Abstract
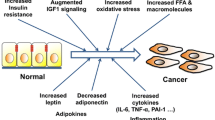
In the late years, both obesity and overweight have been proposed to be the cause for approximately 20 % of all cancers (Wolin, Oncologist 15(6):556–565, 2010). Considering that both obesity and thyroid cancers have been presenting a growing incidence, and literature has been showing that these conditions seem to be associated, it is necessary to better understand and link recent studies in order to characterize and understand the contribution of obesity-related factors that might influence thyroid cancer development and progression. It is well known that many vital processes, such as insulin sensitivity, angiogenesis control, activation of the complement system and responses like inflammation are mediated by products and processes of the adipose tissue. Although these processes have their own molecular pathways, they involve the same molecules through which obesity and adipose tissue might exert their role in carcinogenesis, not only affecting MAPK and PI3K or even insulin pathways, but also recruiting local inflammatory responses that could result in disease formation and progression. In this chapter, we describe three important factors that may explain the connection between obesity and thyroid cancer: thyroid hormones, inflammation and, adipokines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Although during the last one decade literature has been consistently showing associations between obesity and cancers, since excessive weight is an extremely varied condition with different factors involved and a wide range of molecular factors might be implicated in this associations [1]. In addition, cancers present extremely complex and diverse pathways implicated in their occurrence and progression. Solid evidence has been presented connecting endometrial and postmenopausal breast cancers to obesity by endogenous estrogen levels [2–6]. On the other hand, literatures suggest that gallbladder, esophagus, lymphomas and myelomas might be influenced by inflammation, a very important factor in the obesity [2, 7–10]. Besides, pancreatic and colon cancers have been linked with obesity through insulin-related pathways [2, 11, 12].
Regarding thyroid tumors, the observational studies linking obesity to these neoplasms are quite convincing, showing a clear relationship between excessive weight (i.e. overweight and obesity) and thyroid cancers, especially differentiated thyroid cancers (DTCs) [13, 14]. However, literature has not yet unveiled the mechanisms behind this association and causal factor(s) has not yet been established linking DTCs and obesity.
In order to understand the possible mechanisms linking these two conditions, it is necessary to revisit some epidemiological data and molecular pathways involved in DTC and obesity; thus trying to create a rationale on how these two conditions are linked as well the possible factors that might justify this association.
Introduction of Thyroid Cancer
Obesity and overweight have long been recognized as triggers for many metabolic complications, such as hypertension, hypercholesterolemia, insulin resistance leading to Type 2 diabetes and different types of cancer. Importantly, thyroid cancer has been recoded to be worldwide increasing during the last few decades [15–19].
Thyroid cancer presents different histological types. The vast majority of thyroid carcinomas consist of two types of tumors: Papillary Thyroid Carcinomas (PTCs) and Follicular Thyroid Carcinomas (FTCs). These two types are derived from follicular cells and are classified as DTCs. The other types are considered rare and more aggressive. Among these are, undifferentiated or Anaplastic Thyroid Cancers (ATCs) represent approximately 1 % of all thyroid carcinomas, while Medullary Thyroid Cancers (MTCs) are derived from parafollicular cells, represent only 3 % of all thyroid carcinomas [20]. The most common are PTCs representing approximately 85 % of epithelial thyroid malignancies, and these are mostly responsible for the increase in the incidence of thyroid cancers in general [21]. They are indolent cancers and most of them do not have a considerable clinical evolution that would lead patients to death [22]. This is especially noticeable when we look into recent data showing that although the incidence of thyroid cancers has significantly increased in recent years, mortality rates remained stable, suggesting that many of these tumors possibly would not present a clinical evolution [23].
However, the reasons why DTCs’ incidence has increased are not fully comprehended and lead to controversies [24]. Several authors suggest that this increased incidence is solely related to the improvement in diagnostic methods and the population’s access to them, since tumors that present the highest incidence rates are those with small size, and in the past they could not be detected by the clinical examination then existed involving on neck palpation [17, 25, 26]. However, other clinicians have shown that the increased incidence is not only due to tumors size smaller than 1 cm (favored by a better image scanning) but also includes larger tumors, making it difficult to affirm that changes in DTC incidence were taking place exclusively due to improvements in diagnostic techniques and improvements in health care [27].
Hence, it is necessary to investigate other factors that may be contributing to this remarkable increase in thyroid cancer incidence. Exposure to ionizing radiation, iodine intake, family history of thyroid disease, hormonal and reproductive factors and altered thyroid stimulating hormone (TSH) levels are well-established and recognized risk factors for thyroid cancers. Recent studies moreover suggest that the genetic profile, presence of inflammation in the peritumoral area and also body mass index (BMI) should be considered as potential risk factors for DTCs [13, 19, 28]. It is to be noted that based on BMI, two terminologies have now been globally accepted for differentiating overweight and obesity. Those people with BMI of between 25 and 30 are considered to be overweight and those between 30 and 40 are obese and weight over 40 are morbidly obese. In this chapter terminology “obesity” will be used for both.
Relationship Between Obesity and Thyroid Cancer
The first obesity boom was reported during 1980s, leading the scientific community to conduct deeper investigations on obesity and its association with other diseases, mainly through observational studies. It is important to remember that there are two different types of observational studies concerning the relationship between the two conditions, obesity and thyroid cancer: (a) thyroid cancer prevalence is investigated in obese patients; (b) patients with thyroid cancer are screened for obesity. Although both approaches to give relevant information and pictures about the two conditions, the second studies needs to be evaluated more thoroughly. This is because obesity could be linked as an epiphenomenon of thyroid cancers, albeit there is no evidence that these cancers induce important metabolic syndrome. The second type of study, however, is necessary when the objectives of the study include the investigation of clinic-pathological features and their modification by other factors, such as the presence of obesity. Below the results of the studies will be presented in chronological orders with special attention to the latest findings.
1990–2000
Earliest studies showing a relationship between obesity and thyroid cancer dates back to from 1980 [29]. Then in 1990, Dal Maso et al. performed a meta-analysis including 12 case-control studies, analyzing a total of 2056 females and 417 males with thyroid cancer and demonstrated that at diagnosis there was a relationship between BMI and thyroid cancer in women [30]. However, more serious studies with large cohorts, inclusion/exclusion factors and well-described statistical analysis appear to have started only after year 2000. During the following years, several authors described the relationship between obesity and thyroid cancer, mainly through observational studies.
2001–2010
The first strong evidences for a link between obesity and thyroid cancer came from large cohort studies of 30 years by Samanic et al. which included a cohort of 3,668,486 white and 832,214 black male US veterans. They found that obese persons presented a higher risk of developing thyroid cancer. These authors also demonstrated that this increased risk was independent of racial factors, since in this study, white men presented a 1.4 relative risk (RR) and black men presented a 1.9 RR for thyroid cancer [31]. Similarly, Engeland et al. carried out another study with a large cohort (2,000,947 individuals) for 23 years. These authors were able to identify 3046 individuals who developed thyroid cancer during this period, out of which 1415 were obese [32]. Also it was concluded that both men and women had equally increased risk for thyroid cancer. However, when these authors stratified thyroid cancer into its histological subtypes, they confirmed that the association between BMI and thyroid cancer was stronger in females, who presented an increased risk for PTC (RR = 1.19) and FTC (RR = 1.63) and a lower risk of MTC (RR = 0.35) [32], suggesting that the association between thyroid cancer and obesity was due to DTCs. All these data regarding an exclusive association between obese women and thyroid cancer need to be carefully re-analyzed, especially considering two factors: (i) hormones and hormone synthesis might be affected by obesity, and they play an important role on thyroid carcinogenesis, justifying these associations; (ii) it is widely known that women are more susceptible to thyroid cancer, therefore the number of male cases included in thyroid cancer studies is commonly low, leading to the absence of any significant statistical analysis [33]. By the year 2010, higher BMI and obesity had been consistently reported as risk factors for thyroid cancer, but literatures were still scarce on providing mechanisms that would possibly link these conditions, except a few studies pointing out to insulin-related pathways as the major factors that would justify the reported associations [1, 34–42].
2011-Present
In this decade, many authors have been investigating factors that would link obesity and thyroid cancer; these include clinical and molecular features which require further studies.
Between 2011 and 2012, Kitahara et al. published three articles addressing the link between obesity and thyroid cancers. These authors studied a very large cohorts, demonstrating that obese individuals presented higher risks {Hazard Ratios (HRs): 1.20 and 1.53, respectively} of developing thyroid cancer when compared with eutrophic individuals [43]. They also described that both men and women with large waist circumference (>102 cm in men and >88 cm in women) presented an increased risk for thyroid cancer (HR = 1.79 and HR = 1.54, respectively) [45]. Kitahara et al. subsequently reported that individuals with excessive weight who were practicing greater amount of physical activity were at higher risk of thyroid cancer. Additionally in 2012, Rinaldi et al. demonstrated an association between high BMI and thyroid cancer in women, when they analyzed a cohort of 343,765 females and 146,824 males, with 566 incident thyroid cancers [44]. In a meta-analysis of five large cohort studies, which included 8,099,411 individuals and 5154 thyroid cancer patients, Zhao et al. described that excessive weight was associated with an increased risk (Odds ratio – OR = 1.18) of thyroid cancers [13]. Our group has also confirmed these results, in that in 2012 we demonstrated that excessive weight was associated with increased risk of DTCs (OR = 3.787). We also suggested that this association could be linked to excessive caloric ingestion (OR = 5.89), mainly due to the excess ingestion of proteins (OR 4.60) and carbohydrates (OR 4.90) [44].
Later Kim et al. showed that obesity was not only associated with an increased risk of thyroid cancers, but could also exert an influence on tumor presentation. These authors reported that a 5-kg/m2 increase in BMI was associated with PTCs >1 cm (OR = 1.31), microscopic extrathyroidal invasion (OR = 1.23), and with advanced tumor node metastasis (TNM) stage (OR = 1.30) [45]. In an interesting study, Han et al. demonstrated that out of 15,068 subjects that underwent a routine health checkup and when screened by thyroid ultrasonography, 7472 presented cystic or solid nodules and 267 patients were confirmed with thyroid cancer after further investigation. Among these cases, the authors found that the prevalence of thyroid cancer in women was associated with a high BMI (OR = 1.63). Also during this year, Pellegriti et al. included obesity as a potential risk factor that would justify the remarkable increase in thyroid cancer incidence in the latest years, given the convincing results what literature has been canvasing since year 2000 [21].
The year 2014 was especially fruitful to explore the relationship between thyroid cancer and obesity in that several articles appeared addressing this issue and, even though a considerable part of them focused on mechanisms justifying this relationship, there were also interesting results in observational studies. Kitahara et al. analyzed 321,085 children from the Copenhagen School Health Records Register including measurements of height and weight from 7 to 13 years of age. These children were followed-up for a median of 38 years, and during this period 171 women and 64 men were diagnosed with thyroid cancer. Both height and increased BMI were positively associated with thyroid cancer risk, suggesting that not only obesity is a risk factor for thyroid cancer, but also it may impact thyroid cancer risk in adult life [46]. In a pooled analysis of three case-control studies, including 1917 patients with PTC and 2127 controls, Xu et al. demonstrated an increased risk of PTC when patients presented greater weight/BMI (OR = 1.72 for overweight vs. normal weight and OR = 4.17 for obese vs. normal weight). This increase was also reported for body fat percentage (OR = for women and OR = for men, considering the lowest quartile vs. the highest quartile) [47]. In the same year Arduc et al., using fine-needle aspiration biopsy, suggested that the presence of obesity and large waist circumference can be used as predictors of thyroid carcinoma in patients with Hurtle-cell lesions. These authors studied 224 women with these lesions, who had underwent thyroidectomy and found that malignancy risk was 3.819 higher in the obese group. Besides, large waist circumference was also shown to be linked with increased risk for malignant lesions (OR = 5.593) [48]. Zhang et al. employing a meta-analysis of large cohort studies, including 16 studies with 12,616,154 subjects showed that the link between obesity and thyroid cancer was higher in males (RR = 1.35) than in females (RR = 1.29). This association was maintained when these authors analyzed the other factors such as age (RR = 1.34), smoking (RR = 1.36), alcohol use (RR = 1.40), and history of benign thyroid disease (RR = 1.51), confirming that data presented by literature so far is consistent and points out to obesity as risk factors for thyroid cancer [49].
Molecular Mechanisms of Thyroid Cancers in Obesity
Both obesity and thyroid cancers are multifactorial diseases that lead to many systematic modifications in patients. Although a considerable part of these modifications have been clinically detected by physical and laboratorial examinations, but some of them can only be detected by molecular analysis. The latest findings, based on studies at molecular levels have been making it possible to hypothesize that obesity and thyroid cancers have more in common than it was ever speculated. There are specific points that need to be considered if we look into molecular mechanisms connecting obesity and thyroid cancers: Factors involved with these studies include thyroid hormones, adipokines and factors inducing inflammation
Thyroid Hormones
The thyroid gland plays a crucial role in the control of energy metabolism through action of thyroid hormones. There is evidence that the abdominal obesity and a tendency to weight gain are associated with small variations of thyroid hormone levels in euthyroid subjects [50, 51]. Also an association has been reported between low thyroxine (T4) levels and fat accumulation [50, 52].
A positive link has also been reported between free triiodothyronine (T3) levels and both, waist circumference and higher BMI in obese subjects [53]. A moderate increased T3 level in obese individuals has been explained as a compensatory higher conversion of T4 to T3 in order to improve energy expenditure and fat accumulation [53]. The TSH, which promotes the secretion of thyroid hormones to regulate energy expenditure, has also been shown to be altered in obese euthyroid subjects, and a positive link between its levels and increased BMI has been observed [51, 54].
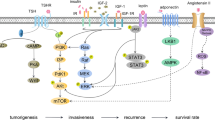
To explain the increase of thyroid stimulating hormone (TSH) in obese individuals, hormonal mediators of adipose tissue, especially leptin, has been suggested as potential stimulators of the hypothalamic-pituitary-thyroid axis [55, 56]. Leptin modulates food intake and energy expenditure and acts as a neuroendocrine regulator, regulating thyrotropin releasing hormone (TRH) expression in the paraventricular nucleus; TSH will then stimulate leptin secretion by adipose tissue [57–60]. In addition, leptin may affect deiodinases, activating the T4 to T3 conversion [61, 62].
Interestingly, besides the association between TSH and BMI, there is a clinical evidence that high TSH levels are linked to increased risk of malignancy in human thyroid nodules at advanced stage of the disease [63]. TSH being the major stimulator of thyrocytes proliferation, we hypothesize a direct role of this hormone in thyroid carcinogenesis in obese individuals [64]. In fact, the mitogenic effects of TSH on follicular cells have been demonstrated using in vitro and animal studies [65–67]. The binding of TSH to its receptor, TSHR, increases the intracellular levels of cAMP and activates proliferation pathways, including PI3K–AKT and RAS–BRAF pathways [68, 69]. The interaction of TSH with insulin represents another possible mechanism linking obesity and thyroid cancer [70]. In insulin resistance, a clinical condition frequently present in obesity is insulin stimulates TSH production promoting proliferation of thyroid cancer cells [64, 71].
Although the importance of TSH in thyroid cell proliferation has been clearly demonstrated, the role of serum TSH levels in tumor growth and anaplastic changes have not been found in animal models [72]. The increased risk of thyroid cancer in obese subjects has also shown to be unrelated to serum TSH levels in human subjects [48, 73]. A clinical study nevertheless has also shown that, in spite of the significantly high TSH levels found in morbidly obese women compared with normal weight and/or slightly overweight women, the prevalence of thyroid nodules has shown to be significantly lower in obese women [74].
In conclusion, there is evidence pointing to a possible involvement of TSH as etiopathogenic element linking obesity and thyroid cancer. However, the present studies and the current data do not allow us to confirm or exclude the possibility of this involvement.
Cytokines, Adipokines and Inflammation
Adipose tissue was once considered as a simple aggregation of cells that are able to store fat in our body. However, the advancement of molecular and cell biology gave us an insight that probably a link exist between obesity and inflammation. In fact, fat cells may be considered a component of the immune system, as it expresses receptors for many cytokines and also produce many proteins and hormones that modulate immune response [75–77]. It has been demonstrated that immune cells infiltrate adipose tissue at the onset of weight gain and directly contribute to and perpetuate the inflammatory state of fat, systemic insulin resistance, and the promotion of obesity [78].
The altered production or dysfunction of adipokines has been implicated in the metabolic syndrome of obesity [79]. In fact, adipose tissue in obese persons produce more proinflammatory substances (such as TNF-alpha, IL-6, iNOS, TGF-β1 and C-reactive protein) than adipose tissue in lean individuals [80–84]. Uysal et al. were able to generate obese mice with a targeted null mutation in the gene encoding TNF-α and those encoding the two receptors for TNF-alpha (Ref). The absence of TNF-α in the null mutant mice resulted in significantly improved insulin sensitivity in both diet-induced obesity and that resulting for the ob−/ob− obese mice. The TNF- α deficient obese mice had lower levels of circulating free fatty acids and were protected from the obesity-related reduction in the insulin receptor signaling in muscle and fat tissues. These results indicate that TNF- α is an important mediator of insulin resistance in obesity through its effects on several important sites of insulin action, suggesting that adipose tissue of obese patients is inflamed [85]. Weisberg et al. studied the transcript profile of adipose tissue of obese animals and found that the expression of 1304 transcripts correlated significantly with body mass. Of the 100 most significantly correlated genes, 30 % encoded proteins that were characteristic of macrophages and are positively correlated with body mass. Immunohistochemical analysis of perigonadal, perirenal, mesenteric, and subcutaneous adipose tissue revealed that the percentage of cells expressing the macrophage marker F4/80 was significantly and positively correlated with both adipocyte size and body mass [79]. Similar relationship was found in human subcutaneous adipose tissue stained for the macrophage antigen CD68 [85]. These results suggests that not only proinflammatory proteins are produced but also there is an enrichment of macrophages in adipose tissue of patients with obesity. How this inflamed state associate with obesity and cancer is the question remains to be asked.
TNF- α is a hormone that is thought to mediate tumor cytotoxicity as well as new blood vessel growth [86]. Liu et al. investigated whether the Wnt pathway, an intracellular signaling cascade that plays a critical role in colorectal carcinogenesis, is activated by obesity-induced elevation of the inflammatory cytokine TNF- α (Ref). The phosphorylation of glycogen synthase kinase 3 β (GSK3β), an important intermediary inhibitor of Wnt signaling and a potential target of TNF- α, was quantitated by immunohistochemistry. The inactivated (phosphorylated) form of glycogen synthase kinase 3 β was elevated in the colonic mucosa of obese mice. Moreover, β-catenin, the key effector of canonical Wnt signaling also was elevated in the colons of obese mice, as was the expression of a downstream target gene, c-myc (Ref). These data demonstrate that diet-induced obesity produces an elevation in colonic TNF- α and instigates a number of alterations of key components within the Wnt signaling pathway that are pro-transformational in nature.
In thyroid cancer a particular mechanism may be elicited. Pang et al. demonstrated that TNF- α has an anti-proliferative action in human papillary thyroid cancer cell line through a receptor-mediated mechanism [87]. However, the exposure of papillary thyroid cancer cell to TNF- α resulted in the development of progressively increasing loss of the TNF- α-induced anti-proliferation, termed resistance [88]. Probably, the high TNF- α exposure provided by obesity may be inducing TNF- α resistance that facilitates thyroid tumor progression [13]. Interestingly, Rotondi et al. recently investigated whether metformin inhibits the secretion of CXCL8, induced by TNF- α in primary cultures of normal and tumor human thyroid cells as well as in thyroid cancer cell lines. They found that metformin significantly and dose-dependently inhibited the TNF- α-induced CXCL8 secretion in both normal thyrocytes and papillary thyroid cancer cells [89]. CXCL8 directly stimulates the proliferation of thyroid tumor cells via autocrine and paracrine mechanisms beside the fact that CXCL8 also plays a crucial role in promoting the invasiveness of thyroid tumor cells [90]. Thus, the inhibitory effect of metformin on TNF- α-induced CXCL8 secretion could be considered as an additional indirect anticancer property of the drug.
Adipokines – Link Between Obesity and Inflammation
Adipokines or adipocytokines are a subset of cytokines produced by the adipose tissue [91]. They are involved in several crucial processes for human metabolic systems including immunity, regulation of appetite and energy balance, insulin sensitivity, angiogenesis, blood pressure regulation and lipid metabolism [92]. It is well known that obesity is intimately linked with inflammation. Obese individuals also run higher risk to develop insulin resistance. Recent research suggests that when both insulin resistance and inflammation are present they alter the inflammatory profile in that it induces the production of anti-inflammatory factors such as adiponectin which leads to the production of pro-inflammatory adipokines, such as leptin and resistin [93]. Also adipokines can promote tumorigenesis as they have already been implicated in the regulation of inflammation and insulin sensitivity, hence representing the link between inflammation, obesity and cancer [49].
Adiponectin is an adipokine with strong anti-inflammatory properties. It is exclusively produced by adipocytes, and promotes the cells’ and adipocyte differentiation and increase insulin sensitivity [94, 95]. Pro-inflammatory factors such as TNF-α, IL-6 and ROS which can play a regulatory role in adiponectin expression. However, recent evidence show that there exists a regulatory feedback loop through which adiponectin controls its own production and the expression of its receptor [96]. Adiponectin also acts as an autocrine and paracrine factor to inhibit the secretion by adipocytes of pro-inflammatory factors such as TNF-α, IL-10, macrophages, T-cells, NK-cells, inducing effects on the storage of lipids and insulin sensitivity in adipocytes. Adipokine is also able to influence cell proliferation and regulate the balance of anti-?what? and always aiming to control inflammation [96–99]. In order to develop these functions, adiponectin binds to two different receptors, AdipoR1 and AdipoR2. These receptors have an important role in improving the insulin signaling on target cells, through the increase in AMPK activity, and PPARα and PGC-α. These molecules might also lead to a reflex on AKT/mTOR/PI3K and MAPK pathways, well known for regulation of cell proliferation [100].
In addition, adiponectin also influences the immune system through NF-κB regulation [101]. Due to its complex antiproliferative and inflammation-restraining functions, this hormone has been linked to breast, endometrial, prostate, colorectal, liver, pancreatic and gastric cancers, as well as some hematological types of leukemia, lymphoma, and myeloma [101]. Recently, evidence presented that adiponectin plays role in developing thyroid cancers. Mitsiades et al. demonstrated that adiponectin serum levels are inversely correlated with DTC, exerting a protective effect against the development of this cancer. Furthermore, these authors demonstrated that thyroid tissues express AdipoR1 and AdipoR2, facilitating the entrance and the functioning of adiponectin in the thyroid [102]. Thus, suggesting that not only adiponectin is expressed in thyroid cells, but it is also functional in them. Another recent study has demonstrated that adiponectin receptors might be important for DTCs (Ref). Comparing tissues of primary papillary thyroid carcinomas with metastatic tissues, Cheng et al. reported that 27 % of primary tumors expressed AdipoR1 and 47 % expressed AdipoR2. When tissues were negative for both receptors, tumors were significantly associated with extrathyroidal invasion, multicentricity, and higher TNM stage, suggesting that the expression of adiponectin receptors can be employed for better prognosis [103].
Leptin is structurally similar to cytokines, IL-2, IL-6, and granulocyte-colony stimulating factor (G-CSF), a characteristic that makes leptin capable of participating in similar cellular and organic processes, such as the control of food intake through satiety sensation, regulation of energy expenditure, activation of monocytes and macrophages, stimulation of VEGF, angiogenesis, cell proliferation, and the suppression of anti-inflammatory cytokines [92]. It is predominantly secreted by adipose tissue, although it can also be produced by skeletal muscle, stomach and blood? plasma [104]. Leptin acts as an endogenous sensing factor, providing a critical link between the environment, metabolism, and immune function [105].
The mechanisms of leptin’s action involve its binding to leptin receptor b (ObR or LEPR), leading to the activation of intracellular signals through JAK2, STAT3 and AMPK [92]. These factors regulate AKT/mTOR/PI3K and ERK/MAPK pathways, involved in cell growth and survival as well in COX2, IL-1 and NF-κB, induced inflammation and VEGFs, involved in angiogenesis [106]. Thus, leptin interplays with several factors that participate in diverse carcinogenic stages, and its association with breast, prostate, colorectal, hepatocellular, pancreatic and lung cancers, as well as thyroid cancer, has consistently been presented in the literature [107, 108]. Concerning thyroid cancers, and more specifically DTCs, leptin and ObR expression was first demonstrated by Cheng et al., who found them associated with a high risk of lymph node metastases [109]. In a recent study, our group demonstrated that patients with AA genotype of rs7799039 in LEP (the gene that codes for leptin) had higher serum levels of leptin (9.22 ± 0.98 ng/mL) than those with AG genotype (10.07 ± 0.60 ng/mL).
We have also shown that the AG genotype of rs2167270 in LEP also produce higher serum leptin (10.05 ± 0.59 ng/mL) than the subjects with GG genotype (9.52 ± 0.79 ng/mL). The AG genotype of rs7799039 in LEP was an independent risk factor for DTC (OR = 11.689). Similarly, AG and GG genotypes of rs1137101 in LEPR (the gene that codes for leptin receptor) increased the susceptibility to DTC (OR = 3.747 and OR = 5.437, respectively). In this study, we did not find any association between polymorphisms and clinic-pathological features of DTC [110]. Other groups reported leptin’s involvement in the clinical phenotype of DTC, and suggested that leptin may affect the migration of thyroid cells, proposing for a worse prognosis and metastasis formation [111–115].
Resistin is an adipokine produced by human monocytes and macrophages, as well as adipocytes [92]. This adipokine was first linked with insulin resistance by the suppression of insulin-mediated signaling in rat adipocytes, but in humans this association is not always found [116]. In fact, resistin presents diverse functions in humans, such as proliferative, antiapoptotic, pro-inflammatory and pro-angiogenicity [104, 117]. Inflammatory cytokines such as IL-1β, IL-6, TNF-α, and LPS can induce resistin expression, but conversely resistin has been demonstrated to stimulate the production of IL-6 and TNF-α through the NF-κB signaling pathway [118]. In addition to its action on the immune system, resistin can also bind to TLR4, activating JNK and p38 MAPK to induce insulin resistance [119]. Due to its ability to regulate immune factors production and its indirect regulation of MAPK pathway and other proliferative events, resistin has been investigated in human cancers. Its expression has been linked to the increased proliferation of prostate cancer by AKT/mTOR pathway stimulation [72]. Resistin has also been linked to breast, endometrial, colorectal, hepatocellular, pancreatic and lung cancers [117, 120–124].
Our group has studied serum concentrations of leptin, adiponectin, resistin and ghrelin, showing that these adipokines may represent excellent markers for malignancy in thyroid nodules. We further showed that DTC patients presented lower adiponectin serum levels when compared with patients with benign nodules. Leptin, on the other hand was higher in DTC than in benign cases. Similarly, resistin levels were higher in DTC than in patients with benign nodules. When we created ROC curves to investigate the accuracy of using these cytokines levels as diagnostic test, we showed that the concentrations of serum adiponectin, leptin and resistin distinguished benign and malignant nodules with 76 %, 100 % and 100 % accuracy, respectively. These cytokines serum levels also helped to discriminate follicular patterned lesions. The follicular variant of papillary thyroid cancer (FVPTC) could be distinguished from follicular adenomas (FA) by adiponectin and leptin levels and from goiters by serum leptin and resistin levels. FA could be differentiated from FTC and from classic PTC (CPTC) by leptin levels. On the other hand, CPTC differentiated from FA by leptin levels and from goiters by leptin and resistin levels. In conclusion, we found that serum concentrations of adiponectin, leptin and resistin may represent a new alternative approach to the diagnosis of thyroid nodules, especially for cases where fine needle aspiration biopsy cannot give a definitive diagnosis, thus, avoiding more aggressive and unnecessary surgeries and interventions [125].
Conclusion
There is no doubt that adipose tissue is involved in many vital processes and its existence facilitates and improves several crucial events, such as insulin regulation, angiogenesis, energy balance, and the production of many immune system proteins and hormones. Although the processes that involve the adipose tissue and/or its products have their own molecular pathways, they also have the same common proteins through which obesity and adipose tissue might exert their role in carcinogenesis. Additionally they not only affect MAPK and PI3K insulin pathways, but also recruiting local inflammatory responses that could result in disease formation and progression. These are the main mechanisms through which obesity and the metabolic changes that it induces might be linked to thyroid cancers. Understanding these mechanisms might lead to different disease-preventing strategies, not only helping patients, but also sparing health systems worldwide to save money and direct money to more complicated cases, which require more complex treatment and care.
References
Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–65. PubMed Pubmed Central PMCID: 3227989. Epub 2010/05/29. eng.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. PubMed Epub 2008/02/19. eng.
Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43. PubMed Epub 2002/12/24. eng.
Begg L, Kuller LH, Gutai JP, Caggiula AG, Wolmark N, Watson CG. Endogenous sex hormone levels and breast cancer risk. Genet Epidemiol. 1987;4(4):233–47. PubMed Epub 1987/01/01. eng.
Hvidtfeldt UA, Gunter MJ, Lange T, Chlebowski RT, Lane D, Farhat GN, et al. Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1203–12. PubMed PMID: ISI:000306210100025. English.
Strong AL, Strong TA, Rhodes LV, Semon JA, Zhang X, Shi Z, et al. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;15(5):R102. PubMed Epub 2013/11/02. Eng.
Li Y, Zhang J, Ma H. Chronic inflammation and gallbladder cancer. Cancer Lett. 2014;345:242–8. PubMed Epub 2013/08/29. Eng.
Kavanagh ME, O’Sullivan KE, O’Hanlon C, O’Sullivan JN, Lysaght J, Reynolds JV. The esophagitis to adenocarcinoma sequence; the role of inflammation. Cancer Lett. 2014;345:182–9. PubMed Epub 2013/09/03. Eng.
Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. PubMed Epub 2013/08/29. Eng.
Lodh M, Goswami B, Gupta N, Patra SK, Saxena A. Assessment of oxidative stress and inflammatory process in patients of multiple myeloma. Indian J Clin Biochem. 2012;27(4):410–3. PubMed Pubmed Central PMCID: 3477461. Epub 2013/10/02. eng.
Sun A, Liu R, Sun G. Insulin therapy and risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Curr Med Res Opin. 2014;30:423–30. PubMed Epub 2013/10/26. Eng.
Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105(14):1027–35. PubMed Pubmed Central PMCID: 3714020. Epub 2013/07/13. eng.
Marcello MA, Cunha LL, Batista FA, Ward LS. Obesity and thyroid cancer. Endocr Relat Cancer. 2014;21(5):T255–71. PubMed.
Zhao ZG, Guo XG, Ba CX, Wang W, Yang YY, Wang J, et al. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J Int Med Res. 2012;40(6):2041–50. PubMed Epub 2013/01/17. eng.
Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983–2007. Asia Pac J Public Health. 2015;27:NP223–9. PubMed Epub 2012/02/22. Eng.
Jung CK, Lubin JH, Brenner AV, Little MP, Sigurdson AJ, Nikiforov YE. The increase in papillary thyroid cancer incidence in the US during the last four decades is accompanied by a high and stable frequency of BRAF mutations and a sharp increase in NRAS mutations. Mod Pathol. 2012;25:146a-a. PubMed PMID: ISI:000299986900603. English.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7. PubMed Epub 2006/05/11. eng.
Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115(16):3801–7. PubMed Epub 2009/07/15. eng.
Agate L, Lorusso L, Elisei R. New and old knowledge on differentiated thyroid cancer epidemiology and risk factors. J Endocrinol Invest. 2012;35(6 Suppl):3–9. PubMed Epub 2012/10/04. eng.
Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1092–100. PubMed Pubmed Central PMCID: 2667567. Epub 2009/03/19. eng.
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. PubMed Pubmed Central PMCID: 3664492. Epub 2013/06/06. eng.
Brito JP, Morris JC, Montori VM. TOO MUCH MEDICINE Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347:f4706. PubMed PMID: ISI:000323880100006. English.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7. PubMed PMID: ISI:000237391300026. English.
Ward LS, Graf H. Thyroid cancer: increased occurrence of the disease or simply in its detection? Arq Bras Endocrinol Metabol. 2008;52(9):1515–6. PubMed Epub 2009/02/07. Cancer da tiroide: aumento na ocorrencia da doenca ou simplesmente na sua deteccao? por.
Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144(6):1038–43. PubMed PMID: ISI:000261581600054. English.
Sprague BL, Andersen SW, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19(6):585–93. PubMed PMID: ISI:000257327300005. English.
Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–91. PubMed Pubmed Central PMCID: 2676561. Epub 2009/02/26. eng.
Marcello MA, Malandrino P, Almeida JF, Martins MB, Cunha LL, Bufalo NE, et al. The influence of the environment on the development of thyroid tumors: a new appraisal. Endocr Relat Cancer. 2014;21(5):T235–54. PubMed.
Albanes D. Caloric intake, body weight, and cancer: a review. Nutr Cancer. 1987;9(4):199–217. PubMed Epub 1987/01/01. eng.
Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000;11(2):137–44. PubMed Epub 2000/03/10. eng.
Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni Jr JF. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35–43. PubMed Epub 2004/02/19. eng.
Engeland A, Tretli S, Akslen LA, Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95(3):366–70. PubMed Pubmed Central PMCID: 2360634. Epub 2006/07/13. eng.
Pappa T, Alevizaki M. Obesity and thyroid cancer: a clinical update. Thyroid. 2014;24:190–9. PubMed Epub 2013/07/25. Eng.
Mijovic T, How J, Pakdaman M, Rochon L, Gologan O, Hier MP, et al. Body mass index in the evaluation of thyroid cancer risk. Thyroid. 2009;19(5):467–72. PubMed Epub 2009/05/07. eng.
Brindel P, Doyon F, Rachedi F, Boissin JL, Sebbag J, Shan L, et al. Anthropometric factors in differentiated thyroid cancer in French Polynesia: a case-control study. Cancer Causes Control. 2009;20(5):581–90. PubMed Epub 2008/12/02. eng.
Clero E, Leux C, Brindel P, Truong T, Anger A, Teinturier C, et al. Pooled analysis of two case-control studies in New Caledonia and French Polynesia of body mass index and differentiated thyroid cancer: the importance of body surface area. Thyroid. 2010;20(11):1285–93. PubMed Epub 2010/10/12. eng.
Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, et al. Prospective study of body mass index, physical activity and thyroid cancer. Int J Cancer. 2010;126(12):2947–56. PubMed Pubmed Central PMCID: 2919690. Epub 2009/10/02. eng.
Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008;18(4):461–4. PubMed Epub 2008/03/19. eng.
Rezzonico JN, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Increased prevalence of insulin resistance in patients with differentiated thyroid carcinoma. Metab Syndr Relat Disord. 2009;7(4):375–80. PubMed Epub 2009/03/27. eng.
Haddad FH, Malkawi OM, Omari AA, Izzat AS, Khassrof HM, Faiad LM, et al. Diabetes and infarcted papillary thyroid cancer. Saudi Med J. 2002;23(4):467–70. PubMed Epub 2002/04/16. eng.
Akinci M, Kosova F, Cetin B, Aslan S, Ari Z, Cetin A. Leptin levels in thyroid cancer. Asian J Surg. 2009;32(4):216–23. PubMed Epub 2009/11/07. eng.
Cheng SP, Yin PH, Chang YC, Lee CH, Huang SY, Chi CW. Differential roles of leptin in regulating cell migration in thyroid cancer cells. Oncol Rep. 2010;23(6):1721–7. PubMed Epub 2010/04/30. eng.
Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–72. PubMed Pubmed Central PMCID: 3079276. Epub 2011/01/27. eng.
Marcello MA, Sampaio AC, Geloneze B, Vasques AC, Assumpcao LV, Ward LS. Obesity and excess protein and carbohydrate consumption are risk factors for thyroid cancer. Nutr Cancer. 2012;64(8):1190–5. PubMed Epub 2012/11/21. eng.
Kim HJ, Kim NK, Choi JH, Sohn SY, Kim SW, Jin SM, et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol (Oxf). 2013;78(1):134–40. PubMed Epub 2012/07/21. eng.
Kitahara CM, Gamborg M, Berrington de Gonzalez A, Sorensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014;74(1):235–42. PubMed Pubmed Central PMCID: 3891884.
Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014;24(6):966–74. PubMed Pubmed Central PMCID: 4046192.
Arduc A, Dogan BA, Tuna MM, Tutuncu Y, Isik S, Berker D, Guler S. Higher body mass index and larger waist circumference may be predictors of thyroid carcinoma in patients with Hürthle-cell lesion/neoplasm fine-needle aspiration diagnosis. Clin Endocrinol (Oxf). 2015;83(3):405–11. doi:10.1111/cen.12628. Epub 2014 Nov 26.
Zhang W, Bai X, Ge H, Cui H, Wei Z, Han G. Meta-analysis in the association between obesity and risk of thyroid cancer. Int J Clin Exp Med. 2014;7(12):5268–74. PubMed Pubmed Central PMCID: 4307477.
Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–24. PubMed.
Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–92. PubMed.
Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol. 2009;161(3):459–65. PubMed.
De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf). 2007;67(2):265–9. PubMed Epub 2007/06/06. eng.
Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf). 2006;64(2):125–8. PubMed Epub 2006/01/25. eng.
Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71(6):1421–32. PubMed Epub 2000/06/06. eng.
Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol (Oxf). 2003;59(2):258–62. PubMed Epub 2003/07/17. eng.
Menendez C, Baldelli R, Camina JP, Escudero B, Peino R, Dieguez C, et al. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7–12. PubMed Epub 2003/01/15. eng.
Oge A, Bayraktar F, Saygili F, Guney E, Demir S. TSH influences serum leptin levels independent of thyroid hormones in hypothyroid and hyperthyroid patients. Endocr J. 2005;52(2):213–7. PubMed Epub 2005/05/03. eng.
Feldt-Rasmussen U. Thyroid and leptin. Thyroid. 2007;17(5):413–9. PubMed Epub 2007/06/05. eng.
Santini F, Galli G, Maffei M, Fierabracci P, Pelosini C, Marsili A, et al. Acute exogenous TSH administration stimulates leptin secretion in vivo. Eur J Endocrinol. 2010;163(1):63–7. PubMed Epub 2010/04/16. eng.
Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. Eur J Endocrinol. 2003;149(4):257–71. PubMed Epub 2003/09/30. eng.
Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316(2):165–71. PubMed Epub 2009/06/23. eng.
Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. 2012;97(4):1134–45. PubMed.
Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect. 1998;106(8):427–36. PubMed Pubmed Central PMCID: 1533202. Epub 1998/07/29. eng.
Farid NR, Shi Y, Zou M. Molecular basis of thyroid cancer. Endocr Rev. 1994;15(2):202–32. PubMed Epub 1994/04/01. eng.
Zielke A, Hoffmann S, Plaul U, Duh QY, Clark OH, Rothmund M. Pleiotropic effects of thyroid stimulating hormone in a differentiated thyroid cancer cell line. Studies on proliferation, thyroglobulin secretion, adhesion, migration and invasion. Exp Clin Endocrinol Diabetes. 1999;107(6):361–9. PubMed Epub 1999/10/30. eng.
Rivas M, Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol. 2003;213(1):31–45. PubMed.
Takada K, Amino N, Tada H, Miyai K. Relationship between proliferation and cell cycle-dependent Ca2+ influx induced by a combination of thyrotropin and insulin-like growth factor-I in rat thyroid cells. J Clin Invest. 1990;86(5):1548–55. PubMed Pubmed Central PMCID: 296902. Epub 1990/11/01. eng.
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–99. PubMed Pubmed Central PMCID: 3791171. Epub 2013/02/23. eng.
Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49(5):945–52. PubMed Epub 2006/03/25. eng.
Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22(4):659–69. PubMed Epub 2008/10/31. eng.
Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, et al. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108(2 Pt 2):E77–83. PubMed Epub 2010/11/06. eng.
Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, et al. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol. 2013;168(6):879–86. PubMed Epub 2013/03/21. eng.
Cappelli C, Pirola I, Mittempergher F, De Martino E, Casella C, Agosti B, et al. Morbid obesity in women is associated to a lower prevalence of thyroid nodules. Obes Surg. 2012;22(3):460–4. PubMed Epub 2011/04/15. eng.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55. PubMed.
Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31(6):228–35. PubMed.
Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20. PubMed Pubmed Central PMCID: 1383660.
Johnson AR, Makowski L. Nutrition and metabolic correlates of obesity and inflammation: clinical considerations. J Nutr. 2015;145:1131S–6. PubMed.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. PubMed Pubmed Central PMCID: 296995.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. PubMed.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. PubMed.
Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7(10):1138–43. PubMed.
Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3(1):37–48. PubMed Pubmed Central PMCID: 2230108.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. PubMed.
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–4. PubMed.
Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–2. PubMed.
Pang XP, Yoshimura M, Wang J, Dubinett SM. TNF-alpha-induced antiproliferation is not dependent on the autocrine action of TGF-beta 1 in a thyroid cancer cell line. Lymphokine Cytokine Res. 1994;13(2):93–7. PubMed.
Pang XP, Ross NS, Hershman JM. Alterations in TNF-alpha signal transduction in resistant human papillary thyroid carcinoma cells. Thyroid. 1996;6(4):313–7. PubMed.
Rotondi M, Coperchini F, Pignatti P, Magri F, Chiovato L. Metformin reverts the secretion of CXCL8 induced by TNF-alpha in primary cultures of human thyroid cells: an additional indirect anti-tumor effect of the drug. J Clin Endocrinol Metab. 2015;100(3):E427–32. PubMed.
Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35(8):1780–7. PubMed.
Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. 2014;21:R85–103. PubMed.
Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:71. PubMed Pubmed Central PMCID: 3679475. Epub 2013/06/20. eng.
Catalan V, Gomez-Ambrosi J, Rodriguez A, Fruhbeck G. Adipose tissue immunity and cancer. Front Physiol. 2013;4:275. PubMed Pubmed Central PMCID: 3788329. Epub 2013/10/10. Eng.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88. PubMed Epub 2009/07/09. eng.
Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46(7):1369–79. PubMed.
Balsan GA, Vieira JL, De Oliveira AM, Portal VL. Relationship between adiponectin, obesity and insulin resistance. Rev Assoc Med Bras. 2015;61(1):72–80.
Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96(5):1723–32. PubMed Epub 2000/08/29. eng.
Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109(17):2046–9. PubMed PMID: ISI:000221173900002. English.
Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117(2):375–86. PubMed PMID: ISI:000244051500017. English.
Hall J, Roberts R, Vora N. Energy homoeostasis: the roles of adipose tissue-derived hormones, peptide YY and Ghrelin. Obes Facts. 2009;2(2):117–25. PubMed Epub 2010/01/08. eng.
Obeid S, Hebbard L. Role of adiponectin and its receptors in cancer. Cancer Biol Med. 2012;9(4):213–20. PubMed Pubmed Central PMCID: 3643674. Epub 2013/05/22. eng.
Mitsiades N, Pazaitou-Panayiotou K, Aronis KN, Moon HS, Chamberland JP, Liu X, et al. Circulating adiponectin is inversely associated with risk of thyroid cancer: in vivo and in vitro studies. J Clin Endocrinol Metab. 2011;96:E2023–8. PubMed Epub 2011/09/23. Eng.
Cheng SP, Liu CL, Hsu YC, Chang YC, Huang SY, Lee JJ. Expression and biologic significance of adiponectin receptors in papillary thyroid carcinoma. Cell Biochem Biophys. 2013;65(2):203–10. PubMed.
Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216(1):T1–15. PubMed Epub 2012/11/20. eng.
Andrade-Oliveira V, Câmara NOS, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:1–11.
Surmacz E. Leptin and adiponectin: emerging therapeutic targets in breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):321–32. PubMed Epub 2013/10/19. eng.
Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–32. PubMed Pubmed Central PMCID: 3630242. Epub 2013/01/29. eng.
Dutta D, Ghosh S, Pandit K, Mukhopadhyay P, Chowdhury S. Leptin and cancer: pathogenesis and modulation. Indian J Endocrinol Metab. 2012;16 Suppl 3:S596–600. PubMed Pubmed Central PMCID: 3602989. Epub 2013/04/09. eng.
Cheng SP, Chi CW, Tzen CY, Yang TL, Lee JJ, Liu TP, et al. Clinicopathologic significance of leptin and leptin receptor expressions in papillary thyroid carcinoma. Surgery. 2010;147(6):847–53. PubMed Epub 2010/01/05. eng.
Marcello MA, Calixto AR, de Almeida JF, Martins MB, Cunha LL, Cavalari CA, et al. Polymorphism in LEP and LEPR may modify leptin levels and represent risk factors for thyroid cancer. Int J Endocrinol. 2015;2015:173218. PubMed Pubmed Central PMCID: 4355553.
Zhang GA, Hou S, Han S, Zhou J, Wang X, Cui W. Clinicopathological implications of leptin and leptin receptor expression in papillary thyroid cancer. Oncol Lett. 2013;5(3):797–800. PubMed Pubmed Central PMCID: 3576217. Epub 2013/02/22. Eng.
Cheng SP, Liu CL, Hsu YC, Chang YC, Huang SY, Lee JJ. Regulation of leptin receptor expression in human papillary thyroid cancer cells. Biomed Pharmacother. 2012;66(6):469–73. PubMed Epub 2012/05/09. eng.
Cheng SP, Yin PH, Hsu YC, Chang YC, Huang SY, Lee JJ, et al. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26(5):1265–71. PubMed PMID: ISI:000295389600030. English.
Cheng SP, Yin PH, Chang YC, Lee CH, Huang SY, Chi CW. Differential roles of leptin in regulating cell migration in thyroid cancer cells. Oncol Rep. 2010;23(6):1721–7. PubMed PMID: ISI:000277526800031. English.
Kim WG, Park JW, Willingham MC, Cheng SY. Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology. 2013;154(8):2936–47. PubMed Pubmed Central PMCID: 3713208. Epub 2013/06/12. eng.
Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25(4):1569–75. PubMed PMID: ISI:000226908000030. English.
Hlavna M, Kohut L, Lipkova J, Bienertova-Vasku J, Dostalova Z, Chovanec J, et al. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58(2):124–8. PubMed Epub 2011/02/01. eng.
Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174(9):5789–95. PubMed Epub 2005/04/22. eng.
Benomar Y, Gertler A, De Lacy P, Crepin D, Hamouda HO, Riffault L, et al. Central resistin overexposure induces insulin resistance through toll-like receptor 4. Diabetes. 2013;62(1):102–14. PubMed PMID: ISI:000312824700020. English.
Stewart PA, Luks J, Roycik MD, Sang QX, Zhang J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One. 2013;8(12):e82460. PubMed Epub 2013/12/11. eng.
Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, et al. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis. 2013;34(11):2600–9. PubMed Epub 2013/08/21. eng.
Duan XF, Tang P, Li Q, Yu ZT. Obesity, adipokines and hepatocellular carcinoma. Int J Cancer. 2013;133(8):1776–83. PubMed PMID: ISI:000322908600002. English.
Dalamaga M, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46(7–8):584–90. PubMed PMID: ISI:000317878100005. English.
Jiang CY, Wang W, Yin YL, Yuan ZR, Wang LB. Expression of the adipocytokine resistin and its association with the clinicopathological features and prognosis of pancreatic ductal adenocarcinoma. Oncol Lett. 2012;4(5):960–4. PubMed PMID: ISI:000310040300021. English.
Marcello MA, Calixto A, Cunha LL, Martins MB, Vasques AC, Geloneze B, et al. Serum cytokines levels identify thyroid nodules malignancy. 2014.
Declaration of Interest
The authors have nothing to disclose. None of the authors has any competing interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Marcello, M.A., Cunha, L.L., De Assis Batista, F., Ward, L.S. (2016). Obesity and Thyroid Cancer. In: Ahmad, S., Imam, S. (eds) Obesity. Springer, Cham. https://doi.org/10.1007/978-3-319-19821-7_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-19821-7_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19820-0
Online ISBN: 978-3-319-19821-7
eBook Packages: MedicineMedicine (R0)