Abstract
Apolipoproteins are a key component of lipid transport in the circulatory system and share a number of structural features that facilitate this role. When bound to lipoprotein particles, these proteins are relatively stable. However, in the absence of lipids they display conformational instability and a propensity to aggregate into amyloid fibrils. Apolipoprotein C-II (apoC-II) is a member of the apolipoprotein family that has been well characterised in terms of its misfolding and aggregation. In the absence of lipid, and at physiological ionic strength and pH, apoC-II readily forms amyloid fibrils with a twisted ribbon-like morphology that are amenable to a range of biophysical and structural analyses. Consistent with its lipid binding function, the misfolding and aggregation of apoC-II are substantially affected by the presence of lipid. Short-chain phospholipids at submicellar concentrations significantly accelerate amyloid formation by inducing a tetrameric form of apoC-II that can nucleate fibril aggregation. Conversely, phospholipid micelles and bilayers inhibit the formation of apoC-II ribbon-type fibrils, but induce slow formation of amyloid with a distinct straight fibril morphology. Our studies of the effects of lipid at each stage of amyloid formation, detailed in this chapter, have revealed complex behaviour dependent on the chemical nature of the lipid molecule, its association state, and the protein:lipid ratio.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Apolipoproteins belong to a highly conserved family of lipid-binding proteins involved in lipid transport. An inauspicious feature of the members of this family is that they account for a substantial proportion of proteins known to form amyloid in vivo. For instance, apoA-I, apoA-II and apoA-IV form amyloid fibrils that are associated with several hepatic, systemic, and renal amyloid diseases (Bergstrom et al. 2001, 2004; Coriu et al. 2003; Yazaki et al. 2003; Obici et al. 1999, 2006). In addition, apoA-I, apoA-II and apoC-II accumulate in amyloid deposits of atherosclerotic lesions and have been suggested to contribute to the progression of cardiovascular diseases (Westermark et al. 1995; Mucchiano et al. 2001a, b; Medeiros et al. 2004). The high propensity of apolipoproteins to form amyloid fibrils was postulated to stem from their low conformational stability in the absence of bound lipids (Gursky and Atkinson 1998; Hatters and Howlett 2002).
The present review focuses on human apoC-II, a 79 amino-acid apolipoprotein that activates lipoprotein lipase and is involved in the remodelling of very-low density lipoproteins and chylomicrons in the circulation. Immunohistochemical analysis of atherosclerotic plaques showed that apoC-II co-localises with serum amyloid P, an in vivo marker of amyloid (Stewart et al. 2007a). ApoC-II amyloid fibrils also activate macrophages in a CD36 receptor-dependent process that has been proposed as an early step in foam cell formation and the development of atherosclerosis (Medeiros et al. 2004).
ApoC-II shares high sequence similarity and structural homology with other members of the apolipoprotein family. In particular, the apoC-II sequence comprises 11 residue tandem repeats, which form the characteristic amphipathic helical motifs that facilitate lipid surface binding by apolipoproteins (Segrest et al. 1990, 1994; see also Chap. 8 by Das and Gursky in this volume). These shared properties make apoC-II an excellent model for understanding the role of lipids in the folding and misfolding of apolipoproteins in general.
In the presence of micellar lipid and lipid mimetics, apoC-II adopts a predominantly α-helical structure (MacRaild et al. 2004), consistent with its role in binding lipid surfaces. Conversely, lipid-free apoC-II contains relatively little α-helical secondary structure that is not significantly altered in the presence of 5 M guanidine hydrochloride (Hatters and Howlett 2002), suggesting that in the absence of lipid surfaces, apoC-II is natively unfolded. In vitro, this lipid-free form of apoC-II readily self-assembles into homogeneous fibrils with increased levels of β-structure and all the hallmarks of amyloid (Hatters et al. 2000). This process occurs in a reproducible and concentration-dependent manner at physiological ionic strength and pH, resulting in somewhat soluble, “twisted-ribbon” fibrils (Fig. 7.1a) that are amenable to a range of biophysical analyses.
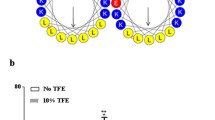
The structure of apoC-II fibrils. (a) Transmission electron micrograph of apoC-II fibrils formed by incubation of apoC-II at 0.3 mg/mL in 100 mM sodium phosphate, pH 7.4, for 2 days. Note the flat, twisted, ribbon-like morphology. Scale bar represents 200 nm (Figure adapted with permission from Griffin et al. (2008)). (b) A structural model for apoC-II amyloid fibrils. ApoC-II monomer assembles into amyloid fibrils with a ‘letter-G-like’ β-strand-loop-β-strand structure. The model includes residues 21–79 of apoC-II with each β-strand of the monomer contributing to each of the two β-sheets, giving rise to a parallel, in-register structure (Figure adapted with permission from Teoh et al. (2011a))
ApoC-II fibrils formed in the absence of lipid have been investigated using a range of structural techniques, including fibre diffraction, scanning transmission electron microscopy (STEM), fluorescence resonance energy transfer (FRET), analytical ultracentrifugation and hydrogen/deuterium exchange NMR. Fibre diffraction experiments show a classical cross-β diffraction pattern with reflections at 4.67 Å and 9.46 Å, indicating the spacing between β-strands in the fibril axis and the average spacing between β-sheets in the fibril cross section, respectively (Teoh et al. 2011b) STEM revealed that the fibrils contain approximately one molecule of apoC-II per 4.7 Å rise in the long axis of the fibril, and hydrogen/deuterium exchange data indicated two protected regions in residues 20–36 and 58–74 (Wilson et al. 2007) that are implicated in formation of the amyloid core. Recently, these and other observations led to the development of a “letter-G-like” β-strand-loop-β-strand structural model for an apoC-II unit within a mature amyloid fibril (Fig. 7.1b) (Teoh et al. 2011b).
Studies of the kinetics of apoC-II fibril formation indicate that it proceeds via a reversible pathway that includes elongation, dissociation, and fibril breaking and rejoining (Binger et al. 2008; Yang et al. 2012). In addition, the concentration dependence of apoC-II aggregation has been analysed (Hatters et al. 2000, 2002a, b). These analyses collectively show that apoC-II forms amyloid fibrils in a time-dependent manner at 0.1–1 mg/mL apoC-II (approximately 10–100 μM), with times for the completion of fibril formation ranging from less than 1 h (at 1 mg/mL apoC-II) to ~7 days (at 0.1 mg/mL). ApoC-II concentrations less than 0.1 mg/mL form amyloid fibrils very slowly (>7 days), facilitating the study of the early events of amyloid fibril formation (Ryan et al. 2008).
Given its role in lipid transport and lipoprotein remodelling, it is perhaps unsurprising that submicellar lipids/detergents and lipid surfaces can substantially affect the misfolding of apoC-II and amyloid formation. Lipids alter the rate and pathway of amyloid formation, as well as the structural features of mature fibrils. This chapter reviews our studies of the effects of lipids and lipid-like detergent molecules on amyloid fibril formation by full-length apoC-II and its fibrillogenic peptides.
7.2 The Structure of ApoC-II Bound to Lipid Micelles
Investigating the role of lipids in protein folding is complicated by a range of factors, including, but not limited to, the diverse range of lipids that are present in biological samples, their general insolubility and their tendency to self-associate to form lipid surfaces. These complications render distinguishing the role of individual lipid molecules from the role of lipid surfaces in protein folding difficult, particularly for native lipids that generally form lipid surfaces or complexes. This is compounded by the tendency of molecules that bind to lipid surfaces to alter the properties of the membrane. For example, apoC-II can bind specific phospholipids within lipid membranes, and thereby locally remodel the composition of the lipid surface (Hanson et al. 2003). Conversely, lipid surfaces have a significant effect on apoC-II structure; binding of lipid surfaces almost always results in the stabilisation of a native α-helical structure in this (MacRaild et al. 2001, 2004) and other apolipoproteins [for review see (Wang 2008)]. However, there is evidence that apoC-II can adopt an amyloid structure in atherosclerotic plaques, which are highly enriched in a range of modified lipids (Medeiros et al. 2004; Teoh et al. 2011a). This dichotomy between the effects of lipids in vitro and in in vivo disease lesions suggest that the role of lipids in apoC-II amyloid formation might be much more complex than previously thought.
Initial circular dichroism (CD) and NMR spectroscopy investigations of the structure of apoC-II focused on the protein in complex with micelles of the detergents sodium dodecyl sulphate (SDS) and dodecylphosphocholine (DPC). These lipid-like detergents induced ~60 % α-helical conformation in apoC-II and prevented its aggregation (MacRaild et al. 2001, 2004). NMR structural analyses of apoC-II in complex with SDS micelles showed two broad regions of α-helical structure, corresponding to the N- and C-terminal regions of the molecule. These helices correspond well to the regions of amphipathic helix predicted from primary sequence analysis, linked by a flexible region, which also has some helical character (Fig. 7.2). The N-terminal helical region includes residues 16–36, and forms a curved helix, postulated by MacRaild et al. (2001) to be important for binding to the large-radius surface of chylomicrons and very low density lipoproteins (diameters 50–1,000 nm), while the C-terminal helix in residues 50–54 and 63–76 contains the binding site for lipoprotein lipase. Subsequent 15N NMR relaxation measurements coupled with molecular dynamics simulations of apoC-II bound to SDS micelles largely confirmed these findings (Zdunek et al. 2003). ApoC-II in the presence of DPC micelles displayed very similar structural characteristics (MacRaild et al. 2004). While these NMR structures elucidated several conformational features of natively folded apoC-II bound to lipid-like surfaces, they provided little information on the role of individual lipid interactions.
The structure of apoC-II bound to SDS micelles. Averaging of 20 NMR structures results in the main chain trace and reveals two regions of well-defined α-helices (represented by narrow line and cylinders) connected by a region of less well-defined structure (represented by the thicker Cα trace). Arrows and numbers indicate the amino acid position (Adapted with permission from MacRaild et al. (2001); Copyright (2001) American Chemical Society)
7.3 The Effects of Submicellar Lipid on the Misfolding of ApoC-II
In an attempt to separate the roles of individual phospholipid molecules and lipid surfaces on apoC-II misfolding and aggregation, we have used a range of simple short-chain phospholipids and detergents. These lipid derivatives have the advantage that they exist in two states, a “monomeric” state at low concentrations and a self-associated micellar state at higher concentrations. The critical micelle concentration (CMC) at which these molecules transition from one state to the other depends on structural features of the detergent molecule, including its size and shape, charge distribution on the head group, length and hydrophobicity of the acyl chains and buffer conditions, including pH, salt concentration, and hydrophobicity. By selecting particular lipid derivatives, a range of factors that affect protein folding can be investigated in the absence of lipid surface.
Investigation of the effect of individual lipids on the process of amyloid formation by apoC-II was initially conducted through the use of the short-chain phospholipid 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), which has a CMC of approximately 10 mM in 100 mM sodium phosphate at pH 7.4 (Hatters et al. 2001). At micellar concentrations this lipid stabilised native structure in apoC-II. However, at submicellar concentrations DHPC enhanced the rate of apoC-II amyloid formation sixfold (Hatters et al. 2001; Griffin et al. 2008; Ryan et al. 2008). Investigation of the effect of acyl chain length, and hence hydrophobicity, was conducted using a series of phosphatidylcholine derivatives with acyl chains containing three to nine carbons at a constant detergent concentration of 10 mM (Griffin et al. 2008) (Fig. 7.3), which is below CMC for short-chain lipids containing three to five carbons. This analysis showed that increasing the length of the acyl chain from three to five carbons increased the rate of fibril formation up to a maximum of sevenfold. This result suggests that higher hydrophobicity increases the rate of fibril formation. Lipids with acyl chains containing six to nine carbons, which approach and surpass their CMC at 10 mM concentration, inhibited amyloid fibril formation by apoC-II. CD spectroscopy indicated that the shorter-chain submicellar lipids induced similar conformation in apoC-II, with a slight trend towards increased negative ellipticity at 222 nm with increasing acyl chain length, suggesting some α-helix formation. The longer-chain micellar lipids invariably induced CD spectra with minima at 208 and 222 nm, characteristic of predominant α-helical structure.
Effect of acyl chain length of short-chain diacyl phospholipids on fibril formation by apoC-II. Diacyl phosphocholine derivatives are designated DnPC, where n refers to the acyl carbon chain length. Fibril formation was monitored using a Thioflavin T (ThT) assay, where solutions of apoC-II (0.3 mg/ml in 100 mM phosphate buffer at pH 7.4) were incubated alone (filled triangles) and in the presence of 10 mM D3PC (filled squares), D4PC (open circles), D5PC (filled circles), D6PC (open diamonds), D7PC (filled diamonds, dashed line), D8PC (open squares, dotted line) and D9PC (open triangles). ThT fluorescence was measured at the intervals and nonlinear best fits to the data using a Hill-3 parameter equation (continuous lines) were used to determine the transition rates and maximum ThT fluorescence (Figure adapted with permission from Griffin et al. (2008))
The effect of head group was investigated by using 1,2-dihexanoyl-sn-glycero-3-phospho-L-serine (DHPS), where the choline functionality of the head group is substituted with a serine (Ryan et al. 2008). This lipid has a net negative charge, which allows it to exist in a non-micellar form at significantly higher concentrations (~20 mM) than its zwitterionic DHPC counterpart. When this difference in CMC is taken into account, the effect of submicellar DHPS on amyloid formation by apoC-II is not significantly different from that of DHPC. Investigation of 1,2-dihexanoyl-sn-glycero-3-phospho-L-ethanolamine (zwitterionic) and 1,2-dihexanoyl-sn-glycero-3-phospho-L-glycerol (anionic) at comparable submicellar concentrations indicated very similar effects on the rate of fibril formation to DHPS and DHPC. Taken together, these data indicate that the head group charge has little effect on the misfolding and rate of fibril formation by apoC-II, and that the acyl chain hydrophobicity is the major determinant of the enhancing effects of these submicellar lipids on apoC-II aggregation (Ryan et al. 2008).
7.4 The Effects of Submicellar Lipids on Initial ApoC-II Self-Association
During the studies outlined in the previous section, it became apparent that the short-chain lipids were significantly affecting the lag phase of apoC-II amyloid fibril formation, suggesting that their primary effect was on the initial folding and self-association of apoC-II (Ryan et al. 2008; Griffin et al. 2008). To further investigate this effect, a single cysteine replacement of serine 61 (apoC-II-S61C) was engineered, and this cysteine was labelled with the fluorophore, c5 maleimide Alexa-488 (Alexa-488-apoC-II) (Ryan et al. 2008) (Fig. 7.4a), which was used to characterize the molecular environment and the process of amyloid fibril formation. Alexa-488-apoC-II showed a similar rate of amyloid fibril formation to native apoC-II (Ryan et al. 2008), providing a valuable tool for detailed biophysical analysis of this process.
Fluorescence spectroscopy of Alexa488-labeled apoC-II. (a) Emission spectra of freshly prepared Alexa488-apoC-II (50 μg/ml in 100 mM phosphate buffer, pH 7.4; excitation at 495 nm) in the absence of lipid (curve 2) and in the presence of 10 mM DHPS (curve 1); and of fibrillar Alexa488-labeled apoC-II (0.3 mg/ml in 100 mM phosphate buffer, pH 7.4, and diluted to 50 μg/ml) in the absence (curve 4) and presence (curve 3) of 10 mM DHPS. (b) Fluorescence emission intensity (black line) and anisotropy (blue line) of Alexa488-apoC-II during fibril formation. c Fluorescence emission intensity (black line) and anisotropy (blue line) of Alexa488- apoC-II in the presence of 10 mM DHPS during fibril formation. Data were acquired on a Florolog Tau-2 spectrophotometer using 55° polarization and an emission cutoff of 505 nm. The aggregation kinetics of the labelled and unlabelled apoC-II was similar (Figure adapted with permission from Ryan et al. (2008))
Initial time courses of Alexa-488-apoC-II aggregation indicated that, in the absence of lipids, the protein displayed a time-dependent decrease in fluorescence intensity during fibril formation. Fluorescence anisotropy measurements, which report on the relative rotational motion of a fluorophore, were consistent with this observation, showing a sigmoidal increase to a maximum of approximately 0.1 over 72 h (Fig. 7.4b). This increase in fluorescence anisotropy is consistent with the formation of large aggregates with low tumbling rates, while the decrease in fluorescence intensity can be attributed to homo-fluorescence resonance energy transfer (FRET) occurring when the Alexa-488 fluorophores from different apoC-II molecules are brought into close proximity. Thus, the two fluorescence measures allow analysis of the time course of aggregation and amyloid fibril formation by apoC-II.
Addition of submicellar DHPC or DHPS resulted in significant differences in the time courses of these fluorescence measurements. In both cases, these short-chain lipids at submicellar concentrations induced a rapid increase in the fluorescence intensity and anisotropy of Alexa-488 (Fig. 7.4c). This immediate response was followed by a slower increase in fluorescence intensity during which no significant change in anisotropy was observed. After these initial changes, the quenching of the Alexa-488 fluorescence intensity and further increases in anisotropy due to fibril formation over longer time periods proceeded as for apoC-II alone. The rate of the slower phase of the initial fluorescence changes was independent of protein concentration, and FRET measurements using both Alexa-488 and Alexa-594 labelled apoC-II-S61C indicated significant inter-molecular interactions in the presence of submicellar phospholipids at early time points. These results indicate that submicellar lipids enhance early association events in the apoC-II aggregation pathway.
This hypothesis was further investigated using analytical ultracentrifugation. Sedimentation velocity experiments using Alexa-488-apoC-II enabled the analysis of apoC-II self-association at low protein concentrations, where significant fibril formation does not occur (Ryan et al. 2008). These experiments indicated that addition of submicellar phospholipids shifted the modal sedimentation coefficient of apoC-II from 1 S to approximately 3.1 S, equivalent to the formation of an apoC-II tetramer (Fig. 7.5). The size of this larger species was confirmed using sedimentation equilibrium experiments, which showed an approximate molecular weight of 40 kDa, equivalent to four labelled apoC-II molecules. Solutions of this stabilised tetrameric apoC-II were capable of seeding fibril formation, indicating that this oligomeric structure can act as a nucleus for amyloid aggregates (Ryan et al. 2008).
Sedimentation analysis of apoC-II self-association in the presence of the short-chain phospholipid derivative, DHPS. Sedimentation velocity data were acquired for freshly refolded Alexa-488-apoC-II (50 μg/mL, 100 mM phosphate buffer) in the presence of increasing concentrations of DHPS (0–20 mM) using the fluorescence detection system analytical ultracentrifuge. The use of fluorescence provides sensitivity and specificity, allowing analysis of the low apoC-II concentration in the presence of the relatively large lipid concentration. (a) Sedimentation velocity data, analysed using the c(S) model, revealed the presence of three peaks that depend on the lipid concentration. The peak circa 1 S corresponds to monomeric apoC-II. Increasing submicellar concentrations of DHPS induce a 3.1 S peak corresponding to apoC-II tetramer (Peak 3). Micellar concentrations of DHPS induce a peak at around 2.4 S, consistent with a micelle-associated apoC-II molecule (Peak 2). (b) The integrated area under each peak in panel (a) is plotted as a function of DHPS concentration, revealing the DHPS concentration-dependence of the monomer (peak 1; squares), micelle associated apoC-II (peak 2; circles), and tetrameric apoC-II (peak 3; filled triangles) (Figure adapted with permission from Ryan et al. (2008))
7.5 Kinetics of ApoC-II Amyloid Formation in the Presence of Submicellar Lipids
The results of biophysical studies indicated that lipids affected nucleation of apoC-II amyloid fibrils. To quantify the precise effects of lipids, kinetic modelling of amyloid fibril formation was required. The relatively soluble nature of the apoC-II fibril allows the use of analytical ultracentrifugation (MacRaild et al. 2003) to analyse the time-dependent evolution of the size distribution of these macromolecular structures during aggregation (Binger et al. 2008). This analysis provides both an average aggregate size and the proportion of aggregate over time, which can be used as constraints to mathematically model the aggregation pathway (Binger et al. 2008).
This model showed that apoC-II fibril formation is a reversible process of nucleation, elongation, fibril breaking and rejoining, which resulted in a fairly consistent size distribution for apoC-II amyloid fibrils, and explained the formation of closed loops of apoC-II fibrils (Binger et al. 2008). Application of this method, in conjunction with stopped-flow analysis of the fluorescence changes, indicated that the nucleation phase was increased by several orders of magnitude in the presence of submicellar phospholipid derivatives, while the elongation phase was unaffected (Ryan et al. 2010). This was supported by the observations that the “free pool” of soluble apoC-II in equilibrium with fibrils in the absence and presence of submicellar phospholipids was the same. The size of this free pool is defined solely by the on- and off-rates of elongation.
Further confirmation of the differential effect on nucleation and elongation is provided by two observations. First, submicellar lipids have little effect on the aggregation of synthetic cysteine-crosslinked dimers of apoC-II-S61C, where the initial association events of the apoC-II fibril formation pathway are already complete. Second, submicellar lipids have no effect on apoC-II aggregation when added midway through the elongation phase. These results suggest that the physicochemical processes underlying the nucleation and the elongation of fibrils are different and can be affected by chemical compounds differentially. This conclusion has potentially important implications for the development of therapeutics designed to modulate fibril formation, but can also provide insight into the basis of in vivo fibril formation.
Another aspect of the nucleation elucidated in this analysis was that the protein tetramerisation in the presence of the submicellar lipid did not constitute the formation of a nucleus, and a second step involving a slow zero-order isomerisation of the tetramer was required to fit the data (Ryan et al. 2010). This isomerisation was confirmed using a combination of stopped-flow fluorescence and CD spectroscopy (Fig. 7.6) showing that this step was rate-limiting for amyloid formation in the presence of submicellar lipids. The nature of the slow isomerisation step is unclear; it could involve a structural rearrangement of the tetramer, a loss of the lipid molecules, or some combination thereof (Ryan et al. 2010). CD time courses indicate that the tetramer structure is subtly altered during this isomerisation step, suggesting that a structural rearrangement does indeed occur.
Time-dependent structural changes of the lipid-induced apoC-II tetramer. (a) CD spectra of apoC-II (0.05 mg/mL in 10 mM Na phosphate buffer, dashed line), immediately after addition of 5 mM DHPC (0 h, dotted line), and after 1 h incubation in the presence of 5 mM DHPC (continuous line). The CD spectrum of apoC-II alone (dashed line) is provided for reference. At this apoC-II concentration, fibril formation is negligible over the time course of the experiment. The data are presented in units of mean residue ellipticity (MRE). (b) The change in MRE at 208 nm (indicated in panel a by red arrow) over time for 0.05 mg/mL apoC-II in the presence of 5 mM DHPC (triangles), and for 0.025 mg/mL apoC-II in the presence of 5 mM DHPC (squares) (Figure adapted with permission from Ryan et al. 2010)
Notably, this rate-limiting isomerisation step is probably not unique to fibril formation by apoC-II. For example, elegant studies by Singh et al., Chap. 4 in this volume show that the slow rate-limiting step in amyloid fibril formation by a small lipid-binding protein, amylin, also involves re-arrangement and self-association of low-order protein oligomers.
7.6 Lipid Dynamics During ApoC-II Aggregation
To provide information on the dynamics of lipid molecules during apoC-II fibril formation, further analysis was conducted using a fluorescently labelled single-chain phospholipid, 1-dodecyl-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-2-hydroxy-sn-glycero-3-phosphocholine (NBD-lyso-12-PC) (Ryan et al. 2011a). The unlabelled form of this lipid has a CMC near 0.2 mM, and both the labelled and unlabelled lipids at submicellar concentrations induce apoC-II tetramerisation and enhance fibril formation (Griffin et al. 2008; Ryan et al. 2008, 2011a). FRET experiments utilising this labelled lipid and freshly refolded Alexa-594-apoC-II indicated that the lipid and protein were in close proximity, suggesting the formation of a reasonably stable complex (Ryan et al. 2011a). Stopped-flow analysis of this FRET signal indicated rapid pseudo-first-order association with a rate constant of 156,000 s−1.
Fluorescence-detected analytical ultracentrifugation indicated that the lipid associated with both monomeric and tetrameric apoC-II in a concentration-dependent manner. Analysis of the proportion of tetramer, as measured by the weight average sedimentation coefficient of the apoC-II in the presence of NBD-lyso-12-PC, provided the tetramerisation constant of 3.5 × 10−3 μM−3, consistent with that obtained from the kinetic analysis of apoC-II fibril formation in the presence of DHPC. Analysis of the proportion of NBD-lyso-12-PC that co-sedimented with apoC-II provided a stoichiometry of five NBD-lyso-12-PC molecules bound per one apoC-II molecule, and the concentration dependence of this association indicated a K d ≅ 10 μM.
The time dependence of the protein-lipid interaction during the fibril formation showed that the FRET signal was lost over 12 h, suggesting that as apoC-II aggregated the lipid affinity decreased. Analysis of the final fibrillar product indicated a complete lack of FRET, suggesting that mature, fibrillar apoC-II did not bind submicellar lipids. This analysis was supported by analytical ultracentrifugation, in which no co-sedimentation between the NBD-lyso-12-PC and mature apoC-II fibrils was observed by fluorescence, and in pelleting assays, where no NBD-lyso-12-PC was found in the pellet fraction of fibrils either added to, or formed in the presence of NBD-lyso-12-PC. Together, these results indicate a catalytic role for submicellar lipids in apolipoprotein misfolding and aggregation, and suggest that the presence of only a small amount of lipid could affect initiation of amyloid fibril formation in vivo (Ryan et al. 2011a).
7.7 The Interaction of ApoC-II Peptide Fragments with Lipids
Analysis of lipid binding by the defined peptide regions of apoC-II has elucidated many biophysical features of apolipoprotein – lipid surface binding. The peptide implicated in forming the primary lipid-binding region, apoC-II19–39, was found to adopt a helical structure and bind to lipid surfaces with K d = 5 μM, and to form dimers at very high protein:lipid ratios (MacPhee et al. 1999). Such self-association has been proposed to be important for stabilising apolipoprotein structures and may influence the function of lipoprotein particles (e. g. apoA-I and apoA-IV dimers).
ApoC-II peptides have also been used to elucidate misfolding events and molecular interactions involved in apoC-II aggregation (Wilson et al. 2007; Legge et al. 2007; Griffin et al. 2012). These peptides are derived from the amyloidogenic regions of apoC-II and undergo many of the processes involved in the misfolding and amyloid fibril formation by the full-length protein. However, these peptides do show some distinct differences from the full-length protein, indicating that the amyloid formation by apoC-II involves several regions acting in concert.
Studies of the effect of phospholipids have primarily focused on one of these regions, apoC-II60 –70, which contains the sequence MSTYTGIFTDQ (Hung et al. 2008, 2009) that is largely hydrophobic and is predicted to be particularly amyloidogenic (Chap. 8 by Das and Gursky in this volume). In contrast to the full-length protein, amyloid fibril formation by this peptide is inhibited by the presence of submicellar lipids, including DHPC (Hung et al. 2009). Interestingly, the peptide responds to the presence of submicellar lipids by forming a range of higher-order oligomers, as evidenced by sedimentation equilibrium experiments, which do not show a significant induction of secondary structure observed by CD spectroscopy (Hung et al. 2009). Molecular dynamics simulations indicate that the presence of solvated lipid molecules enhanced inter-peptide association, particularly through improved hydrophobic contacts, which act to kinetically trap this apoC-II peptide in oligomeric structures ranging from dimer to much larger species (Hung et al. 2009). This result, while appearing to contradict the results for the full-length protein, is actually complementary. The observation that lipids can drive the peptide self-association correlates to the initial induction of tetrameric species of the full-length protein; it is perhaps other regions of the apoC-II that drive the second phase of nucleation, which ultimately results in the ejection of the lipid and the formation of cross-β structure in amyloid fibrils.
7.8 The Effects of Micellar Lipids and Lipid Surfaces
As discussed above, apoC-II in the presence of large excesses of micellar lipids adopts an α-helical conformation and does not aggregate. One explanation for this effect, in addition to stabilisation of a native-like structure, is that the micelles effectively separate apoC-II molecules from each other. For example, as each DHPC micelle contains approximately 40 lipid molecules (Atcliffe et al. 2001), a concentration of 10 mM DHPC is equivalent to a micelle concentration of approximately 0.25 mM, resulting in a molar ratio of around 7.5 micelles per protein molecule at standard amyloid-forming concentrations of apoC-II. Thus, on average, a micelle is expected to contain fewer than one apoC-II molecule.
Interestingly, the primary lipid-binding region of apoC-II at very high protein:lipid ratios appears to undergo self-association (MacPhee et al. 1999). Under these conditions apoC-II displays some interesting aggregation phenomena (Griffin et al. 2008). The use of low concentrations of micellar lipids, facilitated by using longer-chain phospholipids with lower CMCs, coupled with higher concentrations of apoC-II, results in an initially inhibited aggregation process over a period of 200 h. Subsequent sample analysis showed a second phase of aggregation, where the maximal thioflavin T (ThT) fluorescence was significantly greater than in the first phase. This behaviour could be replicated with small unilamellar vesicles comprised of more physiological lipids such as 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), indicating that it is not specific to a particular lipid surface configuration.
Transmission electron microscopy (TEM) analysis of the apoC-II fibrils formed during the first phase of aggregation showed the common twisted-ribbon morphology. However, TEM analysis after the second phase revealed a previously unidentified apoC-II fibril morphology (Fig. 7.7). These fibrils displayed a straight, cable-like structure 13–14 nm wide, with helical periodicity of 95–100 nm. CD spectroscopy showed that the two fibril types had distinct secondary structure, strongly suggesting a divergent amyloid formation pathway. Seeding experiments supported this observation and indicated that the cable-like fibrils can be seeded and propagated in a lipid-free environment. This shows that phospholipids not only play an important role in the initiation and enhancement of amyloid fibril formation, but also in determining the ultimate fibril structure. This level of polymorphism may be of critical importance in understanding the initiation of amyloid fibril formation in lipid-rich environments in vivo and for understanding the molecular basis of amyloid diseases.
Lipids induce a distinct, straight apoC-II fibrillar morphology. Transmission electron micrographs showing the fine detail of (a) mature ribbon-type fibrils formed by 1.0 mg/ml apoC-II alone, and straight fibrils formed by 1.0 mg/ml apoC-II in the presence of (b) 500 μM D8PC, (c) 500 μM D9PC, (d) 500 μM Lyso-MPC, and (e) 500 μM DMPC. Circular objects adjacent to fibrils in the presence of DMPC are probably small unilamelar lipid vesicles that may interact with the apolipoprotein fibers as described by Gorbenko et al. in Chap. 6 of this volume (Figure adapted with permission from Griffin et al. 2008)
7.9 The Effects of Oxidized Cholesterol
Several studies have demonstrated that amyloid deposits are present in up to 50–60 % of aortic atherosclerotic lesions (Westermark et al. 1995; Mucchiano et al. 2001a). These amyloid deposits contain various proteins, including apoC-II. ApoC-II has also been shown to colocalize in these plaques with a range of amyloid markers, providing indirect evidence that it is part of the amyloid component of atheroma. Atherosclerotic lesions also contain a large component of lipids, including oxidized cholesterol, which has been shown to enhance amyloid formation by α-synuclein and amyloid-β peptide (Bieschke et al. 2005; Zhang et al. 2004; Bosco et al. 2006).
ApoC-II fibril formation is significantly enhanced in the presence of the oxidized cholesterol derivative, 3ß-hydroxy-5-oxo-5,6-secocholestan-6-al (Stewart et al. 2007b). Further analysis using HPLC and MALDI-TOF mass spectrometry indicated that this oxidized cholesterol derivative formed a Schiff-base covalent bond with apoC-II lysines 19, 30, 39, 48, 55, and 76 (Stewart et al. 2007b). Isolation of the various oxidized cholesterol adducts and analysis of fibril formation by these modified apoC-II molecules showed that in all cases fibril formation was enhanced, and a variety of alternate fibrillar morphologies were produced (Stewart et al. 2007b).
These observations, in conjunction with the studies of amyloid-β and α-synuclein, suggest that oxidized cholesterol may trigger or enhance the amyloid deposition in various tissues, including atheroma (Stewart et al. 2007b). Another possible implication is a feedback loop with the inflammatory response, which can generate oxidized cholesterol that may worsen amyloid deposition by various proteins. These results provide further evidence that altered lipid metabolism can affect protein folding, and that elucidating the ways in which the diverse lipid molecules are involved in normal and aberrant protein folding may be critical to understanding the basis of amyloid diseases.
7.10 The Effects of Hydrophobic “Lipid-Like” and Detergent Molecules
The effects of lipids and their derivatives on amyloid fibril formation can be altered by the self-association state of the lipid and/or its chemical nature, which varies widely for different lipids. To explore the structural specificity of these effects, a library of 96 diverse amphipathic lipids and detergents was screened at submicellar concentrations for their ability to modulate the rate of amyloid fibril formation by apoC-II (Ryan et al. 2011b) (Fig. 7.8). A combination of ThT fluorescence and light scattering was used to monitor fibril formation by apoC-II (1 mg/mL) in the presence of the 96 compounds at a concentration of one half their respective CMC, ensuring that the majority of the compounds were at submicellar concentrations. Some compounds in the screen, termed non-detergent sulfobetaines, did not form micelles, and others were highly hydrophobic and their CMCs could not be determined. These compounds were screened at concentrations twofold greater than that of apoC-II.
The effects of lipid and lipid-like molecules on apoC-II fibril formation. (a) The rate of fibril formation (1.0 mg/mL apoC-II in 100 mM phosphate at pH 7.4, measured using a continuous ThT assay) in the presence of various lipid-like compounds relative to that of apoC-II alone (rate = 1). (b) The hydrophobicity scale of the various molecules is expressed as calculated octanol/water partitioning coefficients (Log P), where Log P >0 indicates preferential partitioning into octanol (increased hydrophobicity). Log P values were acquired using MarvinSketch (http://www.chemaxon.com/products/marvin/marvinsketch/) (Figure adapted with permission from Ryan et al. 2011b)
The initial screen showed that the library could be split into three groups of compounds that activated fibril formation, inhibited fibril formation, or had no significant effect. Surprisingly, most lipids fell into the activator or inhibitor groups, with relatively few having little effect on fibril formation. In general, the results suggested that cholesterol-like molecules inhibit apoC-II fibril formation, while compounds that closely resemble fatty acids, phospholipids or sphingolipids were more likely to activate apoC-II fibril formation.
Interestingly, apparently minor structural differences in these compounds caused very significant shifts in their effects on apoC-II amyloid fibril formation. For example, at submicellar concentrations, 1-dodecyl,2-lyso-sn-glycero-phosphocholine activates fibril formation while dodecylphosphocholine inhibits it. The structural difference between these two compounds is a glycerol linker between the phosphocholine head group and the single 12-carbon acyl chain. While a comprehensive analysis of the structural basis for inhibition is challenging, a general defining feature of activators and inhibitors was their average hydrophobicity, as measured by octanol/water partitioning coefficients at pH 7.0 (LogP) calculated from molecular structure. Inhibitors typically had higher overall hydrophobicity (logP ≅ +2.2) than activators (logP ≅ −0.81). However, examples of both highly hydrophobic activators and hydrophilic inhibitors were also identified (Fig. 7.8).
Structural analysis in the presence of a subset of these lipid-like detergents indicated that, in general, inhibitors induced α-helical structure while activators induced β-sheet structure in apoC-II. Analytical ultracentrifugation analysis indicated induction of tetramers by the activating compounds, further supporting the idea that lipid-induced activation of the amyloidogenic pathway in apoC-II involves tetramer formation. In contrast, the inhibiting compounds induced dimeric, predominantly α-helical apoC-II species, suggesting that the mechanism of inhibition by these molecules involves stabilisation of non-amyloidogenic oligomers that may have native-like structure (Ryan et al. 2011b).
Addition of compounds during the nucleation or elongation phases showed differential modulatory effects of some compounds at different stages of fibrillation. These stage-specific effects, whereby molecules were identified that activate nucleation but inhibit elongation of fibrils, and vice versa, emphasise that fibril formation is a multi-step process with different biophysical and structural processes occurring at each stage. This outcome may be important when considering therapeutic intervention in amyloid formation, as screening processes may bias the results towards modulators of nucleation, which may provide little benefit or even have detrimental effects in patients with pre-existing amyloid deposits. Interestingly, a parallel study using the same compound library with amyloid-β peptides displayed a quite different pattern of activators and inhibitors. This indicates that the pattern of activation and inhibition observed with apoC-II is specific to this protein, and should not be generalised to other amyloid-forming proteins (Ryan et al. 2012).
The results of this screen primarily indicate that the response to lipid-like molecules by apoC-II depends on subtle differences in chemical structure of the molecule, and that the vast majority of lipid-like molecules are likely to have significant modulatory effects on apoC-II folding, misfolding and aggregation. This suggests that a balance between the effects of activating and inhibiting molecules governs the proper maintenance of apoC-II folding in vivo, and that subtle shifts in the level or structure of a critical lipid might result in aggregation.
7.11 Conclusions
This chapter presents a comprehensive review of the lipid effects on various stages of apoC-II misfolding and aggregation. The picture that emerges is a complex range of effects that are dictated by the ratios of lipid to protein, the association state of the lipids, and their chemical nature (Fig. 7.9). Lipids are a key component of the pathological site of apoC-II accumulation in atheroma; at the same time, lipids clearly are essential for maintaining lipoprotein stability, and thereby stabilizing the native apolipoprotein structure against misfolding. The results strongly suggest that modified lipids may play a catalytic role in initiating amyloid fibril formation by apolipoproteins, and could play a significant pathological role in the development of amyloid plaques in tissues. Lipids also play a significant role in determining the fibrillar morphology of apoC-II amyloid and, therefore, in the stability and dynamics of the aggregates. Clearly, understanding the role of lipids in the folding and misfolding of proteins is critical to understanding protein folding and aggregation in vivo, both in healthy and disease states.
The effects of lipids on apoC-II misfolding and aggregation pathways. In the absence of lipid, apoC-II forms a molten globular structure that can self-associate to form ribbon-type fibrils through a nucleation-dependent process (1). At low protein:micelle ratios, apoC-II adopts a stable native-like α-helical structure (2). In the presence of submicellar lipids, apoC-II rapidly forms a tetramer that binds individual lipid molecules (3). This tetramer undergoes a molecular rearrangement, gaining additional structure and ejecting the bound lipid molecules to form a lipid-free tetramer that rapidly forms ribbon-type fibrils (4). At high protein:micelle ratios, apoC-II adopts a non-native conformation that slowly forms fibrils with an alternate, straight cable-like morphology (5). Structures depicted are schematic models for illustrative purposes, and are not intended to specifically represent the structure of apoC-II in each environment (Figure adapted with permission from Griffin et al. 2008)
Abbreviations
- Apo:
-
Apolipoprotein
- CD:
-
Circular dichroism
- CMC:
-
Critical micelle concentration
- DHPC:
-
1,2-dihexanoyl-sn-glycero-3 -phosphocholine
- DHPS:
-
1,2-dihexanoyl-sn-glycero-3-phospho-L-serine
- DMPC:
-
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DnPC:
-
1,2-diacyl-sn-glycero-3- phosphocholine where n = 3–9 carbon acyl chains
- DPC:
-
Dodecylphosphocholine
- FRET:
-
Fluorescence resonance energy transfer
- LysoMPC:
-
1-myristoyl-2-hydroxy-sn-glycero -3-phosphocholine
- NBD:
-
Nitrobenzoxadiazole
- SDS:
-
Sodium dodecyl sulphate
- STEM:
-
Scanning transmission electron microscopy
- TEM:
-
Transmission electron microscopy
- ThT:
-
Thioflavin T
References
Atcliffe BW, MacRaild CA, Gooley PR, Howlett GJ (2001) The interaction of human apolipoprotein C-I with sub-micellar phospholipid. Eur J Biochem/FEBS 268(10):2838–2846
Bergstrom J, Murphy C, Eulitz M, Weiss DT, Westermark GT, Solomon A, Westermark P (2001) Codeposition of apolipoprotein A-IV and transthyretin in senile systemic (ATTR) amyloidosis. Biochem Biophys Res Commun 285(4):903–908
Bergstrom J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, Westermark P (2004) Two different types of amyloid deposits–apolipoprotein A-IV and transthyretin–in a patient with systemic amyloidosis. Lab Invest 84(8):981–988
Bieschke J, Zhang Q, Powers ET, Lerner RA, Kelly JW (2005) Oxidative metabolites accelerate Alzheimer’s amyloidogenesis by a two-step mechanism, eliminating the requirement for nucleation. Biochemistry 44(13):4977–4983
Binger KJ, Pham CL, Wilson LM, Bailey MF, Lawrence LJ, Schuck P, Howlett GJ (2008) Apolipoprotein C-II amyloid fibrils assemble via a reversible pathway that includes fibril breaking and rejoining. J Mol Biol 376(4):1116–1129
Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P Jr, Lerner RA, Kelly JW (2006) Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol 2(5):249–253
Coriu D, Dispenzieri A, Stevens FJ, Murphy CL, Wang S, Weiss DT, Solomon A (2003) Hepatic amyloidosis resulting from deposition of the apolipoprotein A-I variant Leu75Pro. Amyloid 10(4):215–223
Griffin MD, Mok ML, Wilson LM, Pham CL, Waddington LJ, Perugini MA, Howlett GJ (2008) Phospholipid interaction induces molecular-level polymorphism in apolipoprotein C-II amyloid fibrils via alternative assembly pathways. J Mol Biol 375(1):240–256
Griffin MD, Yeung L, Hung A, Todorova N, Mok YF, Karas JA, Gooley PR, Yarovsky I, Howlett GJ (2012) A cyclic peptide inhibitor of apoC-II peptide fibril formation: mechanistic insight from NMR and molecular dynamics analysis. J Mol Biol 416(5):642–655
Gursky O, Atkinson D (1998) Thermodynamic analysis of human plasma apolipoprotein C-1: high-temperature unfolding and low-temperature oligomer dissociation. Biochemistry 37(5):1283–1291
Hanson CL, Ilag LL, Malo J, Hatters DM, Howlett GJ, Robinson CV (2003) Phospholipid complexation and association with apolipoprotein C-II: insights from mass spectrometry. Biophys J 85(6):3802–3812
Hatters DM, Howlett GJ (2002) The structural basis for amyloid formation by plasma apolipoproteins: a review. Eur Biophys J 31(1):2–8
Hatters DM, MacPhee CE, Lawrence LJ, Sawyer WH, Howlett GJ (2000) Human apolipoprotein C-II forms twisted amyloid ribbons and closed loops. Biochemistry 39(28):8276–8283
Hatters DM, Lawrence LJ, Howlett GJ (2001) Sub-micellar phospholipid accelerates amyloid formation by apolipoprotein C-II. FEBS Lett 494(3):220–224
Hatters DM, Minton AP, Howlett GJ (2002a) Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J Biol Chem 277(10):7824–7830
Hatters DM, Wilson MR, Easterbrook-Smith SB, Howlett GJ (2002b) Suppression of apolipoprotein C-II amyloid formation by the extracellular chaperone, clusterin. Eur J Biochem/FEBS 269(11):2789–2794
Hung A, Griffin MD, Howlett GJ, Yarovsky I (2008) Effects of oxidation, pH and lipids on amyloidogenic peptide structure: implications for fibril formation? Eur Biophys J 38(1):99–110
Hung A, Griffin MD, Howlett GJ, Yarovsky I (2009) Lipids enhance apolipoprotein C-II-derived amyloidogenic peptide oligomerization but inhibit fibril formation. J Phys Chem B 113(28):9447–9453
Legge FS, Treutlein H, Howlett GJ, Yarovsky I (2007) Molecular dynamics simulations of a fibrillogenic peptide derived from apolipoprotein C-II. Biophys Chem 130(3):102–113
MacPhee CE, Howlett GJ, Sawyer WH (1999) Mass spectrometry to characterize the binding of a peptide to a lipid surface. Anal Biochem 275(1):22–29
MacRaild CA, Hatters DM, Howlett GJ, Gooley PR (2001) NMR structure of human apolipoprotein C-II in the presence of sodium dodecyl sulfate. Biochemistry 40(18):5414–5421
MacRaild CA, Hatters DM, Lawrence LJ, Howlett GJ (2003) Sedimentation velocity analysis of flexible macromolecules: self-association and tangling of amyloid fibrils. Biophys J 84(4):2562–2569
MacRaild CA, Howlett GJ, Gooley PR (2004) The structure and interactions of human apolipoprotein C-II in dodecyl phosphocholine. Biochemistry 43(25):8084–8093
Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O’Brien KD, Moore KJ (2004) Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem 279(11):10643–10648
Mucchiano GI, Haggqvist B, Sletten K, Westermark P (2001a) Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J Pathol 193(2):270–275
Mucchiano GI, Jonasson L, Haggqvist B, Einarsson E, Westermark P (2001b) Apolipoprotein A-I-derived amyloid in atherosclerosis. Its association with plasma levels of apolipoprotein A-I and cholesterol. Am J Clin Pathol 115(2):298–303
Obici L, Bellotti V, Mangione P, Stoppini M, Arbustini E, Verga L, Zorzoli I, Anesi E, Zanotti G, Campana C, Vigano M, Merlini G (1999) The new apolipoprotein A-I variant leu(174) → Ser causes hereditary cardiac amyloidosis, and the amyloid fibrils are constituted by the 93-residue N-terminal polypeptide. Am J Pathol 155(3):695–702
Obici L, Franceschini G, Calabresi L, Giorgetti S, Stoppini M, Merlini G, Bellotti V (2006) Structure, function and amyloidogenic propensity of apolipoprotein A-I. Amyloid 13(4):191–205
Ryan TM, Howlett GJ, Bailey MF (2008) Fluorescence detection of a lipid-induced tetrameric intermediate in amyloid fibril formation by apolipoprotein C-II. J Biol Chem 283(50):35118–35128
Ryan TM, Teoh CL, Griffin MD, Bailey MF, Schuck P, Howlett GJ (2010) Phospholipids enhance nucleation but not elongation of apolipoprotein C-II amyloid fibrils. J Mol Biol 399(5):731–740
Ryan TM, Griffin MD, Bailey MF, Schuck P, Howlett GJ (2011a) NBD-labeled phospholipid accelerates apolipoprotein C-II amyloid fibril formation but is not incorporated into mature fibrils. Biochemistry 50(44):9579–9586
Ryan TM, Griffin MD, Teoh CL, Ooi J, Howlett GJ (2011b) High-affinity amphipathic modulators of amyloid fibril nucleation and elongation. J Mol Biol 406(3):416–429
Ryan TM, Friedhuber A, Lind M, Howlett GJ, Masters C, Roberts BR (2012) Small amphipathic molecules modulate secondary structure and amyloid fibril-forming kinetics of Alzheimer disease peptide Abeta(1–42). J Biol Chem 287(20):16947–16954
Segrest JP, De Loof H, Dohlman JG, Brouillette CG, Anantharamaiah GM (1990) Amphipathic helix motif: classes and properties. Proteins 8(2):103–117
Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM (1994) The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem 45:303–369
Stewart CR, Haw A 3rd, Lopez R, McDonald TO, Callaghan JM, McConville MJ, Moore KJ, Howlett GJ, O’Brien KD (2007a) Serum amyloid P colocalizes with apolipoproteins in human atheroma: functional implications. J Lipid Res 48(10):2162–2171
Stewart CR, Wilson LM, Zhang Q, Pham CL, Waddington LJ, Staples MK, Stapleton D, Kelly JW, Howlett GJ (2007b) Oxidized cholesterol metabolites found in human atherosclerotic lesions promote apolipoprotein C-II amyloid fibril formation. Biochemistry 46(18):5552–5561
Teoh CL, Griffin MD, Howlett GJ (2011a) Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein Cell 2(2):116–127
Teoh CL, Pham CL, Todorova N, Hung A, Lincoln CN, Lees E, Lam YH, Binger KJ, Thomson NH, Radford SE, Smith TA, Muller SA, Engel A, Griffin MD, Yarovsky I, Gooley PR, Howlett GJ (2011b) A structural model for apolipoprotein C-II amyloid fibrils: experimental characterization and molecular dynamics simulations. J Mol Biol 405(5):1246–1266
Wang G (2008) NMR of membrane-associated peptides and proteins. Curr Protein Pept Sci 9(1):50–69
Westermark P, Mucchiano G, Marthin T, Johnson KH, Sletten K (1995) Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am J Pathol 147(5):1186–1192
Wilson LM, Mok YF, Binger KJ, Griffin MD, Mertens HD, Lin F, Wade JD, Gooley PR, Howlett GJ (2007) A structural core within apolipoprotein C-II amyloid fibrils identified using hydrogen exchange and proteolysis. J Mol Biol 366(5):1639–1651
Yang S, Griffin MD, Binger KJ, Schuck P, Howlett GJ (2012) An equilibrium model for linear and closed-loop amyloid fibril formation. J Mol Biol 421(2–3):364–377
Yazaki M, Liepnieks JJ, Barats MS, Cohen AH, Benson MD (2003) Hereditary systemic amyloidosis associated with a new apolipoprotein AII stop codon mutation Stop78Arg. Kidney Int 64(1):11–16
Zdunek J, Martinez GV, Schleucher J, Lycksell PO, Yin Y, Nilsson S, Shen Y, Olivecrona G, Wijmenga S (2003) Global structure and dynamics of human apolipoprotein CII in complex with micelles: evidence for increased mobility of the helix involved in the activation of lipoprotein lipase. Biochemistry 42(7):1872–1889
Zhang Q, Powers ET, Nieva J, Huff ME, Dendle MA, Bieschke J, Glabe CG, Eschenmoser A, Wentworth P Jr, Lerner RA, Kelly JW (2004) Metabolite-initiated protein misfolding may trigger Alzheimer’s disease. Proc Natl Acad Sci U S A 101(14):4752–4757
Acknowledgments
T.M.R. is the recipient of the Alzheimer’s Australia Dementia Research Foundation Fellowship. M.D.W.G is the recipient of the C.R. Roper Fellowship and an Australian Research Council Post Doctoral Fellowship (project number DP110103528). This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ryan, T.M., Mok, YF., Howlett, G.J., Griffin, M.D.W. (2015). The Role of Lipid in Misfolding and Amyloid Fibril Formation by Apolipoprotein C-II. In: Gursky, O. (eds) Lipids in Protein Misfolding. Advances in Experimental Medicine and Biology, vol 855. Springer, Cham. https://doi.org/10.1007/978-3-319-17344-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-17344-3_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17343-6
Online ISBN: 978-3-319-17344-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)