Abstract
In present study, we investigated the role of hypoxia inducible factor-1 (HIF-1) in regulation of taurine transport through the blood-brain barrier (BBB) under high glucose condition using TR-BBB cells as an in vitro model of BBB. Treatment with high glucose (25 mM) for 48 h induced HIF-1α and VEGF expression, and decreased [3H]taurine uptake in TR-BBB cells. Also, high glucose condition reduced taurine transporter (TauT) mRNA and protein levels. In addition, pre-treatment with HIF-1 inducer, cobalt chloride in TR-BBB cells decreased TAUT expression. Glucose-induced TauT down-regulation could be reversed by inhibition of HIF-1 and VEGF using several HIF-1 and VEGF inhibitors. Also, both HIF-1 inhibitors and VEGF inhibitors induced the decrease of [3H]taurine uptake caused by high glucose. In conclusion, taurine transport through the BBB can be down-regulated by high glucose, which is mediated to induction of HIF-1 and VEGF. Therefore, we suggest that the inhibitors of HIF-1 and VEGF could have the beneficial effects on hyperglycemia by up-regulation of taurine contents in brain.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Taurine
- Taurine transporter
- Hypoxia inducible factor1
- Vascular endothelial growth factor
- Blood-brain barrier
- High glucose
- Uptake
- TR-BBB cells
1 Introduction
Taurine, a free sulfonic acid, acts as a neuromodulator, a neuroprotector, an antioxidant, and an anti-inflammatory agent (Foos and Wu 2002; Pan et al. 2010; Sun et al. 2011). Several studies suggest that taurine also can prevent the hyperglycemia-induced the microvascular complications of diabetes, such as neuropathy, retinopathy, and autonomic dysfunctions (Ito et al. 2012). The taurine supply to the brain from the circulating blood is mediated by Na+, Cl−-dependent taurine transporter, TAUT, in brain microvascular endothelial cells (blood-brain barrier, BBB) (Kang et al. 2002). This TAUT at the BBB is involved in the maintenance of taurine levels in the brain. In hyperglycemia, elevated glucose is believed to contribute to loss of microvascular barrier integrity (Lorenzi et al. 1986; Frank 2004). Accordingly, transport of taurine at BBB may be also changed by hyperglycemia, and this change could intensely affect the neuroprotective effect of taurine by influencing taurine concentration in the brain. In previous studies, it has been reported that TAUT was regulated by glucose, oxidative stress, and changed in osmolarity (Stevens et al. 1999; Kang et al. 2002; Kang et al. 2009). However, the mechanisms involved in the change of taurine transport remain unclear. Hypoxia inducible factor 1 (HIF-1) is an important factor of diabetes neuropathy, and up-regulates vascular endothelial growth factor (VEGF) (Forsythe et al. 1996), which is well defined in promoting new blood vessel formation and causes BBB disruption and increases BBB permeability (Schoch et al. 2002; Yeh et al. 2007). High glucose has been reported to up-regulate HIF-1 activity in isolated hearts of rats (Marfella et al. 2002) and kidney mesangial cells (Isoe et al. 2010). However, it is not known if HIF-1 is involved in change of taurine transport in brain endothelial cells exposed to high glucose. The purpose of the present study was to determine whether or not HIF-1 played a role in taurine transporter activity at high glucose condition. The study was carried out with an in vitro BBB model of conditionally immortalized rat brain microvascular endothelial cells (TR-BBB cells). Previous publications have well characterized TR-BBB cells as an appropriate model of in vitro BBB, which has been shown to express a number of transporters that are expressed in endothelial cells comprising the BBB, allowing for the characterization of their functions in vitro (Hosoya et al. 2000; Lee and Kang 2010).
2 Methods
2.1 Cell Culture
The TR-BBB cells were cultured according to the previous report (Lee et al. 2012). The TR-BBB cells were seeded at 1 × 105 cells/well in rat tail collagen type 1-coated 24 well culture plates (Iwaki, Tokyo, Japan) for the uptake study. After incubation for 2 days at 33 °C, the cultures became confluent and then were used in uptake study.
2.2 [3H]Taurine Uptake Study in TR-BBB Cells
The [3H]taurine uptake was performed according to the previous report (Lee et al. 2012). Briefly, cells were washed three times with 1 mL extracellular fluid (ECF) buffer. Uptake was initiated by addition of ECF buffer containing [3H]taurine (28 nM) and then incubate at 37 °C for 5 min. [3H]Taurine uptake into the cell was terminated by the addition of ice-cold ECF buffer. The cells were then solubilized in 1 N NaOH, and radioactivity was measured in a liquid scintillation counter (LS6500; Beckman, Fullerton, CA). To investigate the change of [3H]taurine uptake under high glucose condition, the TR-BBB cells were pretreated with 25 mM glucose for 12, 24, 36 and 48 h and the uptake study was performed as described above. To test the effect of HIF-1 and VEGF inhibitors on the change of [3H]taurine uptake under high glucose condition, TR-BBB cells were exposed to 10 μM 2-methoxy estradiol (2ME2), 10 μM 3-(5′-hydroxymethyl-2′-furyl-1-benzylindazole) (YC-1), 10 μM betulinic acid (BA), 10 μM alendronate (ALD) and 10 μM methotrexate (MTX) for 1 h under exposing TR-BBB cells to 25 mM glucose for 48 h. Cell to medium ratio (μL/mg protein) was calculated as follows:
Cell to medium ratio (μL/mg protein) = ([3H] dpm in the cell/amount (mg) of cell protein)/([3H] dpm in the injectate/amount (μL) of injectate) × 100.
2.3 Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from cultured TR-BBB cells by the RNeasy kit from Quiagen (Quiagen, Valencia, CA) and according to the manufacturer’s instructions. Total RNA (2 μg) was reverse-transcribed by using oligo (dT) and high capacity RT kit (Applied Biosystems, Foster City, CA). Quantification of TAUT mRNA was performed on an ABI 7500 Sequence Detector System (Applied Biosystems, Foster City, CA). 5 μL of complementary DNA (cDNA) were used for real-time PCR. Gene-specific oligonucleotide primers and probes for TAUT, HIF-1α, VEGF as well as the endogenous control β-actin were obtained from Applied Biosystems. The reaction contained 10 μL TaqMan Master Mix (Applied Biosystems, Foster City, CA) and 1 μL of the specific primer in a final volume of 20 μL. Reaction condition were 30 min at 48 °C, 10 min at 95 °C, 40 cycles for 15 s at 95 °C and 1 min at 60 °C, and a final 5 min incubation at 25 °C.
2.4 Immunocytochemical Staining
First, we quenched endogenous peroxidase activity in TR-BBB cells by incubating tissue for 30 min in Thermo Scientific Peroxidase Suppressor and then TR-BBB cells were washed with PBST. This step was followed by blocking buffer for 30 min, which we have found to be an effective blocker of nonspecific binding. Cells were incubated with the primary antibody [TAUT C-15 (Santa Cruz Biotechnology, Santa Cruz, CA)] in PBST for 60 min at room temperature. Following washes three times for 10 min each with PBST, cells were incubated with HRP-labeled secondary antibody for 30 min. And then we washed cells three times for 10 min each with PBST. Cells were added metal enhanced DAB solution and incubated for 5 min. After washing the cells two times for 3 min each with PBST, cells were examined under a light microscope.
2.5 Statistic Analysis
Statistical significance was determined by one-way ANOVA with Dunnett’s post-hoc test. Each value was expressed as the mean ± SEM. Differences were considered statistically significant when the calculated P value was less than 0.05.
3 Results
3.1 Effect of High Glucose on the [3H]Taurine Uptake in TR-BBB Cells
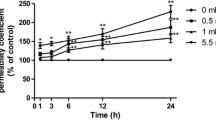
To investigate the effect of excess glucose on taurine uptake at the BBB, [3H]taurine uptake activity was examined in TR-BBB cells at high glucose pre-treatment conditions. In this study, the glucose level of 25 mM was used as high glucose condition against 5.5 mM as normal control. By exposing TR-BBB cells to high glucose for 48 h, [3H]taurine uptake was decreased continuously (Fig. 1).
Change of taurine uptake under high glucose condition by TR-BBB cells. 25 mM glucose was pre-incubated for the time period in the figure. The cells were incubated for 5 min at 37 °C with ECF buffer containing [3H]taurine (28 nM). Each point represents the mean ± SEM (n = 4). *p < 0.05; significantly different from time 0
3.2 Effect of High Glucose on HIF-1α and VEGF mRNA Expression in TR-BBB Cells
HIF-1 is a heterodimer that is composed of α and β subunits. It has been known that the HIF-1α level primarily determines HIF-1 activity (Wang et al. 1995). Therefore, we investigated the change of mRNA levels of HIF-1α and VEGF in TR-BBB cells under high glucose condition. As shown in Fig. 2, high glucose induced HIF-1α and VEGF mRNA expression. Increased HIF-1α and VEGF mRNA expression was effectively reduced by 2ME2 as HIF-1 inhibitor and MTX as VEGF inhibitor, respectively (Fig. 2).
Change of HIF-1α (a) and VEGF (b) mRNA expression under high glucose condition by TR-BBB cells. Cells were pre-treated with glucose at 5.5 (normal) and 25 mM (high) for 48 h. HIF-1 inhibitor (10 μM 2ME2) or VEGF inhibitor (10 μM MTX) were added to culture medium 1 h before the onset of high glucose treatment. Each bar represents the mean ± SEM (n = 3). ***p < 0.001; significantly different from normal control, ###p < 0.001; significantly different from high glucose
3.3 Effect of HIF-1 and VEGF Inhibitors on the [3H]Taurine Uptake in TR-BBB Cells at High Glucose Condition
To further verify the role of HIF-1 and VEGF in high glucose induced taurine uptake decrease, we performed experiments with inhibition of HIF-1 and VEGF by HIF-1 inhibitors (2ME2, YC-1 and BA) and VEGF inhibitors (MTX and ALD). Both HIF-1 inhibitors and VEGF inhibitors induced [3H]taurine uptake decrease caused by high glucose (Fig. 3).
Effect of HIF-1 and VEGF inhibitors on decreased [3H]taurine uptake by high glucose in TR-BBB cells. The cells were pre-treated with 10 μM 2ME2, YC-1, BA, MTX and ALD for 30 min under exposing cells to 25 mM glucose for 48 h. [3H]Taurine uptake was performed with ECF buffer containing [3H]taurine (28 nM) for 5 min at 37 °C. Each point represents the mean ± SEM (n = 3). *p < 0.05; significantly different from normal control; #p < 0.05, ##p < 0.01; significantly different from high glucose
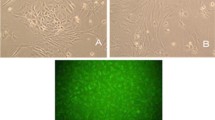
3.4 Change of the Expression of TAUT Involved in HIF-1 in TR-BBB Cells at High Glucose Condition
Next, we tested whether glucose affected expression of taurine transporter, TAUT and the correlation between change of TAUT expression and HIF-1 in TR-BBB cells. TAUT gene expression was significantly reduced at 25 mM glucose, as compared with that of normal control (Fig. 4). Also, TAUT protein level was decreased by pre-treatment 25 mM glucose (Fig. 5). These results were consistent with decrease of [3H]taurine uptake caused by pre-treatment 25 mM glucose in TR-BBB cells. To determine the role of HIF-1 in high glucose induced decrease of TAUT expression, we performed experiments with up-regulation of HIF-1 in cells exposed to normal glucose. We used cobalt chloride as inducer of HIF-1 because cobalt chloride can inhibit HIF-1α degradation and increase HIF-1 activity (Yan et al. 2012). TAUT expression was significantly decreased by cobalt chloride, as compared with that of normal control (Figs. 4 and 5). The decrease of TAUT gene expression by pre-treatment 25 mM glucose was recovered by both HIF-1 inhibitors and VEGF inhibitors (Fig. 4). Also, HIF-1 inhibitor, 2ME2, induced the decrease of TAUT protein expression caused by high glucose (Fig. 5).
Effect of high glucose (25 mM), HIF-1 inhibitors and VEGF inhibitors on TAUT mRNA expression in TR-BBB cells. Real time reverse transcription polymerase chain reaction (RT-PCR) analysis of TAUT mRNA expression in TR-BBB cells without and with pre-treatment with 25 mM glucose for 48 h or 100 μM CoCl2 for 24 h. The cells were pre-treated with 10 μM 2ME2, YC-1, BA, MTX and ALD for 1 h under exposing cells to 25 mM glucose for 48 h. Each point represents the mean ± SEM (n = 3). ***p < 0.001; significantly different from normal control; ###p < 0.001; significantly different from high glucose
Effect of high glucose and HIF-1 inhibitors on TAUT expression in TR-BBB cells. The cells were pre-treated without and with pre-treatment with 25 mM glucose for 48 h, 100 μM CoCl2 for 24 h or 10 μM 2ME2 for 30 min under exposing cells to 25 mM glucose for 48 h. TAUT proteins in TR-BBB cells were immunostained with DAB
4 Discussion
In present study, we demonstrate that HIF-1 contributes to decrease of taurine transport at the BBB under high glucose condition. Actually, the changes in taurine transporter activities at high glucose condition have been reported in human retinal pigment epithelial cells (Stevens et al. 1999; Heller-Stilb et al. 2002). In our previous report, we clarified reduced taurine uptake in TR-iBRB cells as model of inner blood-retinal barrier under high glucose condition (Lee and Kang 2013). Our present result also revealed suppressed [3H]taurine uptake in TR-BBB cells under high glucose condition for 48 h (Fig. 1). Until now, the mechanisms involved in the down-regulation of taurine transport remain to be unknown. HIF-1 is an important factor of diabetes neuropathy. It up-regulates VEGF, which causes BBB disruption and increases BBB permeability (Schoch et al. 2002; Yeh et al. 2007). Also, several evidences suggest that VEGF, and consequently angiogenesis, is related to the pathogenesis of diabetic retinopathy or neuropathy (Nicholson and Schachat 2010). Several reports have found that up-regulation of HIF-1 activity by high glucose in isolated hearts of rats (Marfella et al. 2002) and kidney mesangial cells (Isoe et al. 2010). In vitro exposure to high glucose has been also shown to rapidly increase VEGF expression in various cell types and tissues (Natarajan et al. 1997; Schrufer et al. 2010). Our present result was revealed that high glucose activates expression of HIF-1α and VEGF in TR-BBB cells, brain vascular endothelial cells (Fig. 2). This result agrees with previous findings in other cell lines. Therefore, we hypothesized HIF-1 may be related to reduction of taurine uptake in high glucose in TR-BBB cells. To determine the role of HIF-1 in reduced taurine uptake by high glucose, we tested the change of taurine transport in TR-BBB cells by pre-treatment of HIF-1 inducer, cobalt chloride and HIF-1 inhibitors, 2ME2, YC-1, BA. Cobalt chloride treatments reduced the expression level of TAUT in TR-BBB cells, as in treatment of 25 mM glucose (Figs. 4 and 5). HIF-1 inhibitors recovered both taurine uptake (Fig. 3) and TAUT expression (Figs. 4 and 5) reduced by high glucose. The results verify that HIF-1 played a role in regulation of taurine transport by reducing of TAUT expression in the brain endothelial cells. Bisphosphonate drug, ALD is also used extensively to reduce circulating VEGF thereby reducing metastasis for many forms of cancer (Santini et al. 2002). Pre-treatment of ALD significantly induced [3H]taurine uptake reduced by high glucose in TR-iBRB cells (Lee and Kang 2013). As in previous our result in TR-iBRB cells, ALD recovered both taurine uptake (Fig. 3) and TAUT expression (Fig. 4) in TR-BBB cells. Nonetheless, we could not conclude that HIF-1 is the sole inducer of the VEGF expression. Other factors may also be responsible for the VEGF expression, such as protein kinase C (Poulaki et al. 2002) and peroxisome proliferator-activated receptor γ cofactor-1α (O’Hagan et al. 2009). Further studies about regulation of VEGF by HIF-1 and other factors are needed.
5 Conclusion
In conclusion, taurine transport through the BBB can be down-regulated by high glucose, which is mediated to induction of HIF-1 and VEGF. Therefore, we suggest that the inhibitors of HIF-1 and VEGF could have the beneficial effects on hyperglycemia by up-regulation of taurine contents in brain.
Abbreviations
- 2ME2:
-
2-Methoxy estradiol
- ALD:
-
Alendronate
- BA:
-
Betulinic acid
- BBB:
-
Blood-brain barrier
- HIF-1:
-
Hypoxia inducible factor-1
- MTX:
-
Methotrexate
- TAUT:
-
Taurine transporter
- VEGF:
-
Vascular endothelial growth factor
- YC-1:
-
3-(5′-Hydroxymethyl-2′-furyl-1-benzylindazole)
References
Foos TM, Wu JY (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Frank RN (2004) Diabetic retinopathy. N Engl J Med 350:48–58
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Häussinger D (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16:231–233
Hosoya K, Takashima T, Tetsuka K et al (2000) mRNA expression and transport characterization of conditionally immortalized rat brain capillary endothelial cell lines; a new in vitro BBB model for drug targeting. J Drug Target 8:357–370
Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, Takiyama Y, Itoh H, Haneda M (2010) High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int 78:48–59
Ito T, Schaffer SW, Azuma J (2012) The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 42:1529–1539
Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T (2002) Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem 83:1188–1195
Kang YS, Lee NY, Chung YY (2009) The change of taurine transport in variable stress states through the inner blood-retinal barrier using in vitro model. Biomol Ther 17:175–180.
Lee NY, Kang YS (2010) The inhibitory effect of rivastigmine and galantamine on choline transport in brain capillary endothelial cells. Biomol Ther 18:65–70.
Lee NY, Choi HO, Kang YS (2012) The acetylcholinesterase inhibitors competitively inhibited an acetyl L-carnitine transport through the blood-brain barrier. Neurochem Res 37:1499–1507
Lee NY, Kang YS (2013) The effects of bisphosphonates on taurine transport in retinal capillary endothelial cells under high glucose conditions. Adv Exp Med Biol 776:59–66
Lorenzi M, Healy DP, Hawkins R, Printz JM, Printz MP (1986) Studies on the permeability of the blood-brain barrier in experimental diabetes. Diabetologia 29:58–62
Marfella R, D’Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D (2002) Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 45:1172–1181
Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J (1997) Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol 273:H2224–H2231
Nicholson BP, Schachat AP (2010) A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 248:915–930
O’Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB (2009) PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A 106:2188–2193
Pan C, Gupta A, Prentice H, Wu JY (2010) Protection of taurine and granulocyte colony-stimulating factor against excitotoxicity induced by glutamate in primary cortical neurons. J Biomed Sci 17(Suppl 1):S18
Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP (2002) Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109:805–815
Santini D, Vincenzi B, Avvisati G, Dicuonzo G, Battistoni F, Gavasci M, Salerno A, Denaro V, Tonini G (2002) Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res 8:1080–1084
Schrufer TL, Antonetti DA, Sonenberg N, Kimball SR, Gardner TW, Jefferson LS (2010) Ablation of 4E-BP1/2 prevents hyperglycemia-mediated induction of VEGF expression in the rodent retina and in Muller cells in culture. Diabetes 59:2107–2116
Schoch HJ, Fischer S, Marti HH (2002) Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 125(Pt 11):2549–2557
Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD (1999) Down regulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol 277:760–771
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510–5514
Yan J, Zhang Z, Shi H (2012) HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci 69:115–128
Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM (2007) Inhibition of hypoxia-induced increase of blood–brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol 72:440–449
Acknowledgements
This work was supported by the grant of Sookmyung Women’s University (No. 1-1203-0008).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Lee, NY., Kang, YS. (2015). The Changes by Hypoxia Inducible Factor-1alpha (HIF-1α) on Taurine Uptake in Brain Capillary Endothelial Cells at High Glucose Conditions. In: Marcinkiewicz, J., Schaffer, S. (eds) Taurine 9. Advances in Experimental Medicine and Biology, vol 803. Springer, Cham. https://doi.org/10.1007/978-3-319-15126-7_40
Download citation
DOI: https://doi.org/10.1007/978-3-319-15126-7_40
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15125-0
Online ISBN: 978-3-319-15126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)