Abstract
The study of polarity and its role in cell–cell interactions, such as spermatid polarity and adhesion in the seminiferous epithelium, and blood–testis barrier (BTB) in the testis during the epithelial cycle of spermatogenesis in recent years has yielded some unexpected and interesting observations. Similar to other polarized tissues, Sertoli and germ cells in the seminiferous epithelium express many of the component proteins of the Par-, Scribble- and Crumb-based polarity protein complexes. These polarity proteins are working in concert with non-receptor protein kinases, adhesion proteins, and cytoskeletons to confer spermatid and Sertoli cell polarity, and these proteins are also involved in germ cell transport in the epithelium during the epithelial cycle. In this review, we summarize the latest findings in the field. Based on the available data in the literature, it is increasingly clear that polarity proteins are crucial in (1) conferring spermatid and Sertoli cell polarity, (2) regulating spermatid adhesion and transport, and (3) regulating BTB dynamics in the testis during the epithelial cycle. We also highlight specific areas of research that deserve attention in future years. This information should be helpful to investigators in other blood–tissue and epithelial barriers in the field.
This work was supported by grants from the National Institutes of Health (NICHD, U54 HD029990, Project 5 to C.Y.C.; R01 HD056034 to C.Y.C.); NSFC/RGC Joint Research Scheme (N_HKU 717/12 to W.M.L.), Hong Kong Research Grants Council General Research Fund (771513 to W.M.L.), and University of Hong Kong CRCG Seed Funding (to W.M.L.).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Blood–testis barrier
- Cell polarity
- Cell–cell interactions
- Ectoplasmic specialization
- F-actin
- Germ cell
- Microtubule
- Sertoli cell
- Spermatogenesis
- Testis
1 Introduction
1.1 Concept of Cell–Cell Interactions, Cell Polarity, and the BTB in the Testis
The two major functions of the mammalian testis are to produce testosterone, a hormone synthesized by the interstitial Leydig cells to maintain sexual characteristics of the male, and spermatozoa in the seminiferous tubules for fertilization to safeguard the continuation of a species. One man produces >300 million spermatozoa, as compared to ~10 and 50 million in a male mouse and rat, respectively, via spermatogenesis each day following puberty until death (Auharek et al. 2011; Johnson et al. 1980; Mauss and Hackstedt 1972). This thus illustrates there are tightly regulated and robust cellular events that take place in the testis to maintain this tempo of sperm output. Spermatogenesis is a complex cellular process, and it can be divided into three discrete events: (1) mitosis, for self-renewal of spermatogonial stem cells (SSC) and spermatogonia; (2) meiosis, to ensure that the amount of genetic material carried in each spermatid is half that of spermatocytes; and (3) spermiogenesis, for the development of spermatids into functional spermatozoa, such that during fertilization, the fusion of a single sperm and an ovum reconstitute the genetic material similar to other somatic cells. These events all take place in the seminiferous epithelium of the seminiferous tubule—the functional unit in the testis that produces sperm—via the epithelial cycle of spermatogenesis (Fig. 13.1). These events are supported by testosterone produced by Leydig cells in the interstitium and also estrogen produced by Sertoli and germ cells (Sharpe 1994; O’Donnell et al. 2001; Carreau and Hess 2010; Carreau et al. 2010; Verhoeven et al. 2010; Wang et al. 2009; McLachlan et al. 2002; Mruk and Cheng 2004b). Recent studies have shown that peritubular myoid cells in the tunica propria also play an important role in supporting spermatogenesis (Welsh et al. 2009; Qian et al. 2013). It is conceivable that there are extensive cell–cell interactions in the testis, in particular at the Sertoli–Sertoli and Sertoli–germ cell interface (Fig. 13.2) during the epithelial cycle of spermatogenesis (Fig. 13.1).
Stages of the seminiferous epithelial cycle of spermatogenesis. The epithelial cycle of spermatogenesis in the rat is divided into 14 (I–XIV) stages and lasts 12.8 days, and the duration, in hour (h), of each cycle from I through XIV is shown. It is crucial to note that each stage does not contain one type of germ cell; rather each stage is unique and contains germ cells at different stages in their maturation and development. For instance, in the seminiferous epithelium of a stage VIII tubule, only type A1 spermatogonia, preleptotene spermatocytes, pachytene spermatocytes, and step 8 and 19 spermatids are found. However, a type A1 spermatogonium (at the bottom of the figure on the left panel) will go through the epithelial cycle ~4.5 times until it finally matures into step 19 spermatid, taking a total of ~58 days to complete. Spermatogonia, type A (A) containing A1, A2, A3 and A4, type A1 undergo mitosis (A1 m), type A2 undergo mitosis (A2 m), type A3 undergo mitosis (A3 m), type A4 undergo mitosis (A4 m), intermediate (ln), type In undergo mitosis (Inm), and type B (B), type B undergo mitosis (Bm). Spermatocytes, preleptotene (Pl), leptotene (L), zygotene (Z), pachytene (P), and diplotene (Di). Spermatids, round spermatids (steps 1–8), and elongate spermatids (steps 9–19). This figure was prepared based on the earlier reports and reviews (Dym and Clermont 1970; de Kretser and Kerr 1988; Mruk et al. 2008). Sertoli cell nucleus near the base of the seminiferous epithelium in each stage of the cycle is also shown
A schematic drawing of the cross section of a seminiferous tubule, illustrating different cell junctions at the Sertoli–Sertoli and Sertoli–germ cell interface in the rat testis. The BTB, which is constituted by coexisting actin-based TJ (tight junction), basal ES (ectoplasmic specialization) and gap junction, as well as intermediate filament-based desmosome, physically divides the seminiferous epithelium into the basal and the adluminal compartment. The basal compartment is in close contact with the basement membrane, a modified form of extracellular matrix in the mammalian testis. Apical ES is restricted to the Sertoli–spermatid interface of step 8–19 spermatids, whereas gap junction and desmosome are at the Sertoli–spermatid interface of step 1–7 spermatids. Preleptotene spermatocytes transformed from type B spermatogonia at stage VII of the cycle will be transported across the BTB at late VII–VIII of the cycle (see Fig. 13.1). Thus, the actin-based cytoskeleton undergoes extensive restructuring, and this event is tightly coordinated with the tubulin-based cytoskeleton which serves as the track for the transport of germ cells across the BTB. This mechanism is also used to transport developing spermatids across the adluminal compartment during spermiogenesis
As noted in Fig. 13.2 illustrating the cross section of a typical seminiferous tubule, such as in the rat testis, during the 14 stages of epithelial cycle from I through XIV (Fig. 13.1), elongating/elongated spermatids are highly polarized cells in which their heads are all pointing toward the basement membrane (BM) with their tails toward the tubule lumen (Fig. 13.2), such that the maximal number of developing spermatids can be packed in the tubule to allow the simultaneous development of millions of germ cells. It is noted that spermatid polarity is affected during toxicant- or drug-induced disruption in spermatogenesis such as following treatments with cadmium or adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, a potential male contraceptive under development in our laboratory (Cheng et al. 2005, 2011a)] in which exposure of rats to these toxicants induces misorientation of elongating/elongated spermatids in the epithelium (Cheng et al. 2011b; Wong et al. 2008b). This loss of spermatid polarity appears to be related to the subsequent loss of spermatid adhesion in the epithelium, causing their eventual departure from the testis prematurely (Wong and Cheng 2009; Wong et al. 2008a), thereby leading to infertility (Siu et al. 2009; Mok et al. 2011).
Besides spermatids, Sertoli cells in the epithelium are also highly polarized cells in which their nuclei are located restrictively near the basement membrane (Fig. 13.1). The ultrastructures that constitute the blood–testis barrier (BTB), such as the actin-based tight junction (TJ), basal ectoplasmic specialization (basal ES), and gap junction, as well as the intermediate-based desmosome, are also restricted near the basement membrane which also physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartments (Fig. 13.2). On the other hand, the testis-specific actin-rich anchoring device only found at the Sertoli–spermatid (steps 8–19) interface is restricted to the adluminal compartment (Fig. 13.2). Collectively, these findings illustrate that the Sertoli cell is a highly polarized cell type in the epithelium. Furthermore, Sertoli cells are cyclic in nature during the epithelial cycle. For instance, the protein secretory and phagocytic activities of Sertoli cells are stage-dependent. It is also noted that at each stage of the epithelial cycle, Sertoli cells are catered to specific cellular events that are unique to that specific stage. For example, in the rat testis, meiosis takes place at stage XIV of the cycle (Fig. 13.1), whereas the release of sperm at spermiation and the transit of preleptotene spermatocytes at the BTB take place at stage VIII of the cycle (Figs. 13.1 and 13.3). Thus, at stage VIII, Sertoli cells are working in concert with elongated spermatid to prepare for apical ES degeneration at the adluminal compartment, and Sertoli cells per se are also undergoing restructuring in which a “new” BTB behind and the “old” BTB above the preleptotene spermatocytes (note: preleptotene spermatocytes transformed from type B spermatocytes are the only germ cell type that are transported across the BTB to enter the adluminal compartment for further development) in transit are being assembled and disassembled, respectively. Furthermore, these spermatocytes are connected in “clones” via intercellular bridges (Weber and Russell 1987; Fawcett 1961), making their transport across the BTB a highly coordinated and regulated cellular event. These findings thus illustrate Sertoli cells are highly cyclic in function during the epithelial cycle in which the Sertoli cell performs different functions according to the cycle of the epithelium (Fig. 13.1). As such, in order for spermatogenesis to proceed flawlessly during the epithelial cycle, Sertoli cells must be highly polarized, and perhaps compartmentalized, ultrastructurally and functionally, so that different functions can be performed at discrete domains of a Sertoli cell throughout spermatogenesis. This is particularly important since it is known that a single Sertoli cell is in close contact with ~30–50 germ cells at different stages of their development (Wong and Russell 1983; Weber et al. 1983) to support these germ cells functionally, structurally, and nutritionally, as each type of germ cell has distinct functional needs that are different from each other.
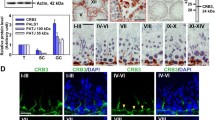
Stage specific expression of actin and α-tubulin. Dual-labeled immunofluorescence analysis was performed in which α-tubulin (red) was found to co-localize with and F-actin (green) throughout stages I–XIV of the seminiferous epithelial cycle in the rat. Stage I–XIV of the epithelial cycle can also be found in Fig. 13.1. Scale bar, 30 μm
2 The Seminiferous Epithelial Cycle of Spermatogenesis and Ectoplasmic Specialization
2.1 The Epithelial Cycle of Spermatogenesis
The seminiferous epithelial cycle refers to the specific pattern of cellular association between Sertoli and germ cells and the cellular events pertinent to spermatogenesis that take place within the epithelium (de Kretser and Kerr 1988; Hess and de Franca 2008; Mruk et al. 2008). The cycle of events is divided into 14 stages (I–XIV) in the rat (Fig. 13.1) versus 12 (I–XII) and 6 (I–VI) stages in the mouse and human testis, respectively (Hess and de Franca 2008; Amann 2008; Mruk et al. 2008; Parvinen and Vanha-Perttula 1972). Each stage has its own defining characteristics; for instance, in the rat testis, preleptotene spermatocytes are found only in stages VII–VIII of the cycle, spermiation is restricted to stage VIII (lasts ~29.1 h), and meiosis is limited to stage XIV (lasts ~17.6 h) (Fig. 13.2). The duration of a complete epithelial cycle (stages I–XIV) is about 12.8 days in the rat testis. However, a type A1 spermatogonium takes ~58 days to develop into a step 19 (mature) spermatid. Thus, a type A1 spermatogonium goes through the epithelial cycle a total of 4.5 times (Fig. 13.1). In short, Sertoli-germ cell associations differ with each stage, as each stage contains specific types of germ cells progressing through the epithelial cycle (Fig. 13.1). If a discrete section of a seminiferous tubule at stage VIII is visualized under a transillumination microscope, one will witness the progression to stages IX through XIV and then I through VIII again in 12.8 days (Fig. 13.1) (Parvinen 1982; Mruk et al. 2008; Lie et al. 2011).
2.2 Ectoplasmic Specialization
When the cross section of a seminiferous tubule, such as in the rat testis, is examined under electron microscope, a testis-specific actin-rich anchoring junction called ectoplasmic specialization (ES) is readily noted which is known to confer cell polarity, cell adhesion, and cellular structural support (Russell and Peterson 1985; Vogl et al. 2008; Cheng and Mruk 2002; Mruk and Cheng 2004a). In the testis, ES is either found in the basal compartment at the Sertoli cell–cell interface at the BTB called basal ES or in the adluminal (apical) compartment at the Sertoli–spermatid interface called apical ES (Fig. 13.2). ES is typified by the presence of actin filament bundles that lie perpendicular to the apposing plasma membranes of either Sertoli–Sertoli (basal ES) or Sertoli–spermatid (apical ES), which are sandwiched in between cisternae of the endoplasmic reticulum and the apposing plasma membranes (Fig. 13.2). Ultrastructurally, apical and basal ES are indistinguishable except that since actin filament bundles are only found in Sertoli cells and not in germ cells, a single array of actin filament bundles is found at the apical ES, whereas a double array of actin microfilaments is found at the basal ES. Once apical ES appears at the interface of Sertoli cells and step 8 spermatids in stage VIII tubules, it becomes the only anchoring device from steps 8 to 19 spermatids, replacing desmosome and gap junction at the Sertoli–spermatid (1–7) interface, and persists until its degeneration at spermiation. Unlike apical ES, basal ES at the Sertoli cell–cell interface never exists alone; rather it coexists with either TJ or gap junction, which together with desmosome constitutes the BTB, one of the tightest blood–tissue barriers in the mammalian body (Franca et al. 2012; Cheng and Mruk 2012; Pelletier 2011; Wong and Cheng 2005) (Fig. 13.2). In fact, when the force that is required to disrupt adhesion induced by apical ES versus desmosome is compared and quantified, ES is at least twice as strong as desmosome to confer steps 8–19 spermatid adhesion to the Sertoli cell (Wolski et al. 2005). This observation is unusual since desmosome, the predominant anchoring junction in skin, is considered to be the strongest adhesive junction in the mammalian body (Green and Simpson 2007; Thomason et al. 2010; Jamora and Fuchs 2002). This unusual strength of the ES apparently is contributed by the extensive network of actin filament bundles (Russell et al. 1988) (Fig. 13.2). It is of interest to note that while the appearance of apical ES at the Sertoli–spermatid (step 8) interface at stage VIII of the cycle replaces desmosome and gap junction between Sertoli cells and step 1–7 spermatids, apical ES is a hybrid atypical adherens junction (AJ) (Wong et al. 2008a; Yan et al. 2007) since it is constituted by proteins that are found in AJ (e.g., nectins, afadins, N-cadherin, β-catenin), TJ (e.g., JAM-C, CAR), gap junction (e.g., connexin 43), and focal adhesion complex (or focal contact) (e.g., α6β1-integrin, laminin-α3,β3,γ3, FAK, c-Src, c-Yes) (Cheng and Mruk 2010). Regardless of the ultrastructural similarity between the apical and basal ES, proteins that are found at the basal ES/BTB are quite different from those at the apical ES (Cheng and Mruk 2010).
2.3 The Uniqueness of the Seminiferous Epithelium Versus Other Epithelia
As noted in Fig. 13.1, the seminiferous epithelium is unique compared to other epithelia due to the seminiferous epithelial cycle of spermatogenesis. This is a unique cellular process in the mammalian body due to the cyclic events of the epithelial cycle such that millions of sperm can be produced from the tubules via spermatogenesis. Furthermore, as germ cells change shape, orientation, and size during maturation, they undergo extensive “adhesion” and “de-adhesion,” which involves major restructuring of junctions at the cell–cell interface. Additionally, germ cells are transported progressively from the basal to the adluminal compartment across the epithelium throughout the epithelial cycle so that step 19 spermatids can be lined up at the adluminal edge of the apical compartment to prepare for their release once they are transformed into spermatozoa at late stage VIII of the cycle (Figs. 13.1 and 13.2). Thus, it is envisioned that actin filament bundles at the ES must undergo cyclic reorganization from their “bundled” to “de-bundled” configuration and vice versa to facilitate the transport of preleptotene spermatocytes and spermatids across the BTB and the epithelium. It is of interest to note that germ cells per se are not motile cells, since they lack cellular structures, such as lamellipodia and filopodia found in fibroblasts, macrophages, and keratinocytes, and they rely solely on the Sertoli cell for their transport across the BTB and the epithelium. Thus, the transport of spermatids across the epithelium during spermiogenesis requires the presence of tubulin-based cytoskeleton/microtubules which serve as the track for cargo (e.g., spermatids) to be transported across the epithelium. In short, the actin- and tubulin-based cytoskeletons are working in concert via yet-to-be defined mechanism(s) to facilitate germ cell transport across the epithelium.
3 Polarity Proteins on Cell Polarity and Cell Adhesion During Spermatogenesis
3.1 Why Is Polarity Important During Spermatogenesis?
Spermatids are highly polarized cells in which the heads of spermatids point toward the basement membrane, while the tails point toward the tubule lumen. Spermatid polarity is crucial to maximize the production of sperm during spermatogenesis since the arrangement of polarized spermatids in the epithelium as noted in Fig. 13.1 allows the maximal number of spermatids that can be packed and developed simultaneously in the epithelium in the tubule. Similarly, polarized Sertoli cells can coordinate cellular events across the epithelium more efficiently so that signals can be sent across the Sertoli cell orderly and Sertoli cell can also communicate with its neighboring cells effectively during the epithelial cycle. In the testis, cell polarity is conferred by the Par (partitioning defective)-based protein complex (e.g., Par6, Par3, Cdc42, aPKC, Pals1, and PatJ), the Scribble-based complex (e.g., Scribble, Dlg, Lgl), and the Crumbs-based complex (e.g., CRB-3, Pals1, Patj) (Wong and Cheng 2009; Wong et al. 2008a). Each of these polarity protein complexes recruits its own binding partners, thus conferring cellular asymmetry; this is because a multiprotein complex can be effectively created for each protein complex and also because there is mutually exclusive distribution between Par-/CRB- and Scribble-based protein complexes across an epithelial cell, such as the Sertoli cell (Iden and Collard 2008; Assemat et al. 2008; Head et al. 2013; Goldstein and Macara 2007). During spermiogenesis, most of the cytosol is eliminated from the developing spermatids and transported to the residual body to be scavenged and cleaned up by the Sertoli cell (Fig. 13.2). Thus, there is scant cytosol remaining in the more mature spermatids, such as step 8–19 spermatids, particularly in the head region where apical ES is present both to anchor spermatids to the Sertoli cell and to confer spermatid polarity (note: acrosome that is found at the spermatid head represents a giant proteasome containing acrosin, a serine protease with trypsin-like specificity, and is to be used by the sperm to penetrate the zona pellucida at fertilization (Honda et al. 2002)). The mechanism(s) through which polarity proteins expressed by germ cells (Wong et al. 2008b; Su et al. 2012b) involved in conferring or regulating spermatid polarity is still not known.
3.2 Role of Par-Based Polarity Proteins on Cell Adhesion and Polarity in the Testis
Studies in the testis have shown that a knockdown of either Par3 or Par6 specifically by RNAi without detectable off-target effects impedes Sertoli cell TJ barrier. In these studies, proteins at the Sertoli cell–cell interface, such as JAM-A and α-catenin, became mis-localized, as these proteins no longer tightly localized to the Sertoli cell BTB, but relocated to the cell cytosol, thereby destabilizing the BTB function via a loss of Sertoli cell adhesion (Wong et al. 2008b). Furthermore, it appears that Par3 and Par6 regulate the localization of different BTB proteins differentially since the knockdown of Par3, but not Par6, induces mis-localization of ZO-1, whereas the knockdown of Par6, but not Par3, induces mis-localization of N-cadherin selectively (Wong et al. 2008b). These changes in protein localization appear to be the result of changes in the kinetics of endocytic vesicle-mediated protein trafficking, since the knockdown of Par5 (also known as 14-3-3) is found to accelerate the endocytosis of JAM-A and N-cadherin, thereby destabilizing the Sertoli cell BTB (Wong et al. 2009), illustrating the role of polarity proteins in cell adhesion function. Par6 was also found to be crucial to confer spermatid polarity in the rat testis. Adjudin induces spermatid loss; however, prior to spermatid loss, there is a loss of spermatid polarity as evidenced by the presence of misoriented spermatids in rats treated with the drug. Treatment of rats with adjudin was associated with considerable loss of Par6 surrounding the spermatid heads, which were pointed in all directions in the epithelium (Wong et al. 2008b). More important, this loss of spermatid polarity occurs before a disruption of spermatid adhesion onto the Sertoli cell in the epithelium is detected, seeming to suggest that spermatid polarity and adhesion are two intimately related events regulated by polarity proteins, such as Par3 and Par6, during spermatogenesis in the testis.
3.3 Role of Scribble-Based Polarity on Cell Polarity and Adhesion Is Mediated by Changes in the Actin-Based Cytoskeleton in the Testis
Scribble, Lgl (lethal giant larvae), and Dlg (discs large) are found to be expressed by both Sertoli and germ cells in the rat testis (Su et al. 2012b). Scribble is localized most notably at the Sertoli cell–cell interface when Sertoli cells establish a functional TJ-permeability barrier in vitro but it is also found in the cell cytosol (Su et al. 2012b). Scribble is also localized predominantly to the BTB in the seminiferous epithelium in vivo in virtually all stages of the epithelial cycle in the rat testis (Su et al. 2012b), illustrating polarity protein Scribble is involved in Sertoli cell polarity and adhesion, and it is involved in BTB dynamics during the epithelial cycle. While the knockdown of Scribble or Dlg1 alone fails to modulate the Sertoli cell TJ-barrier function, the simultaneous knockdown of Scribble and its two integral component proteins Dlg1 and Lgl2 by RNAi using specific siRNA duplexes with no detectable off-target effects is shown to promote the Sertoli cell TJ-permeability barrier, making it “tighter” (Su et al. 2012b), illustrating its role in inducing BTB restructuring during the epithelial cycle. This promoting effect of Scribble on the Sertoli cell BTB function is supported by studies using immunofluorescence microscopy since a considerable increase in occludin and β-catenin localization to the Sertoli cell–cell interface is found in the Sertoli cell epithelium following the simultaneous knockdown of Scribble, Dlg1 and Lgl2 (Su et al. 2012b). More important, when Scribble/Dlg1/Lgl2 is knocked down in the testis in vivo, an increase in occludin localization to the BTB is detected in stage VIII tubules. This change is accompanied by a loss of spermatid polarity in the adluminal compartment in which spermatids no longer orientate properly with their heads pointing toward the basement membrane; instead spermatid alignment is disarrayed, concomitant with a downregulation of laminin-γ3 (Su et al. 2012b), an apical ES protein limited to the spermatid which forms a bona fide adhesion complex with α6β1-integrin in the Sertoli cell (Yan and Cheng 2006; Koch et al. 1999). Interestingly, this loss of spermatid polarity, downregulation of laminin-γ3 at the apical ES, and the increase in occludin at the BTB are associated with changes in the organization of F-actin at these sites; when F-actin is visualized by rhodamine phalloidin, more F-actin is found at the BTB whereas reduced levels of F-actin are detected at the apical ES (Su et al. 2012b). Collectively, these findings thus illustrate that the Scribble-based polarity protein complex supports spermatid polarity and adhesion, while it also promotes BTB dynamics possibly via restructuring, illustrating its antagonistic effects on the apical and basal ES in the testis during the epithelial cycle.
4 Actin- and Microtubule-Based Cytoskeletons and Their Role in Cell–Cell Interactions, Cell Polarity, and BTB Function
4.1 Cross Talk Between Actin and Tubulin Cytoskeletons
Across different cell types, both the actin filament and microtubule networks play critical roles in a variety of processes such as cell division, cell polarization, transport, and migration. Although actin- and tubulin-based cytoskeletons are usually portrayed as functionally independent networks, recent research has begun to unravel the cooperative interaction between them via some routes of communication or cross talk (Gavin 1997; Goode et al. 2000). The Sertoli cell is a highly polarized cell, as earlier described, and also is very dynamic via cyclic changes functionally, adapting to the evolving shape of germ cells as they progress through different stages of the epithelial cycle. This is made possible due to the dynamicity of both actin and microtubule (MT) cytoskeletons, both of which exemplify stage specificity within the seminiferous epithelial cycle. For example, at late stage VIII in the rat, both actin and tubulin (e.g., α-tubulin) levels diminish significantly at the apical ES at the onset of the spermiation (Fig. 13.3). Spermiation is a highly regulated temporal event; if not tightly regulated, it can result in premature or delayed release of spermatids (O’Donnell et al. 2011; Mruk and Cheng 2004b). As shown in Fig. 13.3, actin and α-tubulin co-localize in the majority of the 14 stages of the epithelial cycle in the rat testis (note: α- and β-tubulin are the structural components of tubulin-based cytoskeleton; see Fig. 13.4). Though this is a morphological observation, it lays the foundation for expanding this information on how actin and MT cytoskeletons work in concert to regulate spermatogenesis.
Microtubule structure in mammalian cells including the Sertoli cell in the testis. Microtubules (MTs) are assembled from αβ-tubulin dimers. β-tubulin designates the MT plus (+) end, and α-tubulin the minus (−) end. Polymerization of a MT occurs through the interaction of the α-subunit of an incoming dimer with the β-subunit of a preexisting dimer on a MT protofilament. GTP is bound to both α and β-subunits; however, only the β-tubulin GTP exhibits GTPase activity. GTP hydrolysis occurs as the MT is assembled, leaving most of the MT comprised of GDP-tubulin and the growing plus end as the only region where GTP is still bound to β-tubulin, known as the GTP-cap. γ-tubulin forms a protein complex called the γ-tubulin ring complex and is responsible for nucleation and stabilization of MTs
There are a number of MT regulatory proteins that not only associate with MTs but also with the actin cytoskeleton. For example, CLIP-170 (cytoplasmic linker protein of 170 kDa) is one MT regulatory protein that associates with myosin VI, which is an ATP-dependent actin motor protein, thus linking the actin and MT networks together. CLIP-170 is also known to be involved in the dynein and dynactin pathway (Akhmanova et al. 2005). In general, motor proteins are either plus- or minus-end-directed along MTs. Some formins, such as mDia1, are actin-nucleating proteins that also can stabilize MTs. Both CLIP-170 and mDia1 are present in testis and thus may be the subject of future studies on cross talk between the actin and tubulin networks in regulating key events of spermatogenesis. Table 13.1 summarizes results of findings that illustrate the likely function of microtubule regulatory proteins in microtubule dynamics and their role in male fertility via the use of genetic (such as gene knockout KO or N-ethyl-N-nitrosourea-induced mutation) or knockdown (KD) models. However, the molecular mechanism(s) by which these proteins regulate spermatogenesis remain largely unknown.
4.2 Functional Role of Actin- and Tubulin-Based Cytoskeletons in the Testis
The current understanding of spermatid transport via MTs and the involvement of actin-based cytoskeleon in the seminiferous epithelium remains elusive. Microtubules are abundant in Sertoli cells, and research thus far has implicated their role by serving as the tracks for translocation of spermatids throughout the epithelial cycle. As germ cells mature, they adopt an elongate shape; concomitant with elongation, spermatids are enveloped by the Sertoli cell, and these cells, unlike macrophages, neutrophils, and fibroblasts, are not motile cells. Instead, their transport across the epithelium during the epithelial cycle relies completely on Sertoli cells, and as such, it is logical to speculate that the “cargoes” (i.e., germ cells) require the presence of a “track” (i.e., polarized microtubules) for their transport (Redenbach and Vogl 1991). Indeed, microtubules, which are intrinsically polar, are arranged with their plus and minus ends directed basally and apically, respectively, within the Sertoli cell. Studies have revealed the presence of microtubule motor proteins in the testis, such as dynein and kinesin, which are MT minus and plus-end-directed motor proteins (Hall et al. 1992), supporting the idea that microtubules are in part responsible for the organized movement of spermatids across the seminiferous epithelium. Dynein is classified as a minus-end-directed motor protein, but when it forms a complex with dynactin, an adaptor, it can also be targeted to the plus end (Kardon and Vale 2009). During the epithelial cycle, germ cells are transported progressively up from the basal to the apical region of the seminiferous epithelium; however, during stage V in the rat, developing spermatids actually return to the basal region and are found deep inside the Sertoli cell crypts. This suggests the importance of bidirectional transport along the MTs during spermatogenesis. It has been proposed that the cytoplasmic side of the endoplasmic reticulum (ER) of the apical ES comes into contact with the microtubules, MTs thus confer ER the ability to move along the tracks while the actin filaments of the apical ES anchor the spermatid (Hall et al. 1992; Russell 1977).
Microtubules are polar cylindrical structures made from protofilaments of αβ-tubulin heterodimers. Protofilaments, which are assembled by the head-to-tail addition of αβ-tubulin subunits, arrange laterally to form a hollow tube (Fig. 13.3). MTs possess a property called dynamic instability, which describes both the polymerization and depolymerization that occurs at the plus end of MTs (Wade 2007; Mitchison and Kirschner 1984). As listed in Table 13.1, there are a number of proteins that regulate microtubules. For example, MT plus-end tracking proteins (+TIPs) such as CLIP-170 and EB1 can stabilize MTs. It is conceivable that these proteins are helping to stabilize MTs as spermatids are transported directionally across the epithelium. Stabilizing agents ensure a “steady ride” as the spermatids migrate ultimately toward the apex of the epithelium. Severing proteins, another type of MT regulatory protein, like katanin, which has been studied in the testis (Smith et al. 2012; O’Donnell et al. 2012), may play an important role in regulating transport. Tubulin expression is stage specific, as immunohistological staining show (Fig. 13.3), for instance, in some stages of the epithelial cycle, the length of the MTs that appears as “stalks” in the seminiferous epithelium in late VIII-IX stages are shorter than in others such as at stages V-VII (Wenz and Hess 1998). This observation may be attributed to severing proteins which as the name suggests, sever or cut the MT, to promote generation of new MTs. Transport which occurs across the seminiferous epithelium is a continuous process; thus it is probable that generation of new MTs coincides with spermatid movement.
In epithelia, apicobasal polarity requires specific targeting of proteins to both the apical and basal regions of the cell type. In the testis, protein trafficking in the seminiferous epithelium is crucial for regulation of discrete cellular events of spermatogenesis, such as mitosis, meiosis, spermiogenesis, and spermiation. Most of the current research on protein trafficking and cell–cell communication in the testis has only begun to elucidate the intimate relationship between the actin cytoskeleton and regulation of these cellular events (Su et al. 2013). Although most studies focus on actin dynamics and protein trafficking, there is much yet to be uncovered. In addition, the microtubule cytoskeleton is also at play in regulating the events of spermatogenesis, but how it does so is still the subject of future research.
Cell junction protein recruitment and endocytosis are two types of processes, not only found in the seminiferous epithelium but across all epithelia. Polarity proteins such as Par3/Par6, Scribble, Lgl, 14-3-3, and Cdc42 are involved in regulating these processes in the testis (Wong et al. 2008b; Su et al. 2012b). For example, Par3/Par6, which are established polarity proteins, has been shown to confer spermatid adhesion at the apical ES (Wong et al. 2008a). Changes in cell adhesion regulated by Par proteins may likely coordinate protein endocytosis, which is in part regulated by the actin network (Wong et al. 2009). Microtubules also play a role in endocytosis, via transport of endosomes and lysosomes (Matteoni and Kreis 1987), but the mechanism in the testis has yet to be defined. As previously mentioned, CLIP-170 is one MT regulatory protein that is involved in MT dynamics. This protein was first discovered for its role in linking endocytic vesicles to the MTs (Pierre et al. 1992); in addition to its role as a + TIP, it is a likely player in endocytic trafficking in the testis.
5 Polarity Proteins and Cytoskeletons in the Apical ES–BTB–Basement Membrane (BM) Functional Axis
5.1 The Apical ES–BTB–BM Functional Axis
The concept of a local functional axis that coordinates and regulates cellular events taking place across the seminiferous epithelium during the epithelial cycle was first reported in 2008 (Yan et al. 2008). It was noted that overexpression of fragments of laminin chains at the apical ES or purified recombinant proteins perturbed the Sertoli cell TJ-permeability barrier function by downregulating expression of proteins at the BTB, such as occludin and N-cadherin, but also β1-integrin at the hemidesmosome (HD) at the Sertoli-BM interface (Yan et al. 2008). These findings thus illustrate that matrix metalloproteinase-2 (MMP-2) which is highly expressed at the apical ES at stage VIII of the epithelial cycle (Siu and Cheng 2004) is capable of inducing cleavage of laminin chains at the apical ES during its degeneration to prepare for spermiation to release biologically active fragments. These autocrine-like fragments in turn induce BTB restructuring to facilitate the transit of preleptotene spermatocytes across the BTB. These fragments also perturb HD function, which creates a positive regulatory loop to further potentiate BTB restructuring. This possibility was confirmed by silencing β1-integrin at the HD in Sertoli cells, which indeed was found to perturb the Sertoli cell TJ-permeability function (Yan et al. 2008). Taken collectively, these data illustrate the presence of a functional axis that links cellular events that occur at the apical ES in the adluminal compartment at spermiation with BTB restructuring near the BM in the seminiferous epithelium, and also HD in the BM, at stage VIII of the epithelial cycle. Subsequently studies have shown that MMP-9 is also capable of inducing cleavage of collagen chains, mostly collagen α3(IV), in the BM to release the NC1 (non-collagenous 1) domain peptide, which was shown to perturb the Sertoli cell TJ barrier function (Wong and Cheng 2013). While the purified NC1 recombinant protein was shown not to downregulate the expression of BTB-associated proteins such as CAR-ZO-1 and N-cadherin-β-catenin, it effectively induced mis-localization of these proteins at the Sertoli cell BTB, so that they no longer localized predominantly at the Sertoli cell–cell interface; instead, they were relocalized to the cell cytosol, thereby destabilizing the Sertoli cell TJ-barrier function (Wong and Cheng 2013). Studies using the phthalate-toxicant model have confirmed the presence of this local functional axis in which a disruption of the apical ES by phthalate induces BTB restructuring and can compromise its integrity (Yao et al. 2009, 2010). Collectively, these data demonstrate unequivocally that there is a functional autocrine-based regulatory axis that coordinates cellular events, such as spermiation and BTB restructuring, which take place simultaneously at the opposite ends of the epithelium at stage VIII of the epithelial cycle. In brief, apical ES degeneration as well as HD/BM restructuring contribute to BTB restructuring, which is further induced by re-organization of collagen network in the BM at the tunica propria by generating NC1 domain-containing peptide. A recent study has identified the biologically active domain of the laminin-γ3 chain that induces BTB restructuring, and synthetic peptide based on this functional domain designated F5-peptide is known to perturb BTB integrity in vivo reversibly and it also induces germ cell loss from the epithelium, illustrating its potential to serve as an endogenously produced male contraceptive peptide (Su et al. 2012a). These findings using F5-peptide based on the biologically active fragment of laminin-γ3 chain illustrate that the apical ES-BTB-BM axis can be a target of male contraceptive development. In fact, studies have shown that this functional axis is a target of environmental toxicants, such as phthalates, BPA, cadmium, and PFOS (perfluorooctane sulfonate) (Wan et al. 2013b; Mazaud-Guittot 2011). A disruption of the critical regulatory components in this axis following exposure of men to these toxicants is likely the cause of reduced semen quality and sperm count as recently reported (Rolland et al. 2013; Toft et al. 2012).
5.2 Role of Focal Adhesion Kinase (FAK), Polarity Proteins, and Cytoskeletons at the Apical ES–BTB–BM Functional Axis
While the apical ES–BTB–BM functional is crucial to coordinate cellular events that take place in the seminiferous epithelium during the epithelial cycle, the molecules that are involved in the regulation and the underlying molecular mechanism(s) remain unknown. Recent studies have shown that non-receptor protein tyrosine kinases, such as FAK (Lie et al. 2012) and c-Yes (Xiao et al. 2013); polarity proteins, such as Par6 (Xiao et al. 2013); and actin-based cytoskeleton (Su et al. 2012a) are critical players in this functional axis. For instance, studies using different mutants of FAK have shown that p-FAK-Tyr407 and p-FAK-Tyr397 are crucial to the integrity of the BTB and apical ES, respectively (Lie et al. 2012). In fact, these two phosphorylated forms of FAK are shown to display antagonistic effects on the BTB integrity in which p-FAK-Tyr407 promotes whereas p-FAK-Tyr397 disrupts the Sertoli cell TJ-permeability barrier function (Lie et al. 2012). However, p-FAK-Tyr397 plays a dominant role in maintaining spermatid adhesion via its effects on the adhesive function of the apical ES (Wan et al. 2013a). Thus, p-FAK-Tyr407 and p-FAK-Tyr397 likely serve as the molecular “switches” by turning “on” and “off” cell adhesion function at the Sertoli cell BTB and also Sertoli–spermatid interface along the apical ES–BTB–BM axis. It has been reported that overexpression of p-FAK-Tyr407 that promotes BTB integrity can block the F5-peptide-induced Sertoli cell TJ-permeability barrier disruption (Su et al. 2012a). Also, the F5-peptide-mediated BTB disruption and spermatid loss in vivo is accompanied by a mis-localization of p-FAK-Tyr407 in which this activated form of FAK is no longer restricted tightly to the BTB and the apical ES (Su et al. 2012a). This, in turn, perturbs the organization of F-actin at the apical ES and the BTB (Su et al. 2012a), such that actin filament bundles fail to be properly reorganized at both sites in response to the epithelial cycle of spermatogenesis, likely the result of a disruption in actin polymerization. Thus, a failure in F-actin organization can no longer support adhesive function at the BTB and the apical ES, leading to unwanted BTB and apical ES restructuring or degeneration. This postulate is supported by studies in vitro since the promoting effects of p-FAK-Tyr407 on BTB integrity is mediated, at least in part, via the Arp2/3-N-WASP protein complex that alters the kinetics of branched actin polymerization (Lie et al. 2012; Cheng et al. 2013). In this context, it is of interest to note that since the F5-peptide administered to the testis can be rapidly metabolized and cleared, its disruptive effects on spermatogenesis are reversible and germ cells gradually re-populate the epithelium (Su et al. 2012a).
5.3 The Role of c-Yes, p-FAK-Tyr407, Par6, and F-actin on BTB and Apical ES Function at the Apical ES–BTB Axis
Studies have shown that FAK is the putative substrate of Src family kinases (SFK) such as c-Src and c-Yes in most epithelia including the seminiferous epithelium (Zhao and Guan 2010; Boutros et al. 2008; Xiao et al. 2012). Also, multiple proteins at the BTB and apical ES are binding partners of SFK and/or FAK (Xiao et al. 2012; Li et al. 2013; Cheng and Mruk 2012). In fact, the dual FAK/Src complex is one of the primary targets of chemotherapy (Bolos et al. 2010) and inflammatory and autoimmune diseases (Lowell 2011). Thus, it is not surprising that c-Yes is recently shown to be a crucial player in the apical ES–BTB axis (Xiao et al. 2013). For instance, a knockdown of c-Yes by RNAi was shown to perturb the Sertoli cell TJ-barrier function both in vitro and in vivo, mediated via a disorganization of F-actin at the BTB, in which actin microfilaments no longer tightly restricted to the BTB near the BM (Xiao et al. 2013). These findings are consistent with an earlier report by using SU6656, a selective inhibitor of c-Yes, to probe the role of c-Yes in modulating F-actin organization in Sertoli cells (Xiao et al. 2011). Interestingly, the knockdown of c-Yes in the testis that affects the BTB integrity also perturbs apical ES function, disrupting spermatid polarity and adhesion, which is mediated by a mis-localization of p-FAK-Tyr407 and also polarity protein Par6 (Xiao et al. 2013), illustrating there is a feedback loop between the apical ES and the BTB. These changes, namely, mis-localization of p-FAK-Tyr407 and Par6 at the apical ES, thus impede actin microfilaments at the apical ES, leading to mis-localization of adhesion protein nectin-3, causing defects in spermatid transport and spermiation, so that elongated spermatids are entrapped in the seminiferous epithelium even at the site close to the BM in stage IX tubules (Xiao et al. 2013). These data thus illustrate the intimate functional relationship between FAK/SKF (e.g., p-FAK-Tyr407, c-Yes), polarity proteins (e.g., Par6), and cytoskeletons (e.g., actin microfilaments). Any changes on the cross talk between these proteins would impede cell adhesion function at the apical ES and/or the BTB, illustrating their pivotal role in the apical ES–BTB–BM functional axis.
6 Concluding Remarks and Future Perspectives
Findings discussed herein thus illustrate the intimate relationship between polarity proteins, cell–cell interactions at the Sertoli–Sertoli, and Sertoli–spermatid interface and cytoskeleton in the seminiferous epithelium during the epithelial cycle. It is also noted that non-receptor protein tyrosine kinases, in particular FAK and SFK (e.g., c-Yes and c-Src), are intimately involved in these events. At the time of this writing, no concrete data were found in the literature providing credible information regarding the mechanism(s) by which polarity proteins regulate cytoskeleton and vice versa in the testis. Except for in studies using different animal models, such as the adjudin model, it was shown that the loss of spermatid polarity due to a downregulation of Par6 was likely mediated by adjudin-induced truncation and defragmentation of actin filament bundles at the apical ES (Wong et al. 2008b). This thus destabilized the actin-based adhesion protein complexes at the apical ES, such as integrin–laminin, nectin–afadin, leading to premature loss of spermatids from the epithelium, analogous to “spermiation.” Also, we have yet to integrate the concept regarding the role of tubulin-based cytoskeleton into the biology of spermatid transport using the apical ES and the biology of preleptotene spermatocyte transport at the BTB using the basal ES, and how actin- and tubulin-based cytoskeletons are working in concert to regulate germ cell transport. For instance, several actin regulatory proteins have been identified and studied in the testis; virtually no tubulin regulatory proteins have been investigated in the testis except for several microtubule motor proteins, such as dynein and kinesin. This is an area of research that deserves some attention in future years.
References
Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, Drabek K, van der Wees J, Mommaas M, Onderwater J, van der Meulen H, Tanenbaum ME, Medema RH, Hoogerbrugge J, Vreeburg J, Uringa EJ, Grootegoed JA, Grosveld F, Galjart N (2005) The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev 19:2501–2515. doi:10.1101/gad.344505
Amann RP (2008) The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl 29:469–487
Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D (2008) Polarity complex proteins. Biochim Biophys Acta 1778:614–630
Auharek SA, Avelar GF, Lara NLM, Sharpe RM, Franca LR (2011) Sertoli cell numbers and spermatogenic efficiency are increased in inducible nitric oxide synthase (iNOS) mutant-mice. Int J Androl 34:e621–e629
Bartolini F, Ramalingam N, Gundersen GG (2012) Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 23:4032–4040
Bolos V, Gasent JM, Lopez-Tarruella S, Grande E (2010) The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther 3:83–97
Boutros T, Chevet E, Metrakos P (2008) Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60:261–310
Bruning-Richardson A, Langford KJ, Ruane P, Lee T, Askham JM, Morrison EE (2011) EB1 is required for spindle symmetry in mammalian mitosis. PLoS One 6:e28884
Carreau S, Hess RA (2010) Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365:1517–1535
Carreau S, Wolczynski S, Galeraud-Denis I (2010) Aromatase, estrogens and human male reproduction. Phil Trans R Soc Lond B Biol Sci 365:1571–1579
Cheng CY, Lie PPY, Wong EWP, Mruk DD (2013) Focal adhesion kinase and actin regulatory/binding proteins that modulate F-actin organization at the tissue barrier. Lesson from the testis. Tissue Barriers 1:e24252
Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B (2011a) Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis 1:291–297
Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 82:825–874
Cheng CY, Mruk DD (2010) A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6:380–395
Cheng CY, Mruk DD (2012) The blood-testis barrier and its implication in male contraception. Pharmacol Rev 64:16–64
Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY (2005) AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception 72:251–261
Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, Yan HHN, Mannu J, Mathur PP, Bonanomi M, Silvestrini B, Mruk DD (2011b) Environmental toxicants and male reproductive function. Spermatogenesis 1:2–13
Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci 114(Pt 6):1179–1187
de Kretser DM, Kerr JB (1988) The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW (eds) The physiology of reproduction, vol 1. Raven, New York, pp 837–932
Denk F, Wade-Martins R (2009) Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging 30(1):1–13. doi:10.1016/j.neurobiolaging.2007.05.010
Drabek K, Gutierrez L, Vermeij M, Clapes T, Patel SR, Boisset JC, van Haren J, Pereira AL, Liu Z, Akinci U, Nikolic T, van Ijcken W, van den Hout M, Meinders M, Melo C, Sambade C, Drabek D, Hendriks RW, Philipsen S, Mommaas M, Grosveld F, Maiato H, Italiano JE Jr, Robin C, Galjart N (2012) The microtubule plus-end tracking protein CLASP2 is required for hematopoiesis and hematopoietic stem cell maintenance. Cell Rep 2:781–788
Dym M, Clermont Y (1970) Role of spermatogonia in the repair of the seminiferous epithelium following X-irradiation of the rat testis. Am J Anat 128:265–282
Fawcett DW (1961) Intercellular bridges. Exp Cell Res 8:174–187
Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT (2012) Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 763:237–259
Ganem NJ, Upton K, Compton DA (2005) Efficient mitosis in human cells lacking poleward microtubule flux. Curr Biol 15:1827–1832
Gavin RH (1997) Microtubule-microfilament synergy in the cytoskeleton. Int Rev Cytol 173:207–242
Goldstein B, Macara IG (2007) The PAR proteins: fundamental players in animal cell polarization. Dev Cell 13:609–622
Goode BL, Drubin DG, Barnes G (2000) Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol 12:63–71
Green KJ, Simpson CL (2007) Desmosomes: new perspectives on a classic. J Invest Dermatol 127:2499–2515
Hall ES, Eveleth J, Jiang C, Redenbach DM, Boekelheide K (1992) Distribution of the microtubule-dependent motors cytoplasmic dynein and kinesin in rat testis. Biol Reprod 46:817–828
Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N (1994) Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369:488–491
Head BP, Patel HH, Insel PA (2013) Interaction of membrane/lipid rafts with the cytoskeleton. Impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta (in press), doi: 10.1016/jbbamem201307018
Hess RA, de Franca LR (2008) Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636:1–15
Honda A, Siruntawineti J, Baba T (2002) Role of acrosomal matrix proteases in sperm-zona pellucida interactions. Hum Reprod Update 8:405–412
Iden S, Collard JG (2008) Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9:846–859
Jamora C, Fuchs E (2002) Intercellular adhesion, signaling and the cytoskeletons. Nat Cell Biol 4:E101–E108
Johnson L, Petty CS, Neaves WB (1980) A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod 22:1233–1243
Kardon JR, Vale RD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10:854–865
Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE (2004) Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell 15:1146–1159
Koch M, Olson P, Albus A, Jin W, Hunter D, Brunken W, Burgeson R, Champliaud M (1999) Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol 145:605–618
Li SYT, Mruk DD, Cheng CY (2013) Focal adhesion kinase is a regulator of F-actin dynamics. New insights from studies in the testis. Spermatogenesis 3:e25385
Lie PPY, Cheng CY, Mruk DD (2011) The biology of the desmosome-like junction: a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol 286:223–269
Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY (2012) Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci U S A 109:12562–12567
Liedtke W, Leman EE, Fyffe RE, Raine CS, Schubart UK (2002) Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am J Pathol 160:469–480
Lowell CA (2011) Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol 3:a002352
Lowery LA, Stout A, Faris AE, Ding L, Baird MA, Davidson MW, Danuser G, Van Vactor D (2013) Growth cone-specific functions of XMAP215 in restricting microtubule dynamics and promoting axonal outgrowth. Neural Dev 8:22
Matteoni R, Kreis TE (1987) Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol 105:1253–1265
Mauss J, Hackstedt G (1972) The effect of unilateral orchiectomy and unilateral cryptorchidism on sperm output in the rat. J Reprod Fertil 30:289–292
Mazaud-Guittot S (2011) Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod 85:1091–1093
McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, De Kretser DM, Pratis K, Robertson DM (2002) Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res 57:149–179
Mimori-Kiyosue Y, Grigoriev I, Sasaki H, Matsui C, Akhmanova A, Tsukita S, Vorobjev I (2006) Mammalian CLASPs are required for mitotic spindle organization and kinetochore alignment. Genes Cells 11:845–857
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242
Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY (2011) Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction 141:571–580
Mruk DD, Cheng CY (2004a) Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab 15:439–447
Mruk DD, Cheng CY (2004b) Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25:747–806
Mruk DD, Silvestrini B, Cheng CY (2008) Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 60:146–180
O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG (2011) Spermiation: the process of sperm release. Spermatogenesis 1:14–35
O’Donnell L, Rhodes D, Smith SJ, Merriner DJ, Clark BJ, Borg C, Whittle B, O'Connor AE, Smith LB, McNally FJ, de Kretser DM, Goodnow CC, Ormandy CJ, Jamsai D, O’Bryan MK (2012) An essential role for katanin p80 and microtubule severing in male gamete production. PLoS Genet 8:e1002698
O’Donnell L, Robertson KM, Jones ME, Simpson ER (2001) Estrogen and spermatogenesis. Endocr Rev 22:289–318
Parvinen M (1982) Regulation of the seminiferous epithelium. Endocr Rev 3:404–417
Parvinen M, Vanha-Perttula T (1972) Identification and enzyme quantification of the stages of the seminiferous epithelial wave in the rat. Anat Rec 174:435–450
Pelletier RM (2011) The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 46:49–127
Pierre P, Scheel J, Rickard JE, Kreis TE (1992) CLIP-170 links endocytic vesicles to microtubules. Cell 70:887–900
Qian Y, Liu S, Guan Y, Pan H, Guan X, Qiu Z, Li L, Gao N, Zhao Y, Li X, Lu Y, Liu M, Li D (2013) Lgr4-mediated Wnt/β-catenin signaling in pertibular myoid cells is essential for spermatogenesis. Development 140:1751–1761
Redenbach DM, Vogl AW (1991) Microtubule polarity in Sertoli cells: a model for microtubule-based spermatid transport. Eur J Cell Biol 54:277–290
Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J (2013) Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod 28:462–470
Russell L (1977) Observations on rat Sertoli ectoplasmic (“junctional”) specializations in their association with germ cells of the rat testis. Tissue Cell 9(3):475–498
Russell LD, Goh JC, Rashed RMA, Vogl AW (1988) The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod 39:105–118
Russell LD, Peterson RN (1985) Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 94:177–211
Schober JM, Kwon G, Jayne D, Cain JM (2012) The microtubule-associated protein EB1 maintains cell polarity through activation of protein kinase C. Biochem Biophys Res Commun 417:67–72
Schubart UK, Yu J, Amat JA, Wang Z, Hoffmann MK, Edelmann W (1996) Normal development of mice lacking metablastin (P19), a phosphoprotein implicated in cell cycle regulation. J Biol Chem 271:14062–14066
Sharpe RM (1994) Regulation of spermatogenesis. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 1363–1434
Silva VC, Cassimeris L (2013) Stathmin and microtubules regulate mitotic entry in HeLa cells by controlling activation of both Aurora kinase A and Plk1. Mol Biol Cell 24:3819–3831
Siu ER, Mruk DD, Porto CS, Cheng CY (2009) Cadmium-induced testicular injury. Toxicol Appl Pharmacol 238:240–249
Siu MKY, Cheng CY (2004) Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod 70:945–964
Smith LB, Milne L, Nelson N, Eddie S, Brown P, Atanassova N, O’Bryan MK, O’Donnell L, Rhodes D, Wells S, Napper D, Nolan P, Lalanne Z, Cheeseman M, Peters J (2012) KATNAL1 regulation of sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS Genet 8:e1002697
Stout JR, Rizk RS, Kline SL, Walczak CE (2006) Deciphering protein function during mitosis in PtK cells using RNAi. BMC Cell Biol 7:26
Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY (2012a) A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun 3:1185
Su W, Mruk DD, Cheng CY (2013) Regulation of actin dynamics and protein trafficking during spermatogenesis-insights into a complex process. Crit Rev Biochem Mol Biol 48(2):153–172
Su WH, Wong EWP, Mruk DD, Cheng CY (2012b) The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology 153:6041–6053
Thomason HA, Scothern A, McHarg S, Garrod DR (2010) Desmosomes: adhesive strength and signalling in health and disease. Biochem J 429:419–433
Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, Lenters V, Vermeulen R, Rylander L, Pedersen HS, Ludwicki JK, Zviezdai V, Bonde JP (2012) Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod 27:2532–2540
Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K (2010) Androgens and spermatogenesis: lessons from transgenic mouse models. Phil Trans R Soc Lond B Biol Sci 365:1537–1556
Vogl AW, Vaid KS, Guttman JA (2008) The Sertoli cell cytoskeleton. Adv Exp Med Biol 636:186–211
Wade RH (2007) Microtubules: an overview. Meth Mol Med 137:1–16
Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY (2013a) p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab 305:E687–E699
Wan HT, Mruk DD, Wong CKC, Cheng CY (2013b) The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol Med 19:396–405
Wang RS, Yeh S, Tzeng CR, Chang C (2009) Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30:119–132
Weber JE, Russell LD (1987) A study of intercellular bridges during spermatogenesis in the rat. Am J Anat 180:1–24
Weber JE, Russell LD, Wong V, Peterson RN (1983) Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat 167:163–179
Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB (2009) Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J 23:4218–4230
Wenz JR, Hess RA (1998) Characterization of stage-specific tyrosinated alpha-tubulin immunoperoxidase staining patterns in Sertoli cells of rat seminiferous tubules by light microscopic image analysis. Tissue Cell 30:492–501
Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF (2005) Strength measurement of the Sertoli-spermatid junctional complex. J Androl 26:354–359
Wong CH, Cheng CY (2005) The blood-testis barrier: its biology, regulation and physiological role in spermatogenesis. Curr Topics Dev Biol 71:263–296
Wong EWP, Cheng CY (2009) Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 278:309–353
Wong EWP, Cheng CY (2013) NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis 3:e25465
Wong EWP, Mruk DD, Cheng CY (2008a) Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta 1778:692–708
Wong EWP, Mruk DD, Lee WM, Cheng CY (2008b) Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A 105:9657–9662
Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY (2009) 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology 150:4713–4723
Wong V, Russell LD (1983) Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat 167:143–161
Xiao X, Mruk DD, Cheng CY (2013) c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab 304:E145–E159
Xiao X, Mruk DD, Cheng FL, Cheng CY (2012) c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol 763:295–317
Xiao X, Mruk DD, Lee WM, Cheng CY (2011) c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 43:651–665
Yan HHN, Cheng CY (2006) Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 281:17286–17303
Yan HHN, Mruk DD, Lee WM, Cheng CY (2007) Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays 29:36–48
Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY (2008) An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A 105:8950–8955
Yao PL, Lin YC, Richburg JH (2009) TNFα-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod 80:581–589
Yao PL, Lin YC, Richburg JH (2010) Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod 82:516–527
Zhao X, Guan JL (2010) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63:610–615
Disclosure Statement
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Tang, E.I., Mruk, D.D., Lee, W.M., Cheng, C.Y. (2015). Cell–Cell Interactions, Cell Polarity, and the Blood–Testis Barrier. In: Ebnet, K. (eds) Cell Polarity 1. Springer, Cham. https://doi.org/10.1007/978-3-319-14463-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-14463-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14462-7
Online ISBN: 978-3-319-14463-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)