Abstract
At no time in the history of muscular dystrophy has the future looked brighter. With over 240 studies listed in the U.S.—and at least 11 candidates in the later phases of drug development (e.g., Phase II or Phase III)—the stage is set for positive change. More groundwork is needed, however, such as the conduct of natural history studies, establishment of more global patient registries, and completion of additional genetic and molecular studies, to better understand MD and to identify promising targets. Indeed, it has historically proven difficult to find preclinical and animal models of disease. Several promising approaches to DMD are in the pipeline, including: “exon skipping” drug candidates, which target the mutation that occurs in the gene for dystrophin in individuals with DMD; gene therapy, aimed at introducing a healthy synthetic copy of the dystrophin gene into the muscles to restore production of dystrophin; “reading through stop signals” by targeting a specific type of mistake in the genetic code called a nonsense mutation, which prevents the production of full-length functional proteins; stem cell therapy, where donor cells are injected with the aim of creating healthy muscle fibers; utrophin upregulation, aimed at increasing levels of utrophin, a protein that is functionally similar to dystrophin; and reducing muscle damage. As ongoing studies are completed, it is hoped that the mechanism of disease will become better elucidated, more targets will be identified, and more companies will be willing to invest in clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Muscular Dystrophy
- Cystic Fibrosis Transmembrane Conductance Regulator
- Stem Cell Therapy
- Duchenne Muscular Dystrophy
- Myotonic Muscular Dystrophy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Treatments for Patients with Muscular Dystrophy
Despite being medically recognized for over 150 years, with hundreds of millions of dollars put to work through champion organizations such as the Muscular Dystrophy Association (MDA) and pharmaceutical sponsors, there is currently no cure for any form of muscular dystrophy (MD). However, at no time in the history of MD has the future looked brighter. With over 240 studies listed in the U.S.—and at least 11 candidates in the later phases of drug development (e.g., Phase II or Phase III)—the stage is set for positive change.
It is the author’s opinion that more groundwork needs to be laid, such as the conduct of natural history studies, establishment of more global patient registries, and completion of additional genetic and molecular studies, to better understand MD and to identify promising targets. Indeed, it has historically proven difficult to find preclinical and animal models of disease. However, as these 240+ studies are completed, it is hoped that the mechanism of disease will become better elucidated, more targets will be identified, and more companies will be willing to invest in clinical trials.
It is hoped that pharmaceutical research into gene therapy or stem cell therapy will ultimately provide treatment to stop the progression of some types of MD and possibly provide a cure.
Current treatment is designed to help prevent or reduce deformities in the joints and the spine and to allow people with MD to remain mobile as long as possible. Non-pharmaceutical therapies include range-of-motion exercises, mobility aids, and assisted breathing. Other procedures can include surgery, mainly for contractures, scoliosis, and heart problems. Although current treatments are critically important for patients with MD, this chapter will be mainly limited to a discussion of pharmaceutical treatments.
For those unfamiliar with the (largely Western) milestones within the clinical trial paradigm, a brief overview is provided just below.
Brief Overview of Clinical Trial Development
To understand the pharmaceutical product approval (aka “registration”) process, it is important to be aware of the clinical trial drug development paradigm. The ultimate goal is to put the pieces of the development puzzle together as required by regulatory agencies, in order to get a drug, biologic, or device to market in the shortest time possible, with the fewest resources, and in the safest and most effective manner.
The process starts with testing many molecules in the laboratory—in vitro (using cells outside their normal biological context in a laboratory environment) and in vivo (in living organisms), proceeds to preclinical testing in animals, and then to human testing along the following paradigm:
-
Discovery Phase
-
Preclinical Phase
-
Phase I, II, III clinical trials
-
Marketing Phase
-
Phase IV clinical trials.
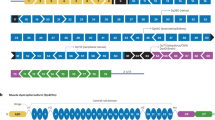
Figure 12.1 was taken from a presentation by the FDA which describes the general plan for overall drug development. Subsequent sections of this chapter will demonstrate how governments, patient advocacy groups, and pharmaceutical companies are currently working in all phases of drug development, with the majority of work being conducted in the lower half of the foundation, but a few promising candidates pushing all the way to later phase clinical testing.
As the scientific (e.g., pathophysiology, mechanism of action [MOA], effect of intervention) and clinical data (post IND phase, such as early and later phase clinical testing) are collected and analyzed, it is sent to regulatory agencies for review and feedback on the progress of the clinical development program. After preclinical testing in the laboratory and in animals, regulatory agencies (the FDA in the U.S.) must give approval before human trials may commence.
In Phase I trials, healthy human volunteers are typically given various doses of the compound in order to study its pharmacokinetics (PK), pharmacodynamics (PD), and, to some extent, its safety. Phase II trials are generally designed to elucidate a safe and effective dosage range in a limited number of patients with the condition the drug was developed to treat. Finally, Phase III clinical trials are conducted on large numbers of patients with the condition for which the drug was developed in order to assess the drug’s safety and efficacy.
Once all of the required information from the multiple trials is collected and analyzed, it is submitted for regulatory approval in the form of written reports. A team of experts from multiple disciplines reviews portions of the data package and determines whether the drug is both safe and effective for the indication for which it was developed. Of interest is the fact that the FDA simply approves the proposed labeling (package insert) for the drug, and not the drug itself, yet it is illegal to market a drug for treatment of any disease without FDA-approved labeling. After the labeling is approved, the product continues to be monitored in an ever-enlarging population of patients as long as it remains on the market. After a drug is in general use, its sponsor may compare it with others used for the same indication either to bolster superiority claims or to study further the safety and efficacy of the product as part of a regulatory commitment at the time of original FDA approval. Such activities are known as Phase IV post-marketing studies.
Although this is the general paradigm for the approval of many drugs, certain exceptions can be made, because of issues such as the ethical conundrum of giving a placebo in lieu of a potential disease modifying treatment to a patient afflicted with MD. In these cases, some groups, such as PMDD, advocate for a comparison between the active drug and the expected natural history course of the disease. Although this is entirely understandable, given the variation with MD—even within families—this may prove difficult unless additional natural history studies are conducted.
Approved Products and Treatments
There is only one product tentatively approved for the treatment of MD anywhere in the world. As mentioned earlier, PTC Therapeutics’ ataluren was granted marketing authorization (tentative approval) in the EU under the trade name Translarna™ for the treatment of DMD in ambulatory patients aged five years and older. Translarna™ is approved to treat the underlying cause of DMD. The European Medicines Agency has designated ataluren as an orphan medicinal product and the FDA has granted orphan drug designation to ataluren for the treatment of DMD.
The ongoing Phase III Ataluren Confirmatory Trial is a randomized, double-blind, placebo-controlled trial designed to confirm the safety and efficacy results seen in an earlier Phase IIb study. The confirmatory study was designed to enroll 220 participants at approximately 58 sites in North America, South America, Europe, Israel, Asia, and Australia. Successful results of this trial would provide the basis for a full approval decision in the EU and the U.S., as well as in other countries.
According to DuchenneConnect: [1]
Based on estimates regarding patient enrollment, initial, top-line data from the Phase III clinical trial were expected in mid-2015. If trial results support approval and FDA approves their application, Translarna™ could be available in the U.S. as early as the second half of 2016. PTC plans to apply for approval in other countries following U.S. approval.
Potential Treatments for MD
A total of 247 MD studies were identified (on October 30, 2014) using the search term “muscular dystrophy” on the Website titled clinicaltrials.gov—a government mandated Website dedicated to posting all clinical trials being conducted in the U.S. It is important to note that other studies may be ongoing—and not recognized on this Website—as they may be conducted in Europe or other non-U.S. regions. Clinical trials conducted in EU and European Economic Area (EEA) can be found at www.clinicaltrialsregister.eu.
It should be emphasized that U.S., Europe, and Japan are the only countries currently following International Conference of Harmonization (ICH) guidelines on good clinical practice and the conduct of clinical trials. This is important to note because a clinical dossier for registration purposes can be filed simultaneously in all ICH countries, though, in the author’s opinion, there are still subtle differences between these geographies meaning that this is not always achievable.
It is also important to note, for those considering participating in a clinical trial outside of ICH countries, that the other countries may not have as rigorous clinical trial requirements and this should be factored into any clinical risk/benefit analysis.
Of these 247 studies, 105 studies were found for “Duchenne muscular dystrophy” and 124 studies were found for “Becker muscular dystrophy,” though it should be noted that most of these studies overlap (because they have a similar pathology), thus are double-counted and not unique. Most DMD and BMD studies focus on treatment of the heart and lungs, where complications can cause early mortality in DMD patients.
Only seven studies were found using the search term “FSH or FSHD”; however, 21 studies were discovered for FSHD using the full search term, “facioscapulohumeral muscular dystrophy,” which reinforces that how one conducts a query may influence the number of studies discovered.
The rest of the studies—about half—are associated with other types of MD as depicted in Table 12.1.
Congenital Muscular Dystrophy
Of the 26 Congenital MD studies cited, most are focused on gathering information to better understand the disease. For example, several trials are listed as natural history or genetic studies and some highlight patient- and family-reported medical information. Most of the other studies included diagnostic tests or physical therapy-type studies (e.g., lung stretch therapy).
Distal Muscular Dystrophy
Distal muscular dystrophy was similar to Congenital MD, except with fewer studies; five out of nine studies were listed as “completed” and only two studies reported as recruiting patients.
Limb Girdle Muscular Dystrophy
Limb Girdle muscular dystrophy was similar to Congenital and Distal MDs—meaning that most studies are focused on gathering information to better understand the disease—except that there are more molecular and genetic studies, including gene transfer and stem cell therapy, though the stem cell therapy was for patients with FSHD, not Limb Girdle MD (as accessed on November 7, 2014).
Myotonic Muscular Dystrophy
Most myotonic muscular dystrophy studies focus on the heart and lungs with two notable exceptions: Iplex from Somaokine and Mexiletine.
According to the ADIS database, Iplex, an insulin-like growth factor, was being studied for multiple indications in the Phase II stage of drug development in Europe; however, clinical development had been suspended or discontinued for multiple indications, including MD.
Mexiletine, an orally active local anesthetic agent structurally related to lidocaine, is an approved drug (sponsor Boehringer Ingelheim). This was studied in Italy in a small number of patients with generalized dystonia, but there has been no further development since 2009, according to the ADIS database (as of November 8, 2014).
Duchenne Muscular Dystrophy: Mechanism of Action
DMD and BMD account for the largest number of clinical trials and have the same MOA, being caused by a defect in the gene for the muscle protein, dystrophin. This may be inherited or may occur without a known family history of the condition [1].
Looking ahead, there is cause for optimism that the approaches being investigated in clinical trials may address the huge unmet medical need for new treatments for DMD and potentially other MDs—ideally by slowing down or halting disease progression, or providing an urgently needed cure.
Most of the clinical studies in the U.S. for DMD involve diagnostic tests to study heart and lung function, stem cell therapies, older, approved products being studied for the treatment of DMD (metformin, carvedilol, prednisone), and protein supplementation (e.g., l-arginine, creatinine, and glutamine).
Current treatments for DMD are aimed at, reducing symptoms and improving quality of life. For example, corticosteroids can help slow down the loss of muscle strength [2]. However, researchers have made great advances in their knowledge of DMD and continue to search for a cure. Several promising approaches to DMD are in the pipeline, according to the MDA [3]:
-
“Exon skipping” drug candidates, which target the mutation that occurs in the gene for dystrophin in individuals with DMD. Also known as molecular patches or antisense oligonucleotides, these are designed to skip the faulty section of this gene so that the muscle protein can be produced, hopefully reducing the symptoms of DMD. Two such products are currently in development: Sarepta’s RNA-based clinical candidate, eteplirsen, and Prosensa’s drisapersen [4, 5].
-
Gene therapy, aimed at introducing a healthy synthetic copy of the dystrophin gene into the muscles to restore production of dystrophin. Challenges include the fact that the dystrophin gene is too big to fit within the virus used to deliver it to muscles; and the body may respond to the virus or dystrophin with an immune reaction. Gene therapy holds great potential if these challenges can be successfully tackled.
-
Reading through stop signals by targeting a specific type of mistake in the genetic code called a nonsense mutation, which prevents the production of full-length functional proteins and affects 10–15% of boys with DMD. For example, PTC Therapeutics’ drug candidate, Ataluren (PTC124) [6], has potential to enable the cell to read through premature nonsense stop signals and make dystrophin.
-
Stem cell therapy, where donor cells are injected with the aim of creating healthy muscle fibers. It may also be possible to isolate the patient’s own stem cells, grow them in the laboratory, correct the genetic defect with gene therapy, and transplant them back into the patient.
-
Utrophin upregulation, aimed at increasing levels of utrophin, a protein that is functionally similar to dystrophin. Summit’s investigational oral small molecule SMT C1100 is in early clinical trials for this use [7].
-
Reducing muscle damage is another goal of ongoing research. For example, Dart Therapeutics’ HT-100 is an orally available, small molecule drug candidate intended to reduce accumulation of scar tissue and inflammation, and to promote healthy muscle fiber regeneration [8].
Selected Case Examples
Hundreds of pharmaceutical candidates, interventions, and studies are posted on clinicaltrials.gov, and, according to a Web search on December 5, 2014, a total of 111 candidates and therapies for the treatment of MD (including protein supplements and drugs previously approved for indications other than MD) have advanced to the Phase II (n = 72) or Phase III (n = 39) stage of clinical drug development. These pharmaceutical candidates represent a significant investment in capital, as well as caregiver and patient time, effort and, in some cases, biological samples.
For Phase II candidates, many trials are studying older, approved drugs and supplements, but in new ways or for new indications (e.g., prednisone, coenzyme Q, metformin, creatine, glutamine), but some candidates appear to be novel, such as GSK’s GSK2402968, idebenone, drisapersen, and eteplirsen, to name a few.
For Phase III candidates, with few exceptions, studies mainly focus on the treatment of DMD/BMD. For example, some Phase III studies are being conducted in patients with Miyoshi myopathy (n = 1), myotonic dystrophy (n = 2), or rare diseases like Bethlem myopathy and Ullrich Congenital MD (n = 1 [same trial to study potential treatment for both diseases]).
Selected novel therapies, in both Phase II and Phase III phases of drug development, are summarized in Table 12.2 below.
Case examples for some of these candidates are provided at the end of this chapter.
Case Example: Ataluren [9]
PTC Therapeutics is developing ataluren (Translarna™) for the treatment of multiple genetic disorders including cystic fibrosis and DMD. Ataluren is formulated as a powder for suspension in water or milk. It is an orally administered, small-molecule compound that targets nonsense mutations. Nonsense mutations are single-point alterations in DNA that introduce a premature translation termination codon, when transcribed into mRNA. This change halts the ribosomal translation process at an earlier site than normal, producing a truncated, non-functional protein. Ataluren allows the cellular machinery to read through premature stop codons in mRNA, and thereby enables the translation process to produce full-length, functional proteins. Specifically, ataluren is designed to mediate the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel for cystic fibrosis and the functional production of dystrophin for DMD. Ataluren is conditionally approved for the treatment of nonsense-mutation DMD (or nmDMD) in the EU, Norway, Iceland, and Lichtenstein.
PTC is conducting a multinational Phase III clinical trial (Ataluren Confirmatory Trial in DMD, or ACT DMD) in support of the conditional approval of ataluren for nonsense mutation DMD. In December 2014, PTC announced that it had commenced a rolling submission of a New Drug Application (NDA) to the FDA for Translarna™ for the treatment of nmDMD. Phase III development in cystic fibrosis is underway in the U.S., Canada, and the EU. Phase II trials were planned for other diseases; however, development for these indications has been suspended to focus on DMD and cystic fibrosis.
According to PTC Therapeutics, ataluren is the first product in the world approved to treat nonsense mutations that cause DMD. Aminoglycosides have also demonstrated an ability to selectively promote read-through of nonsense mutations; however, these agents require parenteral administration and the high doses involved increase the risk of serious toxicity. PTC aims to overcome these limitations by developing an orally administered non-aminoglycoside.
Case Example: Drisapersen [10, 11]
According to ADIS, Prosensa Holding N.V. (aka Prosensa) is developing drisapersen, a 2′-O-methyl antisense oligonucleotide, for the treatment of DMD. DMD is caused by deletion or duplication of exons, or point mutations, in the gene that encodes dystrophin. Because of their capacity to skip an exon by blocking its inclusion during splicing, antisense oligonucleotides have the potential to correct the reading frame of DMD transcripts to yield an internally truncated dystrophin protein. Studies in cultured cells from patients with DMD have demonstrated that drisapersen efficiently induces specific skipping of exon 51. Based on the frequency of mutations in patients with DMD, drisapersen may have the ability to correct the reading frame in up to 25% of deletions, including exon 50, exon 52, exons 45–50, exons 48–50, and exons 49–50. Phase III development of a subcutaneous formulation is underway worldwide for the treatment of DMD.
In the U.S., on October 10, 2014, Prosensa announced that it had initiated the process of submission of a New Drug Application to the FDA. Drisapersen has Breakthrough Therapy Designation and Fast Track Status in the U.S., which made it eligible for a rolling review of the NDA. The submission is expected to be completed before 2015. The company announced on November 24, 2014, that it had been acquired by BioMarin Pharmaceutical Inc. Currently, Prosensa has six DMD candidates in its pipeline, drisapersen being the most advanced one. Notably, all these candidates have orphan drug status in the U.S. and the EU.
Prosensa completed a randomized, double-blind, placebo-controlled Phase III study (results announced in September 2013) on drisapersen for DMD. However, the candidate failed to meet the primary endpoint. The company started re-dosing patients in September 2014.
Prosensa has said that it intends to submit a marketing authorization application in the near future to the European Medicines Agency for conditional approval of drisapersen.
Case Example: Eteplirsen [12]
Sarepta Therapeutics (formerly AVI BioPharma) is developing eteplirsen for the treatment of DMD—where internal exon mutations lead to the formation of truncated dystrophin proteins lacking one of the functional ends. Eteplirsen allows exon 51 to be skipped, providing altered messenger RNA (mRNA), which in turn produces a shortened but functional version of dystrophin. Eteplirsen uses Sarepta’s phosphorodiamidate morpholino oligomer (PMO)-based chemistry and proprietary exon-skipping technology. Oligonucleotides based on this splicing technology do not degrade target RNA and do not lead to down-regulation of the target gene. Previously, AVI BioPharma completed a Phase I/II trial in the UK evaluating intravenously administered eteplirsen in patients with DMD. Phase III development of intravenously infused eteplirsen is underway in the U.S. Sarepta is planning to file for approval with the FDA in the near future, based on the Pre-NDA meeting.
Positive results from a phase I/II trial of intramuscularly administered eteplirsen were reported. However, the company appears to be focusing on the development of eteplirsen for intravenous administration.
A similar approach is being utilized by Sarepta to develop AVI-5038, a therapeutic candidate for DMD with the ability to skip dystrophin exon 50.
Case Example: ATYR-1940 [13, 14]
aTyr Pharma is developing a protein, Resolaris™ (ATYR1940), based on naturally occurring truncated amino acyl-tRNA synthetases, called physiocrines, for the treatment of MDs, including FSHD. Phase I/II development is underway in France and the Netherlands.
Although still early in clinical development, it is interesting to note that in August 2014, aTyr Pharma initiated a Phase I/II trial evaluating the safety and tolerability of ATYR 1940 in patients with molecularly defined genetic facioscapulohumeral muscular dystrophy (ATYR1940-C-002; EudraCT2014-001753-17; NCT02239224). The randomized, double-blind, multiple ascending dose trial is intended to enroll 44 patients in France and the Netherlands.
In February 2015, the company announced that the European Commission had granted orphan drug designation to Resolaris™ for the treatment of FSHD.
References
Duchenne connect FAQ: Ataluran (Translarna™)—an investigational new drug for nonsense mutations by PTC therapuetics. https://www.duchenneconnect.org/en/clinical-trials/study-faq-sheets/646-ataluren-a-novel-drug-for-nonsense-mutations-by-ptc-therapeutics-inc.html. Accessed 10 Dec 2014.
http://www.nlm.nih.gov/medlineplus/ency/article/000705.htm. Accessed 10 Dec 2014.
http://www.mda.org.au/Disorders/Dystrophies/DMD-BMD.asp. Accessed 10 Dec 2014.
http://investorrelations.sareptatherapeutics.com/phoenix.zhtml?c=64231&p=RssLanding&cat=news&id=1904192. Accessed 10 Dec 2014.
http://www.reuters.com/article/2014/01/16/us-prosensa-muscledisorderdrug-idUSBREA0F1IR20140116. Accessed 10 Dec 2014.
http://www.prosensa.eu/technology-and-products/pipeline/drisapersen-pro051. Accessed 10 Dec 2014.
http://www.ptcbio.com/ataluren. Accessed 10 Dec 2014.
http://www.summitplc.com/programmes/duchenne-muscular-dystrophy/. Accessed 10 Dec 2014.
ADIS database, Ataluren. http://bi.adisinsight.com/frames.aspx. Accessed 8 Nov 2014.
ADIS literature search, Drisapersen. http://bi.adisinsight.com/frames.aspx. Accessed 8 Nov 2014.
Diseases and conditions: muscular dystrophy, treatments and drugs. Mayo Clinic. http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/treatment/con-20021240. Accessed 24 Oct 2014.
ADIS database, Eteplirsen. http://bi.adisinsight.com/frames.aspx. Accessed 8 Nov 2014.
ADIS Database, ATYR-1940. http://bi.adisinsight.com/frames.aspx. Accessed 8 Nov 2014.
A placebo-controlled, randomized, multiple ascending dose study to evaluate the safety, tolerability, pharmacokinetics, and biological activity of ATYR1940 in adult patients with molecularly defined genetic muscular dystrophies. http://clinicaltrials.gov/show/NCT02239224. Accessed 8 Nov 2014.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Huml, R.A. (2015). Pharmaceutical Products and Non-pharmaceutical Interventions as Potential Treatments for Patients with Muscular Dystrophy. In: Huml, R. (eds) Muscular Dystrophy. Springer, Cham. https://doi.org/10.1007/978-3-319-17362-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-17362-7_12
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17361-0
Online ISBN: 978-3-319-17362-7
eBook Packages: MedicineMedicine (R0)