Abstract
Typically, proper history and clinical examination can establish the diagnosis of lymphedema and differentiate the type (primary or secondary). Nevertheless, additional tests are sometimes necessary, particularly in the early stages of the disease and in edemas of combined etiology. The imaging studies primarily confirm the presence of impaired lymphatic flow and/or the typical pattern of abnormal fluid distribution within the tissues. Conventional imaging modalities like MRI, CT scan, and US provide valuable information regarding the anatomical distribution and etiology of lymphedema.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Key Points
-
Although the diagnosis of lymphedema is largely clinical, it is crucial to choose the appropriate imaging modalities and have awareness of the common imaging features of lymphedema.
-
Conventional modalities (MRI, CT scan, and Ultrasound) not only show indirect evidence of impaired function of lymphatic channels but also provide anatomical details that may complement the functional assessment provided by lymphoscintigraphy and can be occasionally necessary to establish the diagnosis.
-
These complementary imaging studies may be necessary to rule out the causes of secondary lymphedema and assess the response to therapy.
Introduction
Although the diagnosis of lymphedema is largely clinical, it is crucial to choose the appropriate imaging modalities and have awareness of the common imaging features of lymphedema.
Lymphedema has been divided into primary and secondary (acquired) [1]. Primary lymphedema results mainly from impaired drainage of lymph due to congenital defect in the peripheral lymph transporting system, including collecting lymphatic channels and nodes (i.e., aplasia, hypoplasia, and hyperplasia) [1, 2]. Primary lymphedema is occasionally associated with central conducting lymphatic anomaly. Secondary lymphedema results from obstruction or disruption of the normal collecting lymphatic system due to different pathologic processes such as metastatic disease, radiation, surgical injury, or infection [2–4].
Limb swelling and edema of the extremity, which may simulate lymphedema, can be caused by other local disorders (such as venous hypertension) or systemic disease (such as congestive heart failure, liver disease, renal disease, and hypoalbuminemia) [5]. Conventional imaging modalities are not only helpful in confirming the diagnosis of lymphedema but also in excluding other etiologies of limb swelling.
History and clinical examination can usually establish the diagnosis of lymphedema and differentiate the type (primary or secondary). Nevertheless, additional tests are sometimes necessary, particularly in the early stages of the disease and in edemas of combined etiology.
The imaging studies primarily confirm the presence of impaired lymphatic flow and/or the typical pattern of abnormal fluid distribution within the tissues [4].
The diagnosis of primary lymphedema can be confirmed by functional assessment of the lymphatic channels by modalities like bipedal lymphangiography, lymphoscintigraphy, lymphatic capillaroscopy, and near-infrared (NIR) fluorescence imaging. These modalities are discussed in different chapters.
In this chapter, we discuss the role of conventional imaging modalities (including magnetic resonance imaging (MRI), magnetic resonance lymphangiography, computer tomography (CT) scan, ultrasonography, and plain radiography) in confirming the diagnosis of lymphedema, identifying any underlying causes and gauging response to therapy. These modalities not only show indirect evidence of impaired function of lymphatic channels but also provide anatomical details that complement the data provided by the aforementioned functional tests.
Magnetic Resonance Imaging
MRI relies on the fact that when a radiofrequency pulse is briefly applied to tissues in a magnetic field, the proton relaxation time is dependent on the type of tissue. As the magnetic vector returns to its resting state, this causes a radio wave to be emitted, which is then used to create an image. Usually MRI is performed with a 1.5–3.0 Tesla scanner equipped with high-performance gradients. Imaging of the lower extremity may require more than one station: the calf and the foot region; around the knee region; and proximal thigh and the pelvic region. A dedicated peripheral surface coil is used to examine the upper and lower leg while a phased-array body coil is used to image the pelvic region. MR pulse sequences that are most useful in the imaging of lymphedema include fat-sensitive T1-weighted sequence and fluid-sensitive sequences such as inversion recovery (STIR) or fat-suppressed T2-weighted sequences.
Gadolinium-enhanced, fat-suppressed T1-weighted sequences are required if diagnosis of secondary lymphedema is considered or in the follow up cases of already diagnosed primary lymphedema in context of lymphangitis or cellulitis. Flow-sensitive gradient echo sequences to assess vascularity (magnetic resonance angiography) are not generally necessary.
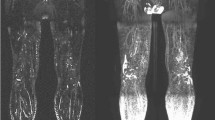
In primary lymphedema, the images reveal a characteristic distribution of edema within the epifascial compartment with a classic reticular (honeycomb) pattern and thickening of the subcutaneous layer (see Fig. 14.1 a, b). These changes are typically circumferential. In chronic primary lymphedema, stasis of lymph stimulates progressive fat deposition and tissue fibrosis [6, 7]. The enlarged lymph channels and fat thickening can be identified on MRI (Fig. 14.1 c, d).
MRI of the right leg in primary lymphedema. Axial T2-W fat-saturated (a) and T1-W fat-saturated, contrast-enhanced (b) MR images of the calf demonstrate extensive circumferential subcutaneous soft tissue thickening and reticular (honeycomb) pattern above the fascia with thickening of the subcutis and dermis. Note reticular post-contrast enhancement suggestive of lymphangitis/cellulitis. Coronal T1-W (c) and T2-W fat-saturated (d) MR images of right calf demonstrate predominantly subcutaneous extrafascial distribution of fluid and fat accumulation; a classic feature of chronic primary lymphedema
MRI can also be useful in the differential diagnosis of lymphedema [8–10]. In edema due to venous disease both the epifascial and subfascial compartments may be affected; the characteristic reticular pattern may or may not present. While in lipidemia, the fat accumulation occurs without signs of lymphatic congestion or reticular appearance [10]. The anatomic details provided by MRI may complement the functional assessment provided by lymphoscintigraphy and can be occasionally necessary to establish the diagnosis [11].
MRI may be helpful in differentiating the various causes of lymphatic obstruction in secondary lymphedema by demonstrating dilated lymphatic trunks and identification of abnormal lymph nodes. Malignant nodal involvement can be assessed further by lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI). These nanoparticles produce a significant susceptibility effect which can be detected as a drop in signal intensity on T2-weighted images [12]. Within a normal lymph node, nanoparticles accumulate within the reticuloendothelial system (phagocytized by macrophages) and show homogeneous uptake resulting in dark signal on T2-weighted images. In a node which is either partially or completely infiltrated by malignant cells, there is absence of functioning macrophages, leading to a lack of nanoparticle uptake resulting in focal or complete area of bright signal intensity on T2-weighted images. This technique is highly effective in identifying metastases in non-enlarged and partially replaced nodes; however, due to the negative-contrast nature of the detection, small lesions can be missed [13]. In one study, sensitivity and specificity of LNMRI was 76.5 % and 98.4 % in diagnosing nodal metastasis [14]. Lymphatic flow velocities can be assessed in lymphedema by visualization of lymphatic flow using principles of spin labeling MR imaging, and thus, lymphedema etiogenesis and therapies may be interrogated without exogenous contrast agents [15].
Magnetic Resonance Lymphography (MRL)
Magnetic resonance lymphangiography (MRL) is a recently added technique in which gadolinium-based, MRI contrast agent is injected for the visualization of lymphatic vessels in patients with primary and secondary lymphedema [16, 17]. A mixture of MRI contrast agent (gadobenate dimeglumine 0.1 mmol per kilogram or gadopentetate dimeglumine 0.2 mmol per kilogram of body weight) and 2 mL of Bupivacaine hydrochloride 0.25 % or Mepivacaine hydrochloride 1 % is injected intracutaneously into the interdigital webs of the dorsal aspect of both feet. Before MR lymphangiography, the extent and distribution of the lymphedema is evaluated using a heavily T2-weighted 3D turbo spin-echo sequence. For MR lymphangiography a 3D spoiled gradient-echo sequence [volumetric interpolated breath hold examination (VIBE)] is used. The three stations are first imaged without contrast material and subsequently repeated at 5, 15, 25, 35, 45, and 55 min after intracutaneous application of contrast. To emphasize the gadolinium-containing structures, baseline images are subtracted before 3D maximum intensity projection (MIP) reconstructions are calculated.
In one study, contrast MRL was capable of evaluating the anatomical and functional status of lymphatic vessels and lymph nodes in primary and secondary lymphedema by real-time visualization of enhanced lymph flow in lymphatic channels and within lymph nodes. In primary lymphedema, there were three major types of lymphatic system malformation: (a) only lymph nodes affected, (b) only lymph vessels affected, and (c) both lymph vessels and lymph nodes affected. In secondary lymphedema, MRL demonstrated tortuous and dilated collecting lymphatics in lymphedematous limbs [18].
In other study, diagnostic accuracy of magnetic resonance imaging (MR-lymphangiography) was calculated relative to the lymphoscintigraphy gold standard for assessment of focal lesions of the peripheral lymphatic system. MR-lymphangiography had sensitivity of 68 %, specificity of 91 %, positive predictive value of 82 %, and negative predictive value of 83 %. There was substantial correlation of results between the two modalities [19].
MR lymphangiography using interstitial injection of gadofosveset trisodium (Ablavar®, Lantheus Medical, North Billerica, MA) alone or premixed with 10 % human serum albumin (HSA) was used to visualize thoracic duct (TD) in a pig model [20]. Intradermal injection of nano-sized gadolinium-labeled dendrimer was also shown to rapidly opacify the deep lymphatic system, including the thoracic duct, in mice and pigs [21].
MRL is relatively noninvasive and can be used to identify anatomic and physiological abnormalities associated with lymphatic dysfunction in order to determine further treatment strategies [16, 17].
Computed Tomography
Although MRI is the preferred modality for assessing lymphedema, computed tomography (CT) can also be used, particularly when MRI cannot be technically or safely performed (e.g., uncooperative patients, unstable cardiovascular or respiratory status, contraindications to MRI). Acquiring CT scan studies is faster and can be performed without sedation or general anesthesia in infants and young children.
In lymphedema (see Fig. 14.2), CT scan demonstrates the characteristic reticular pattern and thickening of the subcutaneous tissue [22, 23]. It also provides anatomic localization of the edema which helps differentiate epifascial versus epifascial and subfascial edema. CT venography can also assess increased interstitial fluid formation due to venous hypertension (incompetent valves, venous obstruction). CT may be used to monitor responses to compression therapy in lymphedema through serial measurements of the cross-sectional area and tissue density in the tissue compartments of interest [24].
Ultrasonography
Ultrasonography (US) is utilized as a noninvasive diagnostic tool for the evaluation of lymphedema. US can be used to rule out the cause of increased interstitial fluid formation due to systemic disease (congestive heart failure, liver disease, renal disease). High-frequency linear-array probes are best for evaluation of superficial tissue. Gray-scale images are routinely obtained in transverse and longitudinal planes. In patients with lymphedema, it shows the thickening of the cutaneous, epifascial tissue compartments, interstitial fluid accumulation and occasionally may allow evaluation of the degree of fibrosis (Fig. 14.3). High frequency sonographic images reveal the characteristic patterns of cutaneous fluid localization in various types of edema [25]. In one study of patients with secondary lymphedema, the relative proportion of fluid and fibrosis identified on sonography correlated well with the clinical findings of soft, medium, hard, or pitting type of edema [26].
Low-flow color Doppler settings permits optimal visualization of small vessels and detection of low-flow vessels and thus help in the evaluation of deep and superficial venous systems, epifascial structure, confirming venous anomalies (i.e., valvular incompetence, obstruction, ectasia,) or excluding venous obstruction [27].
Plain Radiographs
The role of plain radiographs of extremities in the diagnosis of lymphedema is limited. Plain radiographs may show the epifascial soft tissue thickening of affected limb, secondary bone changes due to edema (Fig. 14.4) and limb-length discrepancies.
Conclusion
Conventional imaging modalities like MRI, CT scan, and US can be used to diagnose lymphedema. These studies also may provide information regarding the anatomical distribution of lymphedema, and assess response to therapy. MRI, CT scan, and US also are used to diagnose causes of extremity swelling other than lymphedema.
References
Kinmonth JB, Taylor GW, Tracy GD, Marsh JD. Primary lymphœdema. Clinical and lymphangiographic studies of a series of 107 patients in which the lower limbs were affected. Br J Surg. 1957;45(189):1–10.
Browse NL, Stewart G. Lymphoedema: pathophysiology and classification. J Cardiovasc Surg. 1985;26(2):91–106.
Rockson SG. Lymphedema. Am J Med. 2001; 110(4):288–95.
Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med (Lond, Engl). 1998;3(2):145–56.
Szuba A, Razavi M, Rockson SG. Diagnosis and treatment of concomitant venous obstruction in patients with secondary lymphedema. J Vasc Interv Radiol. 2002;13(8):799–803.
Zampell JC, Aschen S, Weitman ES, Yan A, Elhadad S, De Brot M, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129(4):825–34.
Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129(4):838–47.
Duewell S, Hagspiel KD, Zuber J, von Schulthess GK, Bollinger A, Fuchs WA. Swollen lower extremity: role of MR imaging. Radiology. 1992;184(1):227–31.
Haaverstad R, Nilsen G, Myhre HO, Saether OD, Rinck PA. The use of MRI in the investigation of leg oedema. Eur J Vasc Surg. 1992;6(2):124–9.
Haaverstad R, Nilsen G, Rinck PA, Myhre HO. The use of MRI in the diagnosis of chronic lymphedema of the lower extremity. Int Angiol. 1994;13(2):115–8.
Case TC, Witte CL, Witte MH, Unger EC, Williams WH. Magnetic resonance imaging in human lymphedema: comparison with lymphangioscintigraphy. Magn Reson Imaging. 1992;10(4):549–58.
Guimaraes R, Clement O, Bittoun J, Carnot F, Frija G. MR lymphography with superparamagnetic iron nanoparticles in rats: pathologic basis for contrast enhancement. AJR Am J Roentgenol. 1994;162(1):201–7.
Saksena MA, Saokar A, Harisinghani MG. Lymphotropic nanoparticle enhanced MR imaging (LNMRI) technique for lymph node imaging. Eur J Radiol. 2006;58(3):367–74.
McDermott S, Thayer SP, Fernandez-Del Castillo C, Mino-Kenudson M, Weissleder R, Harisinghani MG. Accurate prediction of nodal status in preoperative patients with pancreatic ductal adenocarcinoma using next-gen nanoparticle. Transl Oncol. 2013;6(6):670–5.
Rane S, Donahue PM, Towse T, Ridner S, Chappell M, Jordi J, et al. Clinical feasibility of noninvasive visualization of lymphatic flow with principles of spin labeling MR imaging: implications for lymphedema assessment. Radiology. 2013;269(3):893–902.
Lohrmann C, Foeldi E, Bartholoma JP, Langer M. Interstitial MR lymphangiography—a diagnostic imaging method for the evaluation of patients with clinically advanced stages of lymphedema. Acta Trop. 2007;104(1):8–15.
Liu NF, Lu Q, Jiang ZH, Wang CG, Zhou JG. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg. 2009;49(4):980–7.
Liu N, Zhang Y (2014) Magnetic Resonance Lymphangiography for the Study of Lymphatic System in Lymphedema. J Reconstr Microsurg (in press).
Weiss M, Burgard C, Baumeister R, Strobl F, Rominger A, Bartenstein P, et al. Magnetic resonance imaging versus lymphoscintigraphy for the assessment of focal lymphatic transport disorders of the lower limb: first experiences. Nuklearmedizin. 2014;53(5):190–6.
Turkbey B, Kobayashi H, Hoyt Jr RF, Choyke PL, Nakajima T, Griffiths GL, et al. Magnetic resonance lymphography of the thoracic duct after interstitial injection of gadofosveset trisodium: a pilot dosing study in a porcine model. Lymphat Res Biol. 2014;12(1):32–6.
Sena LM, Fishman SJ, Jenkins KJ, Xu H, Brechbiel MW, Regino CA, et al. Magnetic resonance lymphangiography with a nano-sized gadolinium-labeled dendrimer in small and large animal models. Nanomedicine (Lond). 2010;5(8):1183–91.
Hadjis NS, Carr DH, Banks L, Pflug JJ. The role of CT in the diagnosis of primary lymphedema of the lower limb. AJR Am J Roentgenol. 1985;144(2):361–4.
Marotel M, Cluzan R, Pascot M, Ghabboun S, Alliot F, Lasry JL. Computerized tomography of 150 cases of lymphedema of the leg. J Radiol. 1998;79(11):1373–8.
Collins CD, Mortimer PS, D’Ettorre H, A’Hern RP, Moskovic EC. Computed tomography in the assessment of response to limb compression in unilateral lymphoedema. Clin Radiol. 1995;50(8):541–4.
Gniadecka M. Localization of dermal edema in lipodermatosclerosis, lymphedema, and cardiac insufficiency. High-frequency ultrasound examination of intradermal echogenicity. J Am Acad Dermatol. 1996;35(1):37–41.
Balzarini A, Milella M, Civelli E, Sigari C, De Conno F. Ultrasonography of arm edema after axillary dissection for breast cancer: a preliminary study. Lymphology. 2001;34(4):152–5.
Lee B, Andrade M, Bergan J, Boccardo F, Campisi C, Damstra R, et al. Diagnosis and treatment of primary lymphedema. Consensus document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2010;29(5):454–70.
Statement of Financial Interest
No financial support or benefits were given to the authors from any source that is related to the scientific work reported in the paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Goyal, P., Chaudry, G., Alomari, A.I. (2015). Conventional Imaging Modalities for the Diagnosis of Lymphedema. In: Greene, A., Slavin, S., Brorson, H. (eds) Lymphedema. Springer, Cham. https://doi.org/10.1007/978-3-319-14493-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-14493-1_14
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14492-4
Online ISBN: 978-3-319-14493-1
eBook Packages: MedicineMedicine (R0)