changes (relative to baseline) observed 30 s after addition of K+. An increase in ΔF/F 0 indicates that K+ can enter the cell and depolarize membrane potential; K+ channels are presumed to be open. No change in ΔF/F 0 indicates that K+ cannot enter cell; channels are presumed to be closed. InsP3 levels were assayed in extracts prepared from similarly treated protoplasts. Values are presented as percent of untreated controls and represent InsP3 levels in protoplasts after 30-s treatment with blue light (10 μM neomycin) or 120-s treatment with 10 μM mastoparan. b extensor responses to darkness at hours 10–12 of light period. Treatments were as above except that darkness, and not blue light, served as a signal. WL, white light (with permission, Moran et al. 1996)
3.3.3 Annexins
Annexins are Ca2+-, phospholipid- and protein-binding proteins, conserved evolutionarily between animals and plants, with increasingly broad range of revealed signalling functions, including extracellular reception (Gerke and Moss 2002; Cantero et al. 2006), and including nucleotide-induced oligo- (possibly tri-) merization of annexin 6 to form active ion channels (of unspecified selectivity Kirilenko et al. 2006). In plants, annexins were predicted to form hyperpolarization-activated Ca channels (Hofmann et al. 2000; White et al. 2002).
Annexins may also mediate Ca2+ effects. Eight annexin genes have been found in Arabidopsis (Cantero et al. 2006). Recently, annexin 1 of Arabidopsis has been suggested to be the ROS-activated Ca2+ channel of the plasma membrane. Arabidopsis annexin 1 mediates a plasma membrane calcium-permeable conductance in roots that is activated by reactive oxygen species . Recombinant annexin 1 forms a very similar conductance in planar lipid bilayers, indicating that this protein could facilitate directly the in vivo conductance (Davies 2014).
Annexin protein isolated from Mimosa was found to bind in vitro to a phospholipid and to F-actin in the presence of calcium , and its amount was developmentally regulated. In the primary pulvinus during daytime, the amount of annexin increased with ABA concentration between 1 and 75 μM (but was not affected by cold or mechanical stimuli, also know to involve Ca2+ signaling). Annexin amount increased also at night, and its distribution changed from the cell periphery during the daytime to cytoplasmic at night (Hoshino et al. 2004). It is thus interesting that while actin (which binds to annexin) is thought to be involved in the seismonastic function of this pulvinus, annexin appears to be rather associated with nyctinastic transitions (but see also Sect. 4.2.2.3).
3.4 Regulation by Other Effectors
3.4.1 Hormones
Auxins (indole-3-acetic acid, IAA) , gibberellins (GA3) and ethylene have been found in gravistimulated leaf-sheath pulvini of grasses (Brock 1993). These hormones, and also jasmonic acid and abscisic acid , affected the long-term growth responses of these tissues (in particular, cell elongation and cell wall production) following exogenous application (Montague 1995, 1997).
In the non-growing pulvini of legumes, only the acute effects of exogenous hormones on the leaf movements have been addressed (Bialczyk and Lechowski 1987; Bourbouloux et al. 1992; Mayer et al. 1997). IAA applied to whole pulvini opened Cassia fasciculata leaflets in darkness, and pharmacological agents aimed to increase the cytoplasmic Ca2+ concentration promoted this opening, and those aimed to decrease it, or to decrease its effect, inhibited (although verapamil and nifedipine, common Ca2+ channel blockers, were ineffective, Bourbouloux et al. 1992).
IAA and ABA applied to protoplasts isolated from the laminar pulvinus of P. vulgaris and bathed in a medium containing KCl as the major salt affected both flexor and extensor cells similarly: protoplasts swelled in response to IAA and shrank in response to ABA. Swelling depended on the presence of K+ and Cl− at acidic pH and shrinking depended on the activity of a functional Cl channel (Iino et al. 2001), in accord with the accepted view of the “osmotic motor”. No receptors for the hormone function are known in pulvini.
Salicylic acid (O-hydroxy benzoic acid, SA), applied in solution to the excised primary pulvinus of M. pudica (at 0.1–1 mM final concentration), triggered a hyperpolarization of the cell membrane in a concentration-dependent manner, as recorded by a microelectrode impaled into the abaxial (extensor) part of the pulvinus (Saeedi et al. 2013). At 1 mM, the hyperpolarization occured in 15 ± 5 s (!), peaked at 15 ± 5 min and lasted approximately 90 min. Benzoic acid, the SA direct biosynthetic precursor, gave the same general result, though with roughly half the amplitude. In contrast, other benzoic acid derivatives induced depolarization and the SA immediate breakdown product did not have any effect on the membrane potential (Saeedi et al. 2013). The observed hyperpolarization could be most simply interpreted here either as due to SA-stimulated activity of the plasma membrane proton pump , or due to the activation of K+-release channels (both would be mutually exclusive in a motor cell). Since the primary pulvinus of Mimosa reacted to SA application by bending , like in a seismonastic response (ibid.), which would be underlain by extensor shrinking, the latter is more plausible. Thus, could SA have activated K+-release channels directly?
Contrasting with this, very rapid and quite specific (albeit not very sensitive) response to SA, was a slower response (on the order of hours) of external alkalinization, monitored in a bath with excised and sliced pulvini, which can be interpreted as cessation of proton pump activity (ibid.). Both responses would be consistent with SA inducing leaf folding.
In contrast to the pulvinus bending in Mimosa, in Cassia fasciculata pulvini, benzoic acid, SA and other benzoic acid derivatives promoted light-induced opening and inhibited dark-induced closure (Saeedi and Roblin 1987). Are these effects plant dependent or conditions dependent?
Nitrous oxide. The involvement of nitrous oxide (NO—the gaseous signalling molecule with well-established physiology in animals) in nyctinastic leaf closure (closure upon the regular light-to-dark transition) was explored in the classic system of leaflets with tertiary pulvini of Albizzia lophanta floating on various experimental solution combined with systematic pharmacological treatment based on the animal system paradigm (Bergareche et al. 2014). NO levels were manipulated by applying, in the experimental solutions, NO donors, NO scavengers and an inhibitor of NO synthase, and an inhibitor of a plant-specific pathway of NO production via nitrate reductase (ibid.). NO partially inhibited leaflet closing. Endogenous NO production in the leaflets (though not necessarily in the pulvini) was demonstrated as the appearance of nitrate + nitrite in the solution. These experiments suggested that the unperturbed nyctinastic leaflet closure achieves a smaller angle due to endogenous NO (ibid.). In addition, cGMP levels (in leaflets, not necessarily in pulvini) were manipulated (also as in an animal paradigm) by applying exogenously cGMP (in a form of its membrane-permeating derivative, 8-bromo-cGMP), or increasing endogenous cGMP by inhibition of phosphodiesterase-5, or decreasing endogenous cGMP by inhibiting guanylate cyclase. This led to the conclusion that cGMP also inhibits leaflet closure (ibid.). Inhibiting leaflet closure means inhibiting extensor shrinking (or promoting extensor swelling) and /or inhibiting flexor swelling (or promoting flexor shrinking). Which of these were effected by NO and/or cGMP? How were NO and cGMP linked?
In guard cells , which, by their light responses, are likened to extensors (Moran 2007a), adding 8-bromo-cGMP (an equivalent of cGMP) initiated swelling (and stomata opening), indeed, like in the extensors of Albizzia lophanta. In contrast to Albizzia, in guard cells, the effect of NO (normally induced by ABA stimulation) is to produce 8-nitro-cGMP and further to elevate cytosolic Ca2+, thereby causing cell shrinking (i.e. stomata closure, Joudoi et al. 2013). In Albizzia lophanta leaflets, Ca2+ also enhanced leaflet closure, i.e. enhanced extensor shrinking, which suggests that Ca2+ does not participate in NO inhibition of leaflet closure (Bergareche et al. 2014). Could NO inhibit leaflet closure by a two-prong effect: causing swelling of the extensor via cGMP without Ca2+ and causing flexor shrinking (concomitant with extensor swelling) via 8-nitro-cGMP and elevation of cytosolic Ca2+ levels in flexors?
3.4.2 Turgorins
Turgorin, PLMF 1 (periodic leaf movement factor 1, sulfonated gallic acid glucoside), induces closure of leaflets in Mimosa with a dose-dependent rate. PLMF 1 has been found in many higher plants with nyctinastic movements, including M. pudica, and in a couple of plants with thigmonastic movements (as reviewed by Schildknecht and Meier-Augenstein 1990). Furthermore, since only one out of two PLMF 1 enantiomers was active, a reaction with a specific receptor has been proposed (Kallas et al. 1990). The colocalization of the enzyme sulfonating the gallic glycoside, along with its end product, to the phloem cells in the motor organ , suggested that this is the site of synthesis and/or accumulation of PLMF-1 in support of the hypothesis that PLMF-1 may be acting as a chemical signal during the seismonastic response of Mimosa (Varin et al. 1997).
3.4.3 A New Jasmonate Cell-Shrinking Signalling Pathway?
In Albizzia julibrissin leaves, Ueda’s group identified a naturally occurring bioactive metabolite of a jasmonic acid, 12-O-β-d-Glucopyranosyljasmonic acid (12-O-Glc-JA), which, when applied in a transpiration stream to detached Albizzia or Samanea saman leaves at a concentration of about 10 μM, induced nyctinastic leaf folding and induced also shrinking of isolated Samanea pulvinar extensor protoplasts (Nakamura et al. 2011, and references therein). Interestingly, the non-glycosylated 12-hydroxyjasmonic acid (12-OH-JA, isolated initially from potato leaves, ibid.) did not evoke any of the typical jasmonate responses, such as tendril coiling, or activation of a variety of genes in several plants (ibid.), but both the aglucon 12-OH-JA and its glucoside 12-O-Glc-JA acted in the same manner as the tuber-inducing factor in potato. Enantiomeric probes synthesized from the glucoside helped discover in the Samanea extensor a 38 kD unidentified membrane protein, which bound selectively only one enantiomer of one of the probes and which the authors named “a membrane target protein of jasmonate glucoside (MTJG)”. None of the proteins known to participate in the “typical” jasmonic acid signalling in plants bound to these probes. Based on this, and, in particular, on the specificity for one enantiomer, the authors suggested that (12-O-Glc-JA) is a signalling molecule, not just a jasmonate degradation metabolite, and that the MTJG is a receptor mediating the extensor shrinking and leaf closure within a hitherto undescribed jasmonate signalling pathway (Nakamura et al. 2011, and references therein).
3.4.4 A “Leaf-Opening Factor”
Isolespedezate (named after its initial source, the “Chinese bushclover”, Lespedeza cuneate), has been identified in Cassia obtusifolia, another leaf-moving legume. When fed via the petiole, the “leaf-opening factor” was also effective at concentrations 1–10 μM, but only in plants within the genus Cassia. A specific binding of its bioactive derivative identified a cytosolic target protein in Cassia, MetE (a 5-methyltetrahydropteroyl-triglutamate-homocysteinemethyltransferase, a cobalamine-independent methyltransferase participating in the final step of methionine biosynthesis; Ueda et al. 2011). The relevance of these findings to the in situ signalling pathways regulating the osmotic machinery in leaves awaits further corroboration.
3.4.5 Temperature
One of the hallmarks of the circadian clock is “temperature compensation ”, i.e. the period and phase remain constant over a rather broad range of temperatures (McClung and Davis 2010; Franklin et al. 2014, and references therein; even if the extent of compensation varies somewhat in different accessions of Arabidopsis Kusakina et al. 2014). This compensation is manifested also in the overt leaf rhythm (Kusakina et al. 2014). The underlying mechanism for this stability versus temperature operates in spite of the strong dependence of the circadian periods on the turnover of the clock mRNA or clock protein, which, by themselves, are strongly temperature dependent; without compensation, a more rapid turnover of clock mRNAs or clock proteins would result in short periods, and a slower turnover—in longer period lengths (Ruoff et al. 1997; Kusakina et al. 2014).
The clock may be entrained separately by temperature pulses, just as by light, since their input pathways to the clock are different. For example, a mutation rendering the plant irresponsive to light entrainment preserved its responsiveness to temperature entrainment (Salome et al. 2002). How are the temperature pulses perceived? The enigma of the plant receptor for temperature entrainment is beginning to unravel: in resemblance to the heat- or cold-sensing TRP channels in mammals (Voets et al. 2004), cyclic nucleotide-gated channels (CNGCs which conduct calcium ) and Ca2+ signalling have been implicated in higher temperature sensing in Arabidopsis (CNGC2) and in the moss Physcomitrella patens (CNGCb and CNGCd) (Finka et al. 2012; Finka and Goloubinoff 2013).
The mechanism of the circadian clock temperature compensation includes counterbalance between the “morning loop” and the “evening loop” of the clock , converging with inputs from red- and blue-light receptors (reviewed by Franklin et al. 2014). This convergence (could it be at the level of Ca2+ signalling? see Sect. 4.3.3.1 above) may underlie the already known phenomenon, whereby elevated temperature is “interpreted by the clock” as daylight signals and cold temperature—as darkness signals (Franklin et al. 2014, and references therein).
4 Unanswered Questions
The light-signalling transduction steps converging on the “osmotic motor” are outlined schematically in Fig. 4.8. The information about pulvini is scarce. In fact, most of the questions below have not yet been answered, or answered adequately, in any plant system.
Ca2+ involvement in the volume changes (a [partial] schematic model). bn are clock output signalling pathways, and cn are the “acute” signalling pathways from the light-activated receptor, R. The acute “shrinking signalling” includes the PtdIns pathway, starting with the activation (c3) of a trimeric G protein (Gαβγ), followed by the activation (c4) of Phospholipase C (PLC) and formation of 2nd messengers by breakdown (c5) of PtdIns2. Kinase (K) can be activated (c6) by the increased (↑) concentration of free cytosolic Ca2+ and phosphorylate (c7) transporters (T) or their modifying protein(s), including those involved in trafficking. PtdInsP2 may affect some of the transporters directly (c8). Some of these elements and links have been demonstrated in the pulvinar cells. The clock may originate Ca2+ oscillations (b3), via the PtdInsP2 pathway or via a Ryanodine receptor cADP-ribose pathway. Ca2+ effects (b4–b5) may resemble those of the acute pathway (c6–c7). The clock effects on the transporters may be “enabling”, modulating its responsiveness to the acute signals. This enabling effect may be exerted also through other levels, marked by dotted arrows. See text for details and Fig. 4.4 for unexplained signs
The “osmotic motor” framework is not very mysterious, but functions still seek transporters: most of the transporters in the plasma membrane and in the tonoplast are yet to be characterized physiologically.
4.1 Acute, Fast Signalling
Signalling pathways are still not well understood, and therefore, there is a long list of questions to be answered.
Do ABA or jasmonate or NO (or their combination) act as physiological mediators of shrinking in pulvinar cells? Are they coupled to the same shrinking cascade as that of a “shrinking light” signal? What signals bring about the hypothesized increases of cytosolic Ca2+? Is InsP3 or InsP6 the actual messenger mobilizing Ca2+ from internal stores? What is its receptor? Are cADP-ribose and the ryanodine receptor part of the Ca2+-mobilizing cascade in the pulvini? Does light signalling affect different enzymes of the PtdIns pathway? Do PtdIns lipids (e.g. PtdInsP2) affect transporters directly in the pulvini? Is a G protein a mediator of light signaling?
The following questions are only a partial list. The acute (i.e., the clock-bypassing) effects on transcription or translation, or membrane trafficking, indicated in the model, all invite, obviously, additional questions.
The signalling cascade leading to swelling is also not known, apart from a requirement for calcium (Mayer et al. 1997) and for a timed “enabling” function, such as the gated activity of the K+-influx channels (Kim et al. 1993). Is it possible that the lysis of the membranal phosphatidylcholine (PtdCh) by PLA2 and the resulting products—lysoPtdCh and free fatty acids—constitute the second messengers for motor cell swelling (Lee et al. 1996)? Exogenous PLA2 caused premature swelling of Samanea flexor protoplasts, but PLA2 inactivated by a short preincubation with its inhibitor, manoalide—was inactive (Lee et al. 1996). As a plausible hypothesis, IAA may be a physiological mediator of the swelling light signals. Other hormones may also be involved. Since the phytohormone, brassinolide, appeared to increase the osmotic water permeability of aquaporins in Arabidopsis hypocotyl protoplasts (Morillon et al. 2001), and since aquaporins are likely a part of the “osmotic motor” of leaf movement, it might be of interest to examine whether brassinolide acts also in pulvini.
How is G protein involved in pulvinar signalling ? What is the role of lipids like sphingosine? The “guard cell paradigm” (see for example Chaps. 9 and 12) is indeed an inspiring framework for studying the fast, acute pulvinar signal transduction .
4.2 The Clock Input and Output
There is also no end to questions which may be addressed with regard to the regulation of pulvinar movements through the clock and by the clock (see Chaps. 8 and 10 for updated descriptions of the circadian plant clockworks). An intriguing question still lingers, posed already several decades ago, and apparently forgotten during the flurry of recent discoveries of the clock molecular components: do membrane ion channels feed back into the oscillator (especially in the light of increasing evidence that rhythmic metabolism contributes to the circadian network, Haydon et al. 2013)?
The future. Given the abundance of basic scientific questions they encrypt, pulvini currently attract insufficient attention as model systems. Large, reversibly moving pulvini, with oppositely functioning flexors and extensors (i.e., with “built-in” “controls”), amenable to easy protoplasts isolation, such as those of Samanea, can afford extensive physiological and biochemical assays of the interactions of the osmotic motor with the clock (roughly suggested in the model, Fig. 4.8). These would be greatly aided by combination with genetic tools, for example via transient protoplast transformation. Studies at the protoplast level could be easily combined with studies on the whole (isolated) pulvinus level and intact leaf level. These could be especially enhanced by automation of measurements of cycling phenomena, which would be of paramount importance to the generation of acceptable quantitative data for meaningful conclusions!
References
Berg VS (1986) Solar tracking: light avoidance induced by water stress in leaves of kidney bean seedlings in the field. Crop Sci 26:980–986
Bergareche C, Moysset L, Angelo AP, Chellik S, Simón E (2014) Nitric-oxide inhibits nyctinastic closure through cGMP in Albizia lophantha leaflets. J Plant Physiol 171:1299–1305
Bialczyk J, Lechowski Z (1987) The effect of abscisic acid and fusicoccin on malic acid concentration in pulvini of Phaseolus coccineus L. New Phytol 105:469–475
Bihler H, Eing C, Hebeisen SRA, Czempinski K, Bertl A (2005) TPK1 Is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol 139:417–424
Blatt MR (1992) K+ channels of stomatal guard cells: characteristics of the inward rectifier and its control by pH. J Gen Physiol 99:615–644
Bourbouloux A, Roblin G, Fleurat-Lessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata. J Exp Bot 43:63–71
Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7:204–210
Brock TG (1993) Hormone trafficking: a case study of growth regulator dynamics. Physiol Plant 89:237–241
Campbell NA, Garber RC (1980) Vacuolar reorganization in the motor cells of Albizzia during leaf movement. Planta 148:251–255
Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP, Clark GB, Roux SJ (2006) Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem 44:13–24
Chen J, Moreau C, Liu Y, Kawaguchi M, Hofer J, Ellis N, Chen R (2012) Conserved genetic determinant of motor organ identity in Medicago truncatula and related legumes. Proc Natl Acad Sci USA 109:11723–11728
Christie JM, Blackwood L, Petersen J, Sullivan S (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol
Cortizo M, Laufs P (2012) Genetic basis of the “sleeping leaves” revealed. Proc Natl Acad Sci 109:11474–11475
Cote GG, Yueh YG, Crain RC (1996) Phosphoinositide turnover and its role in plant signal transduction. In: Biswas BB, Biswas B (eds) Myoinositol-phosphates, phosphoinositides and signal transduction, vol 26. Plenum, London, pp 317–343 Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B (2002) Vacuolar membrane localization of the Arabidopsis 'two-pore' K+ channel KCO1. Plant Journal 29: 809–820
Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B (2002) Vacuolar membrane localization of the Arabidopsis 'two-pore' K+ channel KCO1. Plant Journal 29: 809–820
Davies J (2014) Annexin-mediated calcium signalling in plants. Plants 3:128–140
De Mairan J-JDO (1729) Observation botanique. In: Histoire de l′Academie Royale de sciences. Paris, pp 35–36
Dodd AN, Love J, Webb AAR (2005) The plant clock shows its metal: circadian regulation of cytosolic free Ca2+. Trends Plant Sci 10:15
Donahue R, Berg VS (1990) Leaf orientation of soybean seedlings: II receptor sites and light stimuli. Crop Sci 30:638–643
Dordas C, Chrispeels MJ, Brown PH (2000) Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124:1349–1362
Drobak BK, Dewey RE, Boss WF (1999) Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. Int Rev Cytol Surv Cell Biol 189:95–130
Engelmann W, Antkowiak B (1998) Ultradian rhythms in Desmodium. Chronobiol Int 15:293–307
Eun SO, Lee Y (2000) Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta 210:1014
Fan LM, Zhao Z, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7:537–546
Finka A, Goloubinoff P (2013) The CNGCb and CNGCd genes from Physcomitrella patens moss encode for thermosensory calcium channels responding to fluidity changes in the plasma membrane. Cell Stress Chaperones 1–8
Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24:3333–3348
Fleurat Lessard P, Schmit AC, Vantard M, Stoeckel H, Roblin G (1993) Characterization and immunocytochemical distribution of microtubules and F-actin filaments in protoplasts of Mimosa pudica motor cells. Plant Physiol Biochem 31:757–764
Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain J, Martinoia E (1997) Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol 114:827–834
Franklin KA, Toledo-Ortiz G, Pyott DE, Halliday KJ (2014) Interaction of light and temperature signalling. J Exp Bot 65:2859–2871
Fromm J, Eschrich W (1988a) Transport processes in stimulated and non-stimulated leaves of Mimosa pudica I: the movement of 14C-labelled photoassimilates. Trees Struct Funct 2:7–17
Fromm J, Eschrich W (1988b) Transport processes in stimulated and non-stimulated leaves of Mimosa pudica II: energesis and transmission of seismic stimulation. Trees Struct Funct 2:18–24
Fromm J, Eschrich W (1988c) Transport processes in stimulated and non-stimulated leaves of Mimosa pudica. III. Displacement of ions during seismonastic leaf movements. Trees: Structure and Function 2: 65-72
Garrill A, Tyerman SD, Findlay GP, Ryan PR (1996) Effects of NPPB and niflumic acid on outward K+ and Cl− currents across the plasma membrane of wheat root protoplasts. Austral J Plant Physiol 23:527–534
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371
Gomez LA, Simon E (1995) Circadian rhythm of Robinia pseudoacacia leaflet movements: role of calcium and phytochrome. Photochem Photobiol 61:210–215
Gomez LA, Moysset L, Simon E (1999) Effects of calmodulin inhibitors and blue light on rhythmic movement of Robinia pseudoacacia leaflets. Photochem Photobiol 69:722–727
Gorton HL (1990) Pulvinar water relations in nyctinastic plants. In: Satter RL, Gorton HL, Vogelmann TC (eds) The pulvinus: motor organ for leaf movement, vol 3. ASPP, Rockville, pp 214–222
Gorton HL, Satter RL (1984) Extensor and flexor protoplasts from Samanea pulvini II: X-ray analysis of potassium, chlorine, sulfur, phosphorus, and calcium. Plant Physiol 76:685–690
Harada A, Sakai T, Okada K (2003) phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100:8583–8588
Haydon MJ, Hearn TJ, Bell LJ, Hannah MA, Webb AAR (2013) Metabolic regulation of circadian clocks. Semin Cell Dev Biol 24:414–421
Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu RevPlant Biol 55:401–427
Hofmann A, Proust J, Dorowski A, Schantz R, Huber R (2000) Annnexin 24 from Capsicum annuum: X-ray structure and biochemical characterization. J Biol Chem 275:8072–8082
Hoshino D, Hayashi A, Temmei Y, Kanzawa N, Tsuchiya T (2004) Biochemical and immunohistochemical characterization of Mimosa annexin. Planta 219:867–875
Hudson M, Smith H (1998) The phytochrome B encoded by the HLG locus of Nicotiana plumbaginifolia is required for detection of photoperiod: hlg mutants show altered regulation of flowering and circadian movement. Plant J 15:281–287
Hwang JU, Suh S, Yi HJ, Kim J, Lee Y (1997) Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol 115:335–342
Iglesias A, Satter RL (1983) H+ fluxes in excised Samanea motor tissue I: promotion by light. Plant Physiol 72:564–569
Iino M, Long C, Wang XJ (2001) Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42:1219–1227
Ilan N, Schwartz A, Moran N (1996) External protons enhance the activity of the hyperpolarization-activated K channels in guard cell protoplasts of Vicia faba. J Membr Biol 154:169–181
Irving MS, Ritter S, Tomos AD, Koller D (1997) Phototropic response of the bean pulvinus: movement of water and ions. Bot Acta 110:118–126
Jaensch L, Findlay GP (1998) Ion channels in the plasma membrane of Phaseolus motor cells. In: Tester M, Morris C, Davies J (eds) 11th international workshop on plant membrane biology. Springer, Cambridge, p 148
Jarillo JA, Capel J, Tang RH, Yang HQ, Alonso JM, Ecker JR, Cashmore AR (2001) An Arabidopsis circadian clock component interacts with both CRY1 and phyB. Nature 410:487–490
Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Halen A, Trewavas A (1995) Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269:1863–1864
Joudoi T, Shichiri Y, Kamizono N, Akaike T, Sawa T, Yoshitake J, Yamada N, Iwai S (2013) Nitrated cyclic GMP modulates guard cell signaling in arabidopsis. Plant Cell Online 25:558–571
Kallas P, Meier Augenstein W, Schildknecht H (1990) The structure-activity relationship of the turgorin PLMF 1 in the sensitive plant Mimosa pudica L.: in vitro binding of (carbon-14 carboxyl)-PLMF 1 to plasma membrane fractions from mimosa leaves and bioassays with PLMF 1-isomeric compounds. J Plant Physiol 136:225–230
Kanzawa N, Hoshino Y, Chiba M, Hoshino D, Kobayashi H, Kamasawa N, Kishi Y, Osumi M, Sameshima M, Tsuchiya T (2006) Change in the actin cytoskeleton during seismonastic movement of Mimosa pudica. Plant Cell Physiol 47:531–539
Kayali S, Greppin H, Agosti RD (1997) Effect of EGTA on the diurnal leaf movement of Phaseolus vulgaris. Plant Physiol Biochem 35:915–922
Kim HY, Cote GG, Crain RC (1992) Effects of light on the membrane potential of protoplasts from Samanea saman pulvini: involvement of K+ channels and the H+-ATPase. Plant Physiol 99:1532–1539
Kim HY, Cote GG, Crain RC (1993) Potassium channels in Samanea saman protoplasts controlled by phytochrome and the biological clock. Science 260:960–962
Kim HY, Cote GG, Crain RC (1996) Inositol 1,4,5-trisphosphate may mediate closure of K+ channels by light and darkness in Samanea saman motor cells. Planta 198:279–287
Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18:5548–5558
Kirilenko A, Pikula S, Bandorowicz-Pikula J (2006) Effects of mutagenesis of W343 in human annexin A6 isoform 1 on its interaction with GTP: nucleotide-induced oligomer formation and ion channel activity. Biochemistry 45:4965–4973
Kiyosawa K (1979) Unequal distribution of potassium and anions within the Phaseolus pulvinus during circadian leaf movement. Plant Cell Physiol 20:1621–1634
Koller DV (2001) Dynamic aspects of the response of the pulvinus in the leaf of bean plants (Phaseolus vulgaris L.) to photoexcitation. J Plant Physiol 158:347
Koller D, Zamski E (2002) The phototropic pulvinus of bean Phaseolus vulgaris L. functional features. Plant Biol 4:584–594
Koller D, Ritter S, Fork DC (1996) Light-driven movements of the trifoliate leaves of bean (Phaseolus vulgaris L.): spectral and functional analysis. J Plant Physiol 149:384–392
Kung C (2005) A possible unifying principle for mechanosensation. Nature 436:647–654
Kusakina J, Gould PD, Hall A (2014) A fast circadian clock at high temperatures is a conserved feature across Arabidopsis accessions and likely to be important for vegetative yield. Plant Cell Environ 37:327–340
Lee Y (1990) Ion movements that control pulvinar curvature in nyctinastic legumes. In: Satter RL, Gorton HL, Vogelmann TC (eds) The pulvinus: motor organ for leaf movement, vol 3. ASPB, Rockville, pp 130–138
Lee Y, Satter RL (1989) Effects of white, blue, red light and darkness on pH of the apoplast in the Samanea pulvinus. Planta 178:31–40
Lee Y, Suh S-J, Moran N, Crain RC (1996) Phospholipid metabolism and light regulation of stomatal opening and leaf movement. In: Briggs WR, Heath RL, Tobin EM (eds) Regulation of plant growth and development by light. American Society Plant Physiology, Riverside, pp 89–97
Liu K, Li LG, Luan S (2005) An essential function of phosphatidylinositol phosphates in activation of plant Shaker-type K+ channels. Plant J 42:433–443
Love J, Dodd AN, Webb AAR (2004) Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16:956–966
Lowen CZ, Satter RL (1989) Light-promoted changes in apoplastic K+ activity in the Samanea saman pulvinus, monitored with liquid membrane microelectrodes. Planta 179:421–427
Ma X, Shor O, Diminshtein S, Yu L, Im YJ, Perera I, Lomax A, Boss WF, Moran N (2009) Phosphatidylinositol(4,5)bisphosphate inhibits K+-efflux channel activity in NT1 tobacco cultured cells. Plant Physiol 149:1127–1140
Ma X, Shatil-Cohen A, Ben-Dor S, Wigoda N, Perera I, Im Y, Diminshtein S, Yu L, Boss W, Moshelion M, Moran N (2014) Do phosphoinositides regulate membrane water permeability of tobacco protoplasts by enhancing the aquaporin pathway? Planta 1–15
Mayer WE, Fischer C (1994) Protoplasts from Phaseolus coccineus L. pulvinar motor cells show circadian volume oscillations. Chronobiol Int 11:156–164
Mayer WE, Ruge WA, Starrach N, Hampp R (1987) Chloride availability affects the malate content and its control by the circadian clock in pulvini of Phaseolus-coccineus L. J Biosci 42:553–558
Mayer WE, Hohloch C, Kalkuhl A (1997) Extensor protoplasts of the Phaseolus pulvinus: light-induced swelling may require extracellular Ca2+ influx, dark-induced shrinking, inositol 1,4,5-trisphosphate-induced Ca2+ mobilization. J Exp Bot 48:219–228
Mayer WE, Bok B, Rieger A (1999) Age-dependent changes of the ion content and the circadian leaf movement period in the Phaseolus pulvinus. J Biosci 24:199–206
McClung CR, Davis SJ (2010) Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr Biol 20:1086–1089
Memon AR, Boss WF (1990) Rapid light-induced changes in phosphoinositide kinases and H+-ATPase in plasma membrane of sunflower hypocotyls. J Biol Chem 265:14817–14821
Millet B, Botton AM, Hayoum C, Koukkari WL (1988) An experimental analysis and comparison of 3 rhythms of movements in bean (Phaseolus vulgaris L). Chronobiol Int 5:187–193
Millet B, Coillot L, Agosti RD (1989) The rhythmic leaf movements after regeneration of partially excised pulvinus in Phaseolus vulgaris L. Plant Cell Physiol 30
Montague MJ (1995) Hormonal and gravitropic specificity in the regulation of growth and cell wall synthesis in pulvini and Internodes from shoots of Avena sativa L. (Oat). Plant Physiol 107:553–564
Montague MJ (1997) Exogenous jasmonic and abscisic acids act differentially in elongating tissues from oat stem segments. J Plant Growth Reg 16:11–19
Moran N (1996) Membrane-delimited phosphorylation enables the activation of the outward-rectifying K channels in a plant cell. Plant Physiol 111:1281–1292
Moran N (2007a) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347
Moran N (2007b) Rhythmic leaf movements: physiological and molecular aspects. In: Mancuso S, Shabala S (eds) Rhythms in plants: phenomenology, mechanisms, and adaptive significance. Springer, Berlin, pp 3–38
Moran N, Ehrenstein G, Iwasa K, Mischke C, Bare C, Satter RL (1988) Potassium channels in motor cells of Samanea saman: a patch-clamp study. Plant Physiol 88:643–648
Moran N, Fox D, Satter RL (1990) Interaction of the depolarization-activated K channel of Samanea saman with inorganic ions: a patch-clamp study. Plant Physiol 94:424–431
Moran N, Yueh YG, Crain RC (1996) Signal transduction and cell volume regulation in plant leaflet movements. News Physiol Sci 11:108–114
Morillon R, Catterou M, Sangwan RS, Sangwan BS, Lassalles JP (2001) Brassinolide may control aquaporin activities in Arabidopsis thaliana. Planta 212:199–204
Morse MJ, Satter RL (1979) Relationship between motor cell ultrastructure and leaf movement in Samanea saman. Physiol Plant 46:338–346
Morse MJ, Crain RC, Satter RL (1987) Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci USA 84:7075–7078
Moshelion M, Moran N (2000) K+-efflux channels in extensor and flexor cells of Samanea saman are not identical. Effects of cytosolic Ca2+. Plant Physiol 124:911–919
Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R (2002a) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14:727–739
Moshelion M, Becker D, Czempinski K, Mueller-Roeber B, Attali B, Hedrich R, Moran N (2002b) Diurnal and circadian regulation of putative potassium channels in a leaf moving organ. Plant Physiol 128:634–642
Moyen C, Cognard C, Fleurat Lessard P, Raymond G, Roblin G (1995) Calcium mobilization under a UV-A irradiation in protoplasts isolated from photosensitive pulvinar cells of Mimosa pudica. J Photochem Photobiol 29:59–63
Moysset L, Simon E (1989) Role of calcium in phytochrome-controlled nyctinastic movements of Albizzia lophantha leaflets. Plant Physiol 90:1108–1114
Moysset L, Sugranes SL, Simon E (1991) Changes in morphometry and elemental composition of Robinia pseudoacacia pulvinar motor cells during leaflet movements. J Exp Bot 42:1315–1324
Moysset L, Fernandez E, Cortadellas N, Simon E (2001) Intracellular localization of phytochrome in Robinia pseudoacacia pulvini. Planta 213:565–574
Nakamura Y, Mithöfer A, Kombrink E, Boland W, Hamamoto S, Uozumi N, Tohma K, Ueda M (2011) 12-Hydroxyjasmonic acid glucoside is a COI1-JAZ-independent activator of leaf-closing movement in Samanea saman. Plant Physiol 155:1226–1236
Nishizaki Y (1990) Effects of anoxia and red light on changes induced by blue light in the membrane potential of pulvinar motor cells and leaf movement in Phaseolus vulgaris. Plant Cell Physiol 31:591–596
Nishizaki Y (1994) Vanadate and dicyclohexylcarbodiimide inhibit the blue light-induced depolarization of the membrane in pulvinar motor cells of Phaseolus. Plant Cell Physiol 35:841–844
Nishizaki Y, Kubota M, Yamamiya K, Watanabe M (1997) Action spectrum of light pulse-induced membrane depolarization in pulvinar motor cells of Phaseolus. Plant Cell Physiol 38:526–529
Okazaki Y (2002) Blue light inactivates plasma membrane H+-ATPase in pulvinar motor cells of Phaseolus vulgaris L. Plant Cell Physiol 43:860–868
Okazaki Y, Nishizaki Y, Iwasaki N (1995) Effects of a pulse of blue light on the extracellular pH in the pulvinus of Phaseolus vulgaris L.: measurements with a double-barreled pH-sensitive electrode. Plant Cell Physiol 36:1131–1134
Pfeffer WF (1877) Osmotische Untersuchungen: Studien zur Zell-Mechanik (Osmotic investigations: studies on cell mechanics). Engelmann W, Leipzig
Racusen R, Satter RL (1975) Rhytmic and phytochrome-regulated changes in transmembrane potential in Samanea pulvini. Nature 255:408–410
Roblin G, Fleurat-Lessard P, Bonmort J (1989) Effects of compounds affecting calcium channels on phytochrome—and blue pigment-mediated pulvinar movements of Cassia fasciculata. Plant Physiol 90:697–701
Ruoff P, Rensing L, Kommedal R, Mohsenzadeh S (1997) Modeling temperature compensation in chemical and biological oscillators. Chronobiol Int 14:499–510
Saeedi S, Roblin G (1987) Action of benzoic acid and its o-, m- and p-hydroxy-derivatives on the dark- and light-induced leaflet movements in Cassia fasciculata Michx. Plant Cell Physiol 28:1109–1115
Saeedi S, Rocher F, Bonmort J, Fleurat-Lessard P, Roblin G (2013) Early membrane events induced by salicylic acid in motor cells of the Mimosa pudica pulvinus. J Exp Bot 64:1829–1836
Salome PA, Michael TP, Kearns EV, Fett-Neto AG, Sharrock RA, McClung CR (2002) The out-of-phase 1 mutant defines a role for PHYB in circadian phase control in Arabidopsis. Plant Physiol 129:1674–1685
Satter RL, Galston AW (1974) Potassium flux: a common feature of Albizzia leaflet movement controlled by phytochrome or endogenous rhythm. Science 174:518–520
Satter RL, Galston AW (1981) Mechanisms of control of leaf movements. Annu Rev Plant Physiol 32:83–110
Satter RL, Guggino SE, Lonergan TA, Galston AW (1981) The effects of blue and far-red light on rhythmic movements in Samanea and Albizzia. Plant Physiol 67:965–968
Satter RL, Garber RC, Khairallah L, Cheng YS (1982) Elemental analysis of freeze-dried thin sections of Samanea motor organs: barriers to ion diffusion through the apoplast. J Cell Biol 95:893–902
Satter RL, Xu YJ, Depass A (1987) Effects of temperature on H+-secretion and uptake by excised flexor cells during dark-Iinduced closure of Samanea leaflets. Plant Physiol 85:850–855
Satter RL, Gorton HL, Vogelmann TC (eds) (1990) The pulvinus: motor organ for leaf movement, vol 3. ASPB, Rockville
Schildknecht H, Meier-Augenstein W (1990) Role of turgorins in leaf movement. In: Satter RL, Gorton HL, Vogelmann TC (eds) The pulvinus: motor organ for leaf movement, vol 3. ASPB, Rockville, pp 205–213
Schumaker KS, Sze H (1987) Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem 262:3944–3946
Setty S, Jaffe MJ (1972) Phytochrome-controlled rapid contraction and recovery of contractile vacuoles in the motor cells of Mimosa pudica as an intracellular correlate of nyctinasty. Planta 108:121
Shacklock PS, Read ND, Trewavas AJ (1992) Cytosolic free calcium mediates red-light induced phototomorphogenesis. Nature 358:753–755
Shimazaki K, Iino M, Zeiger E (1985) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319:324–326
S-i Inoue, Kinoshita T, K-i Shimazaki (2005) Possible Involvement of phototropins in leaf movement of kidney bean in response to blue light. Plant Physiol 138:1994–2004
Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R (2004) The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J 37:147–155
Simon E, Satter RL, Galston AW (1976) Circadian rhythmicity in excised Samanea pulvini II: resetting the clock by phytochrome conversion. Plant Physiol 58:421–425
Starrach N, Meyer W-E (1989) Changes of the apoplastic pH and K+ concentration in the Phaseolus pulvinus in situ in relation to rhythmic leaf movements. J Exp Bot 40:865–873
Stevenson JM, Pepera IY, Heilmann I, Persson S, Boss WF (2000) Inositol signaling and plant growth. Trends Plant Sci 5:252–258
Stoeckel H, Takeda K (1993) Plasmalemmal, voltage-dependent ionic currents from excitable pulvinar motor cells of Mimosa pudica. J Membr Biol 131:179–192
Stoeckel H, Takeda K (1995) Calcium-sensitivity of the plasmalemmal delayed rectifier potassium current suggests that calcium influx in pulvinar protoplasts from Mimosa pudica L. can be revealed by hyperpolarization. J Membr Biol 146:201–209
Suh S, Moran N, Lee Y (2000) Blue light activates depolarization-dependent K+ channels in flexor cells from Samanea saman motor organs via two mechanisms. Plant Physiol 123:833–843
Sweeney BM (1987) Rhythmic phenomena in plants, 2nd edn. Academic Press Inc, San Diego
Temmei Y, Uchida S, Hoshino D, Kanzawa N, Kuwahara M, Sasaki S, Tsuchiya T (2005) Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Lett 579:4417–4422
Toriyama H, Jaffe MJ (1972) Migration of calcium and its role in the regulation of seismonasty in the motor cells of Mimosa pudica L. Plant Physiol 49:72–81
Ueda M, Manabe Y, Otsuka Y, Kanzawa N (2011) Cassia obtusifolia MetE as a cytosolic target for potassium isolespedezate, a leaf-opening factor of Cassia plants: target exploration by a compact molecular-probe strategy. Chemistry – An Asian Journal 6: 3286–3297
van Doorn WG, Kamdee C (2014) Flower opening and closure: an update. J Expt Bot 65:5749–5757
van Doorn WG, van Meeteren U (2003) Flower opening and closure: a review. J Exp Bot 54:1801–1812
Varin L, Chamberland H, Lafontaine Jean G, Richard M (1997) The enzyme involved in sulfation of the turgorin, gallic acid 4-O-(beta-D-glucopyranosyl-6′-sulfate) is pulvini-localized in Mimosa pudica. Plant J 12:831–837
Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748
Wang H (2005) Signaling mechanisms of higher plant photoreceptors: a structure-function perspective. In: Schatten GP (ed) Current Topics of Development Biology, vol 68. Academic Press, London, pp 227–261
Wang H, Wang H (2015) Phytochrome signaling: time to tighten up the loose ends. Int Mol Plant. doi:10.1016/j.molp.2014.11.021
Wang X, Haga K, Nishizaki Y, Iino M (2001) Blue-light-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42:1363–1372
Wang X, Wang Q, Nguyen P, Lin C (2014) Cryptochrome-mediated light responses in plants. Enzymes 35:167–189
Wetherell DF (1990) Leaf movements in plants without puilvini. In: Satter RL, Gorton HL, Vogelmann TC (eds) The pulvinus: motor organ for leaf movement, vol 3. ASPB, Rockville, pp 72–78
White PJ, Bowen HC, Demidchik V, Nichols C, Davies JA (2002) Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta Biomembr 1564:299–309
Wigoda N, Moshelion M, Moran N (2014) Is the leaf bundle sheath a “smart flux valve” for K+ nutrition? J Plant Physiol 171:715–722
Wood NT, Haley A, Viry-Moussaid M, Johnson CH, van der Luit AH, Trewavas AJ (2001) The calcium rhythms of different cell types oscillate with different circadian phases. Plant Physiol 125:787–796
Yanovsky MJ, Mazzella MA, Casal JJ (2000) A quadruple photoreceptor mutant still keeps track of time. Curr Biol 10:1013–1015
Yu L, Moshelion M, Moran N (2001) Extracellular protons inhibit the activity of inward-rectifying K channels in the motor cells of Samanea saman pulvini. Plant Physiol 127:1310–1322
Yu L, Becker D, Levi H, Moshelion M, Hedrich R, Lotan I, Moran A, Pick U, Naveh L, Libal Y, Moran N (2006) Phosphorylation of SPICK2, an AKT2 channel homologue from Samanea motor cells. J Expt Bot 57:3583–3594
Zhou C, Han L, Fu C, Chai M, Zhang W, Li G, Tang Y, Wang Z-Y (2012) Identification and characterization of petiolule-like pulvinus mutants with abolished nyctinastic leaf movement in the model legume Medicago truncatula. New Phytol 196:92–100
Acknowledgment
I am grateful for the illuminating comments on the previous version of this chapter from Dr. Virginia S. Berg. The errors, however, are entirely my own.
The work in my laboratory is supported by THE ISRAEL SCIENCE FOUNDATION (grant No 1312/12)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Moran, N. (2015). Rhythmic Leaf Movements: Physiological and Molecular Aspects. In: Mancuso, S., Shabala, S. (eds) Rhythms in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-20517-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-20517-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20516-8
Online ISBN: 978-3-319-20517-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)