Abstract
Preimplantation Genetic Screening (PGS) is a diagnostic technique increasingly used in assisted reproductive technologies (ART) to select chromosomally normal embryos for transfer. For several years, the gold-standard approach for PGS entailed blastomere biopsy at the cleavage stage analyzed by 9-chromosome fluorescent in situ hybridization (FISH). Unfortunately, this first version of PGS failed to show evidence of clinical effectiveness during randomized controlled trials (RCTs). This was mainly due to the limited coverage of FISH-based aneuploidy screening, the high technical error rate when used on singe cells, and the stage at which the biopsy was performed. In fact, blastomere biopsy has a significant impact on embryo development and its screening potential is affected from the phenomenon of chromosomal mosaicism, which reaches its highest incidence at this stage of embryo development, but especially by the technical issues associated with single-cell analysis. Experts started then a pursuit toward new approaches. Moving backwards along preimplantation development, polar bodies (PBs) approach also showed important limitations. In particular, a high false-positive and false-negative diagnosis rates were reported in predicting the chromosomal complement of resulting embryos. This is mainly due to the inability to assess meiosis I (MI) errors balancing at meiosis II (MII), to the relevant proportion of false-positive and false-negative predictions made on single polar bodies, and to the influence of male and especially mitotic-derived aneuploidies undetectable by PBs screening. Moreover, a detrimental impact of the biopsy on preimplantation embryo development was also reported occasionally for the PBs approach. Thus, this time-consuming and poorly cost-effective biopsy strategy is going to be abandoned in favor of blastocyst stage trophectoderm (TE) biopsy. This stage, besides ensuring a more robust genetic analysis and representing the most cost-effective approach, showed also no major impact of chromosomal mosaicism on diagnosis and no impact of the biopsy on embryo implantation potential. Once this new gold-standard approach is set, by exploiting the new technical advances in molecular analysis tools, comprehensive chromosomal screening (CCS) platforms replaced FISH, ensuring a more detailed and reliable diagnosis. All the RCTs published up to date, in different patient populations and through all the CCS platforms currently available, reported an average 30 % increase in sustained implantation rate per embryo transfer (ET) when PGS was performed, and a significant decrease in abortion rate and multiple pregnancy rate. These outcomes testify that CCS-based PGS on TE biopsy is reasonably the only promising approach. The future retains an increase in analysis throughput and a parallel costs reduction by next generation sequencing (NGS) implementation in PGS. A further increase in embryo developmental competence assessment will possibly derive from the novel -omics approaches and the new evidences they will bring about. These new cutting-edge technologies will hopefully help us increasing our predictive power on embryo implantation potential in in vitro fertilization (IVF).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Aneuploidies represent a major barrier for human reproduction, in particular throughout the preimplantation development window when they reach their highest incidence and can affect any chromosome of the karyotype. It is well known that, while in newborn population their incidence is relatively low (approximately 0.3 %) and mainly due to trisomies for the chromosomes 13, 18, and 21 and sex chromosomes’ copy number variation, aneuploidies are responsible for more than 45 % of all spontaneous abortions [1]. When looking at the preimplantation window where no selection mechanisms against the development of chromosomally abnormal embryos are in place, the most recent evidences suggested that the incidence of aneuploidies reaches its highest values. A natural selection against aneuploid embryos from the preimplantation period onward prevents them from resulting in a live birth. These evidences highlighted how most of the couples attending an IVF treatment are subject to a significantly high risk of transferring chromosomally abnormal embryos and that aneuploidies can reasonably be considered as the single most important factor associated with implantation failure and miscarriage during IVF treatments. PGS theory was conceived in this scenario with the ultimate aim of selecting euploid embryos for transfer.
In this context, the main goal of an ideal PGS strategy should be to obtain the same efficacy as conventional IVF, namely the same live birth rate per cycle, while significantly increasing the overall efficiency on an IVF treatment, that is, minimizing related efforts and risks. When an effective PGS strategy is implemented in IVF programs, then many advantages can be expected, ranging from increased sustained implantation rate, because euploid embryos are supposed to implant at a higher rate compared to chromosomally abnormal embryos, and a significant decrease in abortion rate and in the occurrence of abnormal pregnancies. Importantly, the implementation of PGS might lead to adopt a single ET policy also in poor prognosis patient avoiding any kind of obstetrical and neonatal complication associated with multiple pregnancies. Furthermore, it is expected that in PGS programs a lower time to pregnancy can be obtained, since non-useful and potentially detrimental ETs will be avoided. However, it is evident that from the time PGS was theorized in the early 1990s, throughout years, several issues have arisen and have been solved in a progressive evolution of the technique. A fruitful cooperation between embryologists and molecular biologists has represented an important breakthrough, which increased our knowledge of this field of science. Across years different molecular diagnostic techniques, such as several stages of embryo preimplantation development to retrieve the cellular material to be tested, have been investigated. In order to identify a gold-standard approach, all the proposed ones have been thoroughly studied and some of their advantages and/or disadvantages have been described. In particular, the initial gold-standard protocol for PGS clinical application entailed blastomere biopsy at the cleavage stage, namely on day 3 of embryo development, and its analysis by 9-chromosome FISH.
Unfortunately, PGS failed to keep its promises by adopting this approach. In fact, Mastenbroek and colleagues [2] performed a comprehensive meta-analysis of the main nine RCTs produced in order to investigate the clinical effectiveness of PGS and demonstrated that, especially for advanced maternal age (AMA) patients, which theoretically should be the ones benefiting the most from the diagnosis, this technique actually lowered the live birth rate per stimulation cycle. This evidence held the attention on the inefficacy of such an intriguing theory. Supporters of PGS worldwide started to investigate then the causes of such a failure, and concerns were attributed mainly to technical aspects of the procedure. The potential harm to the embryo deriving from the biopsy itself, the biological and genetic features of cleavage stage embryos, and the remarkable limitations of FISH as molecular diagnostic technique especially when applied on single cells were all considered alarming issues. Thus, they started a pursuit toward different stages of preimplantation development to retrieve the biopsy material, such as the first and the second PBs from the oocyte or few cells from the TE at the blastocyst stage. Furthermore, new CCS techniques replaced the limited 9-chromosome FISH, thus extending the possibilities of diagnosis to the whole karyotype. This chapter aims at providing a comprehensive review of the literature focused on these issues, which has been produced in the last years.
Blastomere Biopsy at the Cleavage Stage
Single blastomere analysis is affected by all the concerns related with single-cell diagnostics. From a technical perspective, several artifacts compromising the reliability of the diagnosis can be introduced, thus potentially causing false-positive and false-negative results. In particular, these artifacts can turn out in erroneous copy number assessments, since few loci or whole chromosomes could be under- or overamplified [3] and can be listed as follows: (1) allele drop-out (ADO), namely random loss of alleles; (2) preferential allocation (PA), namely over-amplification of specific genomic region or even a whole chromosome; (3) allele drop-in (ADI), which is an artifact of whole genome amplification substituting an allele with another one; (4) chimerical DNA molecules formation; and (5) failure of DNA amplification occurring more often. Furthermore, none of the contemporary methods for single-cell analysis can distinguish between a cell in G1-, S-, or G2/M-phase of the cell cycle. This can inevitably determine biological false-negative/positive results, in case a cell would be at a specific point of the S-phase of the cell cycle, thus normally replicating the DNA, when it is retrieved for the analysis [4].
Another biological concern acquiring a paramount importance when conducting PGS on a single blastomere at the cleavage stage resides in the phenomenon of chromosomal mosaicism, namely the coexistence of two or more karyotypically different cell lines in the same embryo. Mitotic chromosome errors are responsible for this phenomenon and could be induced mainly by three mechanisms: anaphase lagging, non-disjunction, and structural events of DNA damage of chromatid/chromosome breakage leading to structural rearrangements (e.g., duplications, translocations) [5, 6]. An impressive influence of mosaicism up to 70 % in preimplantation embryos has been reported in some previous studies [7–10]. However, technical variation due to the reasons previously examined in this paragraph could have determined an overestimation of its real incidence. For instance, different papers showed a considerable number of false-positive results when adopting FISH to analyze single blastomere biopsy in comparison with microarray techniques [11, 12], while Mertzanidou and colleagues [13] did not report any meiotic error by analyzing all the blastomeres from 14 normally developing embryos through array Comparative Genomic Hybridization (aCGH). Such a possibility results unlikely and it supplies further evidence that single-cell analysis is not reliable enough, even though CCS by microarray techniques is adopted to perform the diagnosis. Although even if chromosomal mosaicism could potentially affect any stage of embryo preimplantation development and a proper evaluation of its incidence could not be made so far, from a biological perspective it is likely to reach its highest level at the cleavage stage.
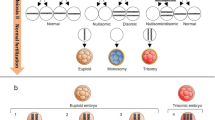
In fact, the origin of mosaicism resides in the early mitotic divisions of cleavage stage embryos. In this time period, the cell cycle control is carried out by the maternal transcripts still present in the ooplasm, but some checkpoint mechanisms are missing a proper control until embryonic genome activation [14]. Finally, mosaic euploid embryos are also likely to self-correct by blastocyst stage [15–17], thus leading to an increased risk of false-positive diagnosis by cleavage stage PGS. Chromosome demolition, non-disjunction, or anaphase lag have been proposed as mechanisms to explain this self-correction, and also a better proliferative rate of euploid cells (or apoptosis of aneuploid ones) may explain this phenomenon [3] (Fig. 7.1).
Evolution of chromosomal mosaicism along embryo preimplantation development. Chromosomal mosaicism is likely to reach its highest level along preimplantation development at the cleavage stage. Its origin is thought to mainly reside in the chromosomal segregation errors occurring during the first mitotic divisions of the early embryo, when cell cycle is under the control of maternal transcripts still present in the ooplasm and some checkpoints are missing a proper regulation. Only at the morula stage, after zygote genome activation, these processes will be reactivated and come mosaic embryos will be prevented from reaching to the blastocyst stage. Furthermore, the embryo can undergo events leading self-correction, so that the incidence of mosaicism at the blastocyst stage has been estimated as ~21 %. However, just ~4 % of the blastocyst are subject to a risk of misdiagnosis, since this is the estimated percentage of mosaic diploid/aneuploid embryos at this stage of preimplantation development showing a mosaic error as the only aneuploidy. As reported in different papers, high-grade mosaic blastocysts, where the incidence of aneuploidies exceeds 40 % of the cells, are likely to be diagnosed as aneuploid during blastocyst stage PGS cycle, thus preventing the transfer of embryos that might have a negative clinical impact on pregnancy
Another consideration when evaluating the effectiveness of a PGS approach is the potential for the harmful effect of the biopsy itself on embryo developmental competence. The only unbiased assessment of this aspect for cleavage stage biopsy was reported by Scott and colleagues [18] in 2013, demonstrating that even a single blastomere removal is sufficient to compromise embryo implantation potential, thus highlighting another noteworthy issue of performing PGS at the cleavage stage. In particular, they ideated an elegant prospective blinded non-selection study. Only double ETs were performed and among the two transferred embryos only one underwent blastomere biopsy before transfer. If a single embryo implanted, DNA fingerprinting was exploited to assess whether it was the biopsied one or not. When comparing the implantation rate of biopsied embryos versus control non-biopsied ones, a significant 19 % relative decrease in implantation rate due to biopsy procedure was reported. All these evidences taken together strongly suggested that the main limitations of cleavage stage PGS could be ascribed to the technical issues of single-cell analysis and to the detrimental effect of the biopsy procedure. No RCT at the moment has been published to assess the clinical effectiveness of this method when used in conjunction with CCS methods. Thus, despite the highest worldwide experience and despite the fact that it is still the mostly used method for PGS and PGD worldwide [19], cleavage stage biopsy is going to be gradually abandoned to explore new approaches, while FISH-based screening has been already replaced by novel comprehensive methods largely more accurate.
Polar Body Approach
Failure to conceive and pregnancy loss, in both natural conception and IVF, are mainly caused by chromosome aneuploidies, whose occurrence exponentially increases with advancing female age [20]. Molecular analyses performed after natural conception or spontaneous miscarriage highlighted that trisomies arise mainly due to an impaired female meiosis, in particular the first meiotic division [21]. Thus, fertility decrease with increasing maternal age is basically ascribable to aneuploidies’ increase due to an oocyte aging issue. This is mainly determined by the long-lasting arrest in the prophase of MI, which ranges from fetal life up to oocyte recruitment for final maturation, occurring between the menarche and the menopause. Unfortunately, despite the unique possibility to perform PGS without directly operating on the embryo, which makes of PB-based PGS the only practice ethically acceptable in some countries, and its compatibility with fresh ET after molecular diagnosis, this approach soon showed important limitations.
One study in particular shed light on the main drawbacks of PBs approach [17]. It was designed as a sequential biopsy associated with aCGH analysis of PBs, blastomere, and TE from the same embryo, which led to an elegant and comprehensive view on chromosomal segregation patterns from female meiosis and throughout preimplantation development up to the blastocysts stage. A unique possibility to infer the etiology of aneuploidies in AMA patient population as well as the accuracy of PB-based chromosome screening in predicting the chromosomal complement of resulting embryos was provided. The results reported in this study firstly confirm the inefficiency of PB1-only approach, because both PBs are needed since approximately half of female-derived aneuploidies in the embryos arose as a consequence of errors originating during the second meiotic division. With the inclusion of the PB2 data, more accurate information inferring oocyte chromosome copy number can be obtained. However, the inability to assess MI errors balanced at MII, the relevant proportion of meiotic errors selected against and corrected during preimplantation development, and the influence of male and mitotic-derived aneuploidies were all important source of errors described in the study and representing inescapable pitfalls of this approach which are sensibly compromising its reliability in predicting embryos’ chromosomal complement.
In this scenario, these intrinsic and technical limitations of PB analysis may result in one case in discarding viable embryos, while in the other in transferring abnormal ones. It is also worth highlighting that Handyside and colleagues found a consistent proportion (21.1 %) of chromosome segregation errors detected as copy number changes in the PBs not resulting in the expected outcome in the corresponding zygote [22], which is also similar to what reported by Capalbo and colleagues in the study previously described. Moreover, also in a recent paper published by Christopikou and colleagues, 17 % of false-positive PB results were observed based on the follow-up analysis performed on the resulting embryos [23]. As previously mentioned, one of the major concerns of PB-based PGS relates to the difficulties in detecting precocious sister chromatid errors balancing in MII that was shown to be one of the major mechanisms contributing to female-derived aneuploidies in embryos [17, 22]. In the matter of this, Forman and colleagues demonstrated in a good prognosis patient population that when reciprocal aneuploidy occurs from MI premature separation of sister chromatids and compensation in MII, the resulting embryo is usually normal for that chromosome [24]. The same authors also showed in a different paper that most of these embryos could result in a chromosomally normal child after ET [25]. Thus, future studies are required to assess whether these embryos should be reanalyzed or the signal intensity of the data is reliable enough to distinguish between chromatid and whole chromosome impairments. In particular, the threshold values should be prospectively set by reanalyzing cases in which PB reciprocal aneuploidies occurred and making blinded predictions of the chromosomal status of the embryo.
At present, all the data reported so far in the scientific literature consistently demonstrate a low accuracy of PB approach in predicting the actual copy number configuration in the embryo and that reciprocal aneuploidies in PBs inevitably require a follow-up analysis in the resulting embryo. Therefore, many concerns related to whether the accuracy achievable using PB screening is good enough to improve the IVF clinical outcome still remain [17, 26, 27]. Another major problem related to PB biopsy is the paucity of material that is available. In our hands as well as in the practice of other qualified centers, around 10 % of the oocytes tested remain without a conclusive diagnosis because of amplification failure in at least one of the two PBs [28]. If PGS aims at improving IVF outcomes, it is crucial that results are obtained from all embryos tested. Economic and logistic issues should then be also considered. In particular, PBs screening results as the most time-consuming and least cost-effective among PGS approaches and it is also independent from oocyte developmental potential, since part of the analyzed oocytes/zygote will never reach to the blastocyst stage and be transferred. In synthesis, all these evidences together resulted in the breakdown of PBs approach, while some investigators proposed to move the biopsy stage forward along preimplantation development. They suggested blastocyst stage TE biopsy, arguing that several advantages could be brought from this novel intriguing approach [29]. Several efforts have been invested then in order to highlight the concrete possibilities offered by this breakthrough and to uncover its technical and clinical opportunities.
Trophectoderm Biopsy at the Blastocyst Stage
Blastocyst stage PGS on TE biopsy ensued the sharp failure of cleavage stage PGS on blastomere biopsy and the betrayed promises of PBs biopsy ones. Understandably, the same issues concerning these previous strategies were moved against this different approach. Thus, in order to prove its efficiency, a fruitful series of evidences were produced and published in literature up to date and more studies are currently in the pipeline. Hereafter, a review of the main evidences reported up to date will be provided. At first it was solved the doubt about a possible impact of the biopsy on embryo developmental potential. A particular concern dealt with the risk of decreasing the pregnancy rate per started cycle by postponing the time of the transfer beyond the cleavage stage, that is, extending embryo culture to the blastocyst stage. This issue in particular arises from the risk of embryo developmental arrest either at the time of compaction or at the time of cavitation, which, especially in poor prognosis patients, can reduce the pool of blastocysts to be screened for aneuploidies and potentially transferred. However, in the scientific literature there is absolutely no evidence that transferring embryos at the cleavage stage can result in higher pregnancy rate per stimulation cycle in poor prognosis patients compared to the use of blastocyst transfer policy and, reasonably, extended culture has not been considered to lead to embryo waste. In this regard, Guerif and colleagues [30] reported on an RCT highlighting that, in a poor prognosis patient population, the pregnancy rates per stimulation cycle was similar after both fresh ETs and frozen ETs when using a cleavage or blastocyst stage ET policy. This suggested that the extension of the culture to the latest stage of preimplantation development does not reduce the number of live births after IVF.
Blastocyst biopsy can be performed in two different ways. In the first one described by Schoolcraft and colleagues in 2010, a zona opening is made at the cleavage stage to prompt TE cells herniation on day 5 or 6 and to facilitate the biopsy procedure [31]. The main drawbacks of this method relate to the extra manipulation of embryos at the cleavage stage, especially in the current trends of contemporary IVF culture that are exploiting closed culture systems from fertilization to the blastocyst stage, and to the risk of Inner Cell Mass (ICM) herniation that may require a second hole in the zona pellucida. A different method for TE biopsy has been then recently described by our group not requiring zona breaching and avoiding any potential stress at the cleavage stage, as well as allowing a more physiological growth of embryos to the blastocyst stage [32]. As far as the impact of biopsy on embryo development is concerned, Scott and colleagues reported, in the same elegant and powerful study previously mentioned in this chapter, no significant differences in implantation rate between untested biopsied and non-biopsied blastocysts [18]. It is not clear whether this is ascribable to a smaller proportion of total cells removed from the blastocyst, to a higher stress tolerance of the blastocyst with respect to other stages of preimplantation development, or to the preservation of the ICM counterpart which originates the fetus, but still this represents a further advantage of postponing the time of the biopsy.
A crucial point of discussion is the accuracy of the analysis and the information that can be obtained from a randomly selected TE sample biopsied at the blastocyst stage. Certainly, blastocyst stage TE biopsy ensures a more accurate assessment of meiotic aneuploidies than previous strategies, since between five and ten cells are retrieved and analyzed from the embryo compared to the analysis of a single cell that is commonly performed on blastomeres and PBs. This translates in a significant reduction of the incidence of all the misdiagnosis risks derived from a single-cell analysis. In fact, all confirmation studies reported so far based on FISH reanalysis of aneuploid blastocysts following TE biopsy and CCS found between 98 and almost 100 % of correct aneuploidies prediction of meiotic errors [33–35]. Also, in a recent study from our group, we provided the first assessment of the reliability of blastocyst stage aneuploidy screening by the analysis of multiple TE biopsies from the same blastocyst with the use of different CCS methods [36]. The analysis was based on the real-time quantitative Polymerase Chain Reaction (qPCR) blinded reanalysis of 120 s biopsies of aneuploid blastocysts previously screened by TE aCGH and showed a consistent chromosome copy number diagnosis in 99.4 % (2,561/2,576; 95 % CI 99.0–99.7) of the chromosomes analyzed. The remaining 0.6 % was due to either technical variation between CCS techniques or occasionally by biological variation due to the presence of chromosomal mosaicism.
The impact of mosaicism on the reliability of the diagnosis, and especially the possibility of a nonrandom allocation of chromosomally abnormal cells exclusively to TE in case of mosaicism [37–39], is in fact another important point to be considered when blastocyst biopsy is performed on randomly selected TE cells. To this end, high concordance between ICM and TE chromosomal complement has been reported in the most recent literature [11, 35, 40], suggesting no preferential allocation of abnormal cells in a mosaic blastocyst, and that the analysis of a TE sample can be considered diagnostic of the ICM. In order to properly perform this analysis, our group conceived and published a novel method of ICM biopsy, which led to the total absence of TE cells contamination [35]. In the same paper, a preliminary aCGH analysis on a TE biopsy during blastocyst stage PGS cycle was performed, which was followed by a FISH reanalysis of three further TE fragments and of the whole ICM from those embryos diagnosed as aneuploid. The ultimate aim of this study design was to define the real influence of mosaicism on the accuracy of the diagnosis. Constitutional aneuploidies were reported in 79.1 % of cases, while mosaic in 20.9 % of cases. However, the real risk of an uncertain diagnosis due to mosaicism when testing at the blastocyst stage accounts for only 4 % of aneuploid blastocysts that were detected to be mosaic diploid/aneuploid (embryos showing a mosaic error as the only aneuploidy).
A very interesting finding of this study was that all cases of high-grade diploid/aneuploid mosaicism where abnormal cells constituted more than 40 % of the blastocysts were diagnosed as aneuploid by the original blastocyst stage aneuploidy screening cycle. This data suggested that blastocyst stage PGS performed on a randomly selected TE sample is able to avoid also the transfer of mosaic embryos with a very high prevalence of abnormal cells that might have a poor clinical outcome on pregnancies (Fig. 7.1). Indeed it is well known that, when compatible with life, mosaicism can be associated with poor fetal outcomes and neonatal morbidity. Looking at mosaicism data in prenatal diagnosis, the overall incidence of mosaicism is very low and reported to range between 1.22 % and 1.32 % after spontaneous pregnancies and after IVF care, respectively, where true mosaicism accounted only for 0.3–0.44 % of pregnancies [41]. These data suggest that the incidence of mosaicism has been overestimated in preimplantation genetics but also that mosaic embryos can be subjected to a negative selective pressure following ET resulting in failure of implantation or early embryo loss, and at last highlight the potential benefit of blastocyst stage PGS to detect and avoid the transfer of mosaic embryos with a high prevalence of abnormal cells. However, even though all these preclinical studies were sufficient to assess no impairment of implantation potential following TE biopsy and the high diagnostic accuracy and reliability of a CCS approach for embryonic aneuploidy, they were not sufficient themselves to determine whether the test has a true clinical value.
Thus, in order to demonstrate whether a clinical benefit results from application of aneuploidy screening at the blastocyst stage and to determine the specific magnitude of this benefit, four RCTs were published up to date using different CCS methods and investigating the use of this approach in different patient populations [31, 42–44]. Taken together, all these RCTs consistently reported an increased sustained implantation and live birth rate (relative increase between 28 and 40 % with respect to the control group) following the transfer of euploid blastocyst compared to the transfer of untested embryo, suggesting that blastocyst stage aneuploidy screening can be considered today a validated technology to improve embryo selection and clinical outcome per transfer in IVF. What is still missing are RCTs evaluating live birth rate per stimulation cycle to assess whether blastocyst stage PGS can result also in similar efficacy compared to standard care according to an intention to treat analysis. A final interesting argument of discussion deals with the implementation of this PGS approach in clinical practice. Regarding this point from an economic and logistic perspective, blastocyst stage PGS on TE biopsy, conversely to previous strategies and especially to PBs biopsy one, represents the most convenient and easy to implement approach. This is mainly due to the fact that only developmentally competent embryos would reach to this stage, while incompetent ones will arrest at previous stages of development. Thus, only reproductively competent embryos will be screened for aneuploidies. This results in PGS cost reduction with the considerable advantage of being able to increase the patient population that can benefit from this technology during their IVF cycle. To summarize, blastocyst stage PGS approach has passed thorough several preclinical and clinical validation steps and fulfilled so far all the requirements that we may expect from an ideal PGS strategy.
Conclusion
Extensive progresses have been made since PGS was conceived in the early 1990s. The long and difficult pathway that was undertaken up to date conduced to the definition of a new gold-standard approach for PGS entailing CCS platforms-based analysis on TE biopsy at the blastocyst stage. The failure of PGS as it was performed at first did not weaken the conviction of its value. In fact, all the different levels of evidence reported in literature and reviewed here proved the efficiency of this last approach against all the issues causing the failure of previous strategies (Fig. 7.2). Nowadays, we can confidently sustain that CCS-based PGS on TE biopsy is the closest approach to an ideal PGS strategy that is currently available. In the last years, the implementation of this strategy in IVF labs was delayed by the low worldwide experience in blastocyst culture and vitrification protocols. Nowadays instead, a strong impulse to increase the IVF laboratories’ experience with blastocyst culture, handling, and cryopreservation will derive from the application of the freeze-all approach and of the cycle segmentation theory [45], which are increasingly being recognized as effective approaches in IVF. In particular, cycle segmentation theory entails a GnRH agonist triggering in a GnRH antagonist cycle as stimulation protocol, which was reported as free from the risk of ovarian hyperstimulation syndrome (OHSS), associated with oocyte and/or blastocyst vitrification in order to perform ET on a receptive endometrium in a natural cycle, thus also reducing the risk of extrauterine pregnancies and improving obstetrical and neonatal outcomes of IVF-derived pregnancies [46]. Conducting CCS on TE biopsy entails the further advantage to be totally integrated in this protocol. In fact, vitrified euploid elective single embryo transfer (eSET) could be performed following this approach, consequently escaping the risk of multiple pregnancies, while increasing implantation rate and decreasing abortion rate per ET. Next progresses in PGS will be mainly technical advances dealing with a reduction of costs and a parallel increase of throughput. In this regard, NGS-based PGD and PGS represent the most promising tools to be implemented in this field. However, even though PGS conducted through NGS can in prospect become accessible to every couple approaching an IVF cycle and with suitable indications to it, we should pay attention not to be excessively optimistic because several technical and ethical considerations should be made about this technique. In particular, an exhaustive genetic counseling will be required in order to thoroughly describe all the possible advantages and limitations of this novel and potentially higher throughput platforms, especially at the beginning of its implementation in preimplantation genetics. Now that the optimal stage of biopsy to accurately detect chromosomally abnormal embryos has been identified, the future challenges will deal with the implementation of embryo evaluation methods beyond aneuploidy screening to further enhance selection among euploid blastocysts. Noticeably, neither blastocyst morphological grade nor embryo developmental rate to the blastocyst stage do significantly correlate with implantation potential of euploid embryos [32], suggesting that the commonly used parameters of blastocyst evaluation are not good indicators to achieve this aim. Thus, future researches are required to identify noninvasive biomarkers of reproductive potential and to further enhance selection beyond euploidy assessment.
Comparison and level of evidence of effectiveness between biopsy strategies to perform PGS. PB polar body,TE trophectoderm, FP/FN false-positive/false-negative, RCT randomized controlled trial, ADO allele drop-out, PA preferential allocation, ADI allele drop-in, ICM inner cell mass, ET embryo transfer, CCS comprehensive chromosomal screening. [1] Levin et al., Fertil 2012 [47]; (2) Scott et al., Fertil Steril 2013 [18]; (3) Capalbo et al., Hum Reprod 2013a [17]; (4) Handyside et al., Eur J Hum Genet, 2012 [22]; (5) Capalbo et al., Hum Reprod 2013b [35]; (6)Schoolcraft et al., Fertil Steril 2010 [31]; (7) Yang et al., Hum Cytogenet, 2012 [42]; (8) Schoolcraft et al., Fertil Steril 2011 [43]; (9) Forman et al., Fertil Steril 2013 [44]. The biopsy strategy to be adopted in order to perform PGS should ensure the absence of a detrimental impact on embryo development, a reliable and informative diagnosis, and clinical evidences of its effectiveness. Furthermore, an easy implementation in the IVF lab in terms of low workload and high cost-effectiveness of the procedure should also be considered. Currently, according to the data reported in literature up to date, TE biopsy is the only approach fulfilling these criteria and thus it should be considered as a gold-standard approach to perform preimplantation aneuploidy screening
Several studies are currently in the pipeline aiming at investigating the correlation between implantation and the -omic sciences world. Genomic, transcriptomic, exomic, methylomic, miRNomic, proteomic, metabolomic, and spent culture media analysis studies represent an unexplored source of knowledge that can help us filling the gap between failure and success of a PGS cycle. If we expect mainly a decrease in costs and an increase in throughput from new advances in the field of CCS-based PGS, the prospect to further increase the outcomes of a PGS cycle resides in these new tools of analysis. The future in blastocyst developmental competence assessment is yet to come and we expect it to be fruitful.
References
Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13(7):493–504.
Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17(4):454–66.
Johnson DS, Cinnioglu C, Ross R, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16(12):944–9.
Van der Aa N, Cheng J, Mateiu L, et al. Genome-wide copy number profiling of single cells in S-phase reveals DNA-replication domains. Nucleic Acids Res. 2013;41(6), e66.
Coonen E, Derhaag JG, Dumoulin JC, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19(2):316–24.
Daphnis DD, Delhanty JD, Jerkovic S, Geyer J, Craft I, Harper JC. Detailed FISH analysis of day 5 human embryos reveals the mechanisms leading to mosaic aneuploidy. Hum Reprod. 2005;20(1):129–37.
Munné S, Sultan KM, Weier HU, Grifo JA, Cohen J, Rosenwaks Z. Assessment of numeric abnormalities of X, Y, 18, and 16 chromosomes in preimplantation human embryos before transfer. Am J Obstet Gynecol. 1995;172(4 Pt 1):1191–9.
Wells D, Delhanty JD. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod. 2000;6(11):1055–62.
Voullaire L, Slater H, Williamson R, Wilton L. Chromosome analysis of blastomeres from human embryos by using comparative genomic hybridization. Hum Genet. 2000;106(2):210–7.
Bielanska M, Tan SL, Ao A. Chromosomal mosaicism throughout human preimplantation development in vitro: incidence, type, and relevance to embryo outcome. Hum Reprod. 2002;17(2):413–9.
Northrop LE, Treff NR, Levy B, Scott Jr RT. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod. 2010;16(8):590–600.
Treff NR, Su J, Tao X, Levy B, Scott Jr RT. Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94(6):2017–21.
Mertzanidou A, Wilton L, Cheng J, et al. Microarray analysis reveals abnormal chromosomal complements in over 70 % of 14 normally developing human embryos. Hum Reprod. 2013;28(1):256–64.
Los FJ, Van Opstal D, van den Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update. 2004;10(1):79–94.
Baart EB, Van Opstal D, Los FJ, Fauser BC, Martini E. Fluorescence in situ hybridization analysis of two blastomeres from day 3 frozen-thawed embryos followed by analysis of the remaining embryo on day 5. Hum Reprod. 2004;19(3):685–93.
Munné S, Velilla E, Colls P, et al. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil Steril. 2005;84(5):1328–34.
Capalbo A, Bono S, Spizzichino L, et al. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum Reprod. 2013;28(2):509–18.
Scott Jr RT, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100(3):624–30.
Moutou C, Goossens V, Coonen E, et al. ESHRE PGD Consortium data collection XII: cycles from January to December 2009 with pregnancy follow-up to October 2010. Hum Reprod. 2014;29(5):880–903.
Heffner LJ. Advanced maternal age—how old is too old? N Engl J Med. 2004;351(19):1927–9.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–91.
Handyside AH, Montag M, Magli MC, et al. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet. 2012;20:742–7.
Christopikou D, Tsorva E, Economou K, et al. Polar body analysis by array comparative genomic hybridization accurately predicts aneuploidies of maternal meiotic origin in cleavage stage embryos of women of advanced maternal age. Hum Reprod. 2013;28:1426–34.
Forman EJ, Treff NR, Stevens JM, et al. Embryos whose polar bodies contain isolated reciprocal chromosome aneuploidy are almost always euploid. Hum Reprod. 2013;28:502–8.
Scott Jr RT, Treff NR, Stevens J, et al. Delivery of a chromosomally normal child from an oocyte with reciprocal aneuploid polar bodies. J Assist Reprod Genet. 2012;6:533–7.
Angell RR. Possible pitfalls in preimplantation diagnosis of chromosomal disorders based on polar body analysis. Hum Reprod. 1994;9(2):181–2.
Scriven PN, Ogilvie CM, Khalaf Y. Embryo selection in IVF: is polar body array comparative genomic hybridization accurate enough? Hum Reprod. 2012;27(4):951–3.
Geraedts J, Montag M, Magli MC, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011;26(11):3173–80.
McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84(6):1628–36.
Guerif F, Lemseffer M, Bidault R, et al. Single day 2 embryo versus blastocyst-stage transfer: a prospective study integrating fresh and frozen embryo transfers. Hum Reprod. 2009;24(5):1051–8.
Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. 2010;94(5):1700–6.
Capalbo A, Rienzi L, Cimadomo D, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Fragouli E, Alfarawati S, Daphnis DD, et al. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2011;26(2):480–90.
Novik V, Moulton EB, Sisson ME, et al. The accuracy of chromosomal microarray testing for identification of embryonic mosaicism in human blastocysts. Mol Cytogenet. 2014;7(1):18.
Capalbo A, Wright G, Elliott T, et al. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013;28(8):2298–307.
Capalbo A, Ubaldi FM, Rienzi L, Tao X, Treff NR, Scott Jr RT. Comparison of quantitative real-time (q)PCR and array comparative genomic hybridization (aCGH) based 24 chromosome aneuploidy screening in human blastocysts. Fertil Steril. 2013;100(3):S2.
Mottla GL, Adelman MR, Hall JL, Gindoff PR, Stillman RJ, Johnson KE. Lineage tracing demonstrates that blastomeres of early cleavage-stage human pre-embryos contribute to both trophectoderm and inner cell mass. Hum Reprod. 1995;10(2):384–91.
Evsikov S, Verlinsky Y. Mosaicism in the inner cell mass of human blastocysts. Hum Reprod. 1998;13(11):3151–5.
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15(8):1781–6.
Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod. 2008;23(11):2596–608.
Huang A, Adusumalli J, Patel S, Liem J, Williams III J, Pisarska MD. Prevalence of chromosomal mosaicism in pregnancies from couples with infertility. Fertil Steril. 2009;91(6):2355–60.
Yang Z, Liu J, Collins GS, Salem SA, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, Scott Jr RT. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96(3):638–40.
Forman EJ, Hong KN, Ferry KM, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7.
Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593–7.
Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98(6):1490–4.
Levin I, Almog B, Shwartz T, Gold V, Ben-Yosef D, Shaubi M, Amit A, Malcov M. Effects of laser polar-body biopsy on embryo quality. Fertil Steril. 2012;97(5):1085–8.
Conflict of Interest
The authors declare no conflict.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Capalbo, A., Cimadomo, D., Rienzi, L., Ubaldi, F.M. (2015). Comprehensive Chromosomal Screening from Polar Body Biopsy to Blastocyst Trophectoderm Sampling: Evidences and Considerations. In: Sills, E. (eds) Screening the Single Euploid Embryo. Springer, Cham. https://doi.org/10.1007/978-3-319-16892-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-16892-0_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16891-3
Online ISBN: 978-3-319-16892-0
eBook Packages: MedicineMedicine (R0)