Abstract
A better understanding of the neurobiological factors that contribute to relapse to smoking is needed for the development of efficacious smoking cessation medications. Reinstatement procedures allow the preclinical assessment of several factors that contribute to relapse in humans, including re-exposure to nicotine via tobacco smoking and the presentation of stimuli that were previously associated with nicotine administration (i.e., conditioned stimuli). This review provides an integrated discussion of the results of animal studies that used reinstatement procedures to assess the efficacy of pharmacologically targeting various neurotransmitter systems in attenuating the cue- and nicotine-induced reinstatement of nicotine seeking. The results of these animal studies have increased our understanding of the neurobiological processes that mediate the conditioned effects of stimuli that trigger reinstatement to nicotine seeking. Thus, these findings provide important insights into the neurobiological substrates that modulate relapse to tobacco smoking in humans and the ongoing search for novel efficacious smoking cessation medications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Animal models
- Nicotine
- Cue-induced reinstatement
- Nicotine-induced reinstatement
- Drug seeking
- Medication development
1 Introduction
The negative impact of tobacco consumption on health remains one of the most urgent health issues (Alberg et al. 2014) because the global number of tobacco smokers continues to steadily increase (Ng et al. 2014). Although the health risks associated with tobacco smoking are greatly reduced by the cessation of tobacco consumption, fewer than 5 % of all quit attempts result in lifelong abstinence from tobacco smoking (Hughes et al. 2004). The vast majority of current smokers have considered or undertaken at least one quit attempt. Specifically, a recent survey of tobacco smokers in the USA reported that two-thirds of the respondents wished to quit their harmful habit and that over half of the respondents had undertaken one quit attempt in the previous year (Centers for Disease Control and Prevention 2010). However, despite the currently available smoking cessation medications and behavioral intervention treatments, an estimated half of smokers failed to quit their habit, despite multiple quit attempts during their lifetime (Centers for Disease Control and Prevention 2000). Relapse to tobacco seeking is thus one of the most defining features of tobacco dependence. To decrease relapse rates, the identification of novel pharmacological targets is needed. The development of these novel pharmacological targets in the treatment of tobacco dependence requires the use of procedures in experimental animals that mimic aspects of relapse in humans.
Nicotine is the main psychoactive ingredient in tobacco (Stolerman and Jarvis 1995). Therefore, experimental animal research that focuses on identifying pharmacological targets for novel smoking aids primarily assesses the effects of nicotine. The reinstatement procedure is one of the most widely used tools for screening the effects of pharmacological compounds on “relapse” to nicotine seeking in animals. The reinstatement of nicotine-seeking behavior is broadly defined as the continuation of the behavioral response that previously resulted in nicotine delivery after noncontingent exposure to nicotine (nicotine-induced reinstatement) or the presentation of stimuli (e.g., cue light illumination and sounds associated with activation of the pump that delivers nicotine) that were previously associated with nicotine administration (cue-induced reinstatement) after a period of abstinence. In the reinstatement procedure, animals are allowed to self-administer nicotine for a prolonged period of time before undergoing extinction training. During extinction training, the animals are placed in the chambers where they were previously allowed to intravenously self-administer nicotine, while nicotine and its conditioned cues are withheld. Alternatively to extinction training, animals may also undergo a period of forced abstinence. During this period of abstinence, also referred to as the incubation of nicotine seeking (Abdolahi et al. 2010; Bedi et al. 2011), the animals remain in their home cages during the withdrawal period. Nicotine seeking in animals can then be reinstated by manipulations that have been associated with relapse in human smokers. Conditions that induce relapse to tobacco smoking in humans include tobacco smoking itself, conditioned cues, and stress (Doherty et al. 1995). In parallel to humans, rodent studies have shown that noncontingent nicotine administration (Dravolina et al. 2007), conditioned cues (Paterson et al. 2005), and stress (Bruijnzeel et al. 2009) all induce the reinstatement of nicotine-seeking behavior (see chapters entitled Behavioral Mechanisms Underlying Nicotine Reinforcement and The Role of Mesoaccumbens Dopamine in Nicotine Dependence; this volume). Importantly, the overlap in factors that induce relapse in humans and reinstatement in experimental animals suggests good etiological validity for the reinstatement model.
Predictive validity for the reinstatement procedure in terms of pharmacological isomorphism (Geyer and Markou 1995) is provided by various studies that identified compounds that attenuate both relapse in humans and the reinstatement of nicotine seeking in animals. For example, the opioid receptor antagonist naltrexone facilitated smoking cessation and reduced relapse rates in humans (Epstein and King 2004; King 2002; King et al. 2012, 2013; King and Meyer 2000) and effectively attenuated the reinstatement of nicotine seeking in rats (Liu et al. 2009). Another example is the cannabinoid CB1 receptor antagonist rimonabant, which similarly decreased relapse to tobacco consumption in humans and reinstatement to nicotine seeking in rats (Cahill and Ussher 2011; Diergaarde et al. 2008; Forget et al. 2009). In contrast, however, two smoking cessation medications that are currently approved by the United States Food and Drug Administration (FDA) produced mixed results in studies of reinstatement of nicotine seeking in rats. Specifically, bupropion enhanced cue-induced reinstatement of nicotine seeking (Liu et al. 2008), while varenicline attenuated nicotine-induced, but not cue-induced, reinstatement of nicotine seeking (O’Connor et al. 2010). This variability in findings may reflect the different neurocircuits that probably underlie different aspects of the reinstatement of nicotine seeking. Results from reinstatement studies of drug seeking for other psychostimulant drugs, including cocaine, support the postulation that distinct neurocircuits are involved in the regulation of diverse aspects of the reinstatement of drug seeking (for reviews, see Kalivas and McFarland 2003; See et al. 2003). The sections below briefly describe several neurocircuits that regulate diverse aspects of nicotine dependence, followed by discussions of the various neurotransmitter systems that are of interest in studies of the reinstatement of nicotine seeking.
2 Neurocircuits of Interest in Studies of the Reinstatement of Nicotine Seeking
2.1 Mesolimbic System
Dopaminergic projections from the ventral tegmental area (VTA) to nucleus accumbens (NAc) are critically involved in mediating the reinforcing and motivational properties of nicotine (Gerasimov et al. 2000; Laviolette and van der Kooy 2004). Nicotine directly activates these dopaminergic projections through β2-containing nicotinic acetylcholine receptors (nAChRs; see chapters entitled Structure of Neuronal Nicotinic Receptors and Genetics of Smoking Behaviour; volume 23) on VTA dopaminergic neurons (Mameli-Engvall et al. 2006) and indirectly through α7 nAChRs located on VTA glutamatergic neurons (Mansvelder and McGehee 2000). Pharmacological compounds that provide low levels of stimulation of dopaminergic projections to the NAc may therefore result in decreased nicotine-seeking behavior. In fact, the efficacy of the nicotinic receptor partial agonist varenicline , one of the few FDA-approved smoking cessation aids, in attenuating relapse to smoking in humans is assumed to partially result from low stimulatory actions at β2-containing nAChRs located on dopaminergic projections from the VTA to NAc (West et al. 2008). Alternatively, increased glutamatergic neurotransmission through the activation of excitatory α7 nAChRs, located on glutamatergic terminals that synapse on VTA dopamine neurons, activates dopaminergic projections from the VTA to NAc (Mansvelder and McGehee 2000). Moreover, a recent study by Gipson et al. (2013) demonstrated that glutamatergic neurotransmission in the NAc contributed to the cue-induced reinstatement of nicotine-seeking behavior, supporting an important role for the mesolimbic neurocircuit in the reinforcing and motivational effects of nicotine and the reinstatement of nicotine seeking. Findings from rat studies indicated changes in glutamatergic neurotransmission in the NAc during early nicotine withdrawal (i.e., 24 h after the cessation of nicotine self-administration; Knackstedt et al. 2009; Liechti et al. 2007). Subsequent studies by Gipson et al. (2013) revealed that these changes in NAc glutamatergic synaptic plasticity persist when the withdrawal period is extended to 2 weeks. Moreover, the cue-induced reinstatement procedure induced simultaneous increases in glutamatergic neurotransmission in the NAc and nicotine seeking (Gipson et al. 2013), further indicating the importance of glutamatergic neurotransmission in the mediation of cue-induced reinstatement.
2.2 Habenulo-interpeduncular Circuit
Opposite to the role of the corticolimbic neurocircuit in the reinforcing effects of nicotine, the habenulo-interpeduncular circuit appears to be important in mediating the aversive effects of nicotine (Fowler et al. 2011, 2013; Frahm et al. 2011). The medial habenula became of interest in the study of nicotine dependence when genetic linkage studies found that genetic variation in the gene cluster that encodes the α3, α5, and β4 nAChR subunits, which are densely located in the habenular circuit (De Biasi and Salas 2008), was associated with lung cancer and nicotine dependence in humans (Saccone et al. 2007, 2010). Mice null for the α5 nAChR subunit vigorously self-administered very high concentrations of nicotine (Fowler et al. 2011). Moreover, re-expression of the α5 nAChR subunit in the medial habenula in these knockout mice returned their increased self-administration rates of high nicotine doses back to rates observed in wild-type mice (Fowler et al. 2011), highlighting the involvement of the habenula-interpeduncular pathway in the high nicotine intake in these knockout mice. The effects of null mutation of the α5 nAChR subunit on nicotine consumption were explained in a later study by Fowler and colleagues. This study suggested that the reward-suppressing effects that high nicotine doses induce in wild-type mice were absent in α5 knockout mice (Fowler et al. 2013). Similarly, increased activity of β4 nAChR subunits in mice, in which the CHRNA5-CHRNA3-CHRNB4 gene cluster was co-expressed with a bacterial artificial chromosome, resulted in the consumption of markedly less nicotine in a no-choice bottle procedure, presumably because of the aversion to nicotine that these mice exhibit in the conditioned place aversion procedure (Frahm et al. 2011; see also chapter entitled Genetics of Smoking Behaviour; volume 23). Lentiviral expression of the D398N α5 variant, which has been genetically associated with nicotine dependence and lung cancer in humans (Falvella et al. 2009; Hung et al. 2008; Wang et al. 2009), in the medial habenula reversed the aversion to nicotine in these mice (Frahm et al. 2011). These studies suggest the involvement of habenular α5 and β4 nAChR subunits in the aversive effects of nicotine, but the role of the habenulo-interpeduncular circuit in the reinstatement of nicotine-seeking behavior remains to be explored. Nevertheless, the somatic signs of nicotine withdrawal were attenuated in mice null for the both α5 and β4 nAChR subunits (Jackson et al. 2008; Salas et al. 2007; Stoker et al. 2012), suggesting that these nAChR subunits may be involved in mediating at least some of the aversive aspects of the nicotine withdrawal syndrome and that pharmacologically targeting these nAChR subunits may alleviate the negative withdrawal symptoms that ultimately result in the reinstatement of nicotine seeking.
2.3 Insular Cortex and Dorsal Striatum
Compared with the mesolimbic and habenulo-interpeduncular neurocircuits, the insula and dorsal striatum are thought to be recruited in later stages of drug dependence when drug-taking behavior becomes more habitual (for reviews, see Everitt et al. 2008; Naqvi and Bechara 2009). These brain structures are therefore particularly interesting in the study of drug reinstatement. The insular cortex became of interest in the study of relapse to tobacco smoking when Naqvi and colleagues reported that damage to the insular cortex facilitated spontaneous smoking cessation in humans, presumably by relieving symptoms of craving (Naqvi et al. 2007). Similarly, pharmacological or electrical inactivation of the insula attenuated the cue- and nicotine-induced reinstatement of nicotine-seeking behavior in animals (Forget et al. 2010a; Pushparaj et al. 2013). Subsequent studies provided additional evidence that the insula critically regulates different aspects of dependence on various psychostimulants and is particularly important in mediating the effects of conditioned cues associated with drug craving (Abdolahi et al. 2010; Contreras et al. 2007, 2012; Forget et al. 2010a; Hollander et al. 2008; Scott and Hiroi 2011). The insula projects to the dorsal striatum , a brain region implicated in the habitual and compulsive aspects of drug dependence. Interestingly, a recent case report described a patient in whom a lesion of the dorsal striatum resulted in the attenuation of nicotine intake (Muskens et al. 2012), similar to the effects of lesions of the insula on smoking cessation. In animals, studies of the involvement of the dorsal striatum in drug seeking have primarily focused on psychostimulant drugs other than nicotine, most notably cocaine. Lesions or pharmacological inactivation of the dorsal striatum in rats attenuated cocaine-seeking behavior (Fuchs et al. 2006; Fucile et al. 1997; Fung and Richard 1994; Gabriele and See 2011). The effects of conditioned cues associated with cocaine were shown to be mediated by dopaminergic neurotransmission in the dorsal striatum in animals (Ito et al. 2002) and humans (Volkow et al. 2006).
3 Role of Various Neurotransmitter Systems in Cue- and Nicotine-induced Reinstatement of Nicotine-seeking Behavior
3.1 Acetylcholine
All smoking cessation medications that are currently approved by the FDA (i.e., nicotine replacement therapy , varenicline , and bupropion ) have some affinity at nAChRs (Coe et al. 2005; Slemmer et al. 2000; Thompson and Hunter 1998). While these medications are ineffective in approximately 80 % of smokers who attempt to quit smoking (Gonzales et al. 2006; Hughes et al. 2003; Jorenby et al. 2006), nAChRs have remained key targets in the research and development of novel pharmacotherapies for smoking cessation. Bupropion , a medication widely used for smoking cessation, was initially used as an antidepressant therapy and primarily acts as a dopamine and norepinephrine reuptake inhibitor. Interestingly, bupropion was also found to act as an nAChR antagonist after it was marketed as a smoking cessation medication (Slemmer et al. 2000). bupropion has had mixed effects on smoking cessation rates in humans (Hurt et al. 1997) and actually enhanced the cue-induced reinstatement of nicotine seeking in rats (Liu et al. 2008). These results suggest that bupropion may have limited efficacy at treating the full spectrum of relapse and may primarily alleviate depressive-like symptoms during withdrawal (Cryan et al. 2003; Paterson et al. 2007).
Varenicline , a partial agonist at α4β2-containing nAChRs (Coe et al. 2005), was synthesized after studies suggested the crucial involvement of β2 nAChRs in nicotine dependence (Maskos et al. 2005; Picciotto 1998). Preclinical studies that assessed the effects of varenicline on the reinstatement of nicotine-seeking behavior demonstrated mixed effects on cue- and nicotine-induced reinstatement. Specifically, the nicotine-induced reinstatement of nicotine seeking was attenuated by varenicline in rats (see Fig. 1b adapted from O’Connor et al. 2010), similar to the effects of varenicline in humans. In contrast, varenicline did not affect the cue-induced reinstatement of nicotine-seeking behavior in rats (O’Connor et al. 2010; Wouda et al. 2011, see Fig. 1a adapted from Wouda et al. 2011), and higher doses of varenicline even enhanced cue-induced reinstatement (Wouda et al. 2011). Interestingly, varenicline attenuated the cue-induced reinstatement of nicotine seeking with a prolonged pretreatment time (Le Foll et al. 2012). When reinstatement was induced by both nicotine priming and the presentation of cues, varenicline also attenuated the reinstatement of nicotine seeking (O’Connor et al. 2010), presumably because of the attenuating effect of varenicline on nicotine-induced reinstatement. Altogether, preclinical studies on the effects of varenicline on nicotine seeking suggest the differential regulation of cue- and nicotine-induced reinstatement. Furthermore, Liu (2014) demonstrated that α7 nAChR antagonism with methyllycaconitine (MLA) but not α4β2-containing nAChR antagonism with dihydro-β-erythroidine (DHβE) reduced the cue-induced reinstatement of nicotine seeking. The results with varenicline and DHβE suggest that α4β2-containing nAChRs may be involved in the regulation of nicotine-induced, but not cue-induced reinstatement of nicotine seeking. In contrast, α7 nAChRs may be involved in mediating the cue-induced reinstatement of nicotine seeking. The enhancement of cue-induced reinstatement that results from administration of higher doses of varenicline (Wouda et al. 2011) may be explained by the activation of α7 nAChRs by varenicline , which acts as a full agonist at these receptors (Mihalak et al. 2006). This interpretation is further supported by results from the study by Liu (2014), which showed that α7 nAChR blockade attenuated cue-induced reinstatement. Further support for the involvement of α7 nAChRs in nicotine seeking reinstated by the presentation of conditioned cues, but not nicotine, is provided by the results of a study that reported that TAT-α7-pep2, a protein that interferes with the function of the α7nAChR–NMDA receptor complex, reduced the cue-induced reinstatement of nicotine seeking while not affecting reinstatement induced by nicotine priming (Li et al. 2012).
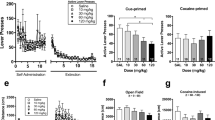
Effects of manipulating various neurotransmitter systems on cue- and nicotine-induced reinstatement of nicotine-seeking behavior. The pharmacological targeting of a wide range of neurotransmitter systems attenuated reinstatement to nicotine seeking induced by the presentation of conditioned cues (left panel) and nicotine priming (right panel). Positive allosteric modulation of α4β2 nAChRs with varenicline had no effect on the cue-induced reinstatement of nicotine seeking (a, modified with permission from O’Connor et al. 2010), while varenicline attenuated the nicotine-induced reinstatement of nicotine seeking (b, modified with permission from O’Connor et al. 2010). The mGlu1 receptor antagonist EMQMCM attenuated both the cue-induced (c, modified with permission from Dravolina et al. 2007) and nicotine-induced (d, modified with permission from Dravolina et al. 2007) reinstatement of nicotine-seeking behavior. The dopamine D4 receptor antagonist L-745,870 also attenuated the cue-induced (e, modified with permission from Yan et al. 2013) and nicotine-induced (f, modified with permission from Yan et al. 2013) reinstatement of nicotine seeking. The GABAB receptor agonists CPG44532 (g, modified with permission from Paterson et al. 2005) and baclofen (h, modified with permission from Fattore et al. 2009) similarly reduced the cue- and nicotine-induced reinstatement of nicotine seeking, respectively. Finally, the CB1 receptor antagonist rimonabant attenuated the cue-induced (i, modified with permission from Forget et al. 2009) and nicotine-induced (j, modified with permission from Forget et al. 2009) reinstatement of nicotine seeking
In addition to the direct activation of nAChRs, cholinergic neurotransmission can be increased by the inhibition of acetylcholinesterase, the enzyme that metabolizes the endogenous nAChR ligand acetylcholine . Galantamine, which acts both as an acetylcholinesterase inhibitor and positive allosteric modulator at α7- and α4β2-containing nAChRs, reduced the cue-induced reinstatement of nicotine seeking, suggesting that acetylcholinesterase inhibitors may be effective tools in the prevention of nicotine reinstatement (Hopkins et al. 2012). The potential therapeutic value of acetylcholinesterase inhibitors was supported by a study that demonstrated that donezipil, which acts exclusively as an acetylcholinesterase inhibitor, attenuated nicotine-induced reinstatement (Kimmey et al. 2012). Combined, the findings with galantamine and donezipil suggest that acetylcholinesterase inhibitors can attenuate both cue- and nicotine-induced reinstatement.
In summary, the pharmacological targeting of acetylcholinergic neurotransmission has been one of the most lucrative avenues in the search for smoking cessation aids to date. However, cue- and nicotine-induced reinstatement appears to be differentially regulated by pharmacological compounds that act on α7- and α4β2-containing nAChRs, potentially limiting their efficacy as pharmacological targets for smoking cessation medication. Exploring the efficacy of pharmacologically targeting diverse nAChR subtypes, including α3-, α5-, and β4-containing nAChR subunits, may thus be an interesting avenue in the identification of novel, highly efficacious smoking cessation medications.
3.2 Glutamate
Glutamatergic neurotransmission is modulated by two different types of receptors: ionotropic glutamate (iGlu) receptors and metabotropic glutamate (mGlu) receptors. iGlu receptors are located postsynaptically and modulate fast glutamatergic neurotransmission. Nicotine self-administration resulted in changes in iGlu and mGlu receptor levels, which likely contributed to the cue-induced reinstatement of nicotine-seeking behavior (Gipson et al. 2013; Liechti et al. 2007). Moreover, pharmacologically targeting iGlu receptors with the NMDA receptor antagonists ifenprodil and acamprosate attenuated the cue-induced reinstatement of nicotine-seeking behavior (Gipson et al. 2013; Pechnick et al. 2011). The development of novel pharmacotherapies for the treatment of drug dependence, however, has focused primarily on mGlu receptors because of the side effects of iGlu receptor antagonists in humans (for review, see Gass and Olive 2008). Metabotropic glutamate receptors have attracted much interest in recent years as targets for novel therapeutics in the treatment of nicotine dependence (Markou 2007). Compared with iGlu receptors, the activity of mGlu receptors is more slow acting and modulatory, presumably resulting in a reduced side effect profile. During nicotine withdrawal, presynaptic mGlu2/3 receptors were downregulated in the VTA and NAc, and mGlu2/3 receptor activation in these brain areas induced by the agonist LY379268 attenuated the cue-induced reinstatement of nicotine seeking (Liechti et al. 2007). N-acetylcysteine, a compound that has been suggested to increase the glutamatergic tone of presynaptic mGlu2/3 receptors (Kupchik et al. 2012), similarly attenuated nicotine reinstatement elicited by environmental cues (Ramirez-Nino et al. 2013). Furthermore, the blockade of postsynaptic mGlu5 receptors (Bespalov et al. 2005) and mGlu1 receptors (see Fig. 1c adapted from Dravolina et al. 2007) decreased cue-induced reinstatement. Specifically, nicotine seeking was attenuated by administration of the mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP; Bespalov et al. 2005) or mGlu1 receptor antagonist 3-ethyl-2-methyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)-methanone methanesulfonate (EMQMCM; Dravolina et al. 2007). In parallel to cue-induced reinstatement, EMQMCM also decreased the nicotine-induced reinstatement of nicotine-seeking behavior in rats (see Fig. 1d adapted from Dravolina et al. 2007). These results of experimental studies in animals on the role of mGlu receptors in nicotine reinstatement demonstrated that pharmacologically targeting glutamatergic neurotransmission effectively attenuates both cue- and nicotine-induced reinstatement and may attenuate relapse to tobacco smoking in humans. In fact, these experimental animal studies resulted in a Phase I clinical trial by Novartis that assessed the efficacy and safety of the mGlu5 receptor antagonist AFQ056 as a treatment option for voluntary smoking cessation. This clinical trial has been completed, but the results of the study have not yet been published (Clinicaltrials.gov 2007).
3.3 Dopamine
As described in Sects. 2.1 and 2.3, dopaminergic neurotransmission, which is mediated by G-protein-coupled dopamine receptors, in the mesolimbic circuit and striatum is critically involved in drug dependence (see chapter entitled The Role of Mesoaccumbens Dopamine in Nicotine Dependence; this volume). The cue-induced reinstatement of nicotine-seeking behavior can be attenuated by pharmacological compounds that decrease dopaminergic tone, including antagonists of dopamine D1 and D2 receptors (Liu et al. 2010), D3 receptors (Khaled et al. 2010), and D4 receptors (see Fig. 1e adapted from Yan et al. 2013). Consistent with these findings, a reduction of dopaminergic tone with the α-type peroxisome proliferator-activated receptor (PPAR-α) agonist clofibrate decreased cue-induced reinstatement in squirrel monkeys (Panlilio et al. 2012). These studies suggest that the pharmacological inhibition of dopaminergic neurotransmission consistently attenuates the cue-induced reinstatement of nicotine-seeking behavior. Furthermore, nicotine-induced reinstatement is similarly attenuated by dopamine D3 and D4 receptor agonists (Andreoli et al. 2003; Yan et al. 2013, see Fig. 1f adapted from Yan et al. 2013) and PPAR-α agonists (Mascia et al. 2011; Panlilio et al. 2012). The inhibition of dopaminergic neurotransmission, therefore, appears to be an interesting possibility in the identification for novel smoking cessation medication targets.
3.4 γ-Aminobutyric Acid (GABA )
GABA is the main inhibitory transmitter in the central nervous system. Inhibitory GABAergic activity attenuates dopaminergic mesocorticolimbic neurotransmission through GABA interneurons located in the VTA, medium spiny GABA neurons in the NAc, and GABAergic projections to the VTA from the NAc, ventral pallidum, and pedunculopontine tegmental nucleus (Klitenick et al. 1992). Inhibitory GABA receptors, therefore, would have to be activated by full agonists or positive allosteric modulators to decrease excitatory neurotransmission in the VTA which, as discussed above, generally attenuates the reinstatement of nicotine seeking. GABAergic neurotransmission is regulated through ionotropic GABAA and GABAC receptors and metabotropic GABAB receptors (Bormann 1986). Of these various GABA receptor subtypes, G-protein-coupled GABAB receptors are primarily of interest in the treatment of nicotine dependence (Li et al. 2014; Vlachou and Markou 2010). GABAB receptor activation induced by the GABAB receptor agonist CPG44532 attenuated the cue-induced reinstatement of nicotine seeking in rats (see Fig. 1g adapted from Paterson et al. 2005). Similar to the GABAB agonist, the GABAB receptor positive allosteric modulator BHF177 also decreased cue-induced reinstatement in rats (Vlachou et al. 2011). The effects of GABAB agonists on the nicotine-induced reinstatement of nicotine seeking have been less extensively explored. One study reported that baclofen decreased reinstatement induced by nicotine priming (see Fig. 1h adapted from Fattore et al. 2009). Furthermore, the GABAB receptor agonist baclofen was suggested to potentially facilitate smoking cessation in humans (Cousins et al. 2001). These studies indicate that GABAB receptors may be a promising target in the treatment of smoking cessation. Moreover, it has been proposed that GABAB positive allosteric modulators may be particularly effective in the treatment of nicotine dependence because of their modulatory actions at GABAB receptors that may result in an improved side effect profile and decreased development of tolerance to these compounds compared with GABAB full receptor agonists (Guery et al. 2007; Vlachou et al. 2011).
3.5 Endocannabinoids
Of the two endocannabinoid receptors cloned to date, CB1 receptors are of primary interest in the treatment of dependence on drugs of abuse (Howlett et al. 2004) because these receptors are found on glutamatergic and GABAergic inputs to dopaminergic neurons (Gardner 2005). In contrast, CB2 receptors are primarily localized on immune cells in both the central and peripheral nervous systems (Howlett 2002). As expected, the reinstatement of nicotine-seeking behavior was unaffected by the CB2 receptor antagonist AM630 or CB2 receptor agonist AM1241 (Gamaleddin et al. 2012b). CB1 receptors located on presynaptic glutamatergic neurons in the VTA are hypothesized to decrease the inhibitory control that GABAergic neurons exert on dopaminergic neurons (Schlicker and Kathmann 2001). Consequently, CB1 receptor activation would result in the increased firing activity of VTA dopamine neurons (French 1997; French et al. 1997) and increased dopamine release in the NAc (Gardner and Vorel 1998; Tanda et al. 1997), suggesting therapeutic potential for CB1 receptor antagonism in attenuating the reinstatement of nicotine seeking. Indeed, antagonism of the CB1 receptor consistently attenuated the cue-induced reinstatement of nicotine-seeking behavior, demonstrated by the administration of rimonabant (Diergaarde et al. 2008; Forget et al. 2009, see Fig. 1i adapted from Forget et al. 2009), SR141716 (Cohen et al. 2005; de Vries et al. 2005), and AM404 (Gamaleddin et al. 2013) in rats. The CB1/2 receptor agonist WIN 55,212-2 facilitated the cue-induced reinstatement of nicotine-seeking behavior (Gamaleddin et al. 2012a), presumably by activating CB1 receptors. Furthermore, antagonism at CB1 receptors attenuated nicotine-induced reinstatement in rats, demonstrated by the administration of rimonabant (see Fig. 1j adapted from Forget et al. 2009), AM251 (Shoaib 2008), and AM404 (Gamaleddin et al. 2013). Additionally, reinstatement induced by the combination of both cue presentation and nicotine priming was attenuated by administration of the CB1 receptor antagonist AM251 (Shoaib 2008).
After various clinical trials assessed the efficacy of rimonabant as a smoking cessation medication, it was approved for this purpose in various European countries in 2006. Inopportunely, treatment of smoking cessation with rimonabant was halted in 2007 after reports of severe side effects that included anxiety and depression (Moreira and Crippa 2009). The development of pharmacological compounds that target the endocannabinoid system in smoking cessation is therefore currently directed toward developing compounds that indirectly target endocannabinoid neurotransmission, including anandamide transport inhibitors and fatty acid amid hydrolase (FAAH). Anandamide is one of the endogenous ligands that act at cannabinoid receptors (Giang and Cravatt 1997) and eliminated by reuptake into cells by anandamide transporters and subsequent hydrolysis by FAAH (Beltramo et al. 1997; Cravatt et al. 1996). The enhancement of endocannabinoid signaling by inhibiting the reuptake or hydrolysis of anandamide attenuated both cue- and nicotine-induced reinstatement of nicotine seeking (Forget et al. 2009; Gamaleddin et al. 2011). Notably, inhibiting the reuptake or hydrolysis of anandamide opposes the effects of CB1 receptor antagonists. That is, CB1 receptor antagonists attenuate endocannabinoid signaling, while FAAH inhibitors and anandamide transport inhibitors enhance endocannabinoid signaling. The same direction of effect (i.e., decrease) on the reinstatement of nicotine seeking by these seemingly opposing mechanisms may be due to the action of CB1 receptor antagonists on neurocircuits that express endocannabinoid ligands other than anandamide (Scherma et al. 2008). Interestingly, compounds that target FAAH may exert dual actions on the reinstatement of nicotine seeking because FAAH also breaks down fatty acid amides that can activate PPAR-α (Fegley et al. 2005). As discussed above, the activation of PPAR-α decreases the cue-induced reinstatement of nicotine seeking, presumably by decreasing dopaminergic neurotransmission, further emphasizing the potential of FAAH-inhibiting compounds in treating the reinstatement of nicotine seeking.
3.6 Other Neurotransmitter Systems
Whereas the aforementioned neurotransmitter systems have been the most extensively explored as targets for pharmacotherapy in attenuating the reinstatement of nicotine seeking, several other neurotransmitter systems have been suggested in the development of novel smoking cessation aids. Serotonergic receptors, for example, modulate dopaminergic neurotransmission and were suggested as potential targets in smoking cessation medications (for review, see Fletcher et al. 2008). The attenuation of serotonergic neurotransmission by the 5-HT2C receptor antagonists Ro60-0175 and locaserin decreased both nicotine- and cue-induced reinstatement (Fletcher et al. 2012; Higgins et al. 2012). Similarly, the modulation of noradrenergic neurotransmission was shown to effectively reduce both cue- and nicotine-induced reinstatement with the noradrenergic α1 receptor antagonist prazosin (Forget et al. 2010b) and β-blocker propranolol, supporting the involvement of noradrenergic neurotransmission in cue-induced reinstatement (Chiamulera et al. 2010). Finally, the T-type calcium channel antagonist TTA-A2 also attenuated both cue- and nicotine-induced reinstatement (Uslaner et al. 2010), potentially by modulating glutamatergic or dopaminergic neurotransmission (Uslaner et al. 2012). Tricyclic antidepressants, which decrease both serotonergic and noradrenergic neurotransmission, have been suggested to facilitate smoking cessation in humans (Edwards et al. 1989; Hall et al. 1998; Prochazka et al. 1998). However, the severe side effects of tricyclic antidepressants, including cardiovascular effects and the severity of overdose symptoms (Biggs et al. 1977; Roose et al. 1991), make these compounds unfavorable as smoking cessation aids. The reversible monoamine oxidase-A (MAO-A) inhibitor moclobemide, which reduces both serotonergic and noradrenergic neurotransmission similarly to tricyclic antidepressants but with a more favorable side effect profile (Stabl et al. 1989), attenuated smoking cessation in a study group of heavy smokers (Berlin et al. 1995). These results suggest that MAO-A inhibitors may be preferred over tricyclic antidepressants as smoking cessation aids. However, bupropion remains the main antidepressant used as a smoking cessation medication (Tables 1 and 2).
4 Concluding Remarks
Relapse is one of the hallmarks of tobacco dependence, but the currently available smoking cessation medications only prevent the occurrence of relapse in a small percentage of people who attempt to quit tobacco consumption. The limited effectiveness of these medications may be explained by the differential regulation of diverse aspects of relapse by various neurotransmitter systems, as discussed in the introduction above, which has been extensively documented for relapse to cocaine seeking. Whereas the possibility of the regulation of the different types of reinstatement by different neurocircuits has not yet been widely explored for relapse to nicotine seeking, specific smoking cessation medications may only target one aspect of relapse, resulting in decreased overall effectiveness compared with pharmacological compounds that target the full spectrum of relapse. This concept is supported by studies of “relapse” to nicotine seeking in animals, which demonstrated that the reinstatement of nicotine seeking is most robust when it is elicited by both nicotine priming and exposure to conditioned cues compared with nicotine priming or conditioned cues themselves (Feltenstein et al. 2012; O’Connor et al. 2010). With less than 5 % of all smoking cessation attempts resulting in lifelong abstinence from tobacco (Hughes et al. 2004), novel medications that attenuate relapse rates are needed. Pharmacologically targeting the neurocircuits or neurotransmitters that are involved in multiple aspects of relapse to tobacco consumption may improve smoking cessation rates. Preclinical models of relapse are greatly important in this pursuit of novel pharmacological targets in the treatment of tobacco dependence. The reinstatement procedure, an animal model widely used to assess “relapse” to drug seeking in animals, has provided important insights into the neurobiological effects of stimuli that trigger relapse in humans, most notably nicotine and its conditioned cues (Shaham et al. 2003; but see Katz and Higgins 2003). The majority of nicotine reinstatement studies have assessed nicotine reinstatement primed by nicotine or its conditioned cues separately, allowing for differentiation of the neurotransmitters that regulate these two different manipulations that induce the reinstatement of drug seeking.
Preclinical studies suggest the limited effectiveness of two widely used smoking cessation medications, varenicline and bupropion, in attenuating the cue-induced reinstatement of nicotine seeking (Liu et al. 2008; O’Connor et al. 2010, Wouda et al. 2011). These results are consistent with the clinical observations that these two FDA-approved medications are not very efficacious in attenuating tobacco smoking in humans. Furthermore, these results suggest that the pursuit of the identification of novel smoking cessation medications would be best served by developing pharmacological compounds that effectively treat the various factors that can induce the reinstatement of nicotine seeking, including nicotine and cues. nAChR subunits other than the α4β2-containing nAChRs (the nAChR subtype on which varenicline acts), including α3, α5, and β4 subunits, may be interesting in the development of more efficacious smoking cessation medications. An increasing body of preclinical studies also suggests that exploring other neurotransmitter systems downstream from nAChRs may be lucrative in the quest for novel pharmacological targets to attenuate nicotine reinstatement.
Glutamatergic neurotransmitter systems are particularly appealing targets for the development of novel smoking cessation medications (Liechti and Markou 2008). Glutamate critically regulates the reinstatement of nicotine seeking (Bespalov et al. 2005; Gipson et al. 2013; Liechti et al. 2007) but also cocaine seeking (for reviews, see Kalivas 2004; Wise 2009), suggesting that pharmacologically targeting glutamatergic neurotransmission may be particularly promising in the identification of novel targets for smoking cessation medications. Nicotine reinstatement studies found that pharmacological compounds that decrease glutamatergic neurotransmission effectively attenuated both the cue- and nicotine-induced reinstatement of nicotine seeking (Bespalov et al. 2005; Dravolina et al. 2007; Gipson et al. 2013; Liechti et al. 2007; Pechnick et al. 2011; Ramirez-Nino et al. 2013). The promise of targeting glutamatergic compounds as smoking cessation medications is further demonstrated by the success of N-acetylcysteine in reducing cigarette consumption in smokers (Knackstedt et al. 2009).
Other than glutamatergic neurotransmission, preclinical studies have suggested that both the cue- and nicotine-induced reinstatement of nicotine seeking can be attenuated by pharmacologically targeting various other neurotransmitters (Table 1). Dopamine receptor antagonists, GABAB receptor agonists or positive allosteric modulators, endocannabinoid receptor antagonists, serotonin receptor agonists, β-blockers, and opioid receptor antagonists have been identified as potentially efficacious as pharmacological smoking cessation medications. These preclinical studies of nicotine seeking have greatly benefited from the synthesis and characterization of novel pharmacological compounds that attenuate relapse to tobacco smoking in humans. Additionally, preclinical studies on the neurobiology of cue- and nicotine-induced reinstatement provide important insights into potential future study directions for clinical trials in ongoing efforts to repurposing medications that have been approved by the FDA for other neurobiological disorders to serve as smoking cessation aids.
References
Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ (2010) Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31:733–741
Alberg AJ, Shopland DR, Cummings KM (2014) The 2014 Surgeon general’s report: commemorating the 50th anniversary of the 1964 report of the advisory committee to the us surgeon general and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol 179:403
Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA (2003) Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 28:1272–1280
Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H (2011) Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69:708–711
Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D (1997) Functional role of high-affinity Anandamide transport, as revealed by selective inhibition. Science 277:1094–1097
Berlin I, Said S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, Lecrubier Y, Puech AJ (1995) A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther 58:444–452
Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A (2005) Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology 49:167–178
Biggs JT, Spiker DG, Petit JM, Ziegler VE (1977) Tricyclic antidepressant overdose: incidence of symptoms. JAMA 238:135–138
Bormann J (1986) Electrophysiology of single GABA receptor chloride channels. Clin Neuropharmacol 9:386–388
Bruijnzeel AW, Prado M, Isaac S (2009) Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry 66:110–117
Cahill K, Ussher MH (2011) Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev 3:CD005353
Centers for Disease Control and Prevention (2000) Cigarette smoking among adults - United States, 1998. Morb Mortal Wkly Rep 49:881–884
Centers for Disease Control and Prevention (2010) Quitting smoking among adults—United States 2001–2010. Morb Mortal Wkly Rep 60:1513–1519
Chiamulera C, Tedesco V, Zangrandi L, Giuliano C, Fumagalli G (2010) Propranolol transiently inhibits reinstatement of nicotine-seeking behaviour in rats. J Psychopharmacol 24:389–395
Clinicaltrials.gov (2007) Effects of AFQ056 and nicotine in reducing cigarette smoking. http://clinicaltrials.gov/ct2/show/NCT00414752?term=Effects+of+AFQ056+and+Nicotine+in+Reducing+Cigarette+Smoking&rank=1. Accessed 23 Mar 2014
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477
Cohen C, Perrault G, Griebel G, Soubrie P (2005) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155
Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, Gonzalez M, Torrealba F (2012) A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology 37:2101–2108
Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318:655–658
Cousins MS, Stamat HM, de Wit H (2001) Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res 3:123–129
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168:347–358
De Biasi M, Salas R (2008) Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med (Maywood) 233:917–929
De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN (2005) Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res 161:164–168
Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ (2008) Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A). Neuropharmacology 55:712–716
Doherty K, Kinnunen T, Militello FS, Garvey AJ (1995) Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology 119:171–178
Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY (2007) mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology 52:263–269
Edwards NB, Murphy JK, Downs AD, Ackerman BJ, Rosenthal TL (1989) Doxepin as an adjunct to smoking cessation: a double-blind pilot study. Am J Psychiatry 146:373–376
Epstein AM, King AC (2004) Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav 77:29–37
Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW (2008) Review neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363:3125–3135
Falvella FS, Galvan A, Frullanti E, Spinola M, Calabro E, Carbone A, Incarbone M, Santambrogio L, Pastorino U, Dragani TA (2009) Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res 15:1837–1842
Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P (2009) Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol 19:487–498
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3’-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Feltenstein MW, Ghee SM, See RE (2012) Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend 121:240–246
Fletcher PJ, Le AD, Higgins GA (2008) Serotonin receptors as potential targets for modulation of nicotine use and dependence. Prog Brain Res 172:361–383
Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Le AD, Higgins GA (2012) Effects of the 5-HT2C receptor agonist Ro60-0175 and the 5-HT2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology 62:2288–2298
Forget B, Coen KM, Le Foll B (2009) Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration–comparison with CB(1) receptor blockade. Psychopharmacology 205:613–624
Forget B, Pushparaj A, Le Foll B (2010a) Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry 68:265–271
Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B (2010b) Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35:1751–1760
Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ (2011) Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471:597–601
Fowler CD, Tuesta L, Kenny PJ (2013) Role of alpha5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology 229:503–513
Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I (2011) Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron 70:522–535
French ED (1997) delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett 226:159–162
French ED, Dillon K, Wu X (1997) Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. NeuroReport 8:649–652
Fuchs RA, Branham RK, See RE (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588
Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alema S, Ballivet M, Eusebi F (1997) Alpha 5 subunit forms functional alpha 3 beta 4 alpha 5 nAChRs in transfected human cells. NeuroReport 8:2433–2436
Fung YK, Richard LA (1994) Behavioural consequences of cocaine withdrawal in rats. J Pharm Pharmacol 46:150–152
Gabriele A, See RE (2011) Lesions and reversible inactivation of the dorsolateral caudate-putamen impair cocaine-primed reinstatement to cocaine-seeking in rats. Brain Res 1417:27–35
Gamaleddin I, Guranda M, Goldberg SR, Le Foll B (2011) The selective Anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol 164:1652–1660
Gamaleddin I, Guranda M, Scherma M, Fratta W, Makriyannis A, Vadivel SK, Goldberg SR, Le Foll B (2013) AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J Psychopharmacol 27:564–571
Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B (2012a) Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol 17:47–61
Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B (2012b) Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS ONE 7:e29900
Gardner EL (2005) Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav 81:263–284
Gardner EL, Vorel SR (1998) Cannabinoid transmission and reward-related events. Neurobiol Dis 5:502–533
Gass JT, Olive MF (2008) Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75:218–265
Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL (2000) Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse 38:432–437
Geyer MA, Markou A (1995) Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven Press, New York, pp 787–798
Giang DK, Cravatt BF (1997) Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci USA 94:2238–2242
Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA 110:9124–9129
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, versus sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296:47–55
Guery S, Floersheim P, Kaupmann K, Froestl W (2007) Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABA B receptors. Bioorg Med Chem Lett 17:6206–6211
Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E (1998) Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 55:683–690
Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37:1177–1191
Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ (2008) Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA 105:19480–19485
Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD (2012) Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology 37:2310–2321
Howlett AC (2002) The cannabinoid receptors. Prostaglandins Other Lipid Mediat 68–69:619–631
Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ (2004) Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 47:345–358
Hughes JR, Keely J, Naud S (2004) Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 99:29–38
Hughes JR, Shiffman S, Callas P, Zhang J (2003) A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control 12:21–27
Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P (2008) A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452:633–637
Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337:1195–1202
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002) Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22:6247–6253
Jackson KJ, Martin BR, Changeux JP, Damaj MI (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325:302–312
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63
Kalivas PW (2004) Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4:23–29
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168:44–56
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B (2010) The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol 13:181–190
Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD (2012) Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addict Biol 19:539–51
King AC (2002) Role of naltrexone in initial smoking cessation: preliminary findings. Alcohol Clin Exp Res 26:1942–1944
King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ (2012) Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol 32:630–636
King AC, Cao D, Zhang L, O’Malley SS (2013) Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry 73:924–930
King AC, Meyer PJ (2000) Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav 66:563–572
Klitenick MA, DeWitte P, Kalivas PW (1992) Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci 12:2623–2632
Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845
Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW (2012) The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71:978–986
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65
Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, Yan Y, Khaled M, Goldberg SR (2012) Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15:1265–1274
Li S, Li Z, Pei L, Le AD, Liu F (2012) The alpha7nACh-NMDA receptor complex is involved in cue-induced reinstatement of nicotine seeking. J Exp Med 209:2141–2147
Li X, Semenova S, D’Souza MS, Stoker AK, Markou A (2014) Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology Part B 76:554–565
Liechti ME, Lhuillier L, Kaupmann K, Markou A (2007) Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27:9077–9085
Liechti ME, Markou A (2008) Role of the glutamatergic system in nicotine dependence : implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs 22:705–724
Liu X (2014) Effects of blockade of alpha4beta2 and alpha7 nicotinic acetylcholine receptors on cue-induced reinstatement of nicotine-seeking behaviour in rats. Int J Neuropsychopharmacol 17:105–116
Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF (2008) Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology 196:365–375
Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE (2007) Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology 32:710–718
Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF (2010) Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 21:153–160
Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, Booth S (2009) Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology 202:589–598
Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50:911–921
Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357
Markou A (2007) Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry 61:17–22
Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR (2011) Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry 69:633–641
Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107
Mihalak KB, Carroll FI, Luetje CW (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805
Moreira FA, Crippa JA (2009) The psychiatric side-effects of rimonabant. Rev Bras Psiquiatr 31:145–153
Muskens JB, Schellekens AF, de Leeuw FE, Tendolkar I, Hepark S (2012) Damage in the dorsal striatum alleviates addictive behavior. Gen Hosp Psychiatry 34: 702 e9-702 e11
Naqvi NH, Bechara A (2009) The hidden island of addiction: the insula. Trends Neurosci 32:56–67
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534
Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 311:183–192
O’Connor EC, Parker D, Rollema H, Mead AN (2010) The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology 208:365–376
Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR (2012) Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology 37:1838–1847
Paterson NE, Balfour DJ, Markou A (2007) Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci 25:3099–3108
Paterson NE, Froestl W, Markou A (2005) Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology 30:119–128
Pechnick RN, Manalo CM, Lacayo LM, Vit JP, Bholat Y, Spivak I, Reyes KC, Farrokhi C (2011) Acamprosate attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 22:222–227
Picciotto MR (1998) Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend 51:165–172
Plaza-Zabala A, Flores Á, Martín-García E, Saravia R, Maldonado R, Berrendero F (2013) A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology 38:1724–36
Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D (1998) A randomized trial of nortriptyline for smoking cessation. Arch Intern Med 158:2035–2039
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, Le Foll B (2013) Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology 38:690–698
Ramirez-Nino AM, D’Souza MS, Markou A (2013) N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology 225:473–482
Roose SP, Dalack GW, Glassman AH, Woodring S, Walsh BT, Giardina EG (1991) Is doxepin a safer tricyclic for the heart? J Clin Psychiatry 52:338–341
Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliovaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nothen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ (2010) Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 6:e1001053
Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16:36–49
Salas R, Main A, Gangitano D, De Biasi M (2007) Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology 53:863–869
Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinova Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR (2008) Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther 327:482–490
Schlicker E, Kathmann M (2001) Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22:565–572
Scott D, Hiroi N (2011) Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry 69:1052–1059
See RE, Fuchs RA, Ledford CC, McLaughlin J (2003) Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci 985:294–307
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20
Shoaib M (2008) The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology 54:438–44
Slemmer JE, Martin BR, Damaj MI (2000) Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 295:321–327
Stabl M, Biziere K, Schmid-Burgk W, Amrein R (1989) Review of comparative clinical trials. Moclobemide vs tricyclic antidepressants and vs placebo in depressive states. J Neural Transm 28:77–89
Stoker AK, Olivier B, Markou A (2012) Role of alpha7- and beta4-containing nicotinic acetylcholine receptors in the affective and somatic aspects of nicotine withdrawal: studies in knockout mice. Behav Genet 42:423–436
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117: 2–10:14–20
Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050
Thompson GH, Hunter DA (1998) Nicotine replacement therapy. Ann Pharmacother 32:1067–1075
Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, Nguyen SJ, Surles NO, Yao L, Barrow JC, Uebele VN, Renger JJ, Clark J, Hutson PH (2012) T-type calcium channel antagonism produces antipsychotic-like effects and reduces stimulant-induced glutamate release in the nucleus accumbens of rats. Neuropharmacology 62:1413–1421
Uslaner JM, Vardigan JD, Drott JM, Uebele VN, Renger JJ, Lee A, Li Z, Le AD, Hutson PH (2010) T-type calcium channel antagonism decreases motivation for nicotine and blocks nicotine- and cue-induced reinstatement for a response previously reinforced with nicotine. Biol Psychiatry 68:712–718
Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, Le AD, Renger JJ (2014) Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res 269:61–5
Vlachou S, Guery S, Froestl W, Banerjee D, Benedict J, Finn MG, Markou A (2011) Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology 215:117–128
Vlachou S, Markou A (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588
Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, Bierut LJ, Goate AM (2009) Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 18:3125–3135
West R, Baker CL, Cappelleri JC, Bushmakin AG (2008) Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 197:371–377
Wise RA (2009) Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking. Neuropharmacology 56:174–176
Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ (2011) Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology 216:267–277
Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, Goldberg SR, Le Foll B (2013) Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology 37:685–696
Acknowledgements
This work was supported by Postdoctoral Fellowship 21FT-0022 from the Tobacco-Related Disease Research Program to AKS and research grant R56 DA011946 from the National Institute on Drug Abuse to AM. The authors would like to thank Ms. Janet Hightower for assistance with preparation of the figure and Mr. Michael Arends for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stoker, A.K., Markou, A. (2015). Neurobiological Bases of Cue- and Nicotine-induced Reinstatement of Nicotine Seeking: Implications for the Development of Smoking Cessation Medications. In: Balfour, D., Munafò, M. (eds) The Neuropharmacology of Nicotine Dependence. Current Topics in Behavioral Neurosciences, vol 24. Springer, Cham. https://doi.org/10.1007/978-3-319-13482-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-13482-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13481-9
Online ISBN: 978-3-319-13482-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)