Abstract
Portal vein embolization (PVE) is used to increase the volume of normal liver tissue, allowing resection of liver malignancy in patients with an insufficient future liver remnant (FLR). PVE is recommended when FLR <20% in normal liver, FLR <30% in liver pretreated with more than 3 months of chemotherapy, and FLR <40% in the cirrhotic liver. Embolization of the right or left portal vein branch causes atrophy of the ipsilateral liver and hypertrophy of the contralateral liver. Right PVE is the most common procedure, as the volume usually favors the right side of the liver. Embolization of segment 4 portal vein branches has proven feasible and effective, in addition to right PVE, to increase regeneration in patients requiring an extended right hepatectomy. Percutaneous transhepatic ipsilateral PVE is the preferred approach, which is safe, with low rates of complications and mortality. We encourage the use of standardized FLR (sFLR) to determine the need for PVE and when measuring the effect of PVE. sFLR is calculated by dividing the measured FLR, determined by contrast-enhanced CT, by the total estimated liver volume (TEL). TEL is calculated based on the BSA. The percent of regeneration can be termed the degree of hypertrophy (DH). The DH divided by the number of weeks can be termed the kinetic growth rate (KGR), and is a functional measurement of the regenerative capacity of the liver. Both are useful predictors when assessing risk of postoperative hepatic insufficiency.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Liver resection
- Portal vein embolization (PVE)
- Two-stage hepatectomy
- Future liver remnant (FLR)
- Failure
- Degree of hypertrophy
- Kinetic growth rate
The Future Liver Remnant and the Rationale for Portal Vein Embolization
Makuuchi et al. performed the first preoperative portal vein embolization (PVE) in 1982 before an extended hepatectomy for bile duct carcinoma [1]. PVE has proven to be an effective method, allowing major liver resections to be performed more safely [2, 3]. The aim of PVE is to increase the future liver remnant (FLR), thereby allowing liver resection with reduced risk of hepatic insufficiency and death. The risk of liver insufficiency is inversely proportional to the FLR.
Surgical Strategy and Preoperative Assessment of FLR
Evaluation of Candidates for Portal Vein Embolization
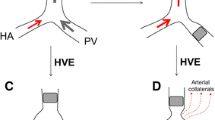
Adequate imaging is essential for staging as well as evaluation of resectability. Liver protocol computed tomography (three-phase CT) and magnetic resonance imaging (MRI) are the most common modalities utilized. In patients being evaluated for liver resection, the FLR is defined as the total liver volume minus the planned resected volume (Fig. 7.1). The volume of the FLR and subsequently risk of postoperative liver insufficiency and death may limit the surgical options in patients with bilateral or centrally placed colorectal liver metastases. Patients with large centrally located lesions involving two hepatic veins can be cleared with extended liver resection performed in one stage, but after PVE if the estimated FLR in insufficient. In patients with bilateral CLM, a two-stage hepatectomy with PVE between the two stages may be required to clear all disease located in the left (partial resection segment I, II and/or III) or right (partial resection of segment V, VIII, VI and/or VII) before performing right or left PVE and subsequently extended right or left resection of the liver with atrophied liver with the remaining disease (Fig. 7.2).
Two-stage hepatectomy may be required to clear all metastatic liver disease in the case of multiple bilateral metastases. The FLR, usually segments 2 and 3 (± parts of 1 and 4), is cleared during the first surgical stage, followed by PVE. The sFLR is re-evaluated and if sufficient, the patient can undergo the planned extended right liver resection in a second surgical stage, usually 4–8 weeks after the portal vein embolization (PVE) was performed
Due to the liver volume alterations occurring during embryology, the right liver represents on average 66% of the total liver volume, and left PVE is rarely indicated, as the right hemiliver to be preserved almost always represents sufficient sFLR. The left liver represents on average 33% of the total liver volume, and PVE is required in approximately 10% of patients undergoing right hepatectomy and in 75% of patients undergoing extended right hepatectomy with preservation of only segment 2 and 3 (Figs. 7.3 and 7.4) [4].
Distributions of FLR volume of according to types of major hepatectomy. Adapted from Abdalla EK et al. Surgery 2004 [4] with permission
Contrast-enhanced CT image of patient with colorectal liver metastases undergoing evaluation for PVE. Segments 2 and 3 represent the FLR, with a total volume of 253 ml. The sFLR was 15%, indicating need for PVE before liver resection. *Estimated total liver volume (TEL) is calculated to be 1,686 with the formula TEL = −794.41 + 1,267.28 × body surface area (BSA). LHV left hepatic vein, MHV middle hepatic vein, RHV right hepatic vein, P portal branch to segment, S segment, R ant PV right anterior portal vein
There are two absolute contraindications for PVE: extensive ipsilateral tumor thrombus because most of the portal flow has already been diverted, and clinically evident portal vein hypertension because of the risk of bleeding varicies of the increased portal pressure from the procedure [5]. Renal insufficiency, coagulopathy, advanced liver fibrosis, and main portal vein thrombosis are conditions with increased risk of complication during or after PVE, and should be assessed as relative contraindications [2].
It is likely that as little as 10% sFLR may be sufficient in some patients with normal liver function [6]. However, a number of studies have demonstrated a significant impact on postoperative complications in patients with preoperative sFLR ≤20% [2, 3, 6]. Therefore, sFLR ≤20% is considered an evidence-based cut-off for PVE in the normal liver. The cut-off for preoperative PVE in the injured liver has not been explored to the same extent. However, studies have showed increased rates of postoperative complications and hepatic insufficiency after resection of liver with steatosis or chemotherapy-induced injury [7,8,9]. Many centers therefore consider the cut-off sFLR <30% an indication for PVE in these patients.
A substantial number of patients with cirrhosis are not candidates for major hepatic resection due to an unacceptable risk of perioperative death. However, patients with Child–Pugh class A cirrhosis are considered for resection if their sFLR is >40%. If sFLR is less than 40% but otherwise resectable, PVE is indicated [10]. This is supported by the findings of a prospective study showing decreased postoperative complications, ICU admissions, and length of hospital stay for patients with chronic liver disease who underwent PVE before resection [11]. Because of the severity of the liver injury occurring, the same cut-off for sFLR (>40%) has been suggested after prolonged biliary obstruction.
Preoperative Assessment of FLR
The FLR must be determined in patients who undergo evaluation for liver resection with a concern for insufficient volume. The most common method of measuring absolute FLR volume is to outline the FLR on axial slices from multiphase contrast-enhanced CT. Based on the area of the outlined FLR and the slice thickness, three-dimensional reconstructions are obtained and the absolute FLR volume can be calculated. However, the absolute FLR volume is inadequate for clinical decision-making, as larger patients require larger FLR. To account for this, most groups now use the ratio FLR to total liver volume (TLV), often termed standardized FLR (sFLR). Only functional non-tumor volume should be included when determining sFLR, and the TLV is calculated directly from three-dimensional computed tomography, subtracting the tumor volume. The main disadvantage with this method is the fact that determining the TLV is time-consuming and may not be accurate in patients with bile duct obstruction.
In our practice, the TLV is based on an estimated TLV (TEL). The TEL is based on the correlation between body surface area (BSA) and total liver volume [12]. Several formulas have been developed, but a meta-analysis found the most accurate to be: TEL =− 794.41 + 1267.28 × BSA [12, 13]. BSA can be calculated using Mosteller’s formula:\( BSA=\sqrt{\frac{\mathrm{height}\kern0.5em \left[ cm\right]\times \mathrm{weight}\kern0.5em \left[ kg\right]}{3600}} \)[14]. Furthermore, this method of calculating sFLR (FLR/TEL) has shown correlation with patient outcomes, and thus proven its clinical relevance [2, 6]. At MD Anderson Cancer Center, a web-based calculator has been design-based on these formulas to calculate the sFLR, degree of hypertrophy, and the kinetic growth rate (Fig. 7.5). The correlation between the BSA and the functional liver volume and the formula presented was developed in Western adults in the United States and Europe [12, 13]. It is important to note that TEL can vary between body size and race. Japanese patients have up to 19% larger livers compared to Caucasians for a given body weight. In some centers, especially in Asia, three-dimensional computer models are increasingly used to calculate FLR and sFLR based on the total liver volume.
Web-based calculator used to determine the degree of hypertrophy and kinetic growth rate (KGR) after PVE. Segment volumes are in ml (cm3). Numbers are representative for a patient undergoing right PVE. The formulas used to calculate body surface area (BSA) [14] and total estimated liver volume (TEL) [12] are:\( BSA=\sqrt{\frac{\mathrm{Height}\kern0.5em \left[ cm\right]\times \mathrm{weight}\kern0.5em \left[ kg\right]}{3600}} \) and TEL = 794.41 + 1267.28 × BSA. The calculations used to generate the output are: \( {}^1 Pre\kern0.5em \mathrm{EMBO}\kern0.5em \mathrm{sFLR}\kern0.5em \left( CT\#1\right)=\frac{Seg2+ Seg3\left(+ Seg4\right)\left(+ Seg1\right)}{Pre\kern0.5emTEL} \) and\( {}^2\mathrm{Post}\kern0.5em \mathrm{EMBO}\kern0.5em \mathrm{sFLR}\kern0.5em \left( CT\#2\right)=\frac{Seg2+ Seg3\left(+ Seg4\right)\left(+ Seg1\right)}{\mathrm{Post}\kern0.5emTEL} \)and 3Degree of Hypertrophy = Post EMBO sFLR − Pre EMBO sFLR and \( {}^4\mathrm{Kinetic}\kern0.5em \mathrm{growth}\kern0.5em \mathrm{rate}\kern0.5em (KGR)=\frac{\mathrm{Degree}\kern0.5em \mathrm{of}\kern0.5em \mathrm{Hypertrophy}}{\mathrm{Weeks}\kern0.5em \mathrm{Between}\kern0.5emCT\#2\kern0.5emand\kern0.5emPVE} \)
The sFLR is estimated before and 3–4 weeks after PVE. If sFLR after PVE meet the resectability criteria, it is generally accepted that the planned resection can be performed within accepted risk of adverse events. At MD Anderson Cancer Center, the following cut-offs are used for FLR resectability criteria: normal liver >20%, liver pretreated with more than 3 months of chemotherapy >30%, cirrhosis >40% (Fig. 7.6) [6, 15,16,17,18,19]. While cirrhosis is rare in patients with CLM, an increasing number are heavily pretreated with chemotherapy. Obesity is increasing worldwide, and hepatic steatosis is also a more common finding which require >30% FLR for safe resection [17].
Requirements for FLR depends on the underlying liver function. In the presence of liver injury, increased FLR is needed to allow safe liver resection with acceptable risk of hepatic insufficiency and death. 1Abdalla et al. Arch Surg 2002, 2Vauthey et al. Ann Surg 2004, 3Azoulay et al. Ann Surg 2000, 4Kubota Hepatology 1997. CTX chemotherapy BMI body mass index
Techniques of Portal Vein Embolization
Accessing the Portal Vein
The portal vein can be accessed for embolization during surgery, but with the increased experience within the field of interventional radiology, the percutaneous technique is currently the method of choice in most centers. Surgical PVE is usually performed via the ileocolic vein, while percutaneous PVE is performed ultrasound-guided transhepatic with catheter access through a distal branch of the ipsilateral or contralateral portal vein. The ipsilateral approach is often chosen due to safety reasons, as the FLR is left without risk of damage [20]. However, the ipsilateral approach may be technically more challenging, and holds a greater potential of peritoneal spillage of tumor cells. Reverse-curve catheters can be used to facilitate access to the segmental branches and cope with the increased technical challenge with the ipsilateral approach (Fig. 7.7).
Ipsilateral right portal vein embolization with embolization of segment 4 portal vein branches. The portal vein is entered via a distal branch of the right posterior portal vein, and the segment 4 embolization (a) is performed before embolization of the right posterior (b) and anterior (c) portal vein branch. The access tract is embolized to prevent capsular hemorrhage. Care should be taken to avoid puncturing tumor tissue due to potential peritoneal spillage of cancer cells when using the ipsilateral approach
Agents Used for Embolization and the Technique
Agents used to embolize the portal vein must be easy and safe to deliver, cause complete occlusion preferably without any recanalization, and be well tolerated by the patient. A number of agents have been used to induce portal vein embolus, including n-butyl cyanoacrylate (NBCA) and ethiodized oil, fibrin glue, ethanol, and microparticles such as polyvinyl alcohol or trisacryl gelatin. To date, no study has convincingly demonstrated the superiority of any those, and the choice of agent is mostly operator-determined. After the PVE catheter has been maneuvered into place, the vascular sheet is secured and a flush portography is performed to assess the portal anatomy. The portal pressure is measured before the embolization takes place. At MD Anderson Cancer Center, a combination of trisacryl gelatin microspheres of various sizes and embolization coils are used. Small caliber microspheres are used initially to embolize smaller distal portal vein branches, followed by larger caliber microspheres in larger proximal portal vein branches. Upon complete stasis, embolization coils are placed proximally to prevent recanalization. Care must be taken in every step not to embolize non-target branches of the portal vein.
Embolization of Segment 4 Portal Vein Branches
Extended right hepatectomy involves resection of the middle hepatic vein and segment 4 or parts of segment 4. In cases where the left lateral section (segment 2 and 3) constitute the FLR, right PVE may not always ensure sufficient volume. This led to the idea that the superior and inferior segment 4 portal vein branches form the left portal vein could be co-embolized to increase atrophy of as much liver tissue to undergo resection as possible, and subsequently induce even further hypertrophy of the FLR (Figs. 7.7 and 7.8). Furthermore, segment 4 is at risk of increased growth in conventional right PVE, which may be unsuitable if segment 4 contains tumor and is planned to undergo resection [21, 22]. Since the late 1990s, several groups have published a significant increase in the degree of hypertrophy when segment 4 portal vein branches were co-embolized with the right portal vein (Fig. 7.9) [23, 24].
Portogram with the catheter in the right portal vein before PVE showing a contrast-filled right portal vein three. Portogram with the catheter placed in the main portal vein after right PVE and embolization of segment 4 branches. White arrows indicate coils in the segment 4 branches. Black arrows indicate the right anterior and right posterior portal vein. Adapted from Madoff DC et al. J Vasc Interv Radiol 2005 [37] with permission
In patients where segment 4 is targeted for portal vein embolization, segment 4 embolization should be performed prior to the right portal vein embolization for safety reasons (Fig. 7.7). If non-target left portal vein embolization or left portal vein injury occurs during the segment 4 embolization, the right portal vein embolization can be aborted. Furthermore, if the ipsilateral method is chosen, the technical aspects of replacing the catheters through the already embolized right portal vein may be challenging and cause dislodging of embolic material when maneuvering to segment 4 branches.
Measuring Effect and Outcome After Portal Vein Embolization
Degree of Hypertrophy
Observational studies have demonstrated that regeneration after PVE occurs slower than after hepatic resection, possibly due to apoptosis, as opposed to frank necrosis, which occurs after PVE [25]. The expected degree of hypertrophy is correlated with the degree of underlying liver disease. The normal liver may regenerate at a pace of up to 21 ml per day, while the same number for the cirrhotic patient may be 9 ml per day [26]. As such, sufficient hypertrophy can occur within 2 weeks in the normal liver, while regeneration may take >6 weeks in the cirrhotic. Degree of hypertrophy (DH) is the term used for the percent increase of the FLR when comparing pre-PVE and post-PVE sFLR. For example, the degree of hypertrophy is 10% if the total volume of segment 2 and 3 increased form 17 to 27% (Fig. 7.5).
Kinetic Growth Rate
Time is an important determinant for the degree of hypertrophy, which is often assessed as primary end-point for PVE. However, time is not included in the equation to determine the degree of hypertrophy. Furthermore, the risk of hepatic insufficiency and postoperative complications is lower in a patient reaching sufficient hypertrophy after 2 weeks, compared to a patient requiring 6 weeks to reach the same hypertrophy.
Kinetic growth rate (KGR) has been proposed as a tool to evaluate regeneration over time. To calculate KGR, the degree of hypertrophy is divided by the number of weeks from the PVE to the date of the post-PVE CT evaluation. A KGR >2% (meaning 2% FLR volume increase per week), is associated with low risk of hepatic insufficiency and mortality after major liver resection, irrespective of the sFLR. As such, KGR represents a functional measurement of the regenerative capacity of the liver after PVE. Caution should be taken if KGR <1% and salvage options after PVE should be considered or the second stage aborted, especially if the sFLR does not meet the resection criteria (Fig. 7.5).
Salvage Options After PVE Failure
In rare patients, PVE fails to cause hypertrophy of the FLR. These patients should be assessed for missed underlying liver disease explaining the absence of regeneration. Furthermore, depending on the technique and agent used to embolize the portal vein branches, the PVE may have been unsuccessful, or recanalization may have occurred. In such cases, a second attempt to embolize the portal vein can be attempted. Embolization of segment 4 portal vein branches can also be tried as a salvage option in patients when the FLR fails to regenerate after conventional right PVE.
There are three main factors limiting liver regeneration: portal inflow, bile outflow, and hepatic outflow. Embolization of the hepatic vein has been reported to induce sufficient liver regeneration in patients that previously have failed to regenerate after PVE (Fig. 7.10) [27, 28].
Patient treated for CLM at MD Anderson Cancer Center. The patient was scheduled to undergo two-stage hepatectomy and PVE. a The liver failed to regenerate with insufficient sFLR at only 15% 6 weeks after right PVE with segment 4 embolization. b and c The patient underwent a second procedure with embolization of the middle hepatic vein. d CT image 8 weeks after embolization of the middle hepatic vein showed sufficient sFLR at 23%, and the patient underwent the planned extended right hepatectomy (d). The patient had an uncomplicated postoperative course, and was discharged at the postoperative day 7
Complications After PVE
The morbidity and procedure-related mortality after PVE is reported at 2.2% and 0%, respectively [29]. Non-target embolization, complete portal vein thrombosis, and recanalization of embolized segment are the most common PVE-specific complications. Subcapsular hematoma, hemobilia, hemoperitoneum, vascular injuries, pneumothorax, and cholangitis are among the most commonly reported complications after transhepatic procedures. The rate of major complications should not exceed 5% [30].
Oncologic Impact of PVE and Effect of Chemotherapy
PVE induces the release of growth factors to generate hypertrophy of normal liver tissue. Investigators have raised the concern that the same growth factors may stimulate tumor growth in the embolized liver, the FLR, or even promote the metastatic process. However, results from studies indicate that PVE does not cause tumor growth [2]. Furthermore, chemotherapy and targeted therapy with anti-angiogenic agent do not appear to affect liver regeneration after PVE [31].
Alternatives to Portal Vein Embolization and Additional Techniques to Induce Liver Hypertrophy
Associating Liver Partition and Portal Vein Ligation
In 2012, a European group reported a short-interval two-stage surgical approach, including initial liver parenchymal transection and right portal vein ligation in the first surgical stage, followed by completion hepatectomy within 7–10 days. The rationale for the method was the concept that parenchymal splitting and thereby division of collaterals would induce more rapid hypertrophy, allowing a shorter time interval between the first and the second stage, the latter where the extended right hepatectomy was completed [29, 32, 33]. In the literature, this method is referred to as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) [34].
However, multiple concerns regarding this method have been raised. First, the rationale is not as obvious as initially believed. Interventional radiology techniques have improved, and with the recent addition of segment 4 embolization, the number of patients aborted from the second surgical stage is very low (3.5%) [35]. Second, the 4–6 week time interval between the procedures in patients undergoing conventional two-stage liver resection and PVE may provide a biological test-of-time, in which patients with rapid progressing disease may not benefit from the second stage. Third, preliminary data suggested a high incidence of major morbidity (40%) and inpatient mortality (12%) associated with ALPPS. One study comparing PVE and ALPPS for patients with small FLR reported that right PVE with segment IV PVE may offer equivalent FLR hypertrophy (62% vs 74%), but reduction in perioperative bile leak (5.8% vs 24%) or sepsis (0% vs 20%) compared to ALPPS [35]. Investigators have also questioned whether the extreme hypertrophy sometimes seen after ALPPS consists of functional liver tissue, or edema due to necrosis. Due to safety concerns, the majority of investigators still emphasize that the ALPPS method should be approached carefully and only in well-designed trials.
Portal Vein Ligation
Portal vein ligation (PVL) is a method that has been used as an alternative to PVE. When performed during the first stage of a planned two-stage hepatectomy, it saves the additional PVE procedure and may therefore be economically beneficial. However, there are several advantages of PVE compared to PVL. First, not all patients requiring portal vein ligation or embolization require two-stage hepatectomy. Second, with PVE the interventional radiologist strives to completely embolize small and larger branches of the portal vein, possibly reducing the potential of collateral circulation from the contralateral liver reaching the portal of the treated liver. In line with this, a retrospective study comparing the two methods suggested that PVE generates more hypertrophy and shorter hospital stay [36]. Third, PVE has proven to be associated with low morbidity and mortality rates, and is therefore considered very safe and minimally invasive for the patient.
Summary
-
PVE is a safe procedure with low rates of major complications and nearly zero mortality.
-
The sFLR takes account of the size of the patient, and is calculated by dividing the FLR determined by contrast-enhanced CT by the TEL, calculated based on the BSA of the patient.
-
Currently, the indication guidelines for PVE are based on the sFLR: <20% in normal liver, <30% in liver pretreated with more than 3 months of chemotherapy, and <40% in the cirrhotic liver.
-
Percutaneous transhepatic ipsilateral approach is the preferred technique due to safety concerns. The agent must be easy to deliver, well tolerated by the patient, and provide occlusion of the target portal branches. It is combined with coils to prevent recanalization.
-
Right PVE is the most common procedure performed, as the left to right volume ratio usually greatly favors the right side of the liver. Left PVE is almost never needed.
-
Embolization of segment 4 may be technically challenging, but has proven to be effective in addition to right PVE to increase the degree of hypertrophy before right hepatectomy is extended to include segment 4.
-
DH and KGR are useful parameters to predict risk of hepatic insufficiency after liver resection. KGR is a functional measurement of the regenerative capacity of the liver after PVE.
References
Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7.
Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94.
Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–8.
Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–10.
Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–90.
Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–80.
de Meijer VE, Kalish BT, Puder M, Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–9.
McCormack L, Petrowsky H, Jochum W, et al. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923–30.
Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–72.
Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–9.
Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–17.
Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–40.
Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481–93.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–86.
Shindoh J, Tzeng CW, Aloia TA, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493–500.
Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–6.
Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30.
Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–81.
Kodama Y, Shimizu T, Endo H, et al. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233–7.
Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–8.
Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–72.
Nagino M, Kamiya J, Kanai M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155–60.
Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–51.
Ikeda K, Kinoshita H, Hirohashi K, et al. The ultrastructure, kinetics and intralobular distribution of apoptotic hepatocytes after portal branch ligation with special reference to their relationship to necrotic hepatocytes. Arch Histol Cytol. 1995;58:171–84.
Madoff DC, Hicks ME, Vauthey JN, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063–76.
Hwang S, Lee SG, Ko GY, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–16.
Munene G, Parker RD, Larrigan J, et al. Sequential preoperative hepatic vein embolization after portal vein embolization for extended left hepatectomy in colorectal liver metastases. World J Surg Oncol. 2013;11:134.
Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57.
Denys A, Bize P, Demartines N, et al. Quality improvement for portal vein embolization. Cardiovasc Intervent Radiol. 2010;33:452–6.
Zorzi D, Chun YS, Madoff DC, et al. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765–72.
Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197:686–90.
Wicherts DA, de Haas RJ, Andreani P, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240–50.
Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14.
Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126–33.
Broering DC, Hillert C, Krupski G, et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6:905–13. discussion 913
Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–25.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Brudvik, K.W., Vauthey, JN. (2017). Portal Vein Embolization. In: de Santibañes, E., Ardiles, V., Alvarez, F., Busnelli, V., de Santibañes, M. (eds) Extreme Hepatic Surgery and Other Strategies. Springer, Cham. https://doi.org/10.1007/978-3-319-13896-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-13896-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13895-4
Online ISBN: 978-3-319-13896-1
eBook Packages: MedicineMedicine (R0)