Abstract
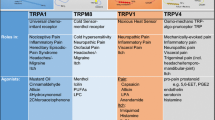
TRP channels are a family of ion channels involved in a plethora of physiological sensory processes. Since their discovery they have attracted the attention of academic and non-academic laboratories with the aim of developing modulators that could be used as pharmacological tools for unveiling their physiological and pathological activities, and as therapeutic compounds for intervening in TRP dysfunction. Intriguingly, TRP pharmacology shows dispersed progress, with vast pharmacology developed for some members of the so-called thermoTRP channel subfamily (TRPV1, TRPV3, TRPM8 and TRPA1), and very little, for all other TRP channels. Pharmacologically, the most investigated TRP channel is undoubtedly TRPV1 for which a large number of agonists and antagonists with in vitro and in vivo activities have been characterized. Recent interest has grown for TRPV3, TRPM8 and TRPA1 because of their implication in several human pathologies and disorders. Similarly, the TRPM3 channel is emerging as important targets for pain transduction. With the development of novel screening methods, the focus is slowly changing to other TRP members for whom we do not have appropriate agonists or antagonists. These include the TRPC family, which has limited our understanding of their role in pathological processes and whether pharmacological intervention in these channels will have a therapeutic benefit. A bright future is anticipated for TRP pharmacology, with the discovery of selective and potent modulators for this important family of sensory channels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- TRP channels
- Agonists
- Antagonists
- Competitive
- Non-competitive
- Uncompetitive
- Pain
- Therapeutic index
- Pathology
2.1 Introduction
TRP channels are a superfamily of ion channels that includes seven subfamilies, namely TRPC, TRPV, TRPP, TRPM, TRPA, TRPML, and TRPN. These channels perform a wide diversity of physiological functions and are present in many tissues, and almost all cell types. Most TRP channels are non-selective cation channels with low voltage dependence. TRP channels use a wide variety of activation and regulatory mechanisms and carry out functions as diverse as thermosensation, phototransduction, pheromone reception, magnesium homeostasis, and vascular tone regulation (Montell 1999). (see Chap. 4 by Bacigalupo et al. in this Book) . Thus, these channels are considered molecular gateways in sensory and regulatory systems.
Structurally, TRP channels are tetrameric assemblies of basic subunits organized around a central aqueous pore. Akin to voltage-gated K + channels, each subunit is composed of a transmembrane region containing 6 transmembrane segments. The recent structural model derived from cryo-electron microscopic images has clearly shown this molecular analogy (Liao et al. 2013) . All TRP channels display this core transmembrane region, and differ in the cytosolic N- and C-termini domains, which are involved in channel gating and mediating intracellular signaling. Indeed, most of TRP channels, if not all, are part of protein complexes known as signalplexes (Devesa et al. 2011; Fernandez-Carvajal et al. 2011; Ferrer-Montiel et al. 2012) .
Some TRP channels have been involved in the pathophysiology of human diseases. This pathological contribution could be the result of channel mutations, giving rise to channelopathies (Devesa et al. 2011; Fernandez-Carvajal et al. 2011; Ferrer-Montiel et al. 2012), or the change in channel function due to alteration of the protein function and/or expression (Devesa et al. 2011; Fernandez-Carvajal et al. 2011; Ferrer-Montiel et al. 2012). The pivotal involvement in the etiology of pathological conditions has signaled members of this large channel family as druggable targets for therapeutic intervention, which has driven discovery programs in academic and non-academic institutions. This concerted effort has notably expanded the pharmacology of TRP channels , although, unfortunately, for a limited number of TRP members. For instance, large families of modulators have been obtained for TRPV1, TRPV3, TRPM8 and TRPA1 , while the pharmacology of other TRP channels is still in its infancy. A plausible reason for the pharmacological progress in these channels is the availability of natural ligands present in food spices. Nonetheless, the development of combinatorial chemistry and the large diversity of vegetal and marine extracts, along with the development of high throughput electrophysiological assays for ion channels will expand the pharmacology of TRP channels to the entire family. Here, we briefly expose the pharmacological data for the most studied TRP channels , namely TRPV1, TRPM8 and TRPA1, and include the data accrued for TRPV2, TRPV4, TRPM3 and the TRPC5, most of them with an important role in sensory transduction. We aim to illustrate the differential pharmacological progress in this exciting field and evidence a drift towards enhancing the pharmacology of other members, if not all, of this pivotal channel family.
2.2 TRPV1
TRP Vanillod 1, TRPV1, a non-selective Ca2 + channel is a TRP channel activated by noxious temperatures ( 43 °C) acidic pH and vanilloid compounds, whose channel activity is highly potentiated by proalgesic mediators in response to inflammation, tissue injury and ischemia (Huang et al. 2006; Ueda et al. 2008) . In addition, TRPV1 expression is markedly up-regulated under acute inflammatory conditions (Camprubi-Robles et al. 2009; Morenilla-Palao et al. 2004; Van Buren et al. 2005) , and in human chronic pain states (Broad et al. 2008; Szallasi and Blumberg 2007) . Consistent with a role in pain signaling, TRPV1 is highly expressed in C-type, peptidergic nociceptors in the peripheral nervous system. Thus, TRPV1 is considered a gateway for pain transduction, and a pivotal target for drug intervention in pain syndromes. In addition, due to a widespread tissue distribution of this TRP channel, it may be involved in the etiology of other human pathologies or disorders (Avelino et al. 2002; Inoue et al. 2002) .
TRPV1 sensitization by inflammatory conditions is produced through two distinct, but complementary mechanisms, namely: (i) covalent modification of the channel by protein kinase A (PKA) and/or protein kinase C (PKC) phosphorylation (Bhave et al. 2003; Tominaga et al. 2001; Varga et al. 2006; Vellani et al. 2001) ; and, (ii) rapid recruitment of a vesicular population of TRPV1 channels to the neuronal surface through a Ca2 + -dependent, SNARE-mediated exocytosis mechanism in response to pro-algesic agents (Camprubi-Robles et al. 2009; Zhang et al. 2005).
Pharmacologically, TRPV1 is primarily activated by a diverse collection of chemical ligands known as vanilloids (Caterina et al. 2000; Khairatkar-Joshi and Szallasi 2009) (Fig. 2.1). The most known agonist of TRPV1 is capsaicin, the pungent compound of chili peppers. Resiniferatoxin (RTX), a vanilloid from Euphorbia resinifera, is also a potent agonist of the receptor. Furthermore, TRPV1 may also be activated by non-vanilloid compounds, such as allicin, piperine, camphor, olvanil, 2-aminoethoxydiphenylborate (2-APB), and tarantula venom peptide toxins (Bohlen et al. 2010) (Table 2.1). In addition, there is a family of endogenous compounds, referred to as endovanilloids, that also act as agonists of TRPV1 (Van Der Stelt and Di 2004) . These compounds may be divided into conjugates of biogenic amines [e.g., N-arachidonoylathanolamine (AEA, anandamide), N-arachidonoyldopamine (NADA), N-oleoylethanolamine (OLEA), N-arachidonolylserine, and various N-acyltaurines and N-acylsalsolinols (Appendino et al. 2008) , and oxygenated eicosatetraenoic acids like the lipoxygenase products 5-, 12-, and 15-hydroperoxyeicosatetraenoic acids (5S-, 12S-, 15S-HPETE), their reduced hydroxyl analogs, prostaglandins, and leukotriene B4 (Ahern 2003; Huang et al. 2006; Wang et al. 2005) (Fig. 2.1) .
Selected examples of activators (1–8) and inhibitors (9–10) of TRPV1. 1 Olvanil (CID 5311093). 2 Piperine (CID 638024). 3 Allicin (CID 65036). 4 Anandamide (CID 5281969). 5 NADA: N-arachidonoyl dopamine. (CID 5282105). 6 OLEA: N-oleoyl ethanolamine (CID 5283454). 7 EMA-6: N-arachidonoyl serine (CID 10596625). 8 15-Hpete: 15-hydroperoxy eicosatetraenoic acid (CID 6437084). 9 BCTC (CID 9929425). 10 I-RTX: 5-iodoresiniferatoxin (CID 16219535)
As expected, the activation of TRPV1 in nociceptors with vanilloids causes a burning pain sensation and irritation. Paradoxically, capsaicin has been in use for many years as anti-nociceptive compound in peripheral neuropathies (e.g., post-herpetic neuralgia, neuropathy, mastectomy, amputation and skin cancer). Capsaicin is used as an analgesic, because in addition to activate the channel, it also induces its desensitization. Furthermore, the repetitive application of the vanilloid produces a rundown of channel activity known as tachyphylaxia that results in a strong anti-nociceptive effect (Knotkova et al. 2008) . This analgesia may be accompanied by reversible and/or irreversible loss of the capsaicin sensitive C-fibers (Hiura 2000) .
Although TRPV1 agonists may have some therapeutic application, their low in vivo activity, along with their poor bioavailability and secondary effects has limited their development as anti-nociceptives, and promoted the research into the design of potent antagonists that display higher therapeutic index . The efforts in developing TRPV1 antagonists have been concentrated in obtaining both competitive and non-competitive (including uncompetitive) inhibitors (Planells-Cases et al. 2003; Szallasi and Appendino 2004) . Uncompetitive antagonists acting as open channel blockers are activity-dependent blockers that preferentially bind to over-activated receptors, with minimal interaction with the physiologically working channels. Accordingly, they are supposed to display lower side-effects than conventional antagonists.
Among the competitive TRPV1 antagonists (Fig. 2.1), capsazepine was the first identified, although with poor in vivo activity (Bevan et al. 1992; Walker et al. 2003) . A vanilloid with better therapeutic potential is 5-iodo-RTX, a potent TRPV1antagonist (IC50 = 3.9 nM) (McDonnell et al. 2002; Wahl et al. 2001) . This compound produced notable analgesic activity in vivo and it is currently under clinical studies.
The family of competitive antagonists grew tremendously thanks to the contribution of pharmaceutical companies that established strong drug discovery programs for TRPV1 channels. As a result, ultra-high affinity synthetic antagonists were discovered for analgesic drug development. However, most of the clinical trials for these compounds had to be cancelled in Phase I because the indiscriminate blockade of TRPV1 channels with these compounds resulted in significant hyperthermia in humans, suggesting that this receptor also plays a pivotal role in core body temperature (Gavva et al. 2008) .
The first non-competitive TRPV1 antagonist was the trinuclear polyamine complex, ruthenium red that was followed by arginine-rich peptides, and peptidomimetic compounds such as peptoids DD00069 and DD01050 (Garcia-Martinez et al. 2002, 2006) . All these compounds resulted in unacceptable in vivo side effects and toxicity that prevented their clinical development. Recently, an uncompetitive antagonist, based in a triazine scaffold (triazine 8aA) that block TRPV1 channel by an activity-dependent mechanism was reported (Vidal-Mosquera et al. 2011) . Triazine 8aA showed a strong voltage-dependent TRPV1 blockade by inhibiting at negative membrane potential, a hallmark of open-channel blockers. This compound holds promise for therapeutic development, although in vivo activity in pain models has not been yet reported.
Allosteric modulators of TRPV1 activity are another class of non-competitive antagonists. These compounds interfere with the allosteric mechanism that gates the channel. Structure-function analysis of TRPV1 channels demonstrated that the intracellular TRP domain, a highly conserved region adjacent to the receptor internal gate (Venkatachalam and Montell 2007) , is essential for subunit tetramerization and allosteric activation (Garcia-Sanz et al. 2004, 2007) . Thus, this protein interface could be used as an allosteric site to modulate channel function. Indeed, compound TRP-p5, a palmitoylated 13-mer peptide patterned after the N-terminus region of the TRP domain, displays in vitro and in vivo inhibitory activity (Valente et al. 2011) . This finding is proof-of-concept that allosteric modulators such as TRPducins represent another family of non-competitive antagonists that could be developed therapeutically as anti-nociceptives.
A complementary approach to reduce the inflammatory sensitization of TRPV1 has been to interfere with the recruitment of the channel to the neuronal surface. This strategy has proven that blockers of neuronal exocytosis such as compound DD04107 display analgesic activity (Ponsati et al. 2012) . In vitro experiments with DD04107 showed that it blocked the inflammatory over expression of TRPV1 channels to the plasma membrane (Camprubi-Robles et al. 2009) . In vivo, this compound displays long-lasting anti-nociceptive activity against inflammatory and neuropathic pain, without apparent side effects, demonstrating that acting on the TRPV1 signalplex may be a valuable pharmacological strategy (Ponsati et al. 2012). This compound is being developed clinically.
2.3 TRPV2
At variance with TRPV1 channels, the pharmacology of its close homologue TRPV2 is still in its infancy (Peralvarez-Marin et al. 2013) . This non-selective Ca2 + channel is also present in the peripheral nervous system and co-localizes with TRPV1 in a subset of nociceptors (Liapi and Wood 2005) . The physiological role of this TRP channel is yet elusive. Initially was considered a thermoTRP channel that activated at 52 °C, and also responded to hypotonicity (Caterina et al. 1999; Muraki et al. 2003) . However, these are still highly debated functions (Park et al. 2011; Peralvarez-Marin et al. 2013) , thus requiring further investigation, including the discovery of agonists and antagonists that could be used as pharmacological tools.
The identification of specific TRPV2 modulators is, surprisingly, inexistent, probably due to the species-specific pharmacology coupled with problems in developing stable recombinant cell lines due to cytotoxic effects of TRPV2 expression (Penna et al. 2006) . Several chemical compounds have been shown to modulate TRPV2, however, virtually all of them are non-specific (Table 2.1 and Fig. 2.2). Indeed, TRPV2 is activated by general TRP channel agonists, such as 2-aminoethoxy-diphenyl borate (2-APB), probenecid, lysophospholipids, and cannabinoids (Juvin et al. 2007; Monet et al. 2009; Qin et al. 2008) . However, the response to these ligands is low and variable and quite species-dependent (Neeper et al. 2007) .
Selected examples of TRPV2-4 effectors. TRPV2 activators (1) and inhibitors (2–5). TRPV3 activators (6–10) and inhibitors (11–12). TRPV4 activators (13–14) and inhibitors (15). 1 probenecid (CID 4911). 2 4-AP: 4-aminopyridine (CID 1727). 3 Trim: 1-(2-(trifluoromethyl)phenyl) imidazole (CID 1359). 4 Amiloride (CID 16231). 5 Tranilast (CID 5282230). 6 Resolvin-D1: 17(R)-resolvin D1 (CID 71434077). 7 Diphenylboronic anhydride (CID 596810). 8 Farnesyl pyrophosphate (CID 44134714). 9 Incensole (CID 44583885). 10 Citral (CID 638011). 11 Icilin (CID 161930). 12 Isopentenyl pyrophosphate (CID 1195). 13 GSK1016790A (CID 23630424). 14 RN-1747 (CID 5068295). 15 RN-1734 (CID 3601086)
To date, only general blockers such as ruthenium red and trivalent cations (La3 + and Gn3 +) (Table 2.1), have been described as blockers of TRPV2 (Leffler et al. 2007) . In addition, the potassium channel blockers tetraethylamonium (TEA), 4-aminopyridine (4-AP), and 1-(2-(trifluoromethyl)phenyl) imidazole are also able to block TRPV2 currents (Vriens et al. 2009) . Other reported inhibitors are SKF96365, amiloride, and Tranilast, an antiallergic drug (Juvin et al. 2007; Mihara et al. 2010) (Fig. 2.2).
2.4 TRPV3
TRPV3 is a non-selective Ca2 + channel that plays a pivotal role in various physiological processes in the skin and hair follicles. This channel displays a moderate sequence homology to TRPV1. TRPV3 is mainly located in keratinocytes and epithelial cells (Nilius and Owsianik 2011; Valdes-Rodriguez et al. 2013) , and marginally in sensory neurons (Nilius et al. 2014) . This TRP channel is a polymodal receptor activated by non-painful temperatures (Peier et al. 2002a; Smith et al. 2002; Xu et al. 2002) , and chemical stimuli (Xu et al. 2006a), including natural irritants and synthetic ligands (Xu et al. 2006a) , and endogenous compounds, some of them involved in the downstream inflammatory cascade (Doerner et al. 2011; Sherkheli et al. 2009) . Stimulation of TRPV3 releases inflammatory mediators from keratinocytes including ATP, prostaglandin E2 and IL-1, which supports its contribution to pain transduction and inflammatory signaling. Indeed, in certain human disease states there are changes in the expression of TRPV3, such as an increase in painful breast tissue (Matta et al. 2008) , or a decrease in keratinocytes in diabetic neuropathy (Facer et al. 2007) .
Some evidence points to phosphatidyl inositol-4,5-bisphosphate and 17(R)-resolvin D1 as putative in vivo modulators of TRPV3 (Bang et al. 2012; Doerner et al. 2011) (Table 2.1). A role of 17(R)-resolvin D1 as potential analgesic mediated by TRPV3 has been described, although a direct evidence is still missing (Bang et al. 2012). Similarly, TRPV3 has been related with the production of nitric oxide via a nitrite independent pathway (Miyamoto et al. 2011) . Furthermore, farnesyl pyrophosphate and isopentenyl pyrophosphate, intermediates of the melanovate pathway, are activator and inhibitor respectively, suggesting a fine-tuning of TRPV3 function (Bang et al. 2010; 2011) . Alfa-hydroxy acids are proton donors commonly used in cosmetics to produce skin exfoliation mediated by TRPV3 activation (Cao et al. 2012) .
The pharmacology of the TRPV family is far from simple, and TRPV3 is not an exception (Table 2.1 and Fig. 2.2). 2-APB also activates TRPV3 (Chung et al. 2004; Hu et al. 2004, 2009) . Prolonged exposure of TRPV3 to 2-APB induced sensitization (Sherkheli et al. 2009) . Structurally related 2-APB compounds such as diphenylboronic anhydride also act as potent TRPV3 agonists (Chung et al. 2005) .
Natural aromatic monoterpenes, such as camphor, carvacrol, eugenol, menthol, thymol, as well as borneol, cresol, and others are an additional class of TRPV3 ligands (Moqrich et al. 2005; Vriens et al. 2009; Xu et al. 2006a) . Camphor is a weak agonist for TRPV3 that activates currents only at concentrations of 10 mM. Carvacrol is responsible for arterial vasodilation by activating TRPV3 channels in the endothelium (Earley et al. 2010) , which may account for some of their attributed cardioprotective effects. In addition to camphor and carvacrol, thymol and eugenol have also been shown to enhance the temperature response of TRPV3 (Macpherson et al. 2006; Xu et al. 2006a) .
Non-aromatic monoterpenes such as carveol and derivatives (monocyclic), or gerianool, propofol and linalool (acyclic) display strong TRPV3 agonism (Vogt-Eisele et al. 2007) . Incensole and incensole acetate are diterpenic cembrenoids found in incense (Boswellia papyrifera) potently activate TRPV3. The traditional use of these natural products is related to anti-inflammatory effects through the activation of TRPV3 in the skin. Interestingly, incensole acetate produces anxiolytic and antidepressive effects in mice (Moussaieff and Mechoulam 2009; Paul and Jauch 2012) . Citral, a bioactive component of lemongrass is also an agonist of TRPV3 (Stotz et al. 2008) , adding to the list of compounds acting on this channel (Fig. 2.2).
Cannabinoids such as cannabidiol or delta-9-tetrahydrocannabinol modulate nonspecifically TRPV3. Other derivatives such as cannabigerovarin or cannabigerolic desensitize TRPV3 (De Petrocellis et al. 2012) . Active research is necessary in this field because, interestingly, the activation of TRPV3 by these compounds may contribute to their described in vivo activity (Anand 2003; Galeotti et al. 2001; Santos and Rao 2001; Umezu et al. 2001; Xu et al. 2005a) .
TRPV3 antagonists include the non-specific ruthenium red, that blocks all TRPV family member at negative potentials (Vennekens et al. 2008) . The compound icilin, which is a strong agonist of TRPM8 channel, is an inhibitor of TRPV3 at low doses (Sherkheli et al. 2012) . Novel inhibitors are under study and have promising analgesic effects, which further suggests the involvement of TRPV3 in pain transduction (Reilly and Kym 2011) . Several pharmaceutical industries have reported strong and selective TRPV3 antagonists including series of chromane-, fused pyrimidine-, fused pyrimidinones-, chromanone- and fused imidazole-derivatives (Ferrer-Montiel et al. 2012) . Some of these antagonists are currently under clinical studies to treat human pain conditions.
2.5 TRPV4
Transient Receptor Potential Vanilloid 4 (TRPV4) a non-selective Ca2 + channel is a homologue of the OSM-9 osmosensory channel first described in C. elegans. TRPV4 is activated by warm temperatures (27–35 °C) (Guler et al. 2002; Liedtke et al. 2000) , and is sensitive to cell swelling and shear stress (Gao et al. 2003; Kohler et al. 2006; Loukin et al. 2010; Strotmann et al. 2000) . Functions include temperature monitoring in skin keratinocytes, osmolarity sensing in the kidney (Pochynyuk et al. 2013) , and shear stress detection in blood vessels, which indicates that TRPV4 functions as a putative mechanosensor (Nilius et al. 2003a, b) , and is involved in nociception (Alessandri-Haber et al. 2005, 2006) . It has been reported that TRPV4 may be activated by hypotonic solutions, and by mechanical forces in membrane patches (Loukin et al. 2009). TRPV4 may contribute to development of mechanical hyperalgesia after inflammation and injury (Alessandri-Haber et al. 2006) . This channel is expressed in several tissues, including primary sensory neurons (Alvarez et al. 2006; Birder et al. 2007; Guler et al. 2002; Pochynyuk et al. 2013; Strotmann et al. 2000; Tabuchi et al. 2005; Watanabe et al. 2002b; Yang et al. 2006) .
TRPV4 is activated by endogenous chemical ligands, such as endocannabinoids, arachidonic acid metabolites and nitric oxide (Birder et al. 2007) (Table 2.1 and Fig. 2.2). Phorbol esters that do not activate PKC, mediate TRPV4 heat responses (Watanabe et al. 2002a) . TRPV4 sensitivity to osmotic and mechanical stimuli may depend on phospholipase A2 activation and the generation of arachidonic acid metabolites (Fernandes et al. 2008; Liedtke et al. 2000; Strotmann et al. 2000; Vriens et al. 2004) . Furthermore, TRPV4 is activated by hypotonicity, diacylglycerol, and PKC-activating phorbol esters (Watanabe et al. 2002a, b, 2003) .
Natural plant extracts (Klausen et al. 2009) , bisandrographolide A (Smith et al. 2006) and synthetic compounds, such as a phorbol derivative (Birder et al. 2007) , or GSK1016790A (Thorneloe et al. 2008) also activate TRPV4 channels. In addition, small molecules such as compound RN-1747 was also found to be a TRPV4 agonist (Vincent et al. 2009) (Table 2.1 and Fig. 2.2).
TRPV4 antagonism is being considered for inflammatory and neuropathic pain treatment (Vincent and Duncton 2011) . However, selective TRPV4 antagonists have not been described appropriately. Ventilator-induced lung injury has emerged as a potential indicator for TRPV4 antagonists (Jin et al. 2011) (Table 2.1). The small molecule RN-1734 2,4-Dichloro-N-isopropyl-N-(2-isopropylaminoethyl)benzenesulfonamide was observed to inhibit ligand- and hypotonicity-activated TRPV4 (Vincent et al. 2009). In addition, the compound showed selective properties for TRPV4 over other TRPs such as TRPV1, TRPV3 and TRPM8, being a valuable pharmacological tool for TRPV4 studies (Vincent et al. 2009) .
2.6 TRPC5
Several mammalian and Drosophila TRP canonical, TRPC proteins (TRPC1-7) have been identified (Plant and Schaefer 2003; Wes et al. 1995; Hardie and Minke 1995) . All mammalian TRPCs seem to be enhanced with G-protein-coupled receptors and tyrosine kinases receptors (Montell 1999) . The channels may be divided in three subgroups according to sequence homology: C1-C4-C5, C3-C6-C7, and C2 (Zufall et al. 2005) . Particularly, TRPC5 is a functional plasma membrane ion channel (Beech 2007) activated by hypo-osmotic stimuli, which is dependent on phosphoinositides (Gomis et al. 2008) . The inhibition of TRPC5 has been shown to suppress inflammatory pain induced by the component of the bee venom mellitin (Ding et al. 2011) . Several studies support the conclusion that TRPC5 plays a role in growth cone extension and axonal guidance (Davare et al. 2009) . A variety of other functions have been assigned to TRPC5, indicating a central role of this channel in physiology (Jiang et al. 2011; Nath et al. 2009; Premkumar and Abooj 2013; Wu et al. 2010; Wuensch et al. 2010; Xu et al. 2008) , although the channel is not essential for life (Riccio et al. 2009) .
TRPC5 modulation is not well known. A common stimulus for TRPC5 is the activation of G protein-coupled receptor. Many different receptors may be involved, including receptors for adenosine 5’-triphosphate, bradykinin, acetylcholine, histamine, prostaglandin E2, thrombin, uridine 5’-triphosphate, sphingosine-1-phosphate, glutamate and cholecystokinin (Meis et al. 2007; Riccio et al. 2009; Xu et al. 2006b; Zeng et al. 2004) . TRPC5 is stimulated by activation of growth factor receptors (Bezzerides et al. 2004) . TRPC5 is also a target for thioredoxin, an endogenous redox protein with established intracellular functions. Reduced thioredoxin activates TRPC5 expressed in secretory fibroblast-like synoviocytes when secreted extracellularly in patients with rheumatoid arthritis (Xu et al. 2008). Lysophosphatidylcholine has been identified as a TRPC5 activator (Flemming et al. 2006) . Current data suggest a complex arrangement between TRPC5 activity and various lipid factors, supporting the hypothesis that a physiological function of TRPC5 channels is to act as lipid signal transducers.
An unusual feature of TRPC5 is its stimulation by external lanthanides (Jung et al. 2003; Schaefer et al. 2000; Xu et al. 2005b; Zeng et al. 2004) . It was reported that ionic lead (Pb2 +) mimics the effect of lanthanides, leading to the hypothesis that TRPC5 may confer survival advantage by acting as a sensor of heavy metal ions (Sukumar and Beech 2010) . Stimulation of TRPC5 by isoflavones like genistein or diadzein has also been reported (Wong et al. 2010) (Table 2.1, Fig. 2.3).
Although no specific or potent exogenous chemical inhibitors of TRPC5 are known, various chemicals have effects on TRPC5 function (Table 2.1). In many of these cases, it is not clear if the agent acts directly on the channel. TRPC5 has been reported to be inhibited by SKF-96365 (Okada et al. 1998) , 3,5-bis(tri-fluoromethyl)pyrazole derivative BTP-2 (He et al. 2005; Kiyonaka et al. 2009) , flufenamic acid (Lee et al. 2003b) , W-13 or chlorpromazine (Shimizu et al. 2006) , W-7 or calmidazolium (Kim et al. 2006) , Pyr2, 2-APB (Xu et al. 2005b) , and the myosin light chain kinase inhibitors ML-7 or ML-9 (Shimizu et al. 2006) (Fig. 2.3).
2.7 TRPM3
TRPM3, TRP melastatin 3, non-selective Ca2 + channel is one of the least investigated proteins of the TRP family of ion channels. In humans, it is highly expressed in the kidney (Grimm et al. 2003; Lee et al. 2003a) , brain (Lee et al. 2003a; Oberwinkler 2007) , sensory neurons, human pituitary (Fonfria et al. 2006) , vascular smooth muscle (Naylor et al. 2010) and pancreatic beta cells (Thiel et al. 2013; Wagner et al. 2008) . However, its physiological role is still under investigation. Activation of TRPM3 has been linked to insulin secretion in pancreatic beta-cells (Wagner et al. 2008), to vascular smooth muscle cell contraction (Naylor et al. 2010), and to potentiating glutamatergic transmission in cerebellar Purkinje neurons of developing rats (Zamudio-Bulcock et al. 2011) . A role in pain transduction has also been reported for TRPM3 (Vriens et al. 2011) .
Recently, pharmacological investigations have been initiated in order to identify substances that influence TRPM3 channel activity. TRPM3 is rapidly and reversibly activated by extracellular pregnenolone sulphate, a neuroactive steroid. Application of pregnenolone sulphate led to a rapid calcium influx and enhanced insulin secretion from pancreatic islets (Wagner et al. 2008) . Pregnenolone sulfate also activates TRPM3 channels in HEK293 cells, vascular smooth muscle cells, and synovial fibroblasts (Ciurtin et al. 2010; Klose et al. 2011; Majeed et al. 2012; Naylor et al. 2010) , confirming the functional relevance of TRPM3 in contractile function. However, the concentration of pregnenolone sulfate required to stimulate TRPM3 channels is in the micromolar range, suggesting that pregnenolone sulfate is not a physiological agonist of TRPM3 and may have only pharmacological relevance. Moreover, the fact that TRPM3 deficient mice did not show alterations in resting blood glucose levels (Vriens et al. 2011) suggests that TRPM3 plays a marginal role in controlling β-cell functions.
Two structural analogs of sphingosine, dihydro-D-erythro-sphingosine and N, N-dimethyl-D-erythro-sphingosine, are able to activate TRPM3 (Grimm et al. 2005) (Table 2.1 and Fig. 2.4). Surprisingly, TRPM3 channels are also activated by the dihydropyridine nifedipine, an inhibitor of voltage-gated Ca2 + channels, while the structurally related compounds nimodipine, nicardipine, and nitrendipine were inactive (Wagner et al. 2008) .
Selected examples of TRPM3 and TRPM8 effectors. TRPM3 activators (1–3) and inhibitors (4–7). TRPM3 activators (8–9) and inhibitors (10–13). 1 Pregnenolone sulphate (CID 105074). 2 Safingol: Dihydro-D-erythro-sphingosine (CID 91486). 3 Nifedipine (CID 4485). 4 Rosiglitazone (CID 77999). 5 Troglitazone (CID 5591). 6 Mefenamic acid (CID 4044). 7 Naringenin (CID 932). 8 Menthol (CID 16666). 9 D-3263 (CID 44137358). 10 AMTB (CID 3036972). 11 JNJ-41876666: Johnson&Johnson patent. 12 Clotrimazole (CID 2812). 13 Econazole (CID 3198)
As for many other members of the TRP ion channel family, 2-APB and Gd3 + have been reported to inhibit Ca2 + influx through TRPM3 channels (Grimm et al. 2003; Harteneck and Schultz 2007; Xu et al. 2005b) . Other TRPM3 channel blockers described thus far include the antidiabetic PPARγ-agonists rosiglitazone and troglitazone (Majeed et al. 2012). Nonsteroidal anti-inflammatory drugs (NSAIDs) of the fenamate group, like mefenamic acid are able to selective block TRPM3-mediated Ca2 + entry as well as insulin release (Klose et al. 2011) . Cholesterol, the precursor metabolite of pregnenolone and progesterone, also prevents TRPM3 channel activation. (Naylor et al. 2010) .
Recently, the screening of a compound library revealed that citrus fruit flavanones, such as naringenin and hesperetin, and fabacea secondary metabolites selectively inhibit TRPM3 channel activation with potencies ranged from upper nanomolar to lower micromolar concentrations (Straub et al. 2013) (Table 2.1).
2.8 TRPM8
TRPM8 is a Ca2 + permeable channel that was first identified in prostate cancer cells (Tsavaler et al. 2001; Bidaux et al. 2007) , but also is present along the male urogenital tract (De Blas et al. 2009) , artery myocytes (Johnson et al. 2009) , and lung epithemium cells (Sabnis et al. 2008) , although its role in many of these tissues still remains unclear. These channels are expressed in primary sensory neurons in skin and mucosae, with a physiological role in detecting low temperature signals (10–33 °C) (Babes et al. 2011) , and in sensing cooling chemicals like menthol and icilin (McKemy et al. 2002; Peier et al. 2002) (Bharate and Bharate 2012; Chuang et al. 2004) . Under pathological conditions, there are increasing experimental evidences that confirm the anomalous over-expression of TRPM8 channels in sensory neurons after nerve injury or inflammation, as well as their involvement in cold allodynia and hyperalgesia (Kapoor 2012; Xing et al. 2007) (Abe et al. 2006; Ramachandran et al. 2013) . The activation of TRPM8 also attenuates pain in certain acute and inflammatory pain states, mediating for instance the analgesic effects of menthol (Liu et al. 2013) . Therefore, both TRPM8 agonists and antagonists could be valuable analgesic agents (Liu and Qin 2011; Maelkiae et al. 2011) . TRPM8 channels are also expressed in corneal afferent neurons implicated in the regulation of ocular surface wetness, and in this respect TRPM8 modulators could have application in dry eye syndrome and excessive lacrimation dysfunction (Fernández-Peña and Viana 2013; Parra et al. 2010) .
On the other hand, TRPM8 is abnormally over-expressed in androgen-sensitive prostate cancer (Gkika and Prevarskaya 2011; Tsavaler et al. 2001) , breast cancer (Dhennin-Duthille et al. 2011; Ouadid-Ahidouch et al. 2012) , skin melanoma cells (Yamamura et al. 2008) , human pancreatic adenocarcinoma (Yee et al. 2010) , oral scamous cell carcinoma (Okamoto et al. 2012) , and osteosarcoma tissues and cell lines (Wang et al. 2014) . Again, both agonists and antagonists of TRPM8 have proved to be valid as pharmacological tools for reducing growth and progression of neoplasias with intense expression of TRPM8 channels. Therefore, TRPM8 may also be considered an attractive target for therapeutic intervention in the search for new antitumor agents (Knowlton and McKemy 2011; Lehen’kyi and Prevarskaya 2011) .
The crucial role of TRPM8 in the human pathologies is behind the intensive drug-discovery programs developed in recent years around this channel (with more than 25 patents filed since 2009). Among the antagonists, different families having benzotiophene, benzimidazole and arylglycine moieties as the central scaffold have been reported (Calvo et al. 2012; Matthews et al. 2012; Parks et al. 2011; Zhu et al. 2013) . Some of these compounds showed excellent in vitro and in vivo profiles, including activity in animal models of inflammatory and neuropathic pain , thus emerging as strong candidates for future development (Table 2.1). The benzothiophene derivative JNJ41876666 has been used, along with other antagonist, AMTB, and RNAi to determine that the inhibition of either the expression or function of TRPM8 channels reduces the proliferation rate of prostate tumor cells, while no effect was observed in non-tumor cells (Valero et al. 2012) .
The commercial antagonist N-(3-Aminopropyl)-2-[(3-methylphenyl)methoxy]-N-(2-thienylmethyl) benzamide hydrochloride AMTB has also served to suggest the potential of TRPM8 channel blocker as a new therapeutic opportunity for treating overactive bladder and painful bladder syndrome (Lashinger et al. 2008) . Some tetrahydroquinoline and aza-analogues are TRPM8 antagonists , and a selected compound from this series reduced icilin-induced wet-dog shakes (WDS) in a dose dependent manner (Tamayo et al. 2012) . Other chemotypes able to inhibit TRPM8 channels include the piperazine urea derivative, BCTC, used to demonstrate that the menthol- and cold-induced allergic responses of mast cells are mediated by TRPM8 (Cho et al. 2010) . A series of spiro-chromene-piperidines endowed with high potency and favorable ADME properties are effective in rodent models of neuropathic pain (Chaudhari et al. 2013) . SKF-96365 and the antifungic drugs clotrimazole and econazole are also effective blockers of TRPM8 (Madrid et al. 2006; Mälkiä et al. 2009; Meseguer et al. 2014; Table 2.1 and Fig. 2.4) .
It has also been described that the Gαq protein, formed after activation of GPCRs, blocks TRPM8 activity by the direct formation of a complex with the channel (Zhang et al. 2012) . This could indirectly arbitrate the inhibition of TRPM8 by the inflammatory mediators bradykinin and histamine in sensory nerves, and could open new strategies for modulating these channels, interfering within protein-protein interactions.
TRPM8 agonists are also therapeutically important for attenuating pain, and may induce apoptosis in TRPM8 expressing cancer cells (Maelkiae et al. 2011) (Table 2.1). The most remarkable result in this field refers to the ability of 3-(2-amino-ethyl)-1(R)-[(2(S)-isopropyl-5(R)-methyl cyclohexanecarbonyl)]− 5-methoxy-1,3-dihydro-benzoimidazol-2-1 hydrochloride D3263 to inhibit the growth of TRPM8 expressing tumors. This new orally bioavailable chemical entity has already completed Phase I clinical trials in healthy individuals. The company also reported clinical studies on patients with advanced solid tumors, for which the preliminary results indicate disease stabilization after treatment. Some preclinical data also demonstrated the potential of D3263 to treat benign prostatic hyperplasia by itself or in combination with the synthetic 5α-reductase inhibitor finasteride.
2.9 TRPA1
Transient receptor potential ankyrin 1 (TRPA1) is a non-selective Ca2 + channel characterized by a high number of ankyrin repeats (14) at the N-terminal domain (Andrade et al. 2012) . This channel is activated by multiple stimuli, including temperature, acids, and numerous chemicals, like different noxious environmental and industrial pollutants, oxidant agents and bacterial endotoxins (Bandell et al. 2004; Bautista et al. 2005; Jordt et al. 2004; Levine and Alessandri-Haber 2007; Peterlin et al. 2007; Meseguer et al. 2014) . Experimental evidence suggests that TRPA1 is activated by low temperatures, near the threshold of harmful cold for humans (Abrahamsen et al. 2008; del Camino et al. 2010; Karashima et al. 2009) , although the categorization of TRPA1 as a cold thermosensor has been controversial.
TRPA1 receptors are vastly expressed in different unmyelinated sensory neurons (Story et al. 2003) . They are also expressed in different organs, including the cardiovascular, gastrointestinal, and urinary systems (Andrade et al. 2012) . TRPA1 co-localizes with TRPV1 channels at least in a subpopulation of C-type nociceptors (Fajardo et al. 2008) . Akin to TRPV1, a number of studies have also established a crucial role of TRPA1 channels in neuronal and non-neuronal neuropathic pain (Barriere et al. 2012; Chen et al. 2011; Wei et al. 2010) .
TRPA1 is also involved in respiratory reflexes due to inhaled pollutants (Bessac et al. 2009) . These results suggest that the activation of this channel might contribute to asthma and chronic obstructive pulmonary disease (Andre et al. 2008; Materazzi et al. 2010) . TRPA1 is also activated by electrophilic products generated during oxidative processes, suggesting that these channels may act as sensors for the tissue damage during inflammatory processes (Taylor-Clark et al. 2008) . Furthermore, TRPA1 channels have been identified as targets of allyl isocyanate, cinnamaldehyde, nifedipine, chlorpromazine, auranofin, as well as of clotrimazole, clioquinol, apomorphine, glibenclamide (Andrade et al. 2012; Babes et al. 2013) , which provides some pharmacological basis for painful side effects of some of these drugs (Table 2.1 and Fig. 2.5).
Selected examples of activators (1–5) and inhibitors (6–10) of TRPA1. 1 Allyl isocyanate (CID15123). 2 Auranofin triethylphosphane (CID 24199313). 3 Clioquinol (CID 2788). 4 Apomorphine (CID 6005). 5 Glibenclamide (CID 3488). 6 HC-030031 (CID 1150897). 7 A-967079 (CID 42641861). 8 Piperazine urea (CID 96934). 9 Eucalyptol: 1,8-cineole (CID 2758). 10 PR-Toxin (CID 56844124)
The TRPA1 channel is considered a promising target for the development of new, clinically relevant drugs in different therapeutic areas (Baraldi et al. 2010) . In this respect, the past few years have seen the emergence of novel TRPA1 antagonists, with more than 30 patents filed by different academic and non-academic institutions. Compounds claimed in these patents belong to different chemical families, but a number of them are related to fused pyrimidindione derivatives displaying efficacy in various models of pain (Table 2.1). Among the pyrimidindione antagonists, we can mention compound HC-030031 and its isobutyl analogue Chembridge-5861528, which are being profusely used as pharmacological tools for studying the implication of TRPA1 channels in pathophysiological pain , among other disorders (Koivisto et al. 2012; Meotti et al. 2013; Samer et al. 2008; Shigetomi et al. 2012) . A small library of related pyrrolo[3,2-d]pyrimidinone derivatives with micromolar antagonist potencies was described (Baraldi et al. 2012) . In addition, compound GRC-17536 has successfully completed Phase I clinical trials and, since 2012, is under Phase II studies in patients with painful diabetic neuropathy. Good efficacy was also observed when this selective compound was administered by the inhalation route, and a Phase IIa study is ongoing in people with refractory chronic cough.
The oxime derivative A-967079 is a TRPA1 antagonist that inhibits Ca2 + influx through this channel at nanomolar concentrations, displayed moderate/good oral biovailability, and was active in models of inflammatory and neuropatic pain (Chen et al. 2011) . Related analogues, showing either modest TRPA1 agonist or antagonist properties, have been described (DeFalco et al. 2010) . Other chemotypes displaying potent TRPA1 antagonist properties include N-arylsufonyl-proline derivatives, piperazineurea, and N-1-Alkyl-2-oxo-2-aryl amide (Vallin et al. 2012) . In addition, a series of 3-ylidenephtahlides and some leucettamol marine products were described to display a dual action, since they are able to activate the TRPA1 channel and to block the related cold sensor TRPM8 (Chianese et al. 2012; Ortar et al. 2013) . On the contrary, the 1,8-cineole, an essential oil from eucalyptus, evoked inward currents through human TRPM8, but inhibited TRPA1 activation (Takaishi et al. 2012) . BCTC, a good blocker of TRPM8 at micromolar concentrations is also a strong activator of TRPA1 at similar concentrations (Madrid et al. 2006) (Table 2.1 and Fig. 2.5). All these structures may be considered versatile templates towards novel TRP channel modulators.
Very recently, toxin ProTx-I was identified as a high-affinity TRPA1 antagonist. This Cys-rich peptide, isolated from the venom of the Peruvian green-velvet tarantula, also behave as an antagonist of voltage-gated sodium (NaV1.2) channels (Gui et al. 2014) . However, mutations by Ala-scan indicated subtle differences in the structural requirements for binding to both ion channels. These findings open the possibility of using this peptide as the starting point for the development of new TRPA1 modulators. The use of ProTx-I, its mutants and the above indicated antagonists as pharmacological tools could certainly contribute to deepen our knowledge of TRPA1 function under physiological and pathological conditions, and to shed light on its gating mechanism.
2.10 Concluding Remarks
TRP channels pharmacology has been evolving a different pace for the members of this channel family, resulting in a plethora of modulators for few TRP proteins and a lack of ligands for the vast majority. However, technical advances in automated electrophysiology, along with an increase in the chemical diversity provided by synthetic and natural libraries most likely will change this conspicuous pharmacological unbalance. Furthermore, the discovery that allosteric modulators may be derived from the protein sequence will probably accelerate the discovery of TRP modulators. Moreover, the finding that TRP channel signalplexes are central to their function open new venues for drug intervention by targeting protein complexes involved in channel expression or signaling. Taken together, all these strategies will expand TRP pharmacology, even to previously considered non-druggable TRP channels.
References
Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S (2006) Ca2+ -dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett 397:140–144
Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN (2008) The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321:702–705
Ahern GP (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278:30429–30434
Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD (2005) TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 118:70–79
Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD (2006) A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci 26:3864–3874
Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI (2006) Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99:988–995
Anand P (2003) Capsaicin and menthol in the treatment of itch and pain: recently cloned receptors provide the key. Gut 52:1233–1235
Andrade EL, Meotti FC, Calixto JB (2012) TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther 133:189–204
Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R (2008) Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118:2574–2582
Appendino G, Minassi A, Pagani A, Ech-Chahad A (2008) The role of natural products in the ligand deorphanization of TRP channels. Curr Pharm Des 14:2–17
Avelino A, Cruz C, Nagy I, Cruz F (2002) Vanilloid receptor 1 expression in the rat urinary tract 83. Neuroscience 109:787–798
Babes A, Ciobanu AC, Neacsu C, Babes R-M (2011) TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Curr Pharm Biotechnol 12:78–88
Babes A, Fischer MJM, Filipovic M, Engel MA, Flonta M-L, Reeh PW (2013) The anti-diabetic drug glibenclamide is an agonist of the transient receptor potential Ankyrin 1 (TRPA1) ion channel. Eur J Pharmacol 704:15–22
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Bang S, Yoo S, Yang TJ, Cho H, Hwang SW (2010) Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem 285:19362–19371
Bang S, Yoo S, Yang TJ, Cho H, Hwang SW (2011) Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 152:1156–1164
Bang S, Yoo S, Yang TJ, Cho H, Hwang SW (2012) 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol 165:683–692
Baraldi PG, Preti D, Materazzi S, Geppetti P (2010) Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem 53:5085–5107
Baraldi PG, Romagnoli R, Saponaro G, Tabrizi MA, Baraldi S, Pedretti P, Fusi C, Nassini R, Materazzi S, Geppetti P, Preti D (2012) 7-Substituted-pyrrolo 3,2-d pyrimidine-2,4-dione derivatives as antagonists of the transient receptor potential ankyrin 1 (TRPA1) channel: a promising approach for treating pain and inflammation. Bioorg Med Chem 20:1690–1698
Barriere DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin M-A, Chapuis L, Salles J, Dubray C, Morio B (2012) Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain 153:553–561
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102:12248–12252
Beech DJ (2007) Canonical transient receptor potential 5. Handb Exp Pharmacol 109–123
Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt S-E (2009) Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23:1102–1114
Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC (1992) Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 107: 544–552
Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE (2004) Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6:709–720
Bharate SS, Bharate SB (2012) Modulation of thermoreceptor TRPM8 by cooling compounds. Acs Chem Neurosci 3:248–267
Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW (2003) Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 100:12480–12485
Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, Roudbaraki M, Bonnal J-L, Mauroy B, Shuba Y, Skryma R, Prevarskaya N (2007) Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest 117:1647–1657
Birder L, Kullmann FA, Lee H, Barrick S, de GW, Kanai A, Caterina M (2007) Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323:227–235
Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D (2010) A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141:834–845
Broad LM, Keding SJ, Blanco MJ (2008) Recent progress in the development of selective TRPV1 antagonists for pain. Curr Top Med Chem 8:1431–1441
Calvo RR, Meegalla SK, Parks DJ, Parsons WH, Ballentine SK, Lubin ML, Schneider C, Colburn RW, Flores CM, Player MR (2012) Discovery of vinylcycloalkyl-substituted benzimidazole TRPM8 antagonists effective in the treatment of cold allodynia. Bioorg Med Chem Lett 22:1903–1907
Camprubi-Robles M, Planells-Cases R, Ferrer-Montiel A (2009) Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J 23:3722–3733
Cao X, Yang F, Zheng J, Wang K (2012) Intracellular proton-mediated activation of TRPV3 channels accounts for the exfoliation effect of alpha-hydroxyl acids on keratinocytes. J Biol Chem 287:25905–25916
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Chaudhari SS, Kadam AB, Khairatkar-Joshi N, Mukhopadhyay I, Karnik PV, Raghuram A, Rao SS, Vaiyapuri TS, Wale DP, Bhosale VM, Gudi GS, Sangana RR, Thomas A (2013) Synthesis and pharmacological evaluation of novel N-aryl-3, 4-dihydro-1'H-spiro chromene-2,4'-piperidine – 1'-carboxamides as TRPM8 antagonists. Bioorg Med Chem 21:6542–6553
Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang X-F, Niforatos W, Bianchi BR, Baker SJ, Zhong C, Simler GH, McDonald HA, Schmidt RG, McGaraughty SP, Chu KL, Faltynek CR, Kort ME, Reilly RM, Kym PR (2011) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152:1165–1172
Chianese G, Fattorusso E, Putra MY, Calcinai B, Bavestrello G, Moriello AS, De Petrocellis L, Marzo V D, Taglialatela-Scafati O (2012) Leucettamols, bifunctionalized marine sphingoids, act as modulators of TRPA1 and TRPM8 channels. Mar Drugs 10:2435–2447
Cho Y, Jang Y, Yang YD, Lee C-H, Lee Y, Oh U (2010) TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium 48:202–208
Chuang HH, Neuhausser WM, Julius D (2004) The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 43:859–869
Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ (2004) 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci 24:5177–5182
Chung MK, Guler AD, Caterina MJ (2005) Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem 280:15928–15941
Ciurtin C, Majeed Y, Naylor J, Sukumar P, English AA, Emery P, Beech DJ (2010) TRPM3 channel stimulated by pregnenolone sulphate in synovial fibroblasts and negatively coupled to hyaluronan. BMC Musculoskelet Disord 11:111
Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA (2009) Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci 29:9794–9808
De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernandez-Gonzalez EO, Chirinos M, Larrea F, Beltran C, Trevino CL (2009) TRPM8, a versatile channel in human sperm. PLos One 4(6):e6095
DeFalco J, Steiger D, Gustafson A, Emerling DE, Kelly MG, Duncton MAJ (2010) Oxime derivatives related to AP18: agonists and antagonists of the TRPA1 receptor. Bioorg Med Chem Lett 20:276–279
del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM (2010) TRPA1 contributes to cold hypersensitivity. J Neurosci 30:15165–15174
De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, Di Marzo V (2012) Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 204:255–266
Devesa I, Planells-Cases R, Fernandez-Ballester G, Gonzalez-Ros JM, Ferrer-Montiel A, Fernandez-Carvajal A (2011) Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J Inflamm Res 4:67–81
Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H (2011) High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem 28:813–822
Ding J, Xiao Y, Lu D, Du YR, Cui XY, Chen J (2011) Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca2+ rise in primary sensory cells. Neurosci Bull 27:135–142
Doerner JF, Hatt H, Ramsey IS (2011) Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol 137:271–288
Earley S, Gonzales AL, Garcia ZI (2010) A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77:612–620
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P (2007) Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 7:11
Fajardo O, Meseguer V, Belmonte C, Viana F (2008) TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci 28:7863–7875
Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA (2008) IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5'-6'-epoxyeicosatrienoic acid. J Gen Physiol 131:i2
Fernandez-Carvajal A, Fernandez-Ballester G, Devesa I, Gonzalez-Ros JM, Ferrer-Montiel A (2011) New strategies to develop novel pain therapies: addressing thermoreceptors from different points of view. Pharmaceuticals 5:16–48
Fernández-Peña C, Viana F (2013) Targeting TRPM8 for pain relief. Open Pain J 6:154–164
Ferrer-Montiel A, Fernandez-Carvajal A, Planells-Cases R, Fernandez-Ballester G, Gonzalez-Ros JM, Messeguer A, Gonzalez-Muniz R (2012) Advances in modulating thermosensory TRP channels. Expert Opin Ther Pat 22:999–1017
Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ (2006) Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem 281:4977–4982
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26:159–178
Galeotti N, Ghelardini C, Mannelli L, Mazzanti G, Baghiroli L, Bartolini A (2001) Local anaesthetic activity of (+)- and (−)-menthol. Planta Med 67:174–176
Gao X, Wu L, O’Neil RG (2003) Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278:27129–27137
Garcia-Martinez C, Humet M, Planells-Cases R, Gomis A, Caprini M, Viana F, De La PE, Sanchez-Baeza F, Carbonell T, De FC, Perez-Paya E, Belmonte C, Messeguer A, Ferrer-Montiel A (2002) Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc Natl Acad Sci U S A 99:2374–2379
Garcia-Martinez C, Fernandez-Carvajal A, Valenzuela B, Gomis A, Van Den Nest W, Ferroni S, Carreno C, Belmonte C, Ferrer-Montiel A (2006) Design and characterization of a noncompetitive antagonist of the transient receptor potential vanilloid subunit 1 channel with in vivo analgesic and anti-inflammatory activity. J Pain 7:735–746
Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A (2004) Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci 24:5307–5314
Garcia-Sanz N, Valente P, Gomis A, Fernandez-Carvajal A, Fernandez-Ballester G, Viana F, Belmonte C, Ferrer-Montiel A (2007) A role of the transient receptor potential domain of vanilloid receptor I in channel gating. J Neurosci 27:11641–11650
Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210
Gkika D, Prevarskaya N (2011) TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl 13:673–676
Gomis A, Soriano S, Belmonte C, Viana F (2008) Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol 586:5633–5649
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278:21493–21501
Grimm C, Kraft R, Schultz G, Harteneck C (2005) Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine [corrected]. Mol Pharmacol 67:798–805
Gui J, Liu B, Cao G, Lipchik AM, Perez M, Dekan Z, Mobli M, Daly NL, Alewood PF, Parker LL, King GF, Zhou Y, Jordt S-E, Nitabach MN (2014) A tarantula-venom peptide antagonizes the TRPA1 nociceptor ion channel by binding to the S1–S4 gating domain. Curr Biol 24:473–483
Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414
Hardie RC, Minke B (1995) Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium 18:256–274
Harteneck C, Schultz G (2007) TRPV4 and TRPM3 as volume-regulated cation channels. In: Liedtke Wb HS (ed) Trp ion channel function in sensory transduction and cellular signaling cascades. CRC Press
He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL (2005) A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem 280:10997–11006
Hiura A (2000) Neuroanatomical effects of capsaicin on the primary afferent neurons. Arch Histol Cytol 63: 199–215
Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX (2004) 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem 279:35741–35748
Hu G, Oboukhova EA, Kumar S, Sturek M, Obukhov AG (2009) Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol Endocrinol 23:689–699
Huang J, Zhang X, McNaughton PA (2006) Inflammatory pain: the cellular basis of heat hyperalgesia. Curr. Neuropharmacol 4:197–206
Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M (2002) Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun 291:124–129
Jiang LH, Gamper N, Beech DJ (2011) Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets 12:724–736
Jin M, Wu Z, Chen L, Jaimes J, Collins D, Walters ET, O’Neil RG (2011) Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLoS One 6:e16713
Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV (2009) Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 296:H1868–H1877
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD (2003) Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278:3562–3571
Juvin V, Penna A, Chemin J, Lin YL, Rassendren FA (2007) Pharmacological characterization and molecular determinants of the activation of transient receptor potential V2 channel orthologs by 2-aminoethoxydiphenyl borate. Mol Pharmacol 72:1258–1268
Kapoor MDS (2012) TRPM8 antagonists and their emerging role in the modulation of pain and allodynia. Biochem Biophys Res Commun 420:937–937
Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 106:1273–1278
Khairatkar-Joshi N, Szallasi A (2009) TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med 15:14–22
Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DS, Yang DK, So I, Kim KW (2006) Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol 290:C1031–C1040
Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y (2009) Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A 106:5400–5405
Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B (2009) Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. J Med Chem 52:2933–2939
Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, Meyerhof W, Harteneck C (2011) Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol 162:1757–1769
Knotkova H, Pappagallo M, Szallasi A (2008) Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin.J. Pain 24:142–154
Knowlton WM, McKemy DD (2011) TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol 12:68–77
Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J (2006) Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26:1495–1502
Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Akerman KE, Lindstedt K, Pertovaara A (2012) Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res 65:149–158
Lashinger ESR, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, Davenport EA, Hoffman BE, Laping NJ, Su X (2008) AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Ren Physiol 295:F803–F810
Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA (2003a) Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278:20890–20897
Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW (2003b) TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol 284:G604–G616
Leffler A, Linte RM, Nau C, Reeh P, Babes A (2007) A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur J Neurosci 26:12–22
Lehen’kyi V, Prevarskaya N (2011) Oncogenic TRP Channels. In: Islam MS (ed) Transient receptor potential channels. Springer, Heidelberg, pp 929–945
Levine JD, Alessandri-Haber N (2007) TRP channels: targets for the relief of pain. Biochimica Biophysica Acta Mole Basis of Dis 1772: 989–1003
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Liapi A, Wood JN (2005) Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci 22:825–834
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Liu Y, Qin N (2011) TRPM8 in health and disease: cold sensing and beyond. In: Islam MS (ed) Transient receptor potential channels, pp 185–208
Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt S-E (2013) TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 154:2169–2177
Loukin SH, Su Z, Kung C (2009) Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett 583:754–758
Loukin S, Zhou X, Su Z, Saimi Y, Kung C (2010) Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem 285:27176–27181
Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell Neurosci 32:335–343
Madrid R, Donovan-Rodriguez T, Meseguer V, Belmonte C, Viana F (2006) Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26(48):12512–12525
Maelkiae A, Morenilla-Palao C, Viana F (2011) The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface. Curr Pharm Biotechnol 12:54–67
Majeed Y, Tumova S, Green BL, Seymour VA, Woods DM, Agarwal AK, Naylor J, Jiang S, Picton HM, Porter KE, O’Regan DJ, Muraki K, Fishwick CW, Beech DJ (2012) Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium 51:1–11
Malkia A, Pertusa M, Fernández-Ballester G, Ferrer-Montiel A, Viana F (2009) Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Molecular Pain 5:62
Materazzi S, Nassini R, Andre E, Gatti R, Trevisani M, Patacchini R, Geppetti P (2010) The contribute of TRPA1 channel in inflammatory respiratory diseases. Neuropeptides 44:522–522
Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP (2008) General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A 105: 8784–8789
Matthews JM, Qin N, Colburn RW, Dax SL, Hawkins M, McNally JJ, Reany L, Youngman MA, Baker J, Hutchinson T, Liu Y, Lubin ML, Neeper M, Brandt MR, Stone DJ, Flores CM (2012) The design and synthesis of novel, phosphonate-containing transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett 22:2922–2926
McDonnell ME, Zhang SP, Dubin AE, Dax SL (2002) Synthesis and in vitro evaluation of a novel iodinated resiniferatoxin derivative that is an agonist at the human vanilloid VR1 receptor. Bioorg Med Chem Lett 12:1189–1192
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416(6876):52–58
Meis S, Munsch T, Sosulina L, Pape HC (2007) Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol. Cell Neurosci 35:356–367
Meotti FC, Forner S, Lima-Garcia JF, Viana AF, Calixto JB (2013) Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact 203:440–447
Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P5, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F (2014) TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 5:3125
Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M (2010) Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci 30:16536–16544
Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A (2011) TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun 2:369
Monet M, Gkika D, Lehen’kyi V, Pourtier A, Vanden Abeele F, Bidaux G, Juvin V, Rassendren F, Humez S, Prevarsakaya N (2009) Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta 1793:528–539
Montell C (1999) Visual transduction in Drosophila. Annu Rev Cell Dev Biol 15:231–268
Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472
Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A (2004) Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem 279:25665–25672
Moussaieff A, Mechoulam R (2009) Boswellia resin: from religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J Pharm Pharmacol 61:1281–1293
Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes 39. Circ Res 93:829–838
Nath AK, Krauthammer M, Li P, Davidov E, Butler LC, Copel J, Katajamaa M, Oresic M, Buhimschi I, Buhimschi C, Snyder M, Madri JA (2009) Proteomic-based detection of a protein cluster dysregulated during cardiovascular development identifies biomarkers of congenital heart defects. PLoS One 4:e4221
Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, Jiang S, Seymour VA, McKeown L, Kumar B, Harteneck C, O’Regan D, Wheatcroft SB, Kearney MT, Jones C, Porter KE, Beech DJ (2010) Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res 106:1507–1515
Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N (2007) Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem 282:15894–15902
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12:218
Nilius B, Droogmans G, Wondergem R (2003a) Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium 10:5–15
Nilius B, Watanabe H, Vriens J (2003b) The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch 446:298–303
Nilius B, Biro T, Owsianik G (2014) TRPV3: time to decipher a poorly understood family member! J Physiol 592:295–304
Oberwinkler J (2007) TRPM3, a biophysical enigma? Biochem Soc Trans 35:89–90
Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y (1998) Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem 273:10279–10287
Okamoto Y, Ohkubo T, Ikebe T, Yamazaki J (2012) Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int J Oncol 40:1431–1440
Ortar G, Moriello AS, Morera E, Nalli M, Marzo V D, De Petrocellis L (2013) 3-Ylidenephthalides as a new class of transient receptor potential channel TRPA1 and TRPM8 modulators. Bioorg Med Chem Lett 23:5614–5618
Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A (2012) TRP calcium channel and breast cancer: expression, role and correlation with clinical parameters. Bull Cancer 99:655–664
Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ (2011) TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci 31:11425–11436
Parks DJ, Parsons WH, Colburn RW, Meegalla SK, Ballentine SK, Illig CR, Qin N, Liu Y, Hutchinson TL, Lubin ML, Stone DJ Jr, Baker JF, Schneider CR, Ma J, Damiano BP, Flores CM, Player MR (2011) Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem 54:233–247
Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Carmen Acosta M, Gallar J, Dhaka A, Viana F, Belmonte C (2010) Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16:1396–1399
Paul M, Jauch J (2012) Efficient preparation of incensole and incensole acetate, and quantification of these bioactive diterpenes in Boswellia papyrifera by a RP-DAD-HPLC method. Nat Prod Commun 7:283–288
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002a) A TRP channel that senses cold stimuli and menthol. Cell 108(5):705–715
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002b) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049
Penna A, Juvin V, Chemin J, Compan V, Monet M, Rassendren FA (2006) PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium 39:495–507
Peralvarez-Marin A, Donate-Macian P, Gaudet R (2013) What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J 280:5471–5487
Peterlin Z, Chesler A, Firestein S (2007) A painful Trp can be a bonding experience. Neuron 53:635–638
Planells-Cases R, Perez-Paya E, Messeguer A, Carreno C, Ferrer-Montiel A (2003) Small molecules targeting the NMDA receptor complex as drugs for neuropathic pain. Mini Rev Med Chem 3:749–756
Plant TD, Schaefer M (2003) TRPC4 and TRPC5: receptor-operated Ca2+ -permeable nonselective cation channels. Cell Calcium 33:441–450
Pochynyuk O, Zaika O, O’Neil RG, Mamenko M (2013) Novel insights into TRPV4 function in the kidney. Pflugers Arch 465:177–186
Ponsati B, Carreno C, Curto-Reyes V, Valenzuela B, Duart MJ, Van den Nest W, Cauli O, Beltran B, Fernandez J, Borsini F, Caprioli A, Serio S D, Veretchy M, Baamonde A, Menendez L, Barros F, de la Pena P, Borges R, Felipo V, Planells-Cases R, Ferrer-Montiel A (2012) An inhibitor of neuronal exocytosis (DD04107) displays long-lasting in vivo activity against chronic inflammatory and neuropathic pain. J Pharmacol Exp Ther 341:634–645
Premkumar LS, Abooj M (2013) TRP channels and analgesia. Life Sci 92:415–424
Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28:6231–6238
Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL, Altier C, Hollenberg MD (2013) TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A 110:7476–7481
Reilly RM, Kym PR (2011) Analgesic potential of TRPV3 antagonists. Curr Top Med Chem 11:2210–2215
Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van't Veer A, Meloni EG, Carlezon WA Jr, Bolshakov VY, Clapham DE (2009) Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137:761–772
Sabnis AS, Shadid M, Yost GS, Reilly CA (2008) Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am J Respir Cell Mol Biol 39:466–474
Samer RE, Eric DC, Eric LM, Hongyu AL, Kar-Chan C, Shelley D, Darrell AH, Stefanie AK, Mark OU (2008) HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain 4:48
Santos FA, Rao VS (2001) 1,8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci 46:331–337
Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G (2000) Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 275:17517–17526
Sherkheli MA, Benecke H, Doerner JF, Kletke O, Vogt-Eisele AK, Gisselmann G, Hatt H (2009) Monoterpenoids induce agonist-specific desensitization of transient receptor potential vanilloid-3 (TRPV3) ion channels. J Pharm Pharm Sci 12:116–128
Sherkheli MA, Gisselmann G, Hatt H (2012) Supercooling agent icilin blocks a warmth-sensing ion channel TRPV3. Sci World J 2012:982725
Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80
Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y (2006) Ca2+ -calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol 570:219–235
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418:186–190
Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE (2006) Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 281:29897–29904
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE (2008) Citral sensing by transient [corrected] receptor potential channels in dorsal root ganglion neurons. PLoS One 3:e2082
Straub I, Krugel U, Mohr F, Teichert J, Rizun O, Konrad M, Oberwinkler J, Schaefer M (2013) Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol 84:736–750
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702
Sukumar P, Beech DJ (2010) Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+). Biochem Biophys Res Commun 393:50–54
Szallasi A, Appendino G (2004) Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse? J Med Chem 47:2717–2723
Szallasi A, Blumberg PM (2007) Complex regulation of TRPV1 by vanilloids In: Liedtke WB, Heller S (eds) TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton, CRC Press
Tabuchi K, Suzuki M, Mizuno A, Hara A (2005) Hearing impairment in TRPV4 knockout mice. Neurosci Lett 382:304–308
Takaishi M, Fujita F, Uchida K, Yamamoto S, Sawada M, Hatai C, Shimizu M, Tominaga M (2012) 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol Pain 8:86
Tamayo NA, Bo Y, Gore V, Ma V, Nishimura N, Tang P, Deng H, Klionsky L, Lehto SG, Wang W, Youngblood B, Chen J, Correll TL, Bartberger MD, Gavva NR, Norman MH (2012) Fused Piperidines as a Novel Class of Potent and Orally Available Transient Receptor Potential Melastatin Type 8 (TRPM8) Antagonists. J Med Chem 55:1593–1611
Taylor-Clark TE, Undem BJ, MacGlashan DW Jr, Ghatta S, Carr MJ, McAlexander MA (2008) Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73:274–281
Thiel G, Muller I, Rossler OG (2013) Signal transduction via TRPM3 channels in pancreatic beta-cells. J Mol Endocrinol 50:R75–R83
Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD (2008) N-((1 S)-1-{[4-((2 S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther 326:432–442
Tominaga M, Wada M, Masu M (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A 98:6951–6956
Tsavaler L, Shapero MH, Morkowski S, Laus R (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61:3760–3769
Ueda K, Tsuji F, Hirata T, Takaoka M, Matsumura Y (2008) Preventive effect of TRPV1 agonists capsaicin and resiniferatoxin on ischemia/reperfusion-induced renal injury in rats. J Cardiovasc Pharmacol 51:513–520
Umezu T, Sakata A, Ito H (2001) Ambulation-promoting effect of peppermint oil and identification of its active constituents. Pharmacol Biochem Behav 69:383–390
Valdes-Rodriguez R, Kaushik SB, Yosipovitch G (2013) Transient receptor potential channels and dermatological disorders. Curr Top Med Chem 13:335–343
Valente P, Fernandez-Carvajal A, Camprubi-Robles M, Gomis A, Quirce S, Viana F, Fernandez-Ballester G, Gonzalez-Ros JM, Belmonte C, Planells-Cases R, Ferrer-Montiel A (2011) Membrane-tethered peptides patterned after the TRP domain (TRPducins) selectively inhibit TRPV1 channel activity. FASEB J 25:1628–1640
Valero ML, de Queiroz FM, Stuehmer W, Viana F, Pardo LA (2012) TRPM8 ion channels differentially modulate proliferation and cell cycle distribution of normal and cancer prostate cells. PLoS One 7(12):e51825
Vallin KSA, Sterky KJ, Nyman E, Bernstrom J, From R, Linde C, Minidis ABE, Nolting A, Narhi K, Santangelo EM, Sehgelmeble FW, Sohn D, Strindlund J, Weigelt D (2012) N-1-Alkyl-2-oxo-2-aryl amides as novel antagonists of the TRPA1 receptor. Bioorg Med Chem Lett 22:5485–5492
Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS (2005) Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain 1:17
Van Der Stelt M, Di MV (2004) Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem 271:1827–1834
Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G (2006) Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo 59. Neuroscience 140:645–657
Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA (2001) Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534:813–825
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Vennekens R, Owsianik G, Nilius B (2008) Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des 14:18–31
Vidal-Mosquera M, Fernandez-Carvajal A, Moure A, Valente P, Planells-Cases R, Gonzalez-Ros JM, Bujons J, Ferrer-Montiel A, Messeguer A (2011) Triazine-based vanilloid 1 receptor open channel blockers: design, synthesis, evaluation, and SAR analysis. J Med Chem 54:7441–7452
Vincent F, Duncton MA (2011) TRPV4 agonists and antagonists. Curr Top Med Chem 11:2216–2226
Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA (2009) Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389:490–494
Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H (2007) Monoterpenoid agonists of TRPV3. Br J Pharmacol 151:530–540
Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A 101:396–401
Vriens J, Appendino G, Nilius B (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75:1262–1279
Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70:482–494
Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10:1421–1430
Wahl P, Foged C, Tullin S, Thomsen C (2001) Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol 59:9–15
Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P (2003) The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 304:56–62
Wang X, Miyares RL, Ahern GP (2005) Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol 564:541–547
Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu T, Wang X (2014) Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci 10:90–102
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B (2002a) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577
Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B (2002b) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277:47044–47051
Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438
Wei H, Koivisto A, Pertovaara A (2010) Spinal TRPA1 ion channels contribute to cutaneous neurogenic inflammation in the rat. Neurosci Lett 479:253–256
Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C (1995) TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A 92:9652–9656
Wong CO, Huang Y, Yao X (2010) Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases. Br J Pharmacol 159:1486–1496
Wu LJ, Sweet TB, Clapham DE (2010) International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404
Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M (2010) High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes 59:844–849
Xing H, Chen M, Ling J, Tan W, Gu JG (2007) TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci 27:13680–13690
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186
Xu H, Blair NT, Clapham DE (2005a) Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci 25:8924–8937
Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ (2005b) Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145:405–414
Xu H, Delling M, Jun JC, Clapham DE (2006a) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635
Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ (2006b) A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98:1381–1389
Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ (2008) TRPC channel activation by extracellular thioredoxin. Nature 451:69–72
Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S (2008) TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol 295:C296–C301
Yang XR, Lin MJ, McIntosh LS, Sham JS (2006) Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290:L1267–L1276
Yee NS, Zhou W, Lee M (2010) Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett 297:49–55
Zamudio-Bulcock PA, Everett J, Harteneck C, Valenzuela CF (2011) Activation of steroid-sensitive TRPM3 channels potentiates glutamatergic transmission at cerebellar Purkinje neurons from developing rats. J Neurochem 119:474–485
Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ (2004) Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol 559:739–750
Zhang X1, Huang J, McNaughton PA (2005) NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24(24):4211–23.
Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA (2012) Direct inhibition of the cold-activated TRPM8 ion channel by G alpha(q). Nat Cell Biol 14:850–168
Zhu B, Xia M, Xu X, Ludovici DW, Tennakoon M, Youngman MA, Matthews JM, Dax SL, Colburn RW, Qin N, Hutchinson TL, Lubin ML, Brandt MR, Stone DJ, Flores CM, Macielag MJ (2013) Arylglycine derivatives as potent transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett 23:2234–2237
Zufall F, Ukhanov K, Lucas P, Liman ER, Leinders-Zufall T (2005) Neurobiology of TRPC2: from gene to behavior. Pflugers Arch 451:61–71
Acknowledgement
We are indebted to the members of our laboratories, to our collaborators, and the funding from the Ministry of Economy and Competitiveness (BFU2012-39092-C02-01/02, and CSD2008-00005), and the Generalitat Valenciana (PROMETEO/2010/046 and ISIC/2012/009).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Fernández-Carvajal, A., Fernández-Ballester, G., González-Muñiz, R., Ferrer-Montiel, A. (2015). Pharmacology of TRP Channels. In: Madrid, R., Bacigalupo, J. (eds) TRP Channels in Sensory Transduction. Springer, Cham. https://doi.org/10.1007/978-3-319-18705-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-18705-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18704-4
Online ISBN: 978-3-319-18705-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)