Abstract
Continuing the theme developed by Shuster and Botton (Estuaries 8:363–372, 1985) on the dynamics of the Delaware Bay population of Limulus polyphemus and by Sekiguchi and Shuster (Limits on the global distribution of horseshoe crabs (Limulacea): lessons learned from two lifetimes of observations: Asia and America. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 5–24, 2009) on global limits on the distribution of horseshoe crabs and by Shuster and Sekiguchi (Basic habitat requirements of the extant species of horseshoe crabs (Limulidae). In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 115–129, 2009) on local habitat requirements, this paper considers why the Delaware Bay is the epicenter of Limulus polyphemus abundance. The emphasis is on how its geologic and geographic characteristics are advantageous to Limulus. Although the species ranges along the east coast of North America from Yucatan, Mexico to Maine, USA (21°N to 44.5°N), only at Delaware Bay (38°40′ N to 39°20′ N) has it produced a population of millions of adult horseshoe crabs, unmatched anywhere. Why? It appears most likely that it was the interaction between the ecological generalist, Limulus polyphemus, and the exceptionally favorable environment that developed in the Delaware Bay area after the past great ice age. However, this productive relationship is short-lived. Chronologically the bay will be shorter-live with a probable existence of some 85,000 years, while Limulus polyphemus may have already existed some 135 million years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Uniqueness of Habitats and Horseshoe Crab Populations
If there ever was another place where a species of horseshoe crabs existed so prominently, it must have been comparable to Delaware Bay and the adjoining continental shelf. Although there is a wide range in the uniqueness of its habitats and of its adaptability, the American species Limulus polyphemus, of all the extant and probably many of the extinct species, has developed the largest numbers in any population at Delaware Bay. Why? This paper offers one interpretation.
Shuster (1955, 1979) interpreted differences in temperature tolerance at two widely separated populations (Florida and Woods Hole; Mayer 1914, later confirmed by Fraenkel 1960), and morphometric data collected on 16 populations of Limulus polyphemus, from Florida to Maine during surveys in 1953 and 1954, as evidence of physiological races. Since then several studies, e.g., by Riska (1981) and by King et al. (2005), reached similar conclusions regarding differences between populations. Therefore, a reasonable conclusion is that all populations of horseshoe crabs are unique, as seen in variations in their habitat and/or in their anatomy, genetics, and behavior. However, regardless of the four extant species considered, some horseshoe crab populations are more unique than others; among these are:
-
Tachypleus tridentatus in the Seto Inland Sea, Japan Once numerous (Sekiguchi 1988; Shuster and Sekiguchi 2009), this species may have approached the great numbers of Limulus seen in Delaware Bay. However, early in the twentieth century the reclamation of land by dikes and polders eliminated most spawning areas in the Seto Sea.
-
Southern-most Populations, Yucatan, Mexico Several populations of Limulus inhabit lagoons around the Yucatan Peninsula, from Campeche Sound on the west around to Bahia de la Ascension on the east coast (Zaldivar-Rae et al. 2009). The geology of the Gulf of Mexico must have been responsible for the ultimate isolation of these populations from all others for a long time – evidenced by tissue samples from northeastern Yucatan crabs, which are highly genetically differentiated from Limulus populations in the United States, suggesting a taxonomic revision of the genus may be due (King et al. 2005).
-
Indian River Lagoon (near Cape Canaveral), Florida, U.S.A. This, a shallow water, hyper-saline habitat lacks significant tides. As a result the reproductive ecology and early life history of Limulus differ markedly from populations inhabiting tidal areas with the usual estuarine salinities (Ehlinger and Tankersly 2009).
-
A Long-Spined Variant, Georgia, U.S.A. The presence of a prominent extra spine on the opercular ridges during the mid-juvenile stages of Limulus in the coastal waterway behind the barrier islands of Georgia, first reported by Say (1818), was rediscovered in 2005 at the aquarium maintained at the Oceanographic Institute, Skidaway, Georgia. All of the dorsal spines are greatly enlarged during development of the juveniles as well as smaller spines arising from the nodules on the opercular ridge (Fig. 2.1). This characteristic is being studied (H.J. Brockman, personal communication). Actually, of all the dorsal spines, only the spine midway along each opercular ridge is the one described by Say (1818).
Fig. 2.1 An exuviae of a juvenile Limulus polyphemus australis (Say 1818), prosomal width 9 cm (Specimen courtesy of Dodie Sanders, University of Georgia Aquarium; photograph by Shuster; the coin is 23 mm in diameter)
-
Northernmost Populations, Maine, U.S.A. In virtually every measurement of quantity, these populations are among the smallest. Within Taunton Bay, lesser bays, Egypt Bay and Hog Bay, with home ranges of 64.1 ha and 61.4 ha, respectively, support discreet populations where the adult mean prosomal width is 15.4 cm in the males and 18.0 cm in the females (Moore and Perrin 2007). The numbers of Limulus spawning at Hog Bay, from 2001 to 2005, averaged 1,082 per year, with a range of 741–1,525 individuals. Lack of any populations to the north of Taunton Bay may be due to the cold, more-northern climate that precluded the establishment of any reproducing Limulus population (Moore and Perrin 2007).
Restricted distributions also exist at Great Bay, New Hampshire (Schaller et al. 2010) and Pleasant Bay, Massachusetts (James-Pirri 2010). These populations apparently rarely utilize resources on the continental shelf, acting as if they were land-locked, except for such influences as those of salinity and the tidal amplitudes. It also appears that it is the stress of the seasonal length of cold weather that keeps population numbers low. Otherwise their local movements are similar to those of other horseshoe crabs except on a much smaller scale. Within their bays they exhibit the annual migration from deeper waters to the shallow waters and spawning beaches, and then back to deeper waters during over-wintering. They differ from nearby populations, at Plum Island Sound, Massachusetts (Baptist et al. 1957) and at Cape Cod Bay (Shuster 1950). The habitats at these locations are more open to deeper waters, and the crabs moved freely from them over relatively long areas. Yet, the dimensions of the annual migrations of the lesser populations and the closeness and sufficient abundance of all the usual resources demonstrate that reproducing populations of Limulus can exist within relatively small reaches of habitats essential in the life cycle of the species. However, the northern-most crabs are probably living at the edge of extinction. It is the combination of the several stresses that puts these populations at a greater local risk (Botton et al. 2010). Otherwise, even a spawning site that is only a small patch of sand might be the start of a population (see Colorplate 14 in Shuster et al. 2003).
2 A Changing Coastline
All estuaries are ephemeral geological events, rare in the history of the earth. (Amos 1979)
The coastline in the vicinity of the present Delaware Bay has been ever-changing and so has the ecology of the area. For example, before the last ice age, ocean waters covered most of the area of present-day Delmarva Peninsula as evidenced by oyster beds that were buried there some 35,000–50,000 years ago by sediments eroded from the uplands that were transported seaward (Fig. 2.2). This is only one of many fossil-bearing geologic formations in the mid-Atlantic coastal areas, e.g., at Calvert Cliffs on Chesapeake Bay or that revealed by the excavation of the Delaware Chesapeake Canal (Shuster 1960a).
Excavation of a reservoir and drainage ditches near Laurel, Delaware, revealed that ancient oyster shells were the most abundant fossils in a layer of blue clay that varied from 1 to 2 ft at a depth of 8 ft below the surface and just above the reservoir water level. At left, Henry Hutchinson lowers a black bucket to Shuster to collect shell samples; on right, a portion of the oyster-laden blue clan layer (Shuster 1960a, photographs by Dr. Frank Daiber)
Fast-forward to some 20,000 years ago when the earth’s climate began an unusually long interglacial interval that is now more than half over (Pielou 1991). In 13,000–11,000 years ago the mass of ice that covered the northeastern portion of North America as far south as Long Island and the northern part of New Jersey began to melt and recede northward. At that time the edge of the continent and the Atlantic Ocean met at the edge of the present continental shelf some 175 km due east of Delaware. Sea level rose rapidly during the initial melting of the ice shield and the ocean moved closer to Delaware. By 7,000–5,000 years ago a long and narrow embayment, the proto-Delaware Bay, was approaching a recognizable shape (Custer 1984). John Kraft and colleagues at the University of Delaware have studied the history of development of the bay including coastline partitioning and physical and chemical attributes that further defined the estuarine nature of the bay (e.g., Chrzastowski 1986; Knebel et al. 1988; Fletcher et al. 1992). If other estuaries were developing at about the same rate along the Atlantic coast, as was the Georgia coast some 5,000 years ago (Henry 2009), then at least most of the coastal area to the south was also nearing environmental conditions in which Limulus could exist. Then, sea level rise escalated 2,000 years ago and has continued (Fletcher et al. 1993; John and Pizzuto 1995).
2.1 Dimensions and Related Features of Delaware Bay
Certain features of the physical configuration of the bay (Shuster 1959) are important in the ecology of Limulus (Table 2.1, see also Fig. 2.3). The bay occupies a relatively large area and has a quantity of habitats favorable to horseshoe crabs, including: (a) extensive tidal marshes that contribute to the base of the food web, (b) many lengthy sandy beaches that support large spawning populations and the incubation of the eggs, (c) shallow-water areas that provide ample nursery areas for the early life stages, (d) prey species are abundant, and (e) the NNW-SSE axis of the channel and the southerly flow of the Delaware River were probably other formative factors. When the interaction of the river and the rising ocean formed the bay, the result was a southern entrance, protected by widely separated capes that provide a less turbulent access to and egress from the bay depending upon the force from westerly or easterly winds.
Areas along Delaware Bay contributing to the shoreline habitats where Limulus is generally found: Submerged contours of the bay (After Shuster 1959); outlines of the watersheds (inland boundary in orange), marshes (inland boundary in green), and tidal streams (in undulating black lines) have been added. The data summarized in Table 2.1 are for the bay area between the heavy dashed lines. Delaware (DNREC 2001): Rivers – Little (LC), St. Jones (SJ), Murderkill (MK), Mispillion (MR), and Broadkill (MR). New Jersey: the state has five watershed regions in its Watershed Management Program: the Cohansey (CR) and Maurice (MA) are within the Lower Delaware Region and Cape May County is within the Atlantic Coastal Region (Shinn 2000). The following beaches (those indicated by the white rectangles on the shoreline are potential annual sampling sites for the U.S. Geological Index of Spawning Activity (ISA) – Smith et al. 2002a, b). Delaware: Pickering (PK), Kitts Hummock (KH), Ted Harvey (TH), No. Bowers (BN), So. Bowers (BS), Bennetts Pier (PB), Big Stone (BG), Slaughter (SB), Fowlers (FO), Primehook (PB) Broadkill (BK), Lewes (LB), and Cape Henlopen (CH). New Jersey: Sea Breeze (SZ), Gandys (GB), Fortesque (FO), Raybins (RB), East Point (EP), Reeds (RD), Kimbles (KI), Pierces Point (PB), Highs B (HI), South Cape Shore (RL = Rutgers Lab), Norburys Landing (NL), Town Bank (TB), North Cape May (NC), Higbee (HI), and Sunset (SS)
In essence, tidal streams, from some eight drainage basins along the Delaware Bay shore (Tiner et al. 2011: New Jersey has a similar geography) break up the shoreline into long sandy stretches. This might divide and double the beaches between the major streams of each basin in Delaware into sub-beaches if different chemical and physical changes occurred at each end (Fig. 2.4). This creates an interesting pattern with the north and south extremes of each beach possibly more influenced by the adjacent stream. If there are effluents in such a stream or the streams and the along-shore currents create a topographical difference in the sub-beaches would these sub-beaches be equally noticed by Limulus as a local homing signal? Regardless, these streams might isolate a discrete spawning population at each sub-beach. This and the tendency of the crabs to spawn at one beach, that between two tributaries, has been the basis for beach-by-beach studies to obtain: (a) the total spawning effort of the bay (Finn et al. 1991), (b) its spawning index (Smith et al. 2002a, b), (c) egg densities (Pooler et al. 2003), or (d) the number of the nests of eggs in the beaches (Weber and Carter 2009) of at least several beaches to establish either a trend or an overview of the activity.
A diagrammatic representation of a portion of the western shore of Delaware Bay (wave symbol) showing four drainage basins. Each basin, in sequence from the bay inland consists of sandy beaches (the white stripes along the bay), tidal streams (black), tidal marshes (shown by clumps of marsh grass), the four drainage basins (one white and three differently shaded), and beyond the upland (tree symbols). This depiction is not to scale, north is to the right
An atlas (NOAA 1980) depicts, on the small scale of about 37.5 mm = 100 statute miles, 13 maps on the physical environments of the Atlantic coast from Florida to Maine showing, on separate maps, that the bay area has: (a) an entrance about 80 miles from the edge of the continental shelf, (b) sedimentary rocks of the Quaternary, (c) mudflats and marshes, (d) a southerly surface drift of ocean currents in January and north and south during July, (e) sea surface temperature in February at 5.0 °C and 22.5 °C in July, (f) prevailing winds in January to the SE and those in July to the NE, and (g) the probability that hurricane force winds of >73 mph will occur 0–4 % a year. The shores of Delaware Bay are lined with non-forested wetlands.
2.2 Significance of the Orientation of Delaware Bay
Due to the southerly axis of Delaware Bay and its shores, prevailing storms usually affect one shore more than the other shore that was more shielded from the winds and higher waves. Whether Limulus moves to the protected shore under such circumstances, Cook (1857) noted that when the crabs were scarce along the Cape May shores they were correspondingly abundant on Delaware shores. Further, it appears that other features may facilitate the coming and going of the “goes with the flow Limulus”: (a) the flooding tides on the New Jersey side arrive sooner and stronger than on the Delaware side (NOAA 1987), (b) the wide entrance to the bay, and (c) the along-shore currents. Crabs tagged to the north enter the bay on the New Jersey side usually arrive earlier on Cape May shores, than those tagged south of Delaware Bay that tend to enter the bay on the Delaware side (Swan 2005). The benthic topography of the bay, with several channels from the shores heading toward the main channel, also may serve in orienting the movements of the crabs. Horseshoe crabs tend to move in the direction of benthic currents (observed by author in the clear waters of Florida and Massachusetts during distribution surveys during the summers of 1949–1950 and 1943–1944). This directional movement was first confirmed by Rudloe and Herrnkind (1976) who released float-tagged adults near breeding beaches. The tagged crabs moved most closely in the direction of the wave surge; even in the absence of a surge they usually moved in relatively straight paths. Similar results were obtained during tests in a large wave-controlled tank (Rudloe and Herrnkind 1980). Moving with the flow was also demonstrated in test runs using large juvenile crabs in a flume (Luckenbach and Shuster 1997) and in observing adults using optics on a benthic sled (Michael Oates, personal communication).
2.3 Considering Climate/Weather
Apparently Xiphosura have mostly inhabited warm-water habitats, tropical and temperate, based on two ancient populations (Barthel et al. 1990; Selden and Nudds 2004) and the present geographic ranges of the extant species (Sekiguchi and Shuster 2009). Two species of Xiphosura existed in the fauna of Mazon Creek, Illinois (ca. 323 million years ago (mya); Shabica and Hay 1997) and one, Mesolimulus walchi (see Fig. 2.8) at Solnhofen, Bavaria, Germany (ca. 150 mya; Barthel et al. 1990).
The three Indo-Pacific species straddle the equator but are limited in their northward range (Yamasaki et al. 1988; Sekiguchi and Shuster 2009). During the last ice age it seems likely that Limulus probably existed only in the Gulf of Mexico area and the eastern coast from Florida to Georgia, in that they served as warm water refuges (deduced from the study by Saunders et al. 1986).
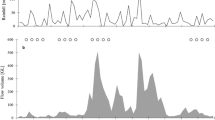
At Delaware Bay, data on the hydroclimate has been accumulating ever since 1927 when the Rutgers oyster research laboratory was established on Cape May, New Jersey. Five years of that data from oyster growing area at Maurice River Cove, a midway area of the bay on the New Jersey side, were selected to construct hydroclimagraphs (Shuster 1960b). These also serve to illustrate a short-term trend in variations in the hydroclimate of horseshoe crabs at mid-bay (Fig. 2.5).
Although water temperatures and salinities are plotted together, they are independent variables, with changes in one not affecting the other. Nevertheless, during the spring and fall seasons, the salinity does seem to vary according to temperature. This variation is due to the difference in evaporation and precipitation as well as to the relative amounts of freshwater and seawater in the bay and to their temperatures (Shuster 1960b). Extremes in salinity, narrow in 1955 (b) and wide in 1956 (c), are “hidden” in the 5-year average of 1955–1959 (a)
2.4 Increasing Beach Erosion
Over the years, after the author’s first trip to Delaware Bay in 1949 to study Limulus, there has been a noticeable sea level rise and significant erosion of the shoreline with several beaches having been swept clean of the sandy deposits by high storm tides, as during hurricane Sandy in October 2010. Whatever the causative factor, erosion of a beach exposed previously covered tidal marshes and usually moved the sand landward covering/eliminating more tidal marshland, e.g., at a severely eroded stretch of beach (Fig. 2.6).
Exposure of intertidal flats at Cape May, New Jersey on 11 September 1951 in the vicinity of the Rutgers oyster research lab (NJORL), showing several collection sites of early life stages of Limulus (nest, quadrats, and transect). The marshy nub extending from the beach developed on a discarded pile of oyster shells (Shuster 1979). In 2014 the entire beach area delineated at the bottom of the picture is now flooded at high tide
Notable changes, especially during heavy storms, are usually associated with certain locations. Other types of change also occur. There has been an increasing accumulation of soupy clay-silt in a relatively quiet water area at least since 1961 (personal observation) off the northern portion of Slaughter Beach (also known as Cedar Beach) where a back-eddy has been created by a mile-long jetty at Mispilion River Inlet. Excessive die-offs of Limulus frequently occur in this area. One instance was after a mass tagging effort (Swan 2005); another was a summer die-off in June 1979 (cause unknown, Shuster and Botton 1985). In 2003 (personal observation) finely ground detritus mixed with the silty-clay created an adverse habitat during the spawning season for the crabs (Fig. 2.7).
(a) Horseshoe crabs have difficulty in surviving and in spawning when mud and finely-ground detritus cover a beach, as in 1999 at the northern portion of Slaughter Beach (sometimes referred to as Cedar Beach), Delaware (Photographs by Shuster). This portion of the beach was covered with a mat of fine organic detritus that contained fine silt (almost a clay – a whitish coating on the crabs). Underneath the mat of detritus the beach was “muddy.” The faint shoreline in the upper photograph is the mile-long Mispillion River that juts out into Delaware Bay creating a back-eddy to the south where fine sediments are collected, (b) In this close up of a portion of the beach (Fig. 2.7a), the crabs are emerging from the covering of detritus; most of the crabs are coated with dried clay
2.5 Defining Environmental Spawning Parameters
After World War II more attention was devoted to two major coastal resources of Delaware Bay, at a time when impacts upon beaches and spawning populations of horseshoe crabs were becoming more obvious. Action revved up in 1992 when the spawning population decreased to a level probably at least 40 % less than in the previous year. Decreases in the annual stop-over of migratory shorebirds that fed on Limulus eggs soon followed. The effect was most intense in the migratory Red Knot, and this sparked a marked increase in the research and budget considerations at Delaware Bay. One consideration led to the mapping and classification of the suitability of beaches totaling 239 km in length as Limulus spawning habitats (Lathrop et al. 2006). Of 31 km out of 91 km (Delaware) and 26 km of 148 km (New Jersey), 24 % received the highest rating (“optimal”) (see Table 2.1 and Fig. 2.3). Only an additional 7 % of the total shoreline was “suitable,” 33 % was “less suitable,” 31 % was “avoided” by the crabs and 5 % was physically “disturbed” habitat (see Table 2.1). Even so, the available spawning habitat (a combined 64 km of optimal, suitable, and less suitable) was either the most suitable in any single embayment, worldwide, or horseshoe crabs at Delaware Bay were the most efficient spawners.
Attention was also directed toward beach restoration. In the late 1980s, Karen Day, US Fish & Wildlife Service, and Shuster developed some preliminary data on beach characteristics that were later researched in detail by Brady and Schrading (1997) as part of a larger study on the effect of beach replenishment upon the suitability/quality of Limulus spawning beaches (Table 2.2). Besides the ranges of these physical parameters, the bay also has the full range of salinity, from 32 to 8 ppt, within which Limulus can survive (the area within the dashed lines shown on Fig. 2.3).
In Delaware the Department of Natural Resources and Environmental Control program of beach restoration and protection of saltwater tidal marshes, sediment replenishment on the beaches (after extensive storm/erosion damage to beaches), did not appear to affect the spawning indices and egg counts of horseshoe crabs (Weber and Carter 2009; Kalasz and Weber 2010; Michels et al. 2010).
Recent studies on the effect of beach dynamics on horseshoe crabs spawning and on distribution of their eggs have expanded an important dimension to research on the environment of Limulus. Jackson et al. (2005) explored the relative impacts of horseshoe crab nest-building versus wave action in redistributing sediments and eggs, with the activities of the crabs generally the more important. On wave and swash transport and redistribution of Limulus eggs, Nordstrom et al. (2006) reported that, because vegetative beach wrack traps the eggs locally, more eggs remain on the surface during flooding tides and when wave energy is low than during ebb tides when more eggs are available in the swash zone.
2.6 Concerning Beach “Fidelity”
Beach fidelity–that horseshoe crabs always spawn at a certain beach (their natal beach) – is most fully expressed by the behavior of the females during the 5–7 days of high tides during a new or full moon. Most female horseshoe crabs rarely completely spawn on one high tide; most often returning on a few more succeeding highs. Acoustic-tag data (Brousseau et al. 2004; Smith et al. 2010) indicate females only move a short distance from the beach and return on a subsequent high tide, usually to a somewhat different spot on the same beach, usually in a up-bay direction (allied to along-shore currents?). Could this short-term/short-distance be interpreted, alternatively to beach fidelity, as conservation of energy?
After completing spawning all of her mature eggs (up to around 100,000 at Delaware Bay), how long does a female remain in the vicinity? Does she actually mature and spawn another full clutch of eggs the same season? If so, remaining in the vicinity would be logical. However, she could leave the bay during the spawning season (presumably after spawning) as has been suggested via benthic sled/video evidence on a large female (Michael Oates, personal observation during an exploratory cruise at the entrance to Delaware Bay on the F/V Maggie S. Myers, Captain Frank “Thumper” Eicherly IV).
But then, how to explain erratic spawning behavior associated with specific sites, as when Limulus tries to spawn in a pile of oyster shells? Did they just tire out when they happened to reach a beach covered with shells or were the shells covering a former spawning site? Or why do they sometimes try to spawn on a beach composed of peat?
Further, tagging data (Swan 2005) demonstrate that adult Limulus wander all around the in-shore continental shelf in the vicinity of Delaware Bay (mostly between Atlantic City, New Jersey, and Toms Cove, Virginia). Also, crabs tagged outside of Delaware Bay are recovered within the bay. How the crabs are guided back to “their native beach” may be as much dependent upon where they enter the bay, as on the first flooding currents that enter the bay on the Cape May side (NOAA 1987) and then follow certain benthic currents within the bay.
Overall, the postulation of rigorous spawning “fidelity” to a beach seems to be an overstatement and contradictory to the concept that horseshoe crabs are ecological generalists. Also, it is inconceivable that Limulus followed the exactly same-beach conditions northward in establishing populations from Florida to Maine after the last great ice age.
2.7 Wave-Protected Beaches and Shallow Waters Essential
Of the environmental components vital to the existence of horseshoe crabs, the weakest links are the intertidal areas suitable for spawning (including incubation of the eggs) and the associated adjacent shallow-water areas suitable as nurseries of the early juvenile stages. However, although observations confirm that sandy beaches have been those largely populated during spawning season, Limulus has spawned in shell heaps along the shore, among gravel and cobble stones, in silty-sand, in muddy detritus, and often are found on marsh banks (Shuster, personal observations).
3 Limulus polyphemus
Apparently, horseshoe crabs long ago came up with a body plan that works well, and have simply stuck tenaciously to it. Yet, internally, their molecular genetic clocks have kept on ticking. (Avise 2002)
In Sect. 2.2 the focus was on the habitat with references to Limulus, here the emphasis is on the adaptations of the species to and relationships with environmental conditions in the Delaware Bay area.
3.1 A Brief Geologic History of Horseshoe Crabs
Horseshoe crabs are ecological/environmental generalists (Eldredge 1991; Loveland et al. 1997) that move freely between oceanic and estuarine environments. This may have been during most of their geologic history. Apparently, millions of years ago, as evidence at least at two coastal environments (Selden and Nudds 2004) – at Mazon Creek (Shabica and Hay 1997) and Solnhofen (Hecht et al. 1985; Barthel et al. 1990) – were inhabited by species of Xiphosura.
Considering the geologic age of Limulus it may have been subjected to adverse conditions during at least two major extinction events that occurred between the Cretaceous and Tertiary Periods some 65 mya and the Pleistocene and the Recent Epochs (ca. 0.01 mya) (Hallam and Wignall 1997). Limulus certainly is a candidate as a survivor.
Mesolimulus walchi was fossilized about 150 mya in the Solnhofen formation, Bavaria, Germany, during the Jurassic Period. If not the ancestral species, it so closely resembles the anatomy of the extant species that when alive could be mistaken for a contemporary species (Fig. 2.8).
The most recent fossilized connection with the American species is Limulus coffini – found in a Cretaceous formation in Colorado, USA, dated at about 80 million years ago (Reeside and Harris 1952). It is represented by a nearly complete dorsal mold of a horseshoe crab opisthosoma, almost identical with that of Limulus polyphemus.
Just when Limulus occupied the Delaware Bay area has not been ascertained, but this bay probably has not been “a Limulus habitat” much more than a few thousand years. During the ocean level rise, Limulus must have moved from Florida waters northward, incrementally (as suggested by the study by Saunders et al. 1986), when each embayment became environmentally hospitable. It seems reasonable to also assume that each successive population produced a population large enough to spin off sufficient “wandering” members to form the next more northward population.
3.2 Delaware Bay as a Habitat and Limulus as a Successful Colonizer
Delaware Bay, after Chesapeake Bay, is the second largest on the east coast of the U.S. Both bays are geologically young, having developed since the end of the last ice age. Both are inhabited by Limulus but the later bay, with more extensive sandy shorelines, is more favorable for horseshoe crabs. Essentially, Delaware Bay was shaped when sea level began rising some 13,000 years ago, when the last great glaciers began to melt and ocean waters began to push into sediments that had been transported seaward from upland erosion. Certain geologic results – size of the bay, its general north-to-south orientation, and the partitioning of the bay shoreline by tidal streams into many beaches – have been of major importance in the spawning and the distribution of the horseshoe crabs. And Limulus was ready, as an ecological generalist produced by a lengthy evolutionary lineage, to take advantage of the resultant favorable environment.
3.3 Life Stage as a Factor in Distribution
At Delaware Bay there is a clear pattern of distribution with the life cycle stages/age of the crabs tracked from the beaches to throughout the bay with many crabs on the continental shelf (Botton et al. 2010). Earlier, in related studies, Botton and Loveland (2003) and Botton et al. (2003a) had also recently added an important part of that information – that plankton tows revealed the newly hatched larvae were most numerous within the immediate shoreline area and the next stage, the first-tailed stage, settled down, also near the shore. As soon as the digestive tract of the hatchling became functional, the next instar (the first tailed stage) of the life stages initiated, albeit slowly, the trek toward deeper water, instar by instar. I do not recall the source of information – that chelicerates, at least spiders, tend to be cannibalistic and disperse once the hatchlings have a functional digestive tract. Is this a partial answer to the dispersion of Limulus?
Early stages of Limulus, usually those in their second and third years, have been relatively common on the intertidal bars and in the sloughs at low tide. The smaller, first-year instars are not always prominent, possibly due to storm dispersal before they become evident. But, when prevalent as at low tide on 11 September 1951, a total of slightly over 2,000 instars, stages IV–VI, were collected from exposed intertidal bars, 1 through 5; instar 6 is the stage usually reached during the year it hatched (Shuster 1979).
The collection efficiency of a suction-dredge (greater) was compared with a trawl (less) in a study of the distribution and abundance of small (mostly first-year instars) juveniles at seven sites (four at Delaware, three at New Jersey) in the upper portion of Delaware Bay (Burton et al. 2009). Sampling was along three parallel transects from 1 to 4 m from shore to about 925 m. In July, juveniles had prosomal widths predominately at 7, 10, and 15 mm; by September/October they were at 13.5, 17, and 24 mm. Densities of the juveniles were greatest at the near-shore transect in July, but by September/October most had gone past the offshore transect into deeper waters.
Beginning in 1992 and continuing through 2008, annual surveys by trawls equipped with appropriate mesh size, depending on the age class to be examined, have compiled data on the distribution of the larger juveniles and adults throughout Delaware waters (Jordan Zimmerman, Delaware Division of Fish & Wildlife, personal communication). Clearly, the early instars increasingly make their way into the deeper waters of the bay and, later, are distributed throughout the area with the mid-sized to almost adult-sized instars common on the continental shelf. This was observed personally off Chincoteague, Virginia in the 1990s on Captain Eustler’s trawler when Steve Doctor, a Maryland fisheries biologist, was conducting a survey of horseshoe crabs and on an exploratory mission with Jim Berkson when a large number of juveniles were trawled. Adults notably congregate during feeding and spawning; otherwise they are widely and unevenly distributed, at least on the continental shelf off Delaware Bay (Botton and Haskin 1984; Botton and Ropes 1987, 1989; Captain Jeff Eutsler, Ocean City, Maryland, personal observation).
3.4 Management of the Limulus Resource
In 2000 the Horseshoe Crab Management Committee of the Atlantic State Marine Fisheries Commission (ASMFC) voted to investigate the protection of horseshoe crabs on the continental shelf. This was due to the abundance of three age classes of Limulus: (a) large adult-sized juvenile females that would molt in the fall and join the spawning migration the next year, (b) those that were first-year spawners, and (c) those older but still active females that would spawn for several more years. The result was the establishment of a large refuge off the mouth of Delaware Bay, 3 miles off the shores of New Jersey, Delaware, and Maryland (NOAA 2001).
3.5 Diet as a Factor in Distribution
The diet of Limulus within the bay and on the continental shelf was an early, important contribution of Mark Botton in his numerous studies on the American horseshoe crab (e.g., Botton 1982, 1984a, b; Botton and Haskin 1984; Botton and Ropes 1987, 1989; Botton et al. 2003b). The ecological importance of the bay/continental shelf to Limulus is evident from the numbers of adult horseshoe crabs on the continental shelf off the entrance to Delaware Bay (Hata and Berkson 2003), where surf clams and whelks are important commercial fisheries, and where tagged Limulus move in and out of the bay (Swan 2005). The shelf not only provides an ample source of food but also an avenue for distribution and a wintering-over area. For example, major concentrations of hard clams, Mercenaria mercenaria, occur in Delaware Bay while the ocean quahog, Arctica islandica, and the surf clam, Spisula solidissima, are abundant on the continental shelf in the mid-Atlantic region (NOAA 1980).
3.6 Abundance and Distribution
Most recently the enormity of the population of horseshoe crabs within Delaware Bay was calculated from a massive, bay-wide tagging/recapture study in 2003 when over 17,500 adult horseshoe crabs were tagged within Delaware Bay (Smith et al. 2006). A total of 7,221 crabs were obtained for tagging by trawl during pre-spawning (26 March to 8 May) and 10,322 crabs from 28 to 30 May prior to peak spawning. Recaptured tags were obtained on 29 May, 31 May, and 2 June 2003, during the annual spawning survey. Resultant estimates were about 20 million within the bay (90 % confidence interval: 13–28 million) of which 6.25 million (90 % confidence interval: 4.0–8.8 million) were females. Earlier, lesser numbers but still impressive numbers of horseshoe crabs were collected by trawl within a 2,912 km2 study area on the continental shelf off Delaware Bay (Hata and Berkson 2003). In their survey the mean abundance for all daytime collections was 6.81 million horseshoe crabs with a confidence interval of 2.29–11.33 million; night-time sampling was greater – 11.4 million with a confidence interval of 5.95–16.85 million.
A preliminary survey of the spawning population of Delaware Bay (Shuster and Botton 1985) was conducted by the senior author in 1977, 1979, and 1980. Realistically this one-man survey was weak in the amount of data collected, so submission was withheld until more was available. By the time of submission of the manuscript to Estuaries in 1984 a different but substantial kind of information was available. Botton had completed most of his studies on the diet of Limulus in the Delaware Bay area. This enabled the authors to expand the paper to consider population dynamics. The added feature that Limulus fed on the surf clam (Spisula solidissima) on the shelf strongly suggested that diet was probably the answer. The source of the crabs also seemed obvious – that the shelf crabs were part of the Delaware Bay spawning population, Thus, the authors designated that portion of the population within the bay as one cohort, that on the shelf as another cohort. This was reinforced by a 15-year tagging program (Swan 2005) that showed adults moved in and out of the bay. All of the above illustrated an enormous population of Limulus and a great amount (and range) of movement. Today this situation is considered to be a fact – that the bay is an enormous incubator producing more horseshoe crabs than can be fed during their life cycle. Migration is the relief value – large numbers of the juveniles move out of the bay onto the shelf where they mature.
Thus, one interpretation of the numbers of the large juvenile and adult horseshoe crabs on the continental shelf is that Delaware Bay is inadequate to support such an enormous population of crabs. Mapping of the distribution of fisheries species such as clams (NOAA 1980) and their harvests strongly indicate that large sources of species eaten by large juveniles and adult horseshoe crabs (Botton and Haskin 1984; Botton and Ropes 1989) are abundant on the shelf. Another reason for the shelf distribution could be that the crabs appear to spread out away from each other (Captain Jeff Eustler, personal observation) or the belief that the crabs do not congregate except when feeding or breeding or at least stop in certain areas to “rest”. Observations also suggest that distribution in the bay and shelf populations may be age-related. For example, since old males tend to be more numerous during the early periods of spawning at Fortesque Beach, is this because they have remained in the upper bay during the cold-water months (Michael Oates, personal communication)? Also, it would be reasonable to expect that more of the younger, more active adults would be on the shelf due to the initial dispersal of the juveniles and to the return of the young adults in search of prey.
Crabs tagged during spawning usually have short-term (i.e., within days) and local movements (Swan 2005). Over longer time periods (months and years) after spawning they show increasing distances and changes in locations (Swan 2005).
4 Delaware Bay: A Multiplicity of Optimal Conditions
The larger-sized crabs found from Georgia to Cape Cod compared with elsewhere within their geographical range suggest that the mid-Atlantic geography and environmental conditions favored Limulus. At Delaware Bay a number of drainage basins and their tidal streams separate the shoreline into numerous, lengthy sandy beaches, each capable of supporting a large spawning aggregation of Limulus (see Figs. 2.3 and 2.4). These beaches extend along a favorable salinity gradient from 32 ppt at the entrance to the bay to 8–10 ppt up-bay, usually the lowest tolerance level of Limulus. The distance from the capes to the last major spawning area upbay is about 50 km.
5 Conclusions
It was and is the juxtaposition of the ecological generalist and consummate survivor behavior of Limulus with the geologic evolution and characteristics of the bay, and the geographic location of Delaware Bay within the mid-range of the species that have contributed to the great success of Limulus at Delaware Bay. It is this juxtaposition that has resulted in an ecological situation that out-produces other populations. How? In essence, each beach supports a seasonally discrete spawning population that contributes to the total production of the bay – with a lesser yield or failure of some beaches and greater success of others that would compensate for the lesser results. This conclusion is supported by the extensive research that has been conducted over the past 30 years at Delaware Bay.
While many changes in the shoreline have probably occurred during the few thousand years since Limulus arrived at Delaware Bay, including the times after the arrival of the early Dutch and Swedish colonists, the species still thrives at this bay. But, in the long run, the ocean will have inundated Delmarva Peninsula, covering both Chesapeake and Delaware Bays within the next 75,000 years (Fig. 2.9). If changes in the shoreline proceed at a pace that include development of shallow-water coastal embayments with sandy beaches within the migration ranges of some populations, Limulus will probably persist. But sea-level rise is not always constant as transgressive facies changes in peat, mud, and sand and muddy sand in tidal wetlands in Delaware suggest a rapid rise 2,000 years ago (Fletcher et al. 1993; John and Pizzuto 1995). The contrast between the persistence of a landform (from about 7,000 years before present to 75,000 years in the future) and the some 135 million years that the species has existed, is more than suggestive that Limulus polyphemus is indeed a survivor.
Long before the next major ice age, seawater will bathe much of the Atlantic coast (Courtesy of Dr. John C. Kraft). Whether the retreating shoreline will also have habitat suitable for Limulus polyphemus is unknown but since this species can spawn in only a patch of sand (Shuster et al. 2003: COLORPLATE 14) and has already existed some 140 million years (Shishikura et al. 1982) it probably will survive whatever occurs within the next 75,000 years
References
Amos WH (1979) The infinite river: a biologist’s vision of the world of water. Random House, New York
Avise JC (2002) Genetics in the wild. Smithsonian Institution Press, Washington, DC
Baptist JP, Smith OR, Ropes JW (1957) Migrations of the horseshoe crab, Limulus polyphemus, in Plum Island Sound, Massachusetts. U.S. Fish & Wildlife Service, Special Scientific Report Fisheries No. 220
Barthel KW, Swinburne NHM, Morris SC (1990) Solnhofen: a study in Mesozoic palaeontology. Cambridge University Press, Cambridge
Botton ML (1982) Predation by adult horseshoe crabs, Limulus polyphemus (L.), and its effect on benthic intertidal community structure of breeding beaches in Delaware Bay, New Jersey. Ph.D. thesis, Rutgers University
Botton ML (1984a) Diet and food preferences of the adult horseshoe crab Limulus polyphemus in Delaware Bay, New Jersey. Mar Biol 81:199–207
Botton ML (1984b) The importance of predation by horseshoe crabs, Limulus polyphemus, to an intertidal flat community. J Mar Res 42:139–161
Botton ML, Haskin HH (1984) Distribution and feeding of the horseshoe crab, Limulus polyphemus, on the continental shelf off New Jersey. Fish Bull 82:383–389
Botton ML, Loveland RE (2003) Abundance and dispersal potential of horseshoe crab (Limulus polyphemus) larvae in the Delaware estuary. Estuaries 26:1472–1479
Botton ML, Ropes JW (1987) Populations of horseshoe crabs, Limulus polyphemus, on the northwestern Atlantic continental shelf. Fish Bull 85:805–812
Botton ML, Ropes JW (1989) Feeding ecology of horseshoe crabs on the continental shelf, New Jersey to North Carolina. Bull Mar Sci 45:637–647
Botton ML, Loveland RE, Tiwari A (2003a) Distribution, abundance, and survivorship of young-of-the-year in a commercially exploited population of horseshoe crabs, Limulus polyphemus. Mar Ecol Prog Ser 265:175–184
Botton ML, Shuster CN Jr, Keinath JA (2003b) Horseshoe crabs in a food web who eats whom? In: Shuster CN Jr, Barlow RB Jr, Brockmann HJ (eds) The American horseshoe crab. University of Harvard Press, Cambridge, MA, pp 133–153
Botton ML, Tankersley RA, Loveland RE (2010) Developmental ecology of the American horseshoe crab Limulus polyphemus. Curr Zool 56:550–562
Brady JT, Schrading EP (1997) Habitat suitability models: horseshoe crab (spawning) Delaware Bay, New Jersey and Delaware (developed for the Cape May, Villas and Reeds Beach Habitat Evaluation Procedures). US Army Corps of Engineers, Philadelphia
Brousseau LJ, Sclafani M, Smith DR et al (2004) Acoustic-tracking and radio-tracking of horseshoe crabs (Limulus polyphemus) to assess spawning behavior and subtidal habitat use in Delaware Bay. N Am J Fish Manag 24:1376–1384
Burton WH, Kelley FS, Franks EA (2009) Distribution of juvenile horseshoe crabs in subtidal habitats of Delaware Bay using a suction-dredge sampling device. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 149–162
Chan S, Schulte S (2008) A plan for monitoring shorebirds during the non-breeding season in bird monitoring region New Jersey–BCR 30. Manoment Center for Conservation Sciences
Chrzastowski MJ (1986) Stratigraphy and geologic history of a Holocene lagoon: Rehoboth Bay and Indian River Bay, Delaware. Ph.D. thesis, University of Delaware
Cook GH (1857) Geology of Cape May, NJ, p 105 (see Fowler HW (1908) The king crab fisheries in Delaware Bay. In: 1907 Annual Report New Jersey State Museum, Trenton, NJ, pp 111–119 + plates 59–65
Custer JF (1984) Delaware prehistoric archaeology, an ecological approach. University of Delaware Press, Newark
DNREC (2001) Inland Bays/Atlantic Ocean Basin assessment report. Delaware Department of Natural Resources and Environmental Control, Dover. 40-01/01/01/02
Ehlinger GS, Tankersley RA (2009) Ecology of horseshoe crabs in microtidal lagoons. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 149–162
Eldredge N (1991) Fossils: the evolution and extinction of species. Harry N. Abrams, Inc., New York
Finn JJ, Shuster CN Jr, Swan BL (1991) Limulus spawning activity on Delaware Bay shore. Finn Tech Industries, Cape May Court House
Fletcher CH III, Knebel HJ, Kraft JC (1992) Holocene depocenter migration and sediment accumulation in Delaware Bay: submerging marginal marine sedimentary basin. Mar Geol 103:165–183
Fletcher CH, Pizzuto JE, John S et al (1993) Sea-level rise acceleration and the drowning of the Delaware Bay coast at 1.8 ka. Geology 21:121–124
Fraenkel G (1960) Lethal high temperatures for three marine invertebrates: Limulus polyphemus, Littorina littorea and Pagurus longicarpus). Oikos 11:171–182
Hallam A, Wignall PB (1997) Mass extinctions and their aftermath. Oxford University Press, Oxford
Hata D, Berkson J (2003) Abundance of horseshoe crabs (Limulus polyphemus) in the Delaware Bay area. Fish Bull 101:933–938
Hecht MK, Ostrom JH, Viohl G, Wellnhofer P (eds) (1985) The beginnings of birds. Freunde des Jura-Museums, Eichstätt
Henry VJ (2009) Geology of the Georgia coast. The New Georgia encyclopedia. University of Georgia Press, Athens
Jackson NL, Nordstrom KF, Smith DR (2005) Influence of waves and horseshoe crab spawning on beach morphology and sediment grain-size characteristics on a sandy estuarine beach. Sedimentology 52:1097–1108
James-Pirri MJ (2010) Seasonal movement of the American horseshoe crab Limulus polyphemus in a semi-enclosed bay on Cape Cod, Massachusetts (USA) as determined by acoustic telemetry. Curr Zool 55:575–586
John SJ, Pizzuto JE (1995) Accelerated sea level rise 2,000 years BP in the Delaware Bay: stratigraphic evidence from the Leipsic River valley, Delaware, USA. J Coastal Res 11(3):573–582
Kalasz KS, Weber RG (2010) 2005–2010 Delaware horseshoe crab (Limulus polyphemus) egg survey project. Report to the Atlantic States Marine Fisheries Commission’s Horseshoe Crab Technical Committee, Washington, DC
King TL, Eackles MS, Spidle AP et al (2005) Regional differentiation and sex-based dispersal among populations of the horseshoe crab Limulus polyphemus. Trans Am Fish Soc 134:441–465
Knebel HJ, Fletcher CH, Kraft JC (1988) Late Wisconsin Holocene paleogeography of Delaware Bay: a large coastal plain estuary. Mar Geol 83:115–133
Lathrop R, Allen M, Love A (2006) Mapping and assessing critical horseshoe crab habitats in Delaware Bay. Rutgers University Center for Remote Sensing and Spatial Analysis. http://deathstar.rutgers.edu/projects/delbay/
Loveland RE, Botton ML, Shuster CN Jr (1997) Life history of the American horseshoe crab (Limulus polyphemus) in Delaware Bay and its importance as a commercial resource. In: Farrell J, Martin C (eds) Proceedings of the horseshoe crab forum status of the resource. University Delaware Sea Grant Program, Lewes
Luckenback M, Shuster CN Jr (1997) Preliminary test runs on the behavior of juvenile Limulus in a flume (unpublished notes and photographs)
Mayer AG (1914) The effects of temperature upon tropical marine animals. Papers Tortugas Laboratory, Carnegie Institute Washington, Publ 183(6):1–24
Michels S, Smith D, Bennet S (2010) Horseshoe crab spawning activity in Delaware Bay 1999–2009. Report to the Atlantic States Marine Fisheries Commission’s Horseshoe Crab Technical Committee, Washington, DC
Moore S, Perrin S (2007) Seasonal movement and resource-use patterns of resident horseshoe crab (Limulus polyphemus) populations in a Maine, USA estuary. Estuar Coast 30:1016–1026
NOAA (1980) Eastern United States coastal and ocean zones data atlas. National Oceanic and Atmospheric Administration, Washington, DC
NOAA (1987) Delaware river and bay: tidal circulation and water level forecast atlas. U.S. Department of Commerce, Washington, DC
NOAA (2001) National Marine Fisheries Service, 50 CFR Part 697, Atlantic Coastal Fisheries Cooperative Management Act Provisions; Horseshoe Crab Fishery; Closed Area. Fed Regist 66(23):8906–8911
Nordstrom KF, Jackson NL, Smith DR (2006) Transport of crab eggs by waves and swash on an estuarine beach: implications for foraging shorebirds. Estuar Coast Shelf Sci 70:438–448
Pielou EC (1991) After the ice age: the return of life to glaciated North America. University Chicago Press, Chicago
Pooler PS, Smith DR, Loveland RE et al (2003) Assessment of sampling methods to estimate horseshoe crab (Limulus polyphemus) egg density in Delaware Bay. Fish Bull 101:698–703
Reeside JB Jr, Harris DV (1952) A cretaceous horseshoe crab from Colorado. J Wash Acad Nat Sci 42:174–187
Riska B (1981) Morphological variation in the horseshoe crab Limulus polyphemus. Evolutionary 35:647–658
Rudloe A, Herrnkind W (1976) Orientation of Limulus polyphemus in the vicinity of breeding beaches. Mar Behav Physiol 4:75–89
Rudloe A, Herrnkind W (1980) Orientation by horseshoe crabs, Limulus polyphemus, in a wave tank. Mar Behav Physiol 7:199–211
Saunders NC, Kessler LG, Avise JC (1986) Genetic variation and geographic differentiation in mitochondrial DNA of the horseshoe crab, Limulus polyphemus. Genetics 112:613–627
Say T (1818) An account of the Crustacea of the United States. J Acad Nat Sci Phila Part II 1(5):423–458
Schaller SW, Chabot CC, Watson WH (2010) Seasonal movements of American horseshoe crabs Limulus polyphemus in the Great Bay estuary, New Hampshire (USA). Curr Zool 56:587–598
Sekiguchi K (1988) Horseshoe crabs of the Japanese coast. In: Sekiguchi K (ed) Biology of horseshoe crabs. Science House, Tokyo, pp 39–45
Sekiguchi K, Shuster CN Jr (2009) Limits on the global distribution of horseshoe crabs (Limulacea): lessons learned from two lifetimes of observations: Asia and America. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 5–24
Selden P, Nudds J (2004) Evolution of fossil ecosystems. Manson Publishing Ltd., London
Shabica CW, Hay AA (eds) (1997) Richardson’s guide to the fossil fauna of Mazon Creek. Northeastern Illinois University, Chicago
Shinn RC (2000) Watershed management and estuary programs: perfect together. In: Kosko K (ed) The Jersey shore line, New Jersey Marine Science Consortium & Sea Grant Special Edition 19(03):5–6
Shishikura F, Nakamura S, Takahashi K et al (1982) Horseshoe crab phylogeny based on amino acid sequences of the fibrino peptide-like C. J Exp Zool 223:89–91
Shuster CN Jr (1950) Observations on the natural history of the American horseshoe crab, Limulus polyphemus. In; 3rd report on investigations of methods of improving the shellfish resources of Massachusetts. Woods Hole Oceanographic Institute, Woods Hole, pp 18–23
Shuster CN Jr (1955) On morphometric and serological relationships within the Limulidae, with particular reference to Limulus polyphemus. Ph.D. Biology dissertation, New York University
Shuster CN Jr (1959) Biological evaluation of the Delaware River Estuary. In: Smith JG, Haber RA, Kaplosky AJ, Simpson CO (coordinating committee) 1959 State of Delaware Intrastate Water Resources Survey, Dover, pp 21–173
Shuster CN Jr (1960a) Oysters in Delaware waters. Estuar Bull Univ Del 5(3):1–15
Shuster CN Jr (1960b) Hydroclimate on the bar grounds. Estuar Bull Univ Del 5(1):7–11
Shuster CN Jr (1979) Distribution of the American horseshoe “crab”, Limulus polyphemus (L.). In: Cohen E (ed) Biomedical applications of the horseshoe crab (Limulidae). Alan R. Liss, Inc., New York, pp 3–26
Shuster CN Jr, Botton ML (1985) A contribution to the population biology of horseshoe crabs, Limulus polyphemus, in Delaware Bay. Estuaries 8:363–372
Shuster CN Jr, Sekiguchi K (2009) Basic habitat requirements of the extant species of horseshoe crabs (Limulidae). In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 115–129
Shuster CN Jr, Barlow RB, Brockmann HJ (eds) (2003) The American horseshoe crab. Harvard University Press, Cambridge, MA
Smith DR, Pooler PS, Swan BL et al (2002a) Spatial and temporal distribution of the horseshoe crab (Limulus polyphemus) spawning in Delaware Bay: implications for monitoring. Estuaries 25(1):115–125
Smith DR, Pooler PS, Loveland RE et al (2002b) Horseshoe crab (Limulus polyphemus) reproductive activity on Delaware Bay beaches: implications for monitoring. J Coast Res 18(4):730–750
Smith DR, Millard MJ, Eyler S (2006) Abundance of adult horseshoe crabs (Limulus polyphemus) in Delaware Bay estimated from a bay-wide mark-recapture study. Fish Bull 104:456–464
Smith DR, Brousseau LJ, Mandt MT et al (2010) Age and sex specific timing, frequency, and special distribution of horseshoe crabs spawning in Delaware Bay: insights from a large-scale radio telemetry array. Curr Zool 56(5):563–574
Swan BL (2005) Migrations of adult horseshoe crabs, Limulus polyphemus, in the Middle Atlantic Bight: a 17-year tagging program. Estuaries 28:28–40
Tiner RW, Biddle AD, Jacobs AD et al (2011) Delaware wetlands: status and changes from 1992 to 2007. Cooperative National Wetlands Inventory Publication, US Fish & Wildlife Service, Northeast Region, Hadley, MA and Delaware Department of Natural Resources & Environmental Control, Dover
Weber RG, Carter DB (2009) Distribution and development of Limulus egg clusters on intertidal beaches in Delaware Bay. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 249–266
Yamasaki T, Makioka T, Saito J (1988) External morphology. In: Sekiguchi K (ed) The biology of horseshoe crabs. Science House, Tokyo, pp 69–132
Zaldivar-Rae J, Sapién-Silva RE, Rosales-Raya M et al (2009) American horseshoe crabs, Limulus polyphemus, in Mexico: open possibilities. In: Tanacredi JT, Botton ML, Smith DR (eds) Biology and conservation of horseshoe crabs. Springer, New York, pp 97–113
Acknowledgements
If no other detailed natural history/ecological studies had been conducted on Limulus polyphemus at Delaware Bay, the contributions by Drs. Mark L. Botton (Fordham University), Robert E. Loveland (Rutgers University), and their colleagues would be notable as being among the first and most significant. The influx of federal scientists during the past score of years, led by Drs. David R. Smith (US Geological Survey) and Michael J. Millard (US Fish and Wildlife Service), that supported the Atlantic States Marine Fisheries Commission Horseshoe Crab Management Program, also added to an understanding of the activities of Limulus at Delaware Bay. Thanks are also due to the hundreds of volunteers who have participated in spawning surveys, in tagging programs, and in development of individual and community-based horseshoe crab sanctuaries and other conservations efforts (in the “Just Flip ‘Me” and community-based horseshoe crab sanctuaries organized by Glenn Gauvry, the Ecological Research and Development Group Inc.), as well as to watermen who shared their opinions on Limulus.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shuster, C.N. (2015). The Delaware Bay Area, U.S.A.: A Unique Habitat of the American Horseshoe Crab, Limulus polyphemus . In: Carmichael, R., Botton, M., Shin, P., Cheung, S. (eds) Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Springer, Cham. https://doi.org/10.1007/978-3-319-19542-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-19542-1_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19541-4
Online ISBN: 978-3-319-19542-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)