Abstract
This chapter focuses on medication reactions relevant to treating patients with inflammatory dermatoses and connective tissue diseases. It is divided into two sections. The first reviews adverse drug reactions to immunomodulatory agents used to manage inflammatory dermatologic conditions. The second section highlights medications that can cause drug-induced lupus erythematosus and drug-induced dermatomyositis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
DMARDs and other anti-inflammatory medications are associated with unique cutaneous and extracutaneous toxicities.
-
In addition to cutaneous hypersensitivity reactions, patients on such medications are potentially at higher risk for infections, hematologic abnormalities, and malignancy.
-
Medication reconciliation is important in recognizing drug-induced connective tissue disease.
Interdisciplinary Introduction
This chapter focuses on medication reactions relevant to treating patients with inflammatory dermatoses and connective tissue diseases. It is divided into two sections. The first reviews adverse drug reactions to immunomodulatory agents used to manage inflammatory dermatologic conditions. The second section highlights medications that can cause drug-induced ·lupus erythematosus and drug-induced dermatomyositis.
Toxicities of Anti-Inflammatory and Immunomodulatory Agents
Improvements in existing disease-modifying anti-rheumatic drugs and the development of novel immunomodulatory agents have significantly augmented the potential for disease control in patients with connective tissue diseases. However, these medications are not without risk. In this section, we will review the side effects of various immunomodulatory agents, with particular attention to toxicities that affect the skin, hair, and nails.
Antimalarials
Antimalarial agents have been used to treat dermatologic conditions since the late nineteenth century. Chloroquine and hydroxychloroquine , the antimalarials most commonly used today, are indicated for a variety of cutaneous and systemic conditions including discoid lupus, subacute cutaneous lupus erythematosus (SCLE), systemic lupus erythematosus, dermatomyositis, sarcoidosis, granuloma annulare, lichen planus, porphyria cutanea tarda, and polymorphous light eruption [1].
Mechanism of Action
The mechanism of action of antimalarial agents is not fully understood. They are believed to have a role in suppressing T-lymphocyte proliferation and leukocyte chemotaxis, as well as stabilizing lysosomal enzymes [2]. Antimalarial agents have an affinity for pigment-containing tissues, including the skin and the melanin-rich iris and choroid of the eye [2].
Cutaneous Side Effects
Cutaneous adverse effects of hydroxychloroquine and chloroquine include dyspigmentation, likely secondary to the binding of hydroxychloriquine or chloroquine to melanin [3]. Classically, patients present with blue-gray to black macules, most commonly involving the anterior legs, arms, face, oral mucosa and nails (Fig. 11.1) [4, 5]. Reversible hypopigmentation or bleaching of hair roots has also been noted with chloroquine [6, 7]. Quinacrine , which is used less frequently, can cause yellowing of the skin, similar to the skin findings of jaundice [8, 9]. Pigmentary changes are usually reversible with cessation of the drug. While the exact incidence and time course of anti-malarial dyspigmentation is unknown, estimates are as high as 25%, with dyspigmentation occurring months to years after initiation of medication [10].
Idiosyncratic cutaneous adverse drug reactions have also been noted with antimalarials. These are often lichenoid eruptions [11, 12]; in addition, more serious drug reactions, including acute generalized exanthematous pustulosis (AGEP), drug-induced hypersensitivity syndrome (DIHS), and toxic epidermal necrolysis (TEN), have been observed [13,14,15]. Of note, recent cases of AGEP induced by hydroxychloroquine have been found to be recalcitrant to drug cessation alone, often requiring systemic treatment [16, 17]. Interestingly, when hydroxychloroquine is used to treat dermatomyositis, evidence suggests that patients are more prone to drug hypersensitivity reactions than when the same agent is used for other indications [18]. Lastly, as antimalarials are well-known photosensitizing agents, phototoxic and photoallergic eruptions can also occur [19].
Extracutaneous and Systemic Side Effects
The most widely reported adverse effect of antimalarial drugs is retinal toxicity. This sequela appears to be agent-specific and dose-dependent: ocular toxicity rarely occurs at dosages greater than 250 mg/d for chloroquine or greater than 6.4 mg/kg/d for hydroxychloroquine, with chloroquine carrying a greater risk for retinal toxicity overall [2]. Concomitant use of hydroxychloroquine and chloroquine confers an additive risk of retinal toxicity. To manage this risk, in patients without a history of maculopathy, a baseline fundus exam is recommended with annual screenings commencing after five years of treatment [20].
Antimalarials may less often cause systemic toxicities, including agranulocytosis, hemolysis in patients with G6-deficiency, gastrointestinal distress, and neuromuscular and neuropsychiatric effects such as irritability, hyperexcitability , and seizures [1]. Cardiac risks including but not limited to QTc prolongation have been reported. While no formal guidelines exist at present, consider baseline ECG in patients with multiple risk factors as well as follow-up ECG and screening for cardiac symptoms in patients with prolonged QTc or those receiving antimalarials in combination with other QTc-prolonging therapies. Hydroxychloroquine-induced cardiomyopathy has also been reported.
Azathioprine
Azathioprine , a derivative of 6-mercaptopurine, is a potent immunosuppressive and anti-inflammatory agent used in dermatology for the treatment of immunobullous diseases, vasculitides, dermatomyositis, scleroderma, and Behcet’s disease [21, 22].
Mechanism of Action
Azathioprine’s active metabolite, 6-thioguanine, is a purine analog whose structure is similar to that of adenine and guanine. Its incorporation into nucleic acid synthesis halts purine metabolism. This leads to decreased cell division and inhibition of B and T cell function [2].
Cutaneous Side Effects
Azathioprine increases patients’ risk of non-melanoma skin cancer (NMSC) [23]. It has also been associated with a severe hypersensitivity syndrome, in which patients present with a morbilliform eruption, fever, leukocytosis, transaminitis, gastrointestinal distress, and in severe cases, cardiogenic shock [24, 25]. Such reactions are typically seen within a month of initiating therapy. In the context of this hypersensitivity reaction, several cases of reactive inflammatory dermatoses, such as Sweet’s syndrome and erythema nodosum, have been reported [26,27,28,29]. Both the azathioprine-associated drug hypersensitivity syndrome and the associated reactive dermatoses typically remit with drug discontinuation. Therapy, as for all other hypersensitivity reactions, is primarily supportive.
Extracutaneous and Systemic Side Effects
The extracutaneous adverse effects most commonly associated with azathioprine are infection, bone marrow suppression, and increased malignancy risk. Specifically, evidence suggests a two to five-fold increased risk of B and T-cell lymphoma [23].
Cyclosporine
Cyclosporine was first used by rheumatologists more than 40 years ago for the treatment of arthritis [30]. Dermatologists have used it for the past two decades to treat inflammatory skin diseases, including atopic dermatitis, immunobullous disorders and pyoderma gangrenosum. Cyclosporine is also now FDA-approved for the treatment of severe, recalcitrant and disabling psoriasis [31].
Mechanism of Action
Cyclosporine works by counteracting the upregulation of pro-inflammatory cytokines, including IL-2, which are required for T-cell activation. This in turn leads to decreased B and T cell function [31]. For the treatment of recalcitrant dermatoses, cyclosporine is used primarily as a short-term temporizing therapy to control flares, acting as a bridge while an alternative immunosuppresive medication takes effect.
Cutaneous Side Effects
The cutaneous adverse effects most commonly attributed to cyclosporine are gingival hyperplasia and hypertrichosis; the latter has been shown to occur in up to 60% patients [32, 33]. Diffuse sebaceous hyperplasia has been reported in transplant patients on long-term cyclosporine [34, 35]. Cutaneous pseudolymphoma secondary to cyclosporine has also been described [36]. While the increased risk of NMSC in patients on long-term cyclosporine therapy has been well described [30], it has not been shown to be increased in patients on low-dose or short term treatments except in those previously treated with PUVA [37].
Extracutaneous and Systemic Side Effects
Extracutaneous toxicities of cyclosporine most commonly include hypertension and kidney injury secondary to the vasoconstrictive effect of cyclosporine on afferent glomerular arterioles [ 30, 37]. Both hypertension and acute kidney injury are dose-dependent. Patients who develop hypertension can be managed either by reducing the cyclosporine dose by 25–50% or adding an antihypertensive agent, preferably a calcium channel blocker of the dihydropyridine class, such as amlodipine or nifedipine [31]. While kidney injury is usually reversible during short-term therapy and may be managed with dose reduction or treatment cessation, irreversible damage to the kidney has been observed in patients on long-term cyclosporine therapy (>2 years) even without previous abnormal blood pressure or kidney function tests [38].
Less frequently, hyperlipidemia, gastrointestinal distress, and neurological symptoms such as headaches, tremor and psychosis have also been reported in patients on cyclosporine [37].
Dapsone
Dapsone is a sulfone drug used for its anti-inflammatory and anti-parasitic properties [39]. It was adopted in the early twentieth century for treatment of infections including those caused by atypical mycobacteria [39]. Prior to the introduction of isotretinoin, dapsone was the drug of choice for the treatment of nodulocystic acne [40]. Today, dapsone is employed primarily in the treatment of neutrophilic and eosinophilic dermatoses [39]. It is also used to treat leprosy, dermatitis herpetiformis, linear IgA dermatosis, IgA pemphigus, subcorneal pustular dermatosis, and erythema elevatum diutinum [41].
Mechanism of Action
Dapsone works by inhibiting neutrophil myeloperoxidase, eosinophil peroxidase and neutrophil chemotaxis [39].
Cutaneous Side Effects
A potentially fatal hypersensitivity reaction consisting of morbilliform eruption associated with fever and hepatitis has been observed in 0.5–3.6% of patients within four to six weeks of dapsone initiation [42, 43]. AGEP has also been reported [43]. When used to treat leprosy, dapsone may also precipitate the development of erythema nodosum leprosum, which can be controlled with thalidomide [2].
Extracutaneous and Systemic Side Effects
Dapsone is metabolized by cytochrome P450 in the liver to form hydroxylamines, metabolites which can lead to methemoglobinemia and promote hemolysis, particularly in patients with a glucose-6-phosphate dehydrogenase (G6PD) deficiency [39]. For this reason, G6PD levels should be checked prior to initiating therapy. In rare circumstances, dapsone has also been associated with other hematologic toxicities, such as agranulocytosis [44]. Other observed toxicities include peripheral neuropathy and hepatoxicity , which appear to be dose-dependent [41].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) , a synthetic derivative of mycophenolic acid (MPA) with greater bioavailability, was originally used as an immunosuppressant in patients who received solid organ transplants. In dermatology, MPA was initially adopted for the treatment of psoriasis. Since the 1990s, MMF has been used as a steroid-sparing agent in the treatment of recalcitrant immunobullous diseases, pyoderma gangrenosum, bullous lichen planus, connective tissue diseases and vasculitides, among other dermatoses [45].
Mechanism of Action
MMF is hydrolyzed to MPA after ingestion. MPA works by selectively inhibiting the enzyme inosine monophosphate dehydrogenase, blocking production of guanosine-5-phosphate [2]. Depletion of the guanosine pool in the cell prevents de novo purine synthesis, leading to a subsequent decrease in B and T lymphocytes. Additionally, MPA suppresses immunoglobulin production by B lymphocytes and plays a role in decreasing intercellular adhesion and leukocyte recruitment [2].
Cutaneous Side Effects
Cutaneous side effects of MMF have not been widely reported.
Extracutaneous and Systemic Side Effects
Given its role as an immunosuppressant, MMF renders patients to higher risk of infections. Overall, however, it has a limited side-effect profile. The most commonly reported side effects are gastrointestinal disturbances including nausea, vomiting, diarrhea and abdominal pain [2]. Mycopenolic acid may be considered in cases of MMF gastrointestinal intolerance. Hematologic abnormalities and hepatotoxicity can occur but are uncommon [46]. Other toxicities reported include neuropsychiatric effects such as headache and insomnia [47].
Methotrexate
Originally used in oncology as a chemotherapeutic agent, low-dose methotrexate (MTX) has been FDA-approved for the treatment of severe, debilitating and recalcitrant psoriasis for nearly half a century [48]. It is also used by dermatologists for the treatment of other inflammatory skin conditions, including pityriasis rubra pilaris, atopic dermatitis and lichen planus [49].
Mechanism of Action
MTX works by inhibiting dihydrofolate reductase, thereby blocking purine production necessary for DNA synthesis [50]. MTX exerts anti-inflammatory effects secondarily, by suppressing neutrophil and macrophage chemotaxis, stimulating apoptosis of T and B lymphocytes, and inhibiting proliferation of proinflammatory cytokines [2].
Cutaneous Side Effects
MTX may rarely cause erosions or ulcerations within psoriatic plaques [51, 52]. Erosions have also been reported in non-psoriatic skin of patients treated with MTX for arthritis and are considered a possible portent of ensuing pancytopenia [53, 54]. MTX therapy also commonly causes increased photosensitivity [54]. Radiation recall has also been reported [55,56,57]. Rheumatoid nodules have been found to develop at an accelerated rate in rheumatoid arthritis patients treated with MTX [58]. Lastly, MTX may cause non-scarring alopecia [2].
Extracutaneous and Systemic Side Effects
Among the most common side effects of MTX is gastrointestinal upset. Increasing doses of folic acid typically help abate this symptom [59]. More serious potential side effects include infection, pancytopenia and hepatotoxicity [49]. Pancytopenia can occur within days to weeks of initiating the medication and is reversible with drug cessation. Severe hepatoxicity in the form of fibrosis or cirrhosis, by contrast, typically develops over years and is irreversible. The risk of hepatotoxicity rises directly with cumulative MTX dose and is compounded by a history of liver disease, alcoholic liver damage, or obesity [49]. Assessment for hepatic fibrosis has traditionally included periodic liver biopsies; more recent investigations have focused on potential serologic markers such as procollagen 3 aminopeptide, alpha-2 macroglobulin, tissue inhibitor of metalloproteinase-1 (TIMP-1) and hyaluronic acid [50, 60]. Patients on long-term MTX therapy should be referred to hepatology for evaluation.
Studies have suggested an increased risk of lymphoproliferative disorders during therapy with MTX [61]. Specifically, methotrexate-induced lymphoproliferative disease (MTX-LPD) has been observed more often in patients with rheumatoid arthritis treated with MTX than in any other patient cohort [62, 63]. However, there is limited evidence linking patients treated with MTX for psoriasis, or any other chronic cutaneous disease, with an increased risk for lymphoproliferative disorders.
Tumor Necrosis Factor Inhibitors
Tumor necrosis factor (TNF)-alpha inhibitors are widely utilized in dermatology for management of psoriasis, with three agents (etanercept, infliximab and adalimumab) FDA-approved for this indication. TNF-alpha inhibitors have also been employed to treat other inflammatory dermatoses, including hidradenitis suppurativa, dermatomyositis, immunobullous disease, neutrophilic dermatoses, and others.
Mechanism of Action
All TNF inhibitors block activation of TNF-alpha, a pro-inflammatory cytokine. Four TNF alpha inhibitors are monoclonal antibodies: infliximab, adalimumab, golimumab, and certolizumab pegol. Etanercept is a dimeric fusion protein.
Cutaneous Side Effects
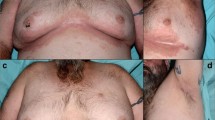
The incidence of cutaneous toxicities secondary to TNF-alpha inhibitors is low. Most commonly, local injection/infusion site reactions are observed [64]. Other reported cutaneous adverse reactions to TNF-alpha inhibitors include cutaneous small-vessel vasculitis, cutaneous infections, interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis, generalized eczematous and lichenoid reactions, psoriasis and psoriasiform lesions (Table 11.1) [64,65,66,67]. Up to one third of patients presenting with psoriatic lesions after starting TNF-alpha inhibitor therapy will present with a palmoplantar pustulosis, a pustular, pruritic eruption on the palms and soles (Figs. 11.2 and 11.3) [64, 68]. Patients may also develop typical psoriatic lesions on the trunk, extremities and scalp. Lastly, TNF-alpha inhibitors can also induce subacute cutaneous and discoid lupus erythematosus, which are reversible within weeks of drug discontinuation [64].
Extracutaneous and Systemic Side Effects
TNF-alpha inhibitors will often lead to the development of antinuclear antibodies (ANA) and antibodies against double-stranded DNA (dsDNA) [69]. They can also induce a reversible syndrome mimicking systemic lupus erythematosus that subsides within weeks of drug cessation [64]. Despite concern to the contrary, patients with psoriasis and/or psoriatic arthritis treated with TNF-alpha inhibitors have not been found to have an increased risk of lymphoma or internal malignancy [70,71,72].
Drug-Induced Lupus Erythematosus and Drug-Induced Dermatomyositis
Though immunomodulatory agents are most often used in dermatology to manage inflammatory and connective tissue diseases, it is important to appreciate that these same agents, along with other common medications, can themselves paradoxically trigger the development of rheumatologic syndromes. Conscientious medication reconciliation is therefore of vital importance when considering a new diagnosis of connective tissue disease. In this section, we will review the important features of drug-induced lupus and drug-induced dermatomyositis, including commonly implicated medications.
Drug-Induced Lupus Erythematosus
The incidence of drug-induced lupus erythematosus, both systemic and cutaneous, has grown in recent years. In drug-induced SCLE, patients develop annular, papulosquamous plaques typically in a photodistribution, which may be clinically indistinguishable from native SCLE (Fig. 11.4) [72]. Additionally, patients typically will have antibodies against SSA/Ro. Evidence suggests that up to 20% of cases of SCLE are drug-induced, necessitating a thorough consideration of medication history when encountering a new diagnosis of SCLE [73].
In drug-induced systemic lupus, cutaneous findings may be absent or may include malar erythema or a photodistributed erythematous eruption [72]. Systemic features often predominate: patients may experience fatigue, fever, weight loss, arthritis, myalgias and serositis [72]. These cases of drug-induced systemic lupus are often associated with positive antinuclear antibody titers, specifically anti-histone antibodies.
The medication profile specific to each entity has been listed in Table 11.2.
Drug-Induced Dermatomyositis
Drug-induced dermatomyositis presents with characteristic skin findings identical to that of idiopathic dermatomyositis. The pathophysiology has not yet been elucidated; the cutaneous findings usually develop after long-term therapy of a potential causative agent, typically at least two years [76].
Drug-induced dermatomyositis has been linked to a variety of medications, including hydroxyurea, penicillamine, statins, cyclophosphamide, BCG vaccine administration, zolendronic acid, TNF-alpha inhibitors, and ipilimumab [76,77,78,79,80]. As with most cases of drug hypersensitivity, identification and cessation of such offending drugs often leads to resolution of symptoms.
Summary
Disease-modifying anti-rheumatic drugs and steroid-sparing agents serve as powerful tools in the management of cutaneous inflammatory and connective tissue disease. However, in addition to their efficacy, such agents are also associated with distinct cutaneous and extracutaneous toxicities. Clinicians who aim to use such medications should have a detailed understanding of such reactions as early recognition and timely intervention could prevent life-threatening sequelae.
References
Kali S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20:160–74.
Katzung BG, Masters SB, Trevor AJ. Basic & clinical pharmacology. New York: Lange Medical Books/McGraw-Hill; 2015.
Puri PK, Lountzis NI, Tyler W, Ferringer T. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35(12):1134–7.
Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:189–94.
Rosen T, Aponte C. Cutaneous hyperpigmentation due to chronic quinine ingestion. Cutis. 2005;75(2):114–6.
Asch PH, Caussade P, Marquart-Elbaz C, Boehm N, Grosshans E. Chloroquine-induced achromotrichia: an ultrastructural study. Ann Dermatol Venereol. 1997;124:552–6.
Donovan JC, Price VH. Images in clinical medicine. Chloroquine-induced hair hypopigmentation. N Engl J Med. 2010;363(4):372.
Vidal D, Altés J, Smandia JA. Yellow skin discoloration induced by quinacrine in a patient with cutaneous lupus erythematosus. Actas Dermosigiliogr. 2013;104(1):89–90.
Sokol RJ, Lichtenstein PK, Farrell MK. Quinacrine hydrochloride induced yellow discoloration of the skin in children. Pediatrics. 1982;69:232–3.
Dereure O. Drug-induced skin pigmentation. Am J Clin Dermatol. 2001;2(4):253–62.
Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993;29:249–55.
Bauer F. Quinacrine hydrochloride drug eruption (tropical lichenoid dermatitis). Its early and late sequelae and its malignant potential: a review. J Am Acad Dermatol. 1981;4(2):239–48.
Paradisi A, Bugatti L, Sisto T, Filosa G, Amerio PL, Capizzi R. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30(5):930–40.
Volpe A, Marchetta A, Caramaschi P, Biasi D, Bambara LM, Arcaro G. Hydroxychloroquine-induced DRESS syndrome. Clin Rheumatol. 2008;27(4):537–9.
Callaly EL, FitzGerald O, Rogers S. Hydroxychloroquine-associated, photo-induced toxic epidermal necrolysis. Clin Exp Dermatol. 2008;33(5):572–4.
Pearson KC, Morrell DS, Runge SR, Jolly P. Prolonged pustular eruption from hydroxychloroquine: an unusual case of acute generalized exanthematous pustulosis. Cutis. 2016;97(3):212–6.
Yalçın B, Çakmak S, Yıldırım B. Successful treatment of hydroxychloroquine-induced recalcitrant acute generalized exanthematous pustulosis with cyclosporine: case report and literature review. Ann Dermatol. 2015;4:431–4.
Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138(9):1231–3.
Lisi P, Assalve D, Hansel K. Phototoxic and photoallergic dermatitis caused by hydroxychloroquine. Contact Dermatitis. 2004;50(4):255–6.
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology. 2016;123:1386–94.
Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55:369–89.
Wise M, Callen JP. Azathioprine: a guide for the management of dermatology patients. Dermatol Ther. 2007;20:206–15.
Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management, expert consult premium edition-enhanced online features. Elsevier Health Sciences; 2010 May 3.
Jeurissen ME, Boerbooms AM, Van de Putte LB, Kruijsen MW. Azathioprine induced fever, chills, rash, and hepatotoxicity in rheumatoid arthritis. Ann Rheum Dis. 1990;49(1):25–7.
Rosenthal E. Azathioprine shock. Postgrad Med J. 1986;62(729):677–8.
Biswas SN, Chakraborty PP, Gantait K, Bar C. Azathioprine-induced bullous Sweet’s syndrome: a rare association. BMJ Case Rep. 2016;2016. https://doi.org/10.1136/bcr-2016-215192. PMID 27090551.
Choonhakarn C, Chaowattanapanit S. Azathioprine-induced Sweet’s syndrome and published work review. J Dermatol. 2013;40(4):267–71.
Turow A, Yong TY, Fok JS, Li JY. Azathioprine hypersensitivity presenting as cardiogenic shock and Sweet’s syndrome in a patient with microscopic polyangiitis. Intern Med. 2012;51(14):1889–92.
De Fonclare AL, Khosrotehrani K, Aractingi S, Duriez P, Cosnes J, Beaugerie L. Erythema nodosum–like eruption as a manifestation of azathioprine hypersensitivity in patients with inflammatory bowel disease. Arch Dermatol. 2007;143(6):744–8.
Bhutani T, Lee CS, Koo JY. Cyclosporine. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. 3rd ed. Edinburgh: Elsevier; 2013. 1991-211.
Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63(6):925–46.
Doufexi A, Mina M, Ioannidou E. Gingival overgrowth in children: epidemiology, pathogenesis, and complications. A literature review. J Periodontol. 2005;76(1):3–10.
Bencini PL, Montagnino G, Sala F, De Vecchi A, Crosti C, Tarantino A. Cutaneous lesions in 67 cyclosporin-treated renal transplant recipients. Dermatology. 1986;172(1):24–30.
de Berker DA, Taylor AE, Quinn AG, Simpson NB. Sebaceous hyperplasia in organ transplant recipients: shared aspects of hyperplastic and dysplastic processes? J Am Acad Dermatol. 1996;35(5):696–9.
Wilken R, Fung MA, Shi VY, Cheng MY, Sultani H, Maverakis E. Cyclosporine-induced sebaceous hyperplasia in a hematopoetic stem cell transplant patient: delayed onset of a common adverse event. Dermatol Online J. 2016;22(1):13030/qt4865202s.
Foley C, Leonard N, Wynne B. Cutaneous pseudolymphoma: A rare side effect of cyclosporine. J Am Acad Dermatol. 2015;72(3):e85–6.
Ryan C, Amor KT, Menter A. The use of cyclosporine in dermatology: part II. J Am Acad Dermatol. 2010;63(6):949–72.
Zachariae H. Renal toxicity of long-term cyclosporine. Scand J Rheumatol. 1999;28(2):65–8.
Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res. 2014;306(2):103–24.
Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel. Am J Clin Dermatol. 2009;10(4):221–7.
Edhegard K, Hall RP. Dapsone. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. 3rd ed. Edinburgh: Elsevier; 2013. p. 228–40.
Hoogeveen RM, van der Bom T, de Boer HH, Thurlings RM, Wind BS, de Vries HJ, van Lent AU, Beuers U, van der Wal AC, Nellen FJ. A lethal case of the dapsone hypersensitivity syndrome involving the myocardium. Neth J Med. 2016;74(2):89–92.
Schulkes KJ, Tervaert JC, Rijken F, Haas LE. Dapsone hypersensitivity syndrome not related to G6PD deficiency. BMJ Case Rep. 2015;2015:bcr2015212742.
Jollow DJ, Bradshaw TP, Mcmillan DC. Dapsone-induced hemolytic anemia. Drug Metab Rev. 1995;27(1–2):107–24.
Narasimharao P, Pratap DV, Suneetha S. Acute generalized exanthematous pustulosis (AGEP) due to dapsone in a patient with leprosy. Lepr Rev. 2009;80(1):81.
Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatol Ther. 2007;20:229–38.
Liu V, Mackool BT. Mycophenolate in dermatology. J Dermatolog Treat. 2003;14:203–11.
Weinstein GD. Methotrexate. Ann Intern Med. 1977;86(2):199–204.
Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. 3rd ed. Edinburgh: Elsevier; 2013. p. 169–81.
Poordad FF. FIBROSpect II: a potential noninvasive test to assess hepatic fibrosis. Expert Rev Mol Diagn. 2004;4(5):593–7.
Souza CF, Suarez OM, Silva TF, Gorenstein AC, Quintella LP, Avelleira JC. Ulcerations due to methotrexate toxicity in a psoriasis patient. An Bras Dermatol. 2016;91(3):375–7.
Koçak AY, Koçak O, Aslan F, Tektaş M. Methotrexate toxicity presenting as cutaneous ulcerations on psoriatic plaques. Cutan Ocul Toxicol. 2013;32(4):333–5.
Shiver MB, Hall LA, Conner KB, Brown GE, Cheung WL, Wirges ML. Cutaneous erosions: a herald for impending pancytopenia in methotrexate toxicity. Dermatol Online J. 2014;20(7):13030/qt46k975h8.
Ben-Amitai D, Hodak E, David M. Cutaneous ulceration: an unusual sign of methotrexate toxicity—first report in a patient without psoriasis. Ann Pharmacother. 1998;32(6):651–3.
Mallory SB, Berry DH. Severe reactivation of sunburn following methotrexate use. Pediatrics. 1986;78(3):514–5.
Guzzo C, Kaidby K. Recurrent recall of sunburn by methotrexate. Photodermatol Photoimmunol Photomed. 1995;11(2):55–6.
Devore KJ. Solar burn reactivation induced by methrotrexate. Pharmacotherapy. 2010;30(4):123e–6e.
Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33(3):361–71.
Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5.
Chalmers RJ, Kirby B, Smith A, Burrows P, Little R, Horan M, Hextall JM, Smith CH, Klaber M, Rogers S. Replacement of routine liver biopsy by procollagen III aminopeptide for monitoring patients with psoriasis receiving long-term methotrexate: a multicentre audit and health economic analysis. Br J Dermatol. 2005;152(3):444–50.
Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol. 2009;60:1001–17.
Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda JI, Tomita Y, Nakatsuka SI, Tamaru JI, Iizuka A, Takeuchi T, Aozasa K. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322–31.
Clarke LE, Junkins-Hopkins J, Seykora JT, Adler DJ, Elenitsas R. Methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis presenting in the skin. J Am Acad Dermatol. 2007;56(4):686–90.
Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti–tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009;61(3):486–504.
Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin Proc. Elsevier. 2012;87(8):739–45.
Stephenson SR, Campbell SM, Drew GS, Magro CM. Palisaded neutrophlic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38(8):644–8.
Jayasekera PS, Walsh ML, Hurrell D, Parslew RG. Case report of lichen planopilaris occurring in a pediatric patient receiving a tumor necrosis factor α inhibitor and a review of the literature. Pediatr Dermatol. 2016;33:e143–6.
Shmidt E, Wetter DA, Ferguson SB, Pittelkow MR. Psoriasis and palmoplantar pustulosis associated with tumor necrosis factor-α inhibitors: the Mayo Clinic experience, 1998 to 2010. J Am Acad Dermatol. 2012;67(5):e179–85.
Eriksson C, Engstrand S, Sundqvist KG, Rantapaa-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005;64:403–7.
Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035–50.
Costa L, et al. Incidence of malignancies in a cohort of psoriatic arthritis patients taking traditional disease modifying antirheumatic drug and tumor necrosis factor inhibitor therapy: an observational study. J Rheumatol. 2016;43:2149–54.
Callen JP. Drug-induced subacute cutaneous lupus erythematosus. Lupus. 2010;19(9):1107–11.
Laurinaviciene R, Sandholdt LH, Bygum A. Drug-induced cutaneous lupus erythematosus: 88 new cases. Eur J Dermatol. 2017;27(1):28–33.
Lowe GC, Henderson CL, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164(3):465–72.
Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149(9):1071–5.
Seidler AM, Gottlieb AB. Dermatomyositis induced by drug therapy: a review of case reports. J Am Acad Dermatol. 2008;59(5):872–80.
Liu SW, Velez NF, Lam C, Femia A, Granter SR, Townsend HB, Vleugels RA. Dermatomyositis induced by anti–tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol. 2013;149(10):1204–8.
Succaria F, Collier M, Mahalingam M. Zoledronic acid–induced interface dermatitis. Am J Dermatopathol. 2015;37(12):933–5.
Tong PL, Yu LL, Chan JJ. Drug-induced dermatomyositis after zoledronic acid. Australas J Dermatol. 2012;53(4):e73–5.
Ali SS, Goddard AL, Luke JJ, Donahue H, Todd DJ, Werchniak A, Vleugels RA. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol. 2015;151(2):195–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rivas, S., Alloo, A. (2022). Iatrogenic Disease and Drug Induced Toxicities Related to Anti-Inflammatory and Immunomodulatory Agents. In: Garg, A., Merola, J.F., Fitzpatrick, L. (eds) Interdisciplinary Approaches to Overlap Disorders in Dermatology & Rheumatology. Springer, Cham. https://doi.org/10.1007/978-3-319-18446-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-18446-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18445-6
Online ISBN: 978-3-319-18446-3
eBook Packages: MedicineMedicine (R0)