Abstract
In addition to being potent chaperones that protect cells against the accumulation of unfolded proteins under stress conditions, mammalian small heat shock proteins (small Hsps) regulate many vital cellular processes in normal and pathological cells. Indeed, these Hsps are constitutively expressed in many tissues and show dramatic changes in their levels of expression in most human pathologies. They are characterized by a large spectrum of activities and are particularly active in protein conformational and inflammatory diseases as well as in cancer pathologies. It is now believed that the immense cellular implications of small Hsps results from their ability to interact, through particular structural changes, with many different client proteins that are subsequently modulated in their activities or half-lifes. Here, we have integrated functionally and structurally the recent data in the literature concerning the interactions of mammalian small Hsps with specific clients. Further analysis with geneMANIA software and database confirmed the incredibly large number of functions associated with these Hsps. The consequences for human pathologies as well as putative therapeutic strategies are discussed, particularly when the expression of small Hsps is harmful (as in some cancer pathologies) or when it appears beneficial for patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mammalian small Hsps
- Oligomeric complexes
- Clients
- Protein interactomes
- Protein predictomes
- Cellular implications
- Pathologies
1 Many Functions Associated with Small Hsps in Addition to Their Protective Role in Stress Condition
The last decade has been characterized by an incredible jump in the interest in the ten mammalian small Hsps. Indeed, until the turn of the century, these stress proteins were considered as exotic chaperones that did not use ATP for their activity. These “forgotten chaperones”, as they were called in 2002 (Solari and Garrido 2002), are now stars among Hsps to judge by the large number of scientific and medical publications dealing with their particular behaviors and functions that fill the current literature. This renewed interest is probably linked to their constitutive expression in normal and pathological conditions as well as to the large number of unrelated functions associated with their over- or under-expression in many different cell types. Interest has also been generated by the growing number of pathological mutations in their genes that induce degenerative or myopathic diseases and by their newly described ability to be secreted.
1.1 Stress Conditions, Chaperone Activity and Anti-aggregation Properties
Early studies dealing with HspB1 and HspB5 revealed their enhanced expression under heat shock conditions as well as their ATP-independent chaperone property (Jakob et al. 1993; Rogalla et al. 1999). It was shown that large oligomeric structures formed by small Hsps store stress-altered polypeptides in a refolding competent state that can interfere with their propensity to aggregate (Bellyei et al. 2007; Carra et al. 2005; Ehrnsperger et al. 1997, 2000; Ganea 2001; Haslbeck et al. 2005; Horwitz et al. 1992; Jakob et al. 1993; Lee et al. 1997; Markossian et al. 2009). These altered polypeptides can subsequently be refolded by the ATP-dependent Hsp70, Hsp90 and co-chaperones “foldase” machines (Buchner 1999; Bukau and Horwich 1998; Freeman and Morimoto 1996; Lee and Vierling 2000) or degraded by the CHIP-ubiquin-26S proteasome machine (McDonough and Patterson 2003). The dynamic oligomerization/phosphorylation status of small Hsps, and particularly HspB1, is an essential factor of this process (Arrigo et al. 1988; Lelj-Garolla and Mauk 2005, 2006; Paul et al. 2010; Preville et al. 1998b; Rogalla et al. 1999; Simon et al. 2013). The cytoskeleton is one of the primary targets protected by HspB1 and HspB5 in response to stress (Bellomo and Mirabelli 1992; Welch and Feramisco 1985) as well as in normal growth conditions. This property probably relies, at least in the case of HspB1, on the fact that phosphorylated small HspB1 oligomers modulate F-actin fiber growth and, indirectly, extracellular matrix organization (Dalle-Donne et al. 2001; Mounier and Arrigo 2002; Perng et al. 1999). Under stress conditions, HspB1 and HspB5 stabilize microtubules (Hino et al. 2000; Preville et al. 1996; Xi et al. 2006). HspB5 is also very active in maintaining intermediate filaments homeostasis, particularly in muscle cells where it associates with desmin (Bennardini et al. 1992; Djabali et al. 1999). Moreover, HspB1 and HspB5 share an intriguing anti-oxidant property which appears linked to the chaperoning of several anti-oxidant enzymes, particularly G6PDH (glucose 6-phosphate dehydrogenase) (Arrigo 2001, 2007b, 2013; Arrigo et al. 2005; Firdaus et al. 2006a; Mehlen et al. 1996a; Paul and Arrigo 2000; Preville et al. 1998a, 1999; Rogalla et al. 1999; Yan et al. 2002). Consequently, damage such as protein and nucleic acid oxidation as well as lipid peroxidation is reduced and the positive effect of these Hsps towards mitochondrial ΔΦm increases ATP levels, which favors the activity of ATP-dependent chaperones (Mehlen et al. 1996a; Preville et al. 1999).
Only HspB1, HspB5 and HspB8 molecular chaperones are induced under stress conditions. Interestingly, constitutively expressed small Hsps, such as HspB2, HspB3, HspB4, HspB6 and HspB7, also display chaperone activities or at least anti-aggregation and pro-degradative functions (Carra et al. 2013). The anti-aggregation and anti-fibrillation properties of mammalian small Hsps are summarized in Table 2.1. Depending on the substrate, some Hsps perform these tasks better than others, suggesting that they do not all have the same chaperone-like activity. For example, HspB4 can chaperone HspB5 once in the alpha-crystallin complex (Andley 2007), while HspB3 (Asthana et al. 2012) and HspB2 exhibit significant chaperone-like activity towards specific target proteins and can attenuate the ordered amyloid fibril formation of α-synuclein (Prabhu et al. 2012). The major substrates recognized by small Hsps can be mutated polypeptides that cause degenerative or myopathic diseases (i.e. desmin, polyQ proteins, SOD, α-synuclein) or proteins that are prone to aggregate. It is also important to mention that small Hsp mutants can induce the aggregation of their substrates, such as the R120G missense mutation in HspB5 which is genetically linked to a desmin-related myopathy consequently of the aggregation of desmin (Bova et al. 1999; Vicart et al. 1998). Similarly, the P182L mutant of HspB1 leads to motor neuronopathies as a result of the formation of aggregates that sequestrate Neurofilament middle chain subunit (NF-M) and p150 Dynactin (Ackerley et al. 2005). Equally, proteins that interact with mutant small Hsps can counteract aggregation, as for example the chaperone-like effect of Bag3 towards aggregated HspB8 mutant (Hishiya et al. 2011). As a result of its interaction with Bag3, HspB8 also has the ability to trigger macroautophagy (Carra 2009; Carra et al. 2008b). This favors the elimination of aggregated polypeptides generated by heat (Nivon et al. 2009) or oxidative stress (Keller et al. 2004; Kiffin et al. 2006). Interestingly, HspB6 also appears to play a role in the Bag-3/HspB8 complex that triggers macroautophagy (Fuchs et al. 2010). Less information is available concerning HspB9 and HspB10 in spite of their ability to interact with particular polypeptides (see Table 2.2).
1.2 Enormous Cellular Implications Associated with Constitutively Expressed Small Hsps
Mammalian small Hsps are expressed in the absence of apparent stress in specific tissues of developing and adult organisms as well as in pathological conditions (Arrigo 2012b; Bhat and Nagineni 1989; Gernold et al. 1993; Huang et al. 2007; Klemenz et al. 1993; Mymrikov et al. 2011; Quraishe et al. 2008; Srinivasan et al. 1992; Tanguay et al. 1993). For example, HspB1 and HspB6 are highly abundant in muscles. However, the overall tissue distribution of these two proteins is different since HspB6 is specific to muscles (Seit-Nebi and Gusev 2010) while HspB1 is expressed in almost all tissues. Similarly, HspB5, which forms with HspB4 the lens alpha-crystallin complex is also expressed in the heart, skeletal muscle fibers, brain and kidney while HspB4 is also present in pancreas. In contrast, HspB9 and HspB10 are restricted to testis expression (de Wit et al. 2004; Yang et al. 2012). Other important points concern the expression of these proteins in pathological conditions as well as the drastic effects (neuropathies, myopathies, cardiomyopathies, cataracts) induced by some of their mutations (i.e. mutations in HspB1, HspB3, HspB4, HspB5, HspB6 and HspB8) (Benndorf et al. 2014; Kwok et al. 2011; Mymrikov et al. 2011; Vicart et al. 1998). So, what is the function of these Hsps in specific tissues? (see Sect. 2.1.2.1).
1.2.1 Small Hsps Client Concept
The recent literature is quite abundant in descriptions of new functions associated with constitutively expressed small Hsps. Moreover, each small Hsp appears to have its own panel of activities (Fig. 2.1). An intriguing point is the unrelated nature of those activities distributed in almost all essential cellular pathways or activities, from cytoskeleton homeostasis to signal transduction pathways, gene expression and cell death (see Fig. 2.1). To understand why so many activities are associated with small Hsps, we must first explain their particular structural organization. Indeed, these proteins share, as a result of their crystallin homology, complex oligomeric structures that allow for the formation of dynamic homo and hetero-oligomeric structures (from 50 to >700 kDa, depending on the small Hsps) (Arrigo 2007a; 2011, Arrigo et al. 1988; Basha et al. 2011; Garrido 2002; Simon et al. 2013). Moreover, phosphorylation plays a key role in the case of HspB1, HspB5 and HspB4. These Hsps bear several serine sites phosphorylated by specific kinases, including stress and MAP kinases. Another key parameter is the cellular environment that modulates, in a dynamic and reversible way, the oligomeric organization and phosphorylation of some of these proteins, such as HspB1 (Arrigo et al. 1988; Arrigo 2000, 2007b, 2011; Arrigo and Gibert 2012; Bruey et al. 2000b; Mehlen and Arrigo 1994; Mehlen et al. 1997a; Paul et al. 2010). This suggests an intracellular sensor activity associated with small Hsps that can record changes in cellular environment. For example, HspB1 reorganizes differently its phosphorylation and oligomerization status in cells exposed to different apoptotic inducers (Paul et al. 2010). What could this mean? Since HspB1 is an anti-apoptotic protein its structural changes could instruct the cell to choose the best strategy to counteract the effects of a particular apoptotic inducer. How can this be done? Do small Hsps have multiple enzymatic activities because of their complex oligomeric organization, and are they thus pleotropic polypeptides, or are they acting via chaperone-like activities towards other polypeptides? Recently published reports revealed that the novel activities of small Hsps often correlate with their ability to interact with different polypeptides. Hence, could the apparent pleotropic effects of small Hsps be indirect and, as previously described for Hsp90 (Georgakis and Younes 2005; Neckers et al. 1999), result from the modulation of the activity and/or half-life of many clients? (list of Hsp90 clients: http://www.picard.ch/downloads). To clarify this point, we analyzed three polypeptides pro-caspase-3, HDAC6 and STAT-2 interacting with HspB1 in HeLa cells and discovered that their half-life was greatly enhanced by interacting with HspB1 (Gibert et al. 2012a), which confirmed that, in the same cell, HspB1 can recognize different protein clients. The updated list of the major proteins interacting with mammalian small Hsps and the cellular consequences mediated by these interactions is presented in Table 2.2, see also (Arrigo 2013; Arrigo and Gibert 2012, 2013; Ciocca et al 2013). Clients are listed according to their activity in major cellular functions, such as transduction pathways, apoptosis, protein degradation, translation, transcription, cytoskeletal organization and homeostasis or cell adhesion. When available, information is given about the structural organization of small Hsps or their corresponding clients involved in the interactions. The little information already available confirms the important role played by the oligomerization and phosphorylation patterns of small Hsps. Several consequences can result from small Hsps/clients interactions, such as modulation of half-life, enzymatic activity, structural organization or modification of the client. For example, some clients interact with HspB1 to increase their half-life and thus avoid their rapid proteolytic degradation (Her2 oncogene, pro-caspase 3, HDM2, the histone deacetylase HDAC6, Androgen Receptor AR and the transcription factors STAT-2 and STAT-3) while the opposite effect occurs for the rapidly degraded PTEN polypeptide when it is bound to HspB1. The transcription factor HSF1 is sumoylated as a result of its interaction with HspB1 coupled to the Ubc-9 like sumoylating enzyme UBE21. Moreover, some cellular effects mediated by small Hsps are well known but the targeted proteins are still not defined. One striking example is the modulation of the TAK-1 inflammation pathway by HspB8 (see Table 2.2).
Two major questions arise from these observations: (i) what are the cellular consequences induced by the interaction of small Hsps to so many protein targets and (ii) how do small Hsps recognize client protein targets?
-
(i)
Concerning the first question one can easily conclude by analyzing Table 2.2 that small Hsps modulate the maturation and activity of a wide range of client proteins including regulators of the life and death of the cell and signal transducer polypeptides, such as kinases and transcription factors. Therefore, by regulating a large repertoire of cellular functions small Hsps have a huge importance on normal biology, disease and evolutionary processes. Hence, as does Hsp90 (McClellan et al. 2007; Moulick et al. 2011; Taipale et al. 2010), these Hsps appear as global regulators of cell systems through their chaperone/client interactome systems. However, it is difficult to obtain a realistic view of the global cellular consequences generated by small Hsps interactomes. To meet this challenge we have performed protein interaction networks analysis using the geneMANIA software and database (Warde-Farley et al. 2010) (http://www.genemania.org/). This web interface shows the relationships between gene products and predicts their functional association in biological processes, pathways or diseases. Such data can help elucidate cellular pathways, create functional links between gene products and diseases, and can enable investigators to extract significantly more information about the cellular impact generated by the expression of small Hsps than by relying solely on primary literature (Table 2.2). However, care must be taken when using these data since some interactions are only predicted. An example presented in Fig. 2.2 illustrates the proteins interacting with HspB1, HspB5, HspB6 and HspB8. Only 100 proteins interacting with the four Hsps are analyzed, so some clients mentioned in Table 2.2 are not listed while new ones are mentioned. Nevertheless, this analysis further confirms that small Hsps interact with a wide spectrum of polypeptides and consequently modulate many different cellular pathways, as for example those dealing with protein kinases, gene expression, cell adhesion and migration, cell death, catabolic processes, responses to stimulation, confirming their broad implications in cell biology.
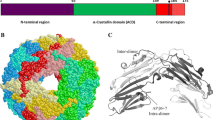
Fig. 2.2 Human HspB1, HspB5, HspB6 and HspB8 protein interactomes and predictomes as proposed by GeneMANIA software and database including BioGRID and PathwayCommons. Analyzed Hsps are indicated in black while interacting proteins are in grey. Physical interactions (red lines) and predicted (orange lines) ones were analyzed. The software was set to analyze up to hundred gene products and at most hundred related attributes. Automatically selected weighting method. Predicted interactions could be for instance, two proteins known to interact in another organism, such as S. cerevisiae. Abbreviations: CRYAB HspB5, CRYAA HspB4, HSPA8 heat shock 70 kDa protein 8, HSPH1 heat shock 105 kDa/110 kDa protein 1, DNAJB1 DnaJ (Hsp40) homolog, subfamily B, member 1, CRYGC crystallin, gamma C, CRYBB2 crystallin, beta B2, CRYZ crystallin, zeta (quinone reductase), F13A1 coagulation factor XIII, A1 polypeptide, BAG3 BCL2-associated athanogene 3, CS citrate synthase, POP7 processing of precursor 7, ribonuclease P/MRP subunit (S. cerevisiae), STAT-3 signal transducer and activator of transcription 3 (acute-phase response factor), SPARCL1 SPARC-like 1 (hevin), RAD51 RAD51 homolog (S. cerevisiae), SPARC secreted protein, acidic, cysteine-rich (osteonectin), USP38 ubiquitin specific peptidase 38, BCL2L1 BCL2-like 1, MAPKAPK5 mitogen-activated protein kinase-activated protein kinase 5, CRYBA1 crystallin, beta A1, TAGLN3 transgelin 3, CASP3 caspase 3, apoptosis-related cysteine peptidase, BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase), CYCS cytochrome c, somatic, MAPKAPK2 mitogen-activated protein kinase-activated protein kinase 2, TGFB1I1 transforming growth factor beta 1 induced transcript 1, YWHAG tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, gamma polypeptide, PSMA3 proteasome subunit, alpha type, 3, MAPKAPK3 mitogen-activated protein kinase-activated protein kinase 3, POLR2D polymerase (RNA) II (DNA directed) polypeptide D, TAGLN2 transgelin 2, PLCG2 phospholipase C, gamma 2 (phosphatidylinositol-specific), PYROXD1 pyridine nucleotide-disulphide oxidoreductase domain 1, TGM1 transglutaminase 1 (K polypeptide epidermal type I, protein-glutamine-gamma-glutamyltransferase), USP1 ubiquitin specific peptidase 1, EIF4G1 eukaryotic translation initiation factor 4 gamma, 1, HNRNPD heterogeneous nuclear ribonucleoprotein D (AU-rich element RNA binding protein 1, 37 kDa), PRKCE protein kinase C, epsilon, HSPG2 heparan sulfate proteoglycan 2, PRKAA1 protein kinase, AMP-activated, alpha 1 catalytic subunit, DMWD dystrophia myotonica, WD repeat containing, PRKD1 protein kinase D1, ILK integrin-linked kinase; MAGED1 melanoma antigen family D, 1, SAP18 Sin3A-associated protein, 18 kDa, GIT1 G protein-coupled receptor kinase interacting ArfGAP 1, MAPK3 mitogen-activated protein kinase 3, MAGEA6 melanoma antigen family A, 6, BRF2 BRF2, subunit of RNA polymerase III transcription initiation factor, BRF1-like, CCNK cyclin K, IGSF21 immunoglobin superfamily, member 21, MME membrane metallo-endopeptidase, PSMD4 proteasome 26S subunit, non-ATPase, 4, PSMD6 proteasome 26S subunit, non-ATPase, 6, TTN titin, CIAO1 cytosolic iron-sulfur protein assembly 1, DAXX death-domain associated protein, EPB41 erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked), PPA1 pyrophosphatase (inorganic) 1, ACTC1 actin, alpha, cardiac muscle 1. AKT1 v-akt murine thymoma viral oncogene homolog 1, KCNMA1 potassium large conductance calcium-activated channel, subfamily M, alpha member 1, LNX1 ligand of numb-protein X, MED31 mediator complex subunit 31, C7orf64 chromosome 7 open reading frame 64, NFKBIA nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, SLC2A4 solute carrier family 2 (facilitated glucose transporter) member 4, TP53 tumor protein p53, TSC22D1 TSC22 domain family, member 1, ALDH18A1 aldehyde dehydrogenase 18 family, member A1, AMOT angiomotin, APP amyloid beta (A4) precursor protein, BAG1 BCL2-associated athanogene, BBC3 BCL2 binding component 3, BCL2L11 BCL2-like 11 (apoptosis facilitator), BRCA2 breast cancer 2, early onset, COL15A1 collagen, type XV, alpha 1, COL3A1 collagen, type III, alpha 1, CSNK1D casein kinase 1, delta, CSNK1E casein kinase 1,epsilon, CSNK2A1 casein kinase 2, alpha 1 polypeptide, CST3 cystatin C, F13B coagulation factor XIII, B polypeptide, FIGN fidgetin, HDAC1 histone deacetylase 1, LALBA alpha-lactalbumin, LRIF1 ligand dependent nuclear receptor interacting factor 1, MDH2 malate dehydrogenase 2, NAD (mitochondrial), MIP major intrinsic protein of lens fiber, MND1 meiotic nuclear divisions 1 homolog (S. cerevisiae), PIAS3 protein inhibitor of activated STAT-3, PRKCA protein kinase C, alpha RAD51AP1: RAD51 associated protein 1, RPP25 ribonuclease P/MRP 25 kDa subunit, SLX4 SLX4 structure-specific endonuclease subunit homolog (S. cerevisiae), SRRM2 serine/arginine repetitive matrix 2, VEGFA vascular endothelial growth factor A
-
(ii)
As for the second question, we believe that small Hsps act as does Hsp90 to recognize clients by taking advantage of a variety of conformational states to interact with co-chaperones and clients (Hessling et al. 2009; Mickler et al. 2009). Compared to other mammalian small Hsps, HspB1 has the most dynamic phospho-oligomeric organization, a property that could explain its ability to recognize a large number of protein clients probably through the rapid generation of interacting platforms (Arrigo and Gibert 2012, 2013; Ciocca et al. 2013; Gibert et al. 2011, 2012a; Paul et al. 2010). Consequently, HspB1 dynamic interactome may allow cells to respond quickly and mount the most effective response to a particular condition. However, an unanswered question is how small Hsps generate specific interacting platforms to act on client repertoire. At least in the case of HspB1, the phenomenon may depend on the complex patterns of MAPKAPK2,3-dependent phosphorylation of three serines sites located in the N-terminal domain of HspB1 (Arrigo and Gibert 2012, 2013; Paul et al. 2010; Rouse et al. 1994; Simon et al. 2013; Stokoe et al. 1992). Our recent observations favor this hypothesis since in growing HeLa cells pro-caspase-3 interacts mainly with the serine 15 phosphorylated small oligomers of HspB1 while HDAC6 is recovered at the level of the large serine 82 phosphorylated oligomers. In contrast, STAT-2 binds to the medium and large sized HspB1 oligomers (Arrigo and Gibert 2013; Gibert et al. 2012a). Thus, in growing HeLa cells, the specific phospho-oligomeric organization of HspB1 consists of signaling structures that recognize and bind at least three different polypeptides and subsequently modulate their half-life. This observation confirms the hypothesis that the dynamic structural plasticity of small Hsps structure can lead to at least 300 different stoichiometries that favor the recognition of many particular target proteins (Stengel et al. 2010).
An increased complexity arises by taking into account another fundamental property of small Hsps. Once they are expressed in the same cells, they have the ability to interact with each other and form multiple combinatorial oligomeric structures (Table 2.3, see also Arrigo 2013; Bukach et al. 2009; den Engelsman et al. 2009 #3479; Saha and Das 2004; Simon et al. 2007; Zantema et al. 1992). Since interaction between two small Hsps mutually affects the structure and chaperone activity of both partners (Aquilina et al. 2013; Bukach et al. 2009; den Engelsman et al. 2009; Gibert et al. 2013; Mymrikov et al. 2012; Simon et al. 2013; Skouri-Panet et al. 2012), it cannot be excluded that the chimeric oligomers can recognize novel client proteins and/or are unable to bind those interacting with parental small Hsps. Moreover, not all sHsps interact equally efficiently with each other in vitro (Mymrikov et al. 2012). In that respect, the molecular ratio between small Hsp partners is often different (e.g. 3:1 in the case of HspB4:HspB5 and HspB2:HspB3 complexes). In vivo, the phenomenon is probably even more complex since modifications, such as phosphorylation, which depend on the type of cell considered and its physiology are of prime importance (Paul et al. 2010; Simon et al. 2013). For example, in cells expressing an equimolar ratio of HspB1 and HspB5, only 90 % of HspB1 forms chimeric molecules with HspB5. This enhances the phosphorylation of the remaining 10 % of non interacting HspB1 which can now recognize a new client, G6PDH, and can stimulate its detoxicant enzymatic activity (Table 2.4) (Arrigo 2013). Unfortunately, no clear data are yet available concerning the protein targets recognized by chimeric small Hsps (Table 2.4), as for example in the case of HspB2:HspB3 complex involved in the development of muscle cells. Similarly, it is not known whether Bag3, which interacts with HspB8 and HspB6, can bind to HspB8:HspB6 complex to modulate autophagy. Another important consequence of the above mentioned property of small Hsps is the dominant effect of a mutated small Hsp that can dramatically spread between other interacting members of the family (Diaz-Latoud et al. 2005; Fontaine et al. 2006; Simon et al. 2013). These pathological interactions can also lead to the accumulation of cytoplasmic protein aggregates linked to diseases.
2 Examples Illustrating the Broad Spectrum of Positive or Negative Roles of Small Hsps in Human Pathologies
Nowadays, the medical literature is filled with reports explaining that the level of expression of small Hsps is highly modulated, as they are often upregulated in pathological conditions such as protein conformational disorders (neurodegenerative diseases, myopathies, cataracts), inflammatory diseases and cancers. Many functions were attributed to HspB1 and HspB5 and, probably due to their more recent discovery, less frequently to the other small Hsps. As mentioned above, these proteins probably act by interacting with pathology specific clients. Based on earlier observations, we proposed that the upregulation of these proteins had a negative effect (for the patient) in cancer pathologies while it was positive in the case of degenerative diseases (Arrigo and Simon 2010; Arrigo 2005; Arrigo et al. 2007). The most recent studies have complicated this hypothesis since, as described below (Sect. 2.2.3), one small Hsp can be beneficial in one type of cancer and harmful in another. In fact, from a patient point of view, the major effects mediated by these interactions will depend on the friendly or hostile nature of the interacting clients. Thus, more work is needed to increase our knowledge of the pathology-dependent clients that interact with small Hsps, and future therapeutic interventions will have to be carefully planned to avoid dramatic off-target effects for patients.
2.1 Degenerative Diseases
2.1.1 Protective Role of Small Hsps
Elevated levels of Hsps, such as HspB1, HspB5 and high molecular weight Hsps, are observed in cells with altered protein folding homeostasis as a result of the expression of proteins prone to aggregate or fibrillate (see Table 2.1). Hence, high levels of these Hsps are observed in cortical Lewy bodies, Alzheimer’s disease plaques containing β-amyloid peptide, granules of neurones expressing polyQ mutants of Huntingtin polypeptide, Rosenthal fibers of Alexander disease, Creutzfeldt-Jakob altered neurons, neurofibrillary tangles, α-synuclein deposit associated with Parkinson’s disease, SOD1 aggregates in amyotrophic lateral sclerosis, myopathy-associated inclusion body such as muscle cells expressing mutated desmin as well as in neurones from cerebral ischemia or heart cells altered by myocardial infarction or atrial fibrillation (Bruinsma et al. 2011; Brundel et al. 2008; Goldfarb et al. 2004; Muchowski 2002; Muchowski and Wacker 2005; Renkawek et al. 1994; Wyttenbach 2004; Yerbury et al. 2012). In these cells, HspB1 and HspB5 trigger a beneficial protection by reducing the formation of pathological protein aggregates (Eaton et al. 2000; Efthymiou et al. 2004; Latchman 2005; Lewis et al. 1999). Protective activity has recently been reported for other small Hsps, such as HspB2, HspB3, HspB6, HspB7, HspB8 (Bruinsma et al. 2011; Brundel et al. 2008; Carra et al. 2005, 2008a; Ke et al. 2011; Vos et al. 2010). However, these Hsps are effective in their own way in counteracting protein aggregation or fibrillation. For example HspB7, which, unlike HspB1, does not improve the refolding of heat-denatured polypeptides, is nevertheless the most efficient small Hsp in suppressing polyQ aggregation and polyQ-induced cellular toxicity (Vos et al. 2010). Taken together these observations lead to the conclusion that small Hsps are beneficial proteins that interfere with pathological processes leading to neurodegenerative, myopathic, cardiomyopathic, cataract and retinal diseases (Andley 2007; Firdaus et al. 2006a; Lee et al. 2006; Outeiro et al. 2006; Perrin et al. 2007; Wilhelmus et al. 2006a, b; Wyttenbach et al. 2002). This conclusion was further supported by mutations which inhibit the chaperone activity of HspB1, HspB3, HspB4, HspB5, HspB6 and HspB8 and provoke pathological diseases, such as amyotrophic lateral sclerosis (ALS), axonal Charcot-Marie-Tooth disease, inherited peripheral and motor neuropathies, myofibrillar myopathies, cardiomyopathies and cataracts (Ackerley et al. 2005; Benndorf et al. 2014; Bova et al. 1999; Datskevich et al. 2012; Dierick et al. 2007; Elicker and Hutson 2007; Evgrafov et al. 2004; Kijima et al. 2005; Vicart et al. 1998). However, depending on the clients that are recognized by these Hsps, the consequences of their mutations will vary, with HspB1, HspB3, and HspB8 causing motor neuropathies, while HspB5 induces particular myopathies called αB-crystallinopathies (Benndorf et al. 2014).
2.1.2 Oxidative Stress Generated by Aggregated Polypeptides
In addition to their anti-aggregation and fibrillation properties the fact that at least HspB1 and HspB5 can act as anti-oxidant molecules (Arrigo 1998, 2013; Arrigo et al. 2005; Chen et al. 2006; Firdaus et al. 2006a, b; Mehlen et al. 1996a; Wyttenbach et al. 2002) is of prime importance as it can counteracts some of the harmful effects induced by aggregated polypeptides. Indeed, a disregulated intracellular redox leading to permanent oxidative conditions is a common feature observed in many degenerative diseases and in cells bearing aggregated polypeptides (Bharath et al. 2002; Browne et al. 1999; Choi et al. 2005; Firdaus et al. 2006b; Fox et al. 2007; Halliwell 2001; Jenner and Olanow 1996; Tabner et al. 2001; Turnbull et al. 2003). This phenomenon is a consequence of Huntingtin, β-amyloid and α-synuclein being metal homeostasis modulating or direct iron/copper binding polypeptides (Hilditch-Maguire et al. 2000; Huang et al. 2004). Hydroxyl radical over-production through the metal-mediated alteration of the hydroxyl radical generating Fenton reaction is thus a common feature of cells containing these aggregated polypeptides (Halliwell and Gutteridge 1984; Sayre et al. 2000 #1935; Shoham and Youdim 2000). Hydroxyl radicals stimulate protein aggregation and interfere with proteasome function (Firdaus et al. 2006a, b; Janue et al. 2007; Liu et al. 2006; Wyttenbach et al. 2002). These observations lead to the conclusion that some small Hsps, as HspB5 (Bjorkdahl et al. 2008; Ousman et al. 2007), could be considered as therapeutic agents to treat degenerative diseases.
2.2 Inflammation
HspB1 is essential for both IL-1 and TNF-induced pro-inflammatory signaling pathways leading to the expression of pro-inflammatory genes, such as cyclooxygenase-2, IL-6, and IL-8 (Alford et al. 2007). Increased cyclooxygenase-2 and IL-6 expression appears to occur through the stabilisation of their respective mRNAs as a result of the enhanced activation of the kinase downstream of p38 MAPK, MK2 by HspB1. The client(s) targeted by HspB1 to perform this task are still unknown, but may reside at the level or more upstream of the pivotal kinase TAK1. This study also shows that in this context many signaling events depend on HspB1, such as downstream signalling by p38 MAPK, JNK and their activators (MKK-3, -4, -6, -7) and IKKβ. In that respect, it is worth noting that HspB1 can interact with the activating kinases IKKα and IKKβ of the transcription factor NF-κB (Dodd et al. 2009). Another role has been proposed for HspB1 through its association with the AUF1- and signal transduction-regulated complex, ASTRC, that regulates mRNA degradation machinery. This could lead to a mechanism that combines proinflammatory cytokine induction with monocyte adhesion and motility (Sinsimer et al. 2008). HspB5 also plays several roles in inflammation. The first one describes HspB5 as a new regulator of leukocyte recruitment, through its ability to enhance NF-κB pro-inflammatory signaling pathways and the expression of endothelial adhesion molecule during endothelial activation (Dieterich et al. 2013). No putative client has yet been described to support this activity. The second activity concerns a role for HspB5 as an extracellular protein (see Sect. 2.2.4) and deals with its ability, when added to the plasma of patients suffering of multiple sclerosis, rheumatoid arthritis, and amyloidosis as well of mice with experimental allergic encephalomyelitis, to interact with some relative apparent selectivity with at least 70 different pro-inflammatory mediators (acute phase proteins, members of the complement cascade, and coagulation factors) (Rothbard et al. 2012) (see Table 2.2). Of great interest, the presence of exogenous HspB5 decreased inflammation as a result of a reduced concentration of these mediators. Using a similar approach, another study points to the activation of an immune-regulatory macrophage response and inhibition of lung inflammation using HspB5-loaded microparticles (van Noort et al. 2013). These observations, as well as that of Kurnellas et al. (2012), confirm that exogenous HspB5 could be used as an anti-inflammation therapeutic agent. HspB1 and HspB5 also have beneficial protective roles against inflammation since their anti-oxidant properties may favor their interference with tumor necrosis factor (TNFα) signaling pathways, as observed in the case of asthma (Alford et al. 2007; Kammanadiminti and Chadee 2006; Mehlen et al. 1995; Merendino et al. 2002). Taken together, these observations suggest crucial, but different, roles for HspB1 and HspB5 in inflammatory processes.
2.3 Cancers
Multiple molecular alterations are key characteristics of most cancer cells. However, an overall view of the major proteins involved in oncogenic signaling pathways is currently beyond reach. In that respect, small Hsps are among the proteins whose expression is altered in cancer cells. It is now well recognized that they have key roles in cancer biology as a result of their interaction with specific clients that modulate tumor development through their activity at the level of apoptosis, mitotic signaling pathways, angiogenesis, cell escape and survival, senescence, epithelial-to-mesenchymal transition (EMT) and metastasis (Arrigo and Gibert 2014). In recent years, the major small Hsps reported to play important roles in cancer pathologies were HspB1 and HspB5 (Arrigo 2007a; Arrigo and Simon 2010; Arrigo and Gibert 2014; Arrigo et al. 2007; Calderwood et al. 2006; Ciocca and Calderwood 2005). Recent observations now include HspB4, HspB6 and HspB8 as well as the intriguing dual pro- and anti-tumorigenic properties of some small Hsps.
2.3.1 Pro-tumorigenic Effects of Small Hsps
Elevated levels of expression of HspB1 and HspB5 were the first indicators of the putative role of small Hsps in some cancer cells. It was first discovered that a high level of expression of these proteins protects against apoptotic death (Mehlen et al. 1996b) and is pro-tumorigenic (Garrido et al. 1998). Recent studies have analyzed their mode of action favoring tumor development.
2.3.1.1 Protection Against Cell Death, Apoptosis
Protection against apoptotic cell death by HspB1 was discovered in 1996 (Mehlen et al. 1996b, 1997b; Samali and Cotter 1996). This property suggested that the high level of expression of HspB1 observed in many cancer cells could promote carcinogenesis, tumor maintenance and dissemination, an assumption demonstrated two years later (Garrido et al. 1998). HspB1 anti-apoptotic property is a consequence of its interaction with many client proteins in the initiation and execution phases of apoptosis (Arrigo 2012a; Arrigo and Gibert 2014; Ciocca et al. 2013). In fact, based on the signal transduction-dependent dynamic reorganization of its phosphorylation and oligomerization status (Paul et al. 2010; Rogalla et al. 1999), HspB1 can interact with the more appropriate clients to counteract apoptotic processes. This leads to the hypothesis that HspB1 has multiple strategies to counteract inducer-specific intrinsic and extrinsic apoptosis (Arrigo 2011; Paul et al. 2010). For example, by acting towards F-actin and t-Bid translocation, HspB1 reduces cytochrome c (Paul et al. 2002) and Smac-diablo (Chauhan et al. 2003) release from mitochondria. In addition, it also decreases apoptosome and caspase-9 activation by a direct interaction with cytosolic cytochrome c (Bruey et al. 2000a; Garrido et al. 1999). A surprising effect occurs at the level of procaspase-3 whose activation is negatively regulated by phosphorylated small oligomers of HspB1 (Arrigo and Gibert 2013; Gibert et al. 2012a; Pandey et al. 2000). In the meantime, HspB1 increases procaspase-3 half-life by down-regulating its degradation by the ubiquitin-proteasome machinery (Gibert et al. 2012a). Among the death receptor pathways that are under the control of HspB1 are Fas, TNFα and Trail (Mehlen et al. 1995, 1996b; Zhuang et al. 2009). In the Fas signal transduction mechanism, phosphorylated dimers of HspB1 abolished the link between activated Fas receptor and apoptotic signaling kinase1 (Ask1) by interacting with DAXX (Charette et al. 2000). The protection against TNFα mediated transduction death signal is less well documented. Nevertheless, HspB1 may protect cells directly through the classical apoptotic machinery and/or its ability to interfere with the oxidative stress generated by this inflammatory cytokine (Mehlen et al. 1995, 1996a). In contrast (see below section “Stimulation of cell survival pathways, senescence”), the inhibitory effect of HspB1 against Trail induced death does not appear to occur at the level of the apoptotic machinery but rather through the stimulation a cell survival mechanism (Qi et al. 2014).
HspB5 and HspB4 have also been reported as anti-apoptotic proteins (Andley et al. 2000; Kamradt et al. 2005) and several reports mention their action towards tumorigenicity (Arrigo 2007a; Chen et al. 2012; Kase et al. 2009; Mahon et al. 1987; Rigas et al. 2009). Their anti-apoptotic modes of action differ from that of HspB1, however. Indeed, in addition to their action towards caspase-3, these Hsps negatively regulate members of the Bcl-2 family, Bcl-XL, Bcl-XS and Bax, as well as cytoplasmic p53 by interfering with their redistribution into mitochondria in apoptotic conditions (Hu et al. 2012; Liu et al. 2007; Mao et al. 2004). HspB5 was also shown to modulate p53 level (Watanabe et al. 2009). Moreover, both HspB4 and HspB5 can prevent apoptosis through interactions with clients involved in regulating signaling Raf/MEK/ERK and PKCαlpha pathways (Liu et al. 2004). Moreover, HspB5 modulates the activity of XIAP, an endogenous inhibitor of caspases (Lee et al. 2012), and inhibits RAS activation responsive to the calcium-activated Raf/MEK/ERK signaling pathway mediated p53-dependent apoptosis (Li et al. 2005). HspB5 expression can also be correlated with pERK1/2 expression (van de Schootbrugge et al. 2013b). However, it is important to note that these particular properties are usually tissue specific; for example, in pancreatic cancer cells HspB4 has a surprising opposite effect and acts as a negative regulator of carcinogenesis (Deng et al. 2010) (see below section “Anti-tumorigenic Effects”). HspB5 also protects retinal pigment epithelial cells through its association with HDAC1 on SC35 speckles (Noh et al. 2008), which suggests that HspB5 knockout could be beneficial to vitreoretinopathy therapy.
It is also interesting to note that 14-3-3 polypeptide is a client of phosphorylated HspB6. Hence, this Hsp can compete with the large number of regulator proteins interacting with 14-3-3 and indirectly modulate many cellular processes, such as those involved in actin cytoskeleton reorganization or Bad mediated apoptosis (Chernik et al. 2007; Seit-Nebi and Gusev 2010; Sluchanko et al. 2011; Zha et al. 1997).
2.3.1.2 Stimulation of Cell Survival Pathways, Senescence
HspB1 still appears as being the major small Hsp involved in the stimulation of cell survival pathways through its interaction with specific clients. Among those pathways, the Akt signaling cascade is a major one which includes key factors such as Akt, PI3K, PTEN, mitogen-activated protein kinase kinase-3,6, BAD and Forkhead transcription factors. In cancer cells, high expression levels of HspB1 result in its interaction with Akt and PTEN. HspB1 action towards Akt kinase activity and the stimulation of the degradation of the phosphatase PTEN stimulate the PI3K/Akt signaling pathway and thus enhance the survival of these pathological cells (Cayado-Gutierrez et al. 2012; Rane et al. 2003; Wu et al. 2007). An interesting survival pathway also modulated by HspB1 is the PEA-15 molecular switch linking cell proliferation to Fas-induced apoptosis. In that regard, the interaction of HspB1 with PEA-15 inhibits Fas-induced apoptosis and promotes cell survival and proliferation (Hayashi et al. 2012). Another example concerns the Src-Akt/ERK pro-survival signaling transduction triggered by TRAIL death receptor. Analysis of the molecular mechanism revealed that phosphorylated HspB1 activates the pathway by interacting with Src and by scaffolding protein beta-arrestin2 (Qi et al. 2014). The signaling complex made of phospho-HspB1/beta-arrestin2/Src appears therefore to be responsible for activating the TRAIL-triggered Src-Akt/ERK pro-survival pathway. HspB1 also appears to act in signaling pathways promoting survival of gliomas, but the molecular mechanism is not yet known (Golembieski et al. 2008; McClung et al. 2012).
In addition to improving cell survival, HspB1 has a p53 dependent negative action towards the oncogene-induced senescence (OIS) pathway which normally blocks cancer progression (O’Callaghan-Sunol et al. 2007). Indeed, HspB1 depletion usually induces a senescent-like phenotype in cancer cells. Among the morphological changes that were observed one can note a drastic reduction in the mitotic index through induction of p21waf expression (O’Callaghan-Sunol et al. 2007) and a particular cellular multi-nucleation which appears to be the result of the degradation of HDAC6 (Gibert et al. 2012a), an HspB1 client acting as a powerful contributor to oncogenic pathways activation (Lee et al. 2008). HDAC6 is proteolytically stabilized by HspB1 serine 82 phosphorylated oligomers (Arrigo and Gibert 2013; Gibert et al. 2012a). Among the other clients and/or pathways effective in supporting the negative effect of HspB1 towards senescence are the p53 stabilizator HDM2, an ubiquitin ligase (E3) that targets p53 for degradation (O’Callaghan-Sunol et al. 2007; Yang et al. 2005) and the PI3K/AKT induced OIS (Ghosh et al. 2013).
2.3.1.3 Cell Escape, Epithelial-to-Mesenchymal Transition (EMT), Metastasis
In addition to counteracting cell death and promoting cell survival pathways, HspB1 and HspB5 have been shown to bear tumorigenic (Garrido et al. 1998, 2006) and pro-metastatic (Bausero et al. 2006; Lemieux et al. 1997; Nagaraja et al. 2012b) properties. In that regard, several clients interacting with these proteins have been identified (Arrigo and Gibert 2014) that are particularly active at the level of the cytoskeleton and extracellular matrix (Arrigo and Gibert 2013; Gibert et al. 2012a; Lavoie et al. 1993; Mounier and Arrigo 2002; Perng et al. 1999; Wettstein et al. 2012; Xi et al. 2006). For example, in cancer cells, HspB1 is necessary for F-actin mediated cytokinesis and interferes with the accumulation of giant polynucleated cells (Gibert et al. 2012a). Another important client interacting with both HspB1 and HspB5 is β-catenin (Fanelli et al. 2008; Ghosh et al. 2007c) and the resulting effect is a modulation of cadherin-catenin cell adhesion proteins (Fanelli et al. 2008). At least in the case of HspB1, the interaction plays a crucial role in promoting tumor growth. Among the other clients of HspB1, one can cite several metalloproteinases (Bausero et al. 2006; Xu et al. 2006) as well as SPARC (secreted protein, acidic and rich in cysteine), a polypeptide that plays an important role in cell adhesion and migration (Golembieski et al. 2008; McClung et al. 2012; Schultz et al. 2012). In several cancer pathologies, HspB5 also promotes cell migration and invasion. For example, HspB5 induces the EGF- and anchorage-independent growth of human breast basal-like tumors through the constitutive activation of the MAPK kinase/ERK (MEK/ERK) pathway and transforms immortalized human mammary epithelial cells in invasive mammary carcinomas that have the same aspect as basal-like breast tumors (Gruvberger-Saal and Parsons 2006; Moyano et al. 2006). At least in the kidney, HspB5 can participate in maintaining tissue integrity by interacting with Ksp-cadherin-16 and promoting its connection to the cytoskeleton (Thedieck et al. 2008).
HspB1 is still the major small Hsp that stimulates metastasis (Bausero et al. 2004, 2006; Gibert et al. 2012b; Nagaraja et al. 2012a, b). Epithelial-to-mesenchymal transition (EMT) is the major parameter controlling metastasis that appears under the control of HspB1 (Shiota et al. 2013; Wei et al. 2011). Indeed, HspB1 modulates the expression of pro-metastatic genes (Nagaraja et al. 2012b), such as those dependent on STAT3/Twist signaling by enhancing the binding of the transcription factor STAT3 to the promoter of the Twist gene (Shiota et al. 2013). This transcriptional event generates two hallmarks of EMT: N-cadherin up-regulation and E-cadherin downregulation. It is therefore possible that the interaction of HspB1 with phosphorylated and activated STAT3 could be one of the key events regulating this phenomenon (Gibert et al. 2012a). HspB1 also binds to and stabilizes the transcription factor Snail, and consequently induces EMT features (Wettstein et al. 2013). The phenomenon probably occurs via a Snail-induced transcriptional blockage of E-cadherin gene expression (Batlle et al. 2000). E-cadherin downregulation is necessary to trigger epithelial-to-mesenchymal transition and acquisition of metastatic potential at late stages of epithelial tumour progression. Concerning HspB5, a recent study mentions that its expression is associated with distant metastases formation in head and neck squamous cell carcinoma, a link that might relate to the chaperone function of HspB5 in mediating folding and secretion of VEGF and stimulating cell migration (van de Schootbrugge et al. 2013a). Thus, among the different small Hsps, at least HspB1 and HspB5 are considered as potent stimulators of tumor progression. However, we should be cautious before coming to a general conclusion on this topic, since, as indicated below (Sect. 2.2.3.2), in some tumors these Hsps have been recently shown to have an anti-tumor activity that counteracts tumor development.
2.3.1.4 Angiogenesis
Do small Hsps participate in the process triggering the excessive formation of blood vessels that irrigate cancer cells? Until recently, no answer could be given to this question since no data supported such a pro-angiogenic hypothesis. However, recent game-changing reports have clearly demonstrated that small Hsps indeed play a role in this process. First, it was shown that, in addition to their intracellular distribution, small Hsps can also be localized in plasma membrane and can be exported in the extracellular milieu (Chowdary et al. 2006; Rayner et al. 2008; Tsvetkova et al. 2002), a phenomenon that correlates with tumor growth and metastasis formation (Bausero et al. 2004). In addition to a possible immunological role for small Hsps, a first observation was that recombinant HspB1 added to the growth medium has a pro-angiogenic effect mediated by Toll-like receptor 3 (TLR3) at the surface of human microvascular endothelial cells (HMECs). The interaction stimulates NF-κB dependent vascular endothelial growth factor (VEGF) gene transcription and promotes secretion of VEGF-activating VEGF receptor type 2 and angiogenesis (Thuringer et al. 2013). Indeed, the production by endothelial cells of intracellular autocrine (intracrine) VEGF is critical for vasculature homeostasis. A more recent study showed that HspB1 is directly released from endothelial cells (ECs) and confirmed that it modulates angiogenesis via direct interaction with VEGF. However, these authors also showed that HspB1 can be cleaved by MMP9 (Matrix MetalloProteinase 9) and recovered as anti-angiogenic fragments which interfere with VEGF-induced ECs activation and tumor progression (Choi et al. 2014). Thus, it appears that the effect mediated by extracellular HspB1 in cancer pathologies may depend on the efficiency of its cleavage by MMP9. However, the first study used recombinant HspB1 added to culture medium, so that the cleavage activity of endogenous MMP9 could have been overwhelmed by an excess of HspB1 and thus a pro-angiogenic effect was observed. Thus, in vivo, HspB1 released from cells appears as an anti-angiogenic polypeptide. This is also supported by the fact that MMP inhibitors have failed in clinical trials, probably through their efficient knock out of HspB1 fragmentation.
Another small Hsp involved in angiogenesis is HspB5 since it is crucial for endothelial cell survival and is up regulated during vessel morphogenesis. For example, tumor vessels in HspB5 (−/−) mice showed signs of caspase-3 activation and apoptosis and tumors grown in such mice were significantly less vascularized than wild-type tumors and displayed increased areas of apoptosis/necrosis (Dimberg et al. 2008). Recently, it was shown that HspB5 is a VEGF chaperone that protects this growth factor against proteolytic degradation (Kerr and Byzova 2010; Ruan et al. 2011). HspB5 appears therefore strongly involved in the pathway maintaining intracrine VEGF signaling that sustains aberrant tumor angiogenesis (Dimberg et al. 2008; Ruan et al. 2011).
2.3.1.5 Gene Expression
The control by HspB1 of several crucial transcription factors (among them Snail, STAT3, NF-κB and HSF1) can have dramatic consequences particularly towards apoptosis inhibition and EMT promotion. HSF1 (heat shock factor 1), the transcription factor responsible for Hsps expression, has also been shown to play a crucial role in tumorogenesis (Mendillo et al. 2012). HSF1 is SUMO-2/3 modified by HspB1-Ubc9 complex (Brunet Simioni et al. 2009). This modification does not affect HSF1 DNA-binding ability but blocks its transactivation function suggesting that it could act, together with NuRD factors, as a transcriptional inhibitor that represses genes that oppose metastasis. Other hypotheses suggest that it could modulate energy metabolism or permit the development of polyploidy in cancer cells (Calderwood 2012; Mendillo et al. 2012).
HspB1, HspB7 and HspB8 can also favor the expression of pro-tumorigenic proteins though the control of mRNAs. Indeed, some clients of these Hsps regulate mRNA splicing, such as SAM68, Ddx20, EFTUD2 and SC35 (Badri et al. 2006; Hegele et al. 2012; Sun et al. 2010; Vos et al. 2009), while others play a role in translational initiation (eIF4G) (Andrieu et al. 2010) or mRNA stability (AUF1) (Sinsimer et al. 2008).
2.3.2 Anti-tumorigenic Effects
In contrast to the classical view described above favoring a pro-tumorigenic activity for HspB1 and HspB5, recent observations indicate that, in some cancer types, HspB1, HspB5 and HspB4 polypeptides display intriguing tumor suppressive activities. Moreover, recent studies dealing with HspB8 and HspB6 clearly show that these polypeptides promote tumor growth resistance and decrease cell survival.
2.3.2.1 Tumor Suppressive Role of HspB1
As mentioned above, HspB1 released from endothelial cells (ECs) regulates angiogenesis by interacting with VEGF (vascular endothelial growth factor). However, new observations have revealed that MMP9 (matrix metalloproteinase 9) can cleave HspB1 and release anti-angiogenic fragments that inhibit lung and liver tumor progression of B16F10 melanoma cells and lung tumor progression of CT26 colon carcinoma cells. The failure of MMP inhibitors in clinical trials could then be explained by their ability to decrease HspB1 fragmentation leading to pro-tumorigenic effects (Choi et al. 2014).
2.3.2.2 Tumor Suppressive Role of HspB5
In the case of nasopharyngeal carcinoma (NPC), an intriguing observation was that HspB5 downregulation is significantly associated with the progression of NPC while its overexpression interferes with NPC progression-associated phenotypes such as loss of cell adhesion, invasion, interaction with the tumor microenvironment, invasive protrusion formation and expression of epithelial-mesenchymal transition-associated markers. Molecular analysis revealed that HspB5 suppresses NPC progression by interacting with the cadherin/catenin adherens junction. This indirectly decreases the levels of expression of critical downstream targets such as cyclin-D1 and c-myc (Huang et al. 2012)
2.3.2.3 HspB4
The role of HspB4 in tumorigenesis appears rather equivocal (Deng et al. 2010). Indeed, depending of the tumor type the level of this protein is either up- or downregulated. In normal conditions, HspB4 is mainly expressed in the lens and is also detectable in the pancreas. Consequently, many of the lens tumor cells display high levels of HspB4 expression, such as those from retinoblastoma and eyelids with sebaceous carcinoma (Kase et al. 2009; Mahon et al. 1987; Rigas et al. 2009). In these cells, HspB4, like HspB5, can promote tumorigenesis since it bears an anti-apoptotic activity (Andley et al. 2000; Ciocca and Calderwood 2005) whose major property is to negatively regulate the pro-apoptotic members of the Bcl-2 family and caspase-3 (Hu et al. 2012). Contrasting with these observations, the moderate level of expression of HspB4 observed in normal human pancreas samples appears significantly reduced in many cases of pancreatic carcinoma of different types. Unfortunately, to date, the mechanism controlling HspB4 down-regulation in pancreatic carcinoma cells is not known. Another interesting point, as demonstrated by genetically forced expression of this protein, concerns the fact that, in the pancreas, HspB4 can act as a negative regulator that blocks cell transformation and retards cell migration (Deng et al. 2010). However, the mechanism by which HspB4 performs this pancreatic task is not yet solved. It may occur through a modulation of ERK MAP kinase activity regulating AP-1 expression and activity to halt cell transformation and retard cell migration (Chen et al. 2012; Deng et al. 2010). Thus, in spite of some common properties towards apoptosis, cell proliferation and tumor metastasis more work is needed to unravel the particular role of HspB4 in pancreatic carcinogenesis.
2.3.2.4 HspB8
It has been recently shown that in a large fraction of melanoma tumors, which are aggressive and drug-resistant cancers, HspB8 gene is silenced through aberrant DNA methylation. This phenomenon modulates Aza-C (5-Aza-2″-deoxycytidine) treatment efficiency (Smith et al. 2011). The anti-tumor property of HspB8 was then identified by experiments aimed at restoring its expression. Indeed, putting HspB8 back in cells inhibited tumor growth and induced the death of genetically diverse melanoma lines as a result of the activation of TAK1 (TGF-β activated kinase 1)-dependent death pathways (Li et al. 2007; Smith et al. 2012). Among the TAK1 putative down-stream pathways that could be involved is the inflammasome independent activation of caspase-1 resulting from the upregulation of ASC (apoptosis-associated speck-like protein containing a CARD). Apoptosis could then be caused by caspase-1-mediated cleavage of Beclin-1, a polypeptide upregulated in melanoma tumors as a result of mTOR (mammalian target of rapamycin) phosphorylation.
2.3.2.5 HspB6
Recent findings have shown that, in human hepatocellular carcinoma (HCC), HspB6 expression levels are inversely correlated with the progression of HCC. The negative effect mediated by HspB6 appears to result from its interaction with PI3K (phosphoinositide 3-kinase, an upstream kinase of Akt). This interaction suppresses PI3K activity, inhibits the AKT survival pathway and subsequently decreases HCC survival and growth (Matsushima-Nishiwaki et al. 2013).
2.3.2.6 Therapeutic Thoughts About Tumor Suppressive Small Hsps in Cancer
The examples presented above clearly indicate that, in some cancer cells, small Hsps can be associated with anti-tumorigenic activity. Hence, it is intriguing to note that cancer cells can devise strategies to improve their growth and dissemination by down-regulating the expression of these polypeptides. This may open up new therapeutic options aimed at restoring or up regulating the expression or activity of these proteins. However, restoring the specific expression of transcriptionally silenced genes is quite difficult. Moreover, as in the case of HspB8, the approach can be limited by the genetic diversity of the tumors. A better way to improve therapeutic strategies would be to mimic chemically the activation performed by small Hsps, as for example towards the TAK1 pathway in the case of melanoma. Similarly, restoring HspB4 or HspB5 level of expression, up-regulating HspB6 activity towards PI3K or stimulating HspB1 cleavage by MMP9 could be a challenge. In the meantime a better understanding of the role of HspB4 towards ERK MAP kinase activity and AP-1 expression as well as of HspB6 inhibitory interaction with PI3K may help in the discovery of new drugs effective against pancreatic and hepatic cells carcinogenesis.
2.4 Extracellular Roles of Small Hsps
Recently, a major discovery was that HspB1, HspB5 and HspB8 can localize in plasma membrane and be secreted in spite of their major intracellular localization (Chowdary et al. 2006; Rayner et al. 2008; Sreekumar et al. 2010; Tsvetkova et al. 2002). Thus, what could be the functions of these proteins at the cell surface or in the extracellular milieu? Do these circulating proteins share some of the properties of circulating Hsp70 (De Maio 2011)? For example, are they associated with immunogenic peptides which trigger an immune response (Delneste et al. 2002), or are they pro-immunosuppressive polypeptides (Chalmin et al. 2010). Are they involved in anti-inflammation, alarmone or other pathways by interacting with specific cellular receptors? Recent observations suggest that circulating HspB1 is not associated with immunogenic peptides but could have immunoregulatory activity. For example, circulating HspB5 stimulates macrophages through its ability to recognize CD14, TLR1 and TLR2 (Toll-like receptor 1 and 2) at their surface (van Noort et al. 2013). Similarly, HspB8 and HspB4 recognize TLR4 and induce dendritic cells activation (Roelofs et al. 2006). HspB1 was also found to activate NF-κB in macrophages (Salari et al. 2012). In addition, this protein recognizes several cell surface polypeptides such as CD10 (Dall’Era et al. 2007), Plasminogen, Angiostatin (Dudani et al. 2007) and TLR3 (Thuringer et al. 2013). In 4T1 breast adenocarcinoma cells, HspB1 cell surface expression appears correlated with tumor growth and metastasis formation (Bausero et al. 2004, 2006). Moreover, the angiogenic property of HspB1 is regulated by the cleavage efficiency of MMP9 (Choi et al. 2014; Thuringer et al. 2013) (see also Sect. Angiogenesis).
A key aspect of circulating small Hsps is that they can be either beneficial or harmful to patients suffering from different pathologies. In that regard they behave like intracellular small Hsps. For example, a major positive effect of circulating HspB1 is its impressive atheroprotective effect (Rayner et al. 2008; Salari et al. 2012). On the other hand, secreted HspB1 correlates with vascular complications in type 1 diabetic patients (Gruden et al. 2008) and is not a positive signal in cancers. Consequently, major care will have to be taken in case of therapeutic approaches targeting circulating Hsps. More studies are urgently needed to evaluate the multiple roles played by these extracellular proteins in normal and pathological physiological conditions.
2.5 Conclusions
As described here, small Hsps have immense cellular implications as a result of their interaction with many specific client polypeptides whose number is growing exponentially. Their ability to bind polypeptides and modulate their folding is a property that was originally discovered in heat shock treated cells where HspB1 was shown to interact with aberrantly folded polypeptides to prevent their aggregation. It is now well known that small Hsps can modulate folding or induce modifications in interacting clients. They also have the crucial ability to positively or negatively modulate their half-lifes. Taken together, these observations show that small Hsps can have a drastic influence on the level of expression as well as on the activity of interacting clients. Consequently, these Hsps indirectly appear to have a huge number of functions that allow cells to rest, grow or better adapt to changes in their physiology or pathological status. Moreover, by targeting specific clients, small Hsps can be protective and beneficial against cell degeneration. They can also have a disastrous effect by causing some cancer cells to proliferate and create metastasis.
The proteomic analysis presented here confirms our feeling that small Hsps, as Hsp90 (McClellan et al. 2007; Moulick et al. 2011; Taipale et al. 2010), are global regulators of cell systems that exert marked effects on normal biology and diseases through their chaperone/client interactome systems. Hence, we are now facing problems that are even more complex than those encountered by researchers working with Hsp90. The first of these illustrates the complexity associated with small Hsps and deals with the chimeric structures that can form between two small Hsps. These structures appear to have lost the properties associated with parental homo-oligomers, but do they have specific interactomes or are they inert? The second problem is common to small Hsps and Hsp90: what is the structural dynamic that acts on a diverse client repertoire in defined cellular conditions? In the case of HspB1, phosphorylation and oligomerization appear as key factors that dynamically react and provide a recognition platform for specific clients (Arrigo and Gibert 2013; Paul et al. 2010), however nothing is known about the molecular signaling mechanisms involved in this process. Thus, more in-depth structural work, signaling studies as well as analysis of the organization of small Hsps in living cells are necessary to unravel the problem of how these chaperones recognize client polypeptides. The third problem deals with therapeutic strategies aimed at modulating the level or activity of these chaperones. In the case of Hsp90, drugs interfering with its chaperone activity and broad interaction with clients have been clinically tested. Their modest effects and unsuspected side effects resulted in lack of FDA recognition (Whitesell et al. 2012). More specific drugs targeting only a subset of Hsp90-clients may prove more useful (Moulick et al. 2011). Similarly, the use of genetic techniques to invalidate the expression of small Hsps appears efficient (Gibert et al. 2012b; Wettstein et al. 2013) but in the long term they could be disappointing because of the complete disruption of small Hsps protein interactomes. Drugs or genetic techniques altering the structure of small Hsps can lead to interesting results (Gibert et al. 2011; Heinrich et al. 2011) but will require in-depth analysis of their effects on small Hsps interactomes. More work is needed to build comprehensive dynamic interactomes of small Hsps in specific pathologies. This will be necessary in characterizing both the good and pathological clients recognized by these Hsps. The discovery of new drugs or genetic techniques that preserve their interaction with the good clients and destroy those with the ugly ones will probably have a bright future.
References
Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K (2005) A mutation in the small heat shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet 15(2):347–354
Adhikari AS, Singh BN, Rao KS, Rao Ch M (2011) alphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim Biophys Acta 1813(8):1532–1542
Agrawal P, Yu K, Salomon AR, Sedivy JM (2010) Proteomic profiling of Myc-associated proteins. Cell Cycle 9(24):4908–4921
Ahner A, Gong X, Schmidt BZ, Peters KW, Rabeh WM, Thibodeau PH, Lukacs GL, Frizzell RA (2012) Small heat shock proteins target mutant CFTR for degradation via a SUMO-dependent pathway. Mol Biol Cell 24(2):74–84
Alford KA, Glennie S, Turrell BR, Rawlinson L, Saklatvala J, Dean JL (2007) HSP27 functions in inflammatory gene expression and TAK1-mediated signalling. J Biol Chem 282:6232–6241
Al-Madhoun AS, Chen YX, Haidari L, Rayner K, Gerthoffer W, McBride H, O’Brien ER (2007) The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol 270(1–2):33–42
Andley UP (2007) Crystallins in the eye: function and pathology. Prog Retin Eye Res 26(1):78–98
Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S (2000) Differential protective activity of {alpha}A- and {alpha}B-crystallin in lens epithelial cells. J Biol Chem 275:36823–36831
Andrieu C, Taieb D et al (2010) Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene 29(13):1883–1896
Aquilina JA, Shrestha S, Morris AM, Ecroyd H (2013) Structural and functional aspects of hetero-oligomers formed by the small heat-shock proteins alphaB crystallin and HSP27. J Biol Chem 288(19):13602–13609
Arany I, Clark JS, Reed DK, Ember I, Juncos LA (2012) Cisplatin enhances interaction between p66Shc and HSP27: its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer Res 32(11):4759–4763
Arrigo AP (1998) Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem 379(1):19–26
Arrigo AP (2000) sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol (Paris) 48(3):280–288
Arrigo AP (2001) Hsp27: novel regulator of intracellular redox state. IUBMB Life 52(6):303–307
Arrigo AP (2005) Heat shock proteins as molecular chaperones. Med Sci (Paris) 21(6–7):619–625
Arrigo A-P (2007a) Anti-apoptotic, tumorigenic and metastatic potential of Hsp27 (HspB1) and alphaB-crystallin (HspB5): emerging targets for the development of new anti-cancer therapeutic strategies. In: Calderwood SK, Sherman M, Ciocca D (eds) Heat shock proteins in cancer. Springer, New-York, pp 73–92
Arrigo AP (2007b) The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol 594:14–26
Arrigo AP (2011) Structure-functions of HspB1 (Hsp27). Methods Mol Biol 787:105–119
Arrigo AP (2012a) Editorial: heat shock proteins in cancer. Curr Mol Med 12(9):1099–1101
Arrigo AP (2012b) Pathology-dependent effects linked to small heat shock proteins expression. Scientifica 2012:19 (Article ID 185641)
Arrigo AP (2013) Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett 587(13):1959–1969
Arrigo AP, Gibert B (2012) HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr Mol Med 12:1151–1163
Arrigo AP, Gibert B (2013) Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int J Hyperthermia 29:409–422
Arrigo AP, Gibert B (2014) HspB1, HspB5 and HspB4 in human cancers: potent oncogenic role of some of their client proteins. Cancers (Basel) 6(1):333–365
Arrigo A-P, Simon S (2010) Dual, beneficial and deleterious, roles of small stress proteins in human diseases: implications for therapeutic strategies. In: Simon S, Arrigo A-P (eds) Book serie: protein science engineering. Nova Sciences, New York, pp 457–476
Arrigo A-P, Suhan JP, Welch WJ (1988) Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol 8:5059–5071
Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C (2005) Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 7(3–4):414–422
Arrigo AP, Simon S et al (2007) Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett 581(19):3665–3674
Asthana A, Raman B, Ramakrishna T, Rao Ch M (2012) Structural aspects and chaperone activity of human HspB3: role of the “C-terminal extension”. Cell Biochem Biophys 64(1):61–72. doi:10.1007/s12013-012-9366-x
Badri KR, Modem S, Gerard HC, Khan I, Bagchi M, Hudson AP, Reddy TR (2006) Regulation of Sam68 activity by small heat shock protein 22. J Cell Biochem 99(5):1353–1362
Barton KA, Hsu CD, Petrash JM (2009) Interactions between small heat shock protein alpha-crystallin and galectin-related interfiber protein (GRIFIN) in the ocular lens. Biochemistry 48(18):3956–3966
Basha E, O’Neill H, Vierling E (2011) Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci 37(3):106–117
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2(2):84–89
Bausero MA, Page DT, Osinaga E, Asea A (2004) Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol 25(5–6):243–251
Bausero MA, Bharti A et al (2006) Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumour Biol 27(1):17–26
Bellaye PS, Wettstein G et al (2014) The small heat-shock protein alphaB-crystallin is essential for the nuclear localization of Smad4: impact on pulmonary fibrosis. J Pathol 232(4):458–472
Bellomo G, Mirabelli F (1992) Oxidative stress and cytoskeletal alterations. Ann N Y Acad Sci 663:97–109
Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, Farkas R, Sumegi B, Gallyas F Jr (2007) Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol 86(3):161–171
Bennardini F, Wrzosek A, Chiesi M (1992) Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res 71(2):288–294
Benndorf R, Martin JL, Kosakovsky Pond SL, Wertheim JO (2014) Neuropathy- and myopathy-associated mutations in human small heat shock proteins: characteristics and evolutionary history of the mutation sites. Mutat Res. doi:10.1016/j.mrrev.2014.02.004
Beresford PJ, Jaju M, Friedman RS, Yoon MJ, Lieberman J (1998) A role for heat shock protein 27 in CTL-mediated cell death. J Immunol 161(1):161–167
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione, iron and Parkinson’s disease. Biochem Pharmacol 64(5–6):1037–1048
Bhat SP, Nagineni CN (1989) αB subunit of lens-specific protein α-cristallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun 158(1):319–325
Bjorkdahl C, Sjogren MJ, Zhou X, Concha H, Avila J, Winblad B, Pei JJ (2008) Small heat shock proteins Hsp27 or alphaB-crystallin and the protein components of neurofibrillary tangles: tau and neurofilaments. J Neurosci Res 86(6):1343–1352
Boelens WC, Croes Y, de Jong WW (2001) Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim Biophys Acta 1544(1–2):311–319
Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J (1999) Mutation R120G in alphaB-crystallin, which is linked to a desmin- related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A 96(11):6137–6142
Browne SE, Ferrante RJ, Beal MF (1999) Oxidative stress in Huntington’s disease. Brain Pathol 9(1):147–163
Bruey JM, Ducasse C et al (2000a) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2(9):645–652
Bruey JM, Paul C, Fromentin A, Hilpert S, Arrigo AP, Solary E, Garrido C (2000b) Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene 19(42):4855–4863
Bruinsma IB, Bruggink KA et al (2011) Inhibition of alpha-synuclein aggregation by small heat shock proteins. Proteins 79(10):2956–2967
Brundel BJ, Ke L, Dijkhuis AJ, Qi X, Shiroshita-Takeshita A, Nattel S, Henning RH, Kampinga HH (2008) Heat shock proteins as molecular targets for intervention in atrial fibrillation. Cardiovasc Res 78(3):422–428
Brunet Simioni M, De Thonel A et al (2009) Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene 28:3332–3344
Buchner J (1999) Hsp90 & Co. – a holding for folding. Trends Biochem Sci 24(4):136–141
Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB (2009) Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim Biophys Acta 1794(3):486–495
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92(3):351–366
Bullard B, Ferguson C et al (2004) Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem 279(9):7917–7924
Calderwood SK (2012) HSF1, a versatile factor in tumorogenesis. Curr Mol Med 12(9):1102–1107
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31(3):164–172
Carra S (2009) The stress-inducible HspB8-Bag3 complex induces the eIF2alpha kinase pathway: implications for protein quality control and viral factory degradation? Autophagy 5(3):428–429
Carra S, Sivilotti M, Chavez Zobel AT, Lambert H, Landry J (2005) HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum Mol Genet 14(12):1659–1669
Carra S, Seguin SJ, Lambert H, Landry J (2008a) HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem 283(3):1437–1444
Carra S, Seguin SJ, Landry J (2008b) HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 4(2):237–239
Carra S, Rusmini P et al (2013) Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos Trans R Soc Lond B Biol Sci 368(1617):20110409
Cayado-Gutierrez N, Moncalero VL, Rosales EM, Beron W, Salvatierra EE, Alvarez-Olmedo D, Radrizzani M, Ciocca DR (2012) Downregulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress Chaperones 18(2):243–249
Chalmin F, Ladoire S et al (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120(2):457–471
Charette SJ, Landry J (2000) The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci 926:126–131
Charette SJ, Lavoie JN, Lambert H, Landry J (2000) Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 20(20):7602–7612
Chauhan D, Li G et al (2003) Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood 102(9):3379–3386
Chebotareva NA, Makeeva VF, Bazhina SG, Eronina TB, Gusev NB, Kurganov BI (2010) Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol Biosci 10(7):783–789
Chen H, Zheng C, Zhang Y, Chang YZ, Qian ZM, Shen X (2006) Heat shock protein 27 downregulates the transferrin receptor 1-mediated iron uptake. Int J Biochem Cell Biol 38(8):1402–1416
Chen P, Ji W et al (2012) Alpha-crystallins and tumorigenesis. Curr Mol Med 12(9):1164–1173
Chen A, Karolczak-Bayatti M, Sweeney M, Treumann A, Morrissey K, Ulrich SM, Europe-Finner GN, Taggart MJ (2013) Lysine deacetylase inhibition promotes relaxation of arterial tone and C-terminal acetylation of HSPB6 (Hsp20) in vascular smooth muscle cells. Physiol Rep 1(6):e00127
Chernik IS, Seit-Nebi AS, Marston SB, Gusev NB (2007) Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3gamma. Mol Cell Biochem 295(1–2):9–17
Choi YW, Tan YJ, Lim SG, Hong W, Goh PY (2004) Proteomic approach identifies HSP27 as an interacting partner of the hepatitis C virus NS5A protein. Biochem Biophys Res Commun 318(2):514–519
Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L (2005) Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem 280(12):11648–11655
Choi SH, Lee HJ et al (2014) MMP9 processing of HSPB1 regulates tumor progression. PLoS One 9(1):e85509
Chowdary TK, Bakthisaran R, Tangirala R, Rao MC (2006) Interaction of mammalian Hsp22 with lipid membranes. Biochem J 401:437–445
Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10(2):86–103
Ciocca DR, Arrigo AP, Calderwood SK (2013) Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol 87(1):19–48
Cosentino C, Grieco D, Costanzo V (2011) ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J 30(3):546–555
Crippa V, Sau D et al (2010) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19(17):3440–3456
Cuesta R, Laroia G, Schneider RJ (2000) Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev 14(12):1460–1470
Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R (2001) The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med 31(12):1624–1632
Dall’Era MA, Oudes A, Martin DB, Liu AY (2007) HSP27 and HSP70 interact with CD10 in C4-2 prostate cancer cells. Prostate 67(7):714–721
Datskevich PN, Nefedova VV, Sudnitsyna MV, Gusev NB (2012) Mutations of small heat shock proteins and human congenital diseases. Biochemistry (Mosc) 77(13):1500–1514
De Maio A (2011) Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 16(3):235–249
de Thonel A, Vandekerckhove J et al (2010) HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood 116(1):85–96
de Wit NJ, Verschuure P, Kappe G, King SM, de Jong WW, van Muijen GN, Boelens WC (2004) Testis-specific human small heat shock protein HSPB9 is a cancer/testis antigen, and potentially interacts with the dynein subunit TCTEL1. Eur J Cell Biol 83(7):337–345
Del Vecchio PJ, MacElroy KS, Rosser MP, Church RL (1984) Association of alpha-crystallin with actin in cultured lens cells. Curr Eye Res 3(10):1213–1219
Delneste Y, Magistrelli G et al (2002) Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17(3):353–362
den Engelsman J, Gerrits D, de Jong WW, Robbins J, Kato K, Boelens WC (2005) Nuclear import of {alpha}B-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J Biol Chem 280(44):37139–37148
den Engelsman J, Boros S et al (2009) The small heat-shock proteins HSPB2 and HSPB3 form well-defined heterooligomers in a unique 3 to 1 subunit ratio. J Mol Biol 393(5):1022–1032
Deng M, Chen PC et al (2010) The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim Biophys Acta 1802(7–8):621–631
Devlin GL, Carver JA, Bottomley SP (2003) The selective inhibition of serpin aggregation by the molecular chaperone, alpha-crystallin, indicates a nucleation-dependent specificity. J Biol Chem 278(49):48644–48650
Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP (2005) Substitution of the unique cysteine residue of murine hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 7(3–4):436–445
Dierick I, Irobi J et al (2007) Genetic variant in the HSPB1 promoter region impairs the HSP27 stress response. Hum Mutat 28(8):830
Dieterich LC, Huang H, Massena S, Golenhofen N, Phillipson M, Dimberg A (2013) alphaB-crystallin/HspB5 regulates endothelial-leukocyte interactions by enhancing NF-kappaB-induced up-regulation of adhesion molecules ICAM-1, VCAM-1 and E-selectin. Angiogenesis 16(4):975–983 [In eng]
Dimberg A, Rylova S et al (2008) alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood 111(4):2015–2023
Djabali K, de Nechaud B, Landon F, Portier MM (1997) AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci 110(Pt 21):2759–2769
Djabali K, Piron G, de Nechaud B, Portier MM (1999) alphaB-crystallin interacts with cytoplasmic intermediate filament bundles during mitosis. Exp Cell Res 253(2):649–662
Dodd SL, Hain B, Senf SM, Judge AR (2009) Hsp27 inhibits IKK{beta}-induced NF-{kappa}B activity and skeletal muscle atrophy. FASEB J 23:3415–3423
Doppler H, Storz P, Li J, Comb MJ, Toker A (2005) A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem 280(15):15013–15019
Dudani AK, Mehic J, Martyres A (2007) Plasminogen and angiostatin interact with heat shock proteins. Mol Cell Biochem 300(1–2):197–205
Duverger O, Paslaru L, Morange M (2004) HSP25 is involved in two steps of the differentiation of PAM212 keratinocytes. J Biol Chem 279(11):10252–10260
Eaton P, Awad WI, Miller JI, Hearse DJ, Shattock MJ (2000) Ischemic preconditioning: a potential role for constitutive low molecular weight stress protein translocation and phosphorylation? J Mol Cell Cardiol 32(6):961–971
Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM (2004) Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol 99(6):392–394. Epub 2004 Jul 13
Ehrnsperger M, Graber S, Gaestel M, Buchner J (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 16(2):221–229
Ehrnsperger M, Gaestel M, Buchner J (2000) Analysis of chaperone properties of small Hsp’s. Methods Mol Biol 99:421–429
Elicker KS, Hutson LD (2007) Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene 403(1–2):60–69
Evgrafov OV, Mersiyanova I et al (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 36(6):602–606
Fanelli MA, Montt-Guevara M, Diblasi AM, Gago FE, Tello O, Cuello-Carrion FD, Callegari E, Bausero MA, Ciocca DR (2008) P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients; beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones 13(2):207–220
Firdaus WJ, Wyttenbach A, Diaz-Latoud C, Currie RW, Arrigo AP (2006a) Analysis of oxidative events induced by expanded polyglutamine huntingtin exon 1 that are differentially restored by expression of heat shock proteins or treatment with an antioxidant. FEBS J 273(13):3076–3093
Firdaus WJ, Wyttenbach A, Giuliano P, Kretz-Remy C, Currie RW, Arrigo AP (2006b) Huntingtin inclusion bodies are iron-dependent centers of oxidative events. FEBS J 273(23):5428–5441
Fontaine JM, Sun X, Benndorf R, Welsh MJ (2005) Interactions of HSP22 (HSPB8) with HSP20, alphaB-crystallin, and HSPB3. Biochem Biophys Res Commun 337(3):1006–1011
Fontaine JM, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, Benndorf R (2006) Abnormal small heat shock protein interactions involving neuropathy-associated HSP22 (HSPB8) mutants. FASEB J 20:2168–2170
Forsman A, Ruetschi U, Ekholm J, Rymo L (2008) Identification of intracellular proteins associated with the EBV-encoded nuclear antigen 5 using an efficient TAP procedure and FT-ICR mass spectrometry. J Proteome Res 7(6):2309–2319
Fox JH, Kama JA et al (2007) Mechanisms of copper ion mediated Huntington’s disease progression. PLoS One 2(3):e334
Freeman BC, Morimoto RI (1996) The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J 15(12):2969–2979
Fu L, Liang JJ (2002) Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay. J Biol Chem 277(6):4255–4260
Fu L, Liang JJ (2003) Enhanced stability of alpha B-crystallin in the presence of small heat shock protein Hsp27. Biochem Biophys Res Commun 302(4):710–714
Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, Landry J (2010) Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J 425(1):245–255
Ganea E (2001) Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci 2(3):205–225
Gangalum RK, Bhat SP (2009) AlphaB-crystallin: a Golgi-associated membrane protein in the developing ocular lens. Invest Ophthalmol Vis Sci 50(7):3283–5290
Garrido C (2002) Size matters: of the small HSP27 and its large oligomers. Cell Death Differ 9(5):483–485
Garrido C, Fromentin A, Bonnotte B, Favre N, Moutet M, Arrigo AP, Mehlen P, Solary E (1998) Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res 58(23):5495–5499
Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E (1999) HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J 13(14):2061–2070
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5:22
Gastmann O, Burfeind P, Gunther E, Hameister H, Szpirer C, Hoyer-Fender S (1993) Sequence, expression, and chromosomal assignment of a human sperm outer dense fiber gene. Mol Reprod Dev 36(4):407–418
Georgakis GV, Younes A (2005) Heat-shock protein 90 inhibitors in cancer therapy: 17AAG and beyond. Future Oncol 1(2):273–281
Gernold M, Knauf U, Gaestel M, Stahl J, Kloetzel P-M (1993) Development and tissue-specific distribution of mouse small heat shock protein hsp 25. Dev Genet 14:103–111
Ghosh JG, Houck SA, Clark JI (2007a) Interactive domains in the molecular chaperone human alphaB crystallin modulate microtubule assembly and disassembly. PLoS One 2(6):e498
Ghosh JG, Houck SA, Clark JI (2007b) Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol 39(10):1804–1815
Ghosh JG, Shenoy AK Jr, Clark JI (2007c) Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 46(21):6308–6317
Ghosh JG, Houck SA, Clark JI (2008) Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins. Int J Biochem Cell Biol 40(5):954–967
Ghosh A, Lai C, McDonald S, Suraweera N, Sengupta N, Propper D, Dorudi S, Silver A (2013) HSP27 expression in primary colorectal cancers is dependent on mutation of KRAS and PI3K/AKT activation status and is independent of TP53. Exp Mol Pathol 94(1):103–108
Gibert B, Hadchity E, Czekalla A, Aloy MT, Colas P, Rodriguez-Lafrasse C, Arrigo AP, Diaz-Latoud C (2011) Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene 34:3672–3681
Gibert B, Eckel B et al (2012a) Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS One 7(1):e29719
Gibert B, Eckel B et al (2012b) Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. Br J Cancer 107(1):63–70
Gibert B, Simon S, Dimitrova V, Diaz-Latoud C, Arrigo A-P (2013) Peptide aptamers – tools to negatively or positively modulate HspB1 (27) function. Philos Trans R Soc B Biol Sci 368(1617):20120075. doi: 10.1098/rstb.2012.0075
Goldfarb LG, Vicart P, Goebel HH, Dalakas MC (2004) Desmin myopathy. Brain 127(Pt 4):723–734
Golembieski WA, Thomas SL et al (2008) HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia 56(10):1061–1075
Groenen P, Merck K, de Jong W, Bloemendal H (1994) Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem 225:1–19
Gruden G, Bruno G et al (2008) Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes 57(7):1966–1970
Gruvberger-Saal SK, Parsons R (2006) Is the small heat shock protein alphaB-crystallin an oncogene? J Clin Invest 116(1):30–32
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18(9):685–716
Halliwell B, Gutteridge J (1984) Role of iron in oxygen radical reactions. Methods Enzymol 105:47–56
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12(10):842–846
Hatters DM, Lindner RA, Carver JA, Howlett GJ (2001) The molecular chaperone, alpha-crystallin, inhibits amyloid formation by apolipoprotein C-II. J Biol Chem 276(36):33755–33761
Havugimana PC, Hart GT et al (2012) A census of human soluble protein complexes. Cell 150(5):1068–1081
Hayashi N, Peacock JW, Beraldi E, Zoubeidi A, Gleave ME, Ong CJ (2012) Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ 19(6):990–1002
Hegele A, Kamburov A et al (2012) Dynamic protein-protein interaction wiring of the human spliceosome. Mol Cell 45(4):567–5880
Heinrich JC, Tuukkanen A, Schroeder M, Fahrig T, Fahrig R (2011) RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J Cancer Res Clin Oncol 137(9):1349–1361
Hessling M, Richter K, Buchner J (2009) Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol 16(3):287–293
Hilditch-Maguire P, Trettel F, Passani LA, Auerbach A, Persichetti F, MacDonald ME (2000) Huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles. Hum Mol Genet 9(19):2789–2797
Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H (2000) Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun 271(1):164–169
Hishiya A, Salman MN, Carra S, Kampinga HH, Takayama S (2011) BAG3 directly interacts with mutated alphaB-crystallin to suppress its aggregation and toxicity. PLoS One 6(3):e16828
Hook D, Harding J (1996) Alpha-crystallin acting as a molecular chaperone protects catalase against steroid-induced inactivation. FEBS Lett 382:281–284
Horwitz J, Huang Q-L, Ding L-L (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A 89:10449–10453
Hu WF, Gong L et al (2012) alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med 12(2):177–187
Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT (2004) Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann N Y Acad Sci 1012:153–163
Huang L, Min JN, Masters S, Mivechi NF, Moskophidis D (2007) Insights into function and regulation of small heat shock protein 25 (HSPB1) in a mouse model with targeted gene disruption. Genesis 45(8):487–501
Huang Z, Cheng Y et al (2012) Tumor suppressor Alpha B-crystallin (CRYAB) associates with the cadherin/catenin adherens junction and impairs NPC progression-associated properties. Oncogene 31(32):3709–3720
Jakob U, Gaestel M, Engels K, Buchner J (1993) Small heat shock proteins are molecular chaperones. J Biol Chem 268:1517–1520
Janue A, Olive M, Ferrer I (2007) Oxidative stress in desminopathies and myotilinopathies: a link between oxidative damage and abnormal protein aggregation. Brain Pathol 17(4):377–388
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47(6):S161–S170
Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE (2001) Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem 276(43):39911–39918
Jiang T, Altman S (2001) Protein-protein interactions with subunits of human nuclear RNase P. Proc Natl Acad Sci U S A 98(3):920–925
Kammanadiminti SJ, Chadee K (2006) Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem 281(36):26112–26120
Kamradt MC, Lu M et al (2005) The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem 280(12):11059–11066
Kang SH, Kang KW et al (2008) Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer 8(1):286
Kase S, Parikh JG, Rao NA (2009) Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br J Ophthalmol 93(4):541–544
Ke L, Meijering RA, Hoogstra-Berends F, Mackovicova K, Vos MJ, Van Gelder IC, Henning RH, Kampinga HH, Brundel BJ (2011) HSPB1, HSPB6, HSPB7 and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One 6(6):e20395
Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q (2004) Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol 36(12):2376–2391
Kerr BA, Byzova TV (2010) alphaB-crystallin: a novel VEGF chaperone. Blood 115(16):3181–3183
Kiffin R, Bandyopadhyay U, Cuervo AM (2006) Oxidative stress and autophagy. Antioxid Redox Signal 8(1–2):152–162
Kijima K, Numakura C, Goto T, Takahashi T, Otagiri T, Umetsu K, Hayasaka K (2005) Small heat shock protein 27 mutation in a Japanese patient with distal hereditary motor neuropathy. J Hum Genet 50(9):473–476
Kim EH, Lee HJ et al (2007) Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res 67(13):6333–6341
Klemenz R, Andres AC, Fröhli E, Schäfer R, Aoyama A (1993) Expression of the murine small heat shock proteins hsp25 and aB crystallin in the absence of stress. J Cell Biol 120(3):639–645
Knapinska AM, Gratacos FM, Krause CD, Hernandez K, Jensen AG, Bradley JJ, Wu X, Pestka S, Brewer G (2011) Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol 31(7):1419–1431
Koch HB, Zhang R, Verdoodt B, Bailey A, Zhang CD, Yates JR 3rd, Menssen A, Hermeking H (2007) Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle 6(2):205–217
Kurnellas MP, Brownell SE et al (2012) Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem 287(43):36423–36434
Kwok AS, Phadwal K, Turner BJ, Oliver PL, Raw A, Simon AK, Talbot K, Agashe VR (2011) HspB8 mutation causing hereditary distal motor neuropathy impairs lysosomal delivery of autophagosomes. J Neurochem 119(6):1155–1161
Latchman DS (2005) HSP27 and cell survival in neurones. Int J Hyperthermia 21(5):393–402
Lavoie JN, Hickey E, Weber LA, Landry J (1993) Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268(32):24210–24214
Lee HJ, Lee YS (2010) Repeated-dose toxicity of HSP27-binding heptapeptide in mice. Drug Chem Toxicol 33(3):284–290
Lee GJ, Vierling E (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol 122(1):189–198
Lee GJ, Roseman AM, Saibil HR, Vierling E (1997) A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J 16:659–671
Lee S, Carson K, Rice-Ficht A, Good T (2006) Small heat shock proteins differentially affect Abeta aggregation and toxicity. Biochem Biophys Res Commun 347(2):527–533
Lee YS, Lim KH et al (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68(18):7561–7569
Lee JS, Kim HY et al (2012) Expression of alphaB-crystallin overrides the anti-apoptotic activity of XIAP. Neuro Oncol 14(11):1332–1345
Lelj-Garolla B, Mauk AG (2005) Self-association of a small heat shock protein. J Mol Biol 345(3):631–642
Lelj-Garolla B, Mauk AG (2006) Self-association and chaperone activity of Hsp27 are thermally activated. J Biol Chem 281(12):8169–8174
Lemieux P, Oesterreich S, Lawrence JA, Steeg PS, Hilsenbeck SG, Harvey JM, Fuqua SA (1997) The small heat shock protein hsp27 increases invasiveness but decreases motility of breast cancer cells. Invasion Metastasis 17(3):113–123
Lewis SE, Mannion RJ, White FA, Coggeshall RE, Beggs S, Costigan M, Martin JL, Dillmann WH, Woolf CJ (1999) A role for HSP27 in sensory neuron survival. J Neurosci 19(20):8945–8953
Li DW, Liu JP et al (2005) Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell 16(9):4437–4453
Li B, Smith CC, Laing JM, Gober MD, Liu L, Aurelian L (2007) Overload of the heat-shock protein H11/HspB8 triggers melanoma cell apoptosis through activation of transforming growth factor-beta-activated kinase 1. Oncogene 26(24):3521–3531
Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA (2006) Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell 24(3):355–366
Liu BF, Liang JJ (2008) Confocal fluorescence microscopy study of interaction between lens MIP26/AQP0 and crystallins in living cells. J Cell Biochem 104(1):51–58
Liu C, Gilmont RR, Benndorf R, Welsh MJ (2000) Identification and characterization of a novel protein from Sertoli cells, PASS1, that associates with mammalian small stress protein hsp27. J Biol Chem 275(25):18724–18731
Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW (2004) Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res 79(6):393–403
Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X (2006) Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J 20(2):362–364
Liu S, Li J, Tao Y, Xiao X (2007) Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun 354(1):109–114
Mahon KA, Chepelinsky AB, Khillan JS, Overbeek PA, Piatigorsky J, Westphal H (1987) Oncogenesis of the lens in transgenic mice. Science 235(4796):1622–1628
Mao YW, Liu JP, Xiang H, Li DW (2004) Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ 11(5):512–526
Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH (2006) Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell 17(2):886–894
Markossian KA, Yudin IK, Kurganov BI (2009) Mechanism of suppression of protein aggregation by alpha-crystallin. Int J Mol Sci 10(3):1314–1345
Matsushima-Nishiwaki R, Kumada T et al (2013) Direct association of heat shock protein 20 (HSPB6) with phosphoinositide 3-kinase (PI3K) in human hepatocellular carcinoma: regulation of the PI3K activity. PLoS One 8(11):e78440
McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J (2007) Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 131(1):121–135
McClung HM, Golembieski WA, Schultz CR, Jankowski M, Schultz LR, Rempel SA (2012) Deletion of the SPARC acidic domain or EGF-like module reduces SPARC-induced migration and signaling through p38 MAPK/HSP27 in glioma. Carcinogenesis 33(2):275–284
McDonough H, Patterson C (2003) CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8(4):303–308
Mehlen P, Arrigo A-P (1994) The serum-induced phosphorylation of mammalian hsp27 correlates with changes in its intracellular localization and levels of oligomerization. Eur J Biochem 221:327–334
Mehlen P, Préville X, Chareyron P, Briolay J, Klemenz R, Arrigo A-P (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154(1):363–374
Mehlen P, Préville X, Kretz-Remy C, Arrigo A-P (1996a) Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these protein against TNFα−induced cell death. EMBO J 15:2695–2706
Mehlen P, Schulze-Osthoff K, Arrigo AP (1996b) Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271(28):16510–16514
Mehlen P, Hickey E, Weber L, Arrigo A-P (1997a) Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem Biophys Res Commun 241:187–192
Mehlen P, Mehlen A, Godet J, Arrigo A-P (1997b) hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 272:31657–31665
Mendez F, Sandigursky M, Franklin WA, Kenny MK, Kureekattil R, Bases R (2000) Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat Res 153(2):186–195
Mendillo ML, Santagata S et al (2012) HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150(3):549–562
Merendino AM, Paul C, Vignola AM, Costa MA, Melis M, Chiappara G, Izzo V, Bousquet J, Arrigo AP (2002) Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma. Cell Stress Chaperones 7(3):269–280
Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T (2009) The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat Struct Mol Biol 16(3):281–286
Moulick K, Ahn JH et al (2011) Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol 7(11):818–826
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7(2):167–176
Moyano JV, Evans JR et al (2006) AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest 116(1):261–270
Muchowski PJ (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35(1):9–12
Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6(1):11–22
Muchowski PJ, Valdez MM, Clark JI (1999) AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci 40(5):951–958
Mymrikov EV, Seit-Nebi AS, Gusev NB (2011) Large potentials of small heat shock proteins. Physiol Rev 91(4):1123–1159
Mymrikov EV, Seit-Nebi AS, Gusev NB (2012) Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 17(2):157–169
Nagaraja GM, Kaur P, Neumann W, Asea EE, Bausero MA, Multhoff G, Asea A (2012a) Silencing Hsp25/Hsp27 gene expression augments proteasome activity and increases CD8+ T-cell-mediated tumor killing and memory responses. Cancer Prev Res (Phila) 5(1):122–137
Nagaraja GN, Kaur P, Asea A (2012b) Role of human and mouse HspB1 in metastasis. Curr Mol Med 12(9):1142–1150
Neckers L, Mimnaugh E, Schulte TW (1999) Hsp90 as an anti-cancer target. Drug Resist Updat 2(3):165–172
Nemes Z, Devreese B, Steinert PM, Van Beeumen J, Fesus L (2004) Cross-linking of ubiquitin, HSP27, parkin, and alpha-synuclein by gamma-glutamyl-epsilon-lysine bonds in Alzheimer’s neurofibrillary tangles. FASEB J 18(10):1135–1137
Nivon M, Richet E, Codogno P, Arrigo AP, Kretz-Remy C (2009) Autophagy activation by NFkappaB is essential for cell survival after heat shock. Autophagy 5:766–783
Noh SJ, Jeong WJ et al (2008) Sensitization of RPE cells by alphaB-crystallin siRNA to SAHA-induced stage 1 apoptosis through abolishing the association of alphaB-crystallin with HDAC1 in SC35 speckles. Invest Ophthalmol Vis Sci 49(11):4753–4759
O’Callaghan-Sunol C, Gabai VL, Sherman MY (2007) Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res 67(24):11779–11788
Ohto-Fujita E, Fujita Y, Atomi Y (2007) Analysis of the alphaB-crystallin domain responsible for inhibiting tubulin aggregation. Cell Stress Chaperones 12(2):163–171
Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L (2007) Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 448(7152):474–479
Outeiro TF, Klucken J, Strathearn KE, Liu F, Nguyen P, Rochet JC, Hyman BT, McLean PJ (2006) Small heat shock proteins protect against alpha-synuclein-induced toxicity and aggregation. Biochem Biophys Res Commun 351(3):631–638
Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S (2000) Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19(16):1975–1981
Parcellier A, Schmitt E et al (2003) HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol 23(16):5790–5802
Parcellier A, Brunet M et al (2006) HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J 20(8):1179–1181
Patil SB, Pawar MD, Bitar KN (2004) Direct association and translocation of PKC-alpha with calponin. Am J Physiol Gastrointest Liver Physiol 286(6):G954–G963
Paul C, Arrigo AP (2000) Comparison of the protective activities generated by two survival proteins: Bcl-2 and Hsp27 in L929 murine fibroblasts exposed to menadione or staurosporine. Exp Gerontol 35(6–7):757–766
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22(3):816–834
Paul C, Simon S, Gibert B, Virot S, Manero F, Arrigo AP (2010) Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27). Exp Cell Res 316(9):1535–1552
Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA (1999) Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci 112(Pt 13):2099–2112
Perrin V, Regulier E, Abbas-Terki T, Hassig R, Brouillet E, Aebischer P, Luthi-Carter R, Deglon N (2007) Neuroprotection by Hsp104 and Hsp27 in lentiviral-based rat models of Huntington’s disease. Mol Ther 15(5):903–911
Prabhu S, Raman B, Ramakrishna T, Rao Ch M (2012) HspB2/myotonic dystrophy protein kinase binding protein (MKBP) as a novel molecular chaperone: structural and functional aspects. PLoS One 7(1):e29810
Preville X, Mehlen P, Fabre-Jonca N, Chaufour S, Kretz-Remy C, Michel MR, Arrigo A-P (1996) Biochemical and immunofluorescence analysis of the constitutively expressed hsp27 stress protein in monkey CV-1 cells. J Biosci 21(2):1–14
Preville X, Gaestel M, Arrigo AP (1998a) Phosphorylation is not essential for protection of L929 cells by Hsp25 against H2O2-mediated disruption actin cytoskeleton, a protection which appears related to the redox change mediated by Hsp25. Cell Stress Chaperones 3(3):177–187
Preville X, Schultz H, Knauf U, Gaestel M, Arrigo AP (1998b) Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide-induced cell death. J Cell Biochem 69(4):436–452
Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, Ursini MV, Arrigo AP (1999) Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res 247(1):61–78
Qi S, Xin Y, Qi Z, Xu Y, Diao Y, Lan L, Luo L, Yin Z (2014) HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with beta-arrestin2. Cell Signal 26(3):594–602
Quraishe S, Asuni A, Boelens WC, O’Connor V, Wyttenbach A (2008) Expression of the small heat shock protein family in the mouse CNS: differential anatomical and biochemical compartmentalization. Neuroscience 153(2):483–491
Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR (2001) p38 Kinase-dependent MAPKAPK-2 activation functions as 3- phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem 276(5):3517–3523
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278(30):27828–27835
Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER (2008) Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res 103(2):133–141
Renkawek K, Bosman GJ, de Jong WW (1994) Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol (Berl) 87(5):511–519
Rigas PK, Kase S, Rao NA (2009) Expression of alpha-crystallins in human sebaceous carcinoma of the eyelid. Eur J Ophthalmol 19(5):702–707
Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, Bottomley SP (2010) Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proc Natl Acad Sci U S A 107(23):10424–10429
Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, Gleave M (2005) Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res 65(23):11083–11093
Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176(11):7021–7027
Rogalla T, Ehrnsperger M et al (1999) Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem 274(27):18947–18956
Rosenbaum EE, Brehm KS, Vasiljevic E, Liu CH, Hardie RC, Colley NJ (2011) XPORT-dependent transport of TRP and rhodopsin. Neuron 72(4):602–615
Rothbard JB, Kurnellas MP et al (2012) Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem 287(13):9708–9721
Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR (1994) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78(6):1027–1037
Ruan Q, Han S, Jiang WG, Boulton ME, Chen ZJ, Law BK, Cai J (2011) alphaB-crystallin, an effector of unfolded protein response, confers anti-VEGF resistance to breast cancer via maintenance of intracrine VEGF in endothelial cells. Mol Cancer Res 9(12):1632–1643
Saha S, Das KP (2004) Relationship between chaperone activity and oligomeric size of recombinant human alphaA- and alphaB-crystallin: a tryptic digestion study. Proteins 57(3):610–617
Salari S, Seibert T, Chen YX, Hu T, Shi C, Zhao X, Cuerrier CM, Raizman JE, O’Brien ER (2012) Extracellular HSP27 acts as a signaling molecule to activate NF-kappaB in macrophages. Cell Stress Chaperones 18(1):53–63
Samali A, Cotter TG (1996) Heat shock proteins increase resistance to apoptosis. Exp Cell Res 223:163–170
Sayre LM, Perry G, Atwood CS, Smith MA (2000) The role of metals in neurodegenerative diseases. Cell Mol Biol 46:731–741
Schultz CR, Golembieski WA, King DA, Brown SL, Brodie C, Rempel SA (2012) Inhibition of HSP27 alone or in combination with pAKT inhibition as therapeutic approaches to target SPARC-induced glioma cell survival. Mol Cancer 11:20
Seit-Nebi AS, Gusev NB (2010) Versatility of the small heat shock protein HSPB6 (Hsp20). Cell Stress Chaperones 15(3):233–236
Shammas SL, Waudby CA, Wang S, Buell AK, Knowle TP, Ecroyd H, Welland ME, Carver JA, Dobson CM, Meehan S (2011) Binding of the molecular chaperone alphaB-crystallin to Abeta amyloid fibrils inhibits fibril elongation. Biophys J 101:1681–1689
Shimura H, Miura-Shimura Y, Kosik KS (2004) Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem 279(17):17957–17962
Shinder GA, Lacourse MC, Minotti S, Durham HD (2001) Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J Biol Chem 276(16):12791–12796
Shiota M, Bishop JL et al (2013) Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res 73(10):3109–3119
Shoham S, Youdim MB (2000) Iron involvement in neural damage and microgliosis in models of neurodegenerative diseases. Cell Mol Biol (Noisy-le-Grand) 46(4):743–760
Simon S, Fontaine JM, Martin JL, Sun X, Hoppe AD, Welsh MJ, Benndorf R, Vicart P (2007) Myopathy-associated alpha B-crystallin mutants: abnormal phosphorylation, intracellular location, and interactions with other small heat shock proteins. J Biol Chem 82:34276–34287
Simon S, Dimitrova V et al (2013) Analysis of the dominant effects mediated by wild type or R120G mutant of alphaB-crystallin (HspB5) towards Hsp27 (HspB1). PLoS One 8(8):e70545
Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao Ch M (2007) Association of alphaB-crystallin, a small heat shock protein, with actin: role in modulating actin filament dynamics in vivo. J Mol Biol 366(3):756–767
Sinsimer KS, Gratacos FM et al (2008) Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol Cell Biol 28(17):5223–5237
Skouri-Panet F, Michiel M, Ferard C, Duprat E, Finet S (2012) Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie 94(4):975–984
Sluchanko NN, Sudnitsyna MV, Chernik IS, Seit-Nebi AS, Gusev NB (2011) Phosphomimicking mutations of human 14-3-3zeta affect its interaction with tau protein and small heat shock protein HspB6. Arch Biochem Biophys 506(1):24–34
Smith CC, Li B, Liu J, Lee KS, Aurelian L (2011) The Levels of H11/HspB8 DNA methylation in human melanoma tissues and xenografts are a critical molecular marker for 5-Aza-2′-deoxycytidine therapy. Cancer Invest 29(6):383–395
Smith CC, Lee KS, Li B, Laing JM, Hersl J, Shvartsbeyn M, Aurelian L (2012) Restored expression of the atypical heat shock protein H11/HspB8 inhibits the growth of genetically diverse melanoma tumors through activation of novel TAK1-dependent death pathways. Cell Death Dis 3:e371
Solari E, Garrido C (2002) The forgotten chaperones. Nat Cell Biol 4:E125
Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR (2010) alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One 5(10):e12578
Sreelakshmi Y, Sharma KK (2006) The interaction between alphaA- and alphaB-crystallin is sequence-specific. Mol Vis 12:581–587
Srinivas PN, Reddy PY, Reddy GB (2008) Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: chaperone-like activity, structure and hydrophobicity. Biochem J 414(3):453–460
Srinivasan A, Nagineni C, Bhat S (1992) alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem 267:23337–23341
Stengel F, Baldwin AJ, Painter AJ, Jaya N, Basha E, Kay LE, Vierling E, Robinson CV, Benesch JL (2010) Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc Natl Acad Sci U S A 107(5):2007–2012
Stokoe D, Engel K, Campbell D, Cohen P, Gaestel M (1992) Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett 313:307–313
Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MM, Tsui SK, Yoshida S, Ohno S (2000) Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem 275(2):1095–1104
Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R (2004) Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem 279(4):2394–2402
Sun G, Guo M et al (2005) Bovine PrPC directly interacts with alphaB-crystalline. FEBS Lett 579(24):5419–5424
Sun Y, Yi H et al (2007) Identification of differential proteins in nasopharyngeal carcinoma cells with p53 silence by proteome analysis. FEBS Lett 581(1):131–139
Sun X, Fontaine JM et al (2010) Abnormal interaction of motor neuropathy-associated mutant HspB8 (Hsp22) forms with the RNA helicase Ddx20 (gemin3). Cell Stress Chaperones 15(5):567–582
Sun Y, Zhou M, Fu D, Xu B, Fang T, Ma Y, Chen J, Zhang J (2011) Ubiquitination of heat shock protein 27 is mediated by its interaction with Smad ubiquitination regulatory factor 2 in A549 cells. Exp Lung Res 37:568–573
Tabner BJ, Turnbull S, El-Agnaf O, Allsop D (2001) Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr Top Med Chem 1(6):507–517
Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11(7):515–528
Tang G, Perng MD, Wilk S, Quinlan R, Goldman JE (2010) Oligomers of mutant glial fibrillary acidic protein (GFAP) Inhibit the proteasome system in alexander disease astrocytes, and the small heat shock protein alphaB-crystallin reverses the inhibition. J Biol Chem 285(14):10527–10537
Tanguay R, Wu Y, Khandjian E (1993) Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet 14:112–118
Thedieck C, Kalbacher H, Kratzer U, Lammers R, Stevanovic S, Klein G (2008) alpha B-crystallin is a cytoplasmic interaction partner of the kidney-specific cadherin-16. J Mol Biol 378(1):145–153
Thuringer D, Jego G et al (2013) Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J 27(10):4169–4183
Tsvetkova NM, Horvath I et al (2002) Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A 99(21):13504–13509
Turnbull S, Tabner BJ, Brown DR, Allsop D (2003) Copper-dependent generation of hydrogen peroxide from the toxic prion protein fragment PrP106-126. Neurosci Lett 336(3):159–162
van de Schootbrugge C, Bussink J, Span PN, Sweep FC, Grenman R, Stegeman H, Pruijn GJ, Kaanders JH, Boelens WC (2013a) alphaB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer 13:128. doi:10.1186/1471-2407-13-128
van de Schootbrugge C, van Asten F et al (2013b) alphaB-crystallin expression is correlated with phospho-ERK1/2 expression in human breast cancer. Int J Biol Markers 28(4):e365–e370
van Noort JM, Bsibsi M et al (2013) Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials 34(3):831–840
Verschuure P, Croes Y, van den IPR, Quinlan RA, de Jong WW, Boelens WC (2002) Translocation of small heat shock proteins to the actin cytoskeleton upon proteasomal inhibition. J Mol Cell Cardiol 34(2):117–128
Vicart P, Caron A et al (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20(1):92–95
Vos MJ, Kanon B, Kampinga HH (2009) HSPB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta 1793(8):1343–1353
Vos MJ, Zijlstra MP, Kanon B, van Waarde-Verhagen MA, Brunt ER, Oosterveld-Hut HM, Carra S, Sibon OC, Kampinga HH (2010) HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum Mol Genet 19(23):4677–4693
Wang K, Spector A (1996) alpha-crystallin stabilizes actin filaments and prevents cytochalasin- induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem 242(1):56–66
Wang J, Huo K et al (2011) Toward an understanding of the protein interaction network of the human liver. Mol Syst Biol 7:536
Warde-Farley D, Donaldson SL et al (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38(Web Server issue):W214–W220. doi:10.1093/nar/gkq537
Watanabe G, Kato S, Nakata H, Ishida T, Ohuchi N, Ishioka C (2009) alphaB-crystallin: a novel p53-target gene required for p53-dependent apoptosis. Cancer Sci 100(12):2368–2375
Wei L, Liu TT, Wang HH, Hong HM, Yu AL, Feng HP, Chang WW (2011) Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res 13(5):R101
Welch WJ, Feramisco JR (1985) Disruption of the three cytoskeletal networks in mammalian cells does not affect transcription, translation, or protein translocation changes induced by heat shock. Mol Cell Biol 5(7):1571–1581
Wettstein G, Bellaye PS, Micheau O, Bonniaud P (2012) Small heat shock proteins and the cytoskeleton: an essential interplay for cell integrity? Int J Biochem Cell Biol 44(10):1680–1686
Wettstein G, Bellaye PS et al (2013) Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J 27(4):1549–1560
Whitesell L, Santagata S, Lin NU (2012) Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med 12(9):1108–1124
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM (2006a) Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol 111(2):139–149
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM (2006b) Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res 1089:67–78
Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ (2007) Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem 282(30):21598–21608
Wu Y, Liu J, Zhang Z, Huang H, Shen J, Zhang S, Jiang Y, Luo L, Yin Z (2009) HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell Signal 21(1):143–150
Wyttenbach A (2004) Role of heat shock proteins during polyglutamine neurodegeneration: mechanisms and hypothesis. J Mol Neurosci 23(1–2):69–96
Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC (2002) Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet 11(9):1137–1151
Xi JH, Bai F, McGaha R, Andley UP (2006) Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. FASEB J 20(7):846–857
Xu L, Chen S, Bergan RC (2006) MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 25:2987–2998
Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ (2002) Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J 21(19):5164–5172
Yang Y, Ludwig RL et al (2005) Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7(6):547–559
Yang K, Meinhardt A, Zhang B, Grzmil P, Adham IM, Hoyer-Fender S (2012) The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol Cell Biol 32(1):216–225
Yerbury JJ, Gower D, Vanags L, Roberts K, Lee JA, Ecroyd H (2012) The small heat shock proteins alphaB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones 18(2):251–257
Zantema A, Vries MV-D, Maasdam D, Bol S, Avd E (1992) Heat shock protein 27 and αB-cristallin can form a complex, which dissociates by heat shock. J Biol Chem 267(18):12936–12941
Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ (1997) BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem 272(39):24101–24104
Zhu Y, Tassi L, Lane W, Mendelsohn ME (1994) Specific binding of the transglutaminase, platelet factor XIII, to HSP27. J Biol Chem 269(35):22379–22384
Zhuang H, Jiang W et al (2009) Down-regulation of HSP27 sensitizes TRAIL-resistant tumor cell to TRAIL-induced apoptosis. Lung Cancer 68(1):27–38
Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M (2007) Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 67(21):10455–10465
Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L, Gleave ME (2010) Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res 70(6):2307–2317
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Arrigo, AP., Ducarouge, B., Lavial, F., Gibert, B. (2015). Immense Cellular Implications Associated to Small Stress Proteins Expression: Impacts on Human Pathologies. In: Tanguay, R., Hightower, L. (eds) The Big Book on Small Heat Shock Proteins. Heat Shock Proteins, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-319-16077-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-16077-1_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16076-4
Online ISBN: 978-3-319-16077-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)