Abstract
Breast cancer is the most common malignancy in women and significant cause of cancer-related deaths, second only to lung cancer. Amongst different subtypes of breast cancers, triple-negative breast cancer (TNBC) is especially challenging due to fewer treatment options and more aggressive clinical course. Therefore, the development of molecularly targeted therapies for TNBC is crucial. Glycoprotein nonmetastatic B (GPNMB) is overexpressed in many malignancies, including TNBC, and is associated with poor prognosis. The antibody—drug conjugate (ADC) glembatumumab vedotin (CDX-011) selectively target GPNMB, and is being investigated for its efficacy in metastatic breast cancer. This chapter reviews the mechanisms of action, preclinical, and phase I/II results of glembatumumab vedotin, and ongoing studies of its role in the treatment of breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Breast cancer is the most common malignancy in women worldwide and despite advances in screening , diagnosis, and treatment, it remains one of the most significant causes of cancer-related deaths in women (Bray et al. 2013). Of the different subtypes of breast cancers, triple-negative breast cancer (TNBC) poses a special challenge due to its aggressive clinical course, predilection for metastases, and the lack of targeted therapy (Shuen and Foulkes 2012). Molecularly targeted therapies are becoming increasingly prominent as part of the armamentarium of treatments for breast cancer (Muller et al. 2009). Glycoprotein nonmetastatic B (GPNMB ) was identified as an overexpressed gene in many malignancies, including TNBC, and is associated with lower disease-free and overall survival. The antibody -drug conjugate (ADC) glembatumumab vedotin (CDX-011) appears to selectively target GPNMB, and is being investigated in clinical trials for efficacy in patients with metastatic breast cancer and other GPNMB-expressing tumors. This chapter reviews the mechanisms of action, preclinical, and phase I/II results of glembatumumab vedotin, and ongoing studies of its role in the treatment of advanced breast cancer.
2 GPNMB as a Therapeutic Target
Glycoprotein non-metastatic melanoma protein B is a type I transmembrane protein, which is also known as osteoactivin (OA) (Saitoh et al. 1992). It was first described in 1995 by Weterman et al., as a highly expressed protein in melanoma cell line with low metastatic potential (Weterman et al. 1995). Subsequently, GPNMB was discovered to have high expression in a number of malignancies including glioblastoma multiforme (Kuan et al. 2006), cancers of the breast (Rose et al. 2007), prostate (Fiorentini et al. 2014), liver (Haralanova-Ilieva et al. 2005), and colon (Eldai et al. 2013). GPNMB has a complex role in tumor biology. Despite its originally perceived low invasive phenotype, it is associated with increased metastatic tendencies in malignancies such as breast cancer.7
The GPNMB is located on the small arm of chromosome 7 (7q15), and belongs to the Pmel17/NMB family. Pmel17 is the major structural component of melanosomes, and is essential in melanocyte pigment production (Yamaguchi and Hearing 2009). Also important is that GPNMB shares homology to the lysosomal-associated membrane protein (LAMP) glycoproteins that are implicated in cell adhesion and metastasis(Saitoh et al. 1992).
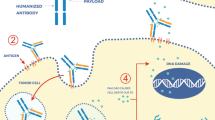
Under normal conditions, GPNMB is situated in intracellular compartments in macrophages and melanocytes (Tomihari et al. 2009; Ripoll et al. 2007). In tumor cells, however, GPNMB expression is enriched on the cell surface (Fig. 13.1; Maric et al. 2013).
Rose et al. found that when GPNMB is overexpressed in parental 4T1 mouse mammary carcinoma cells, they acquire a more invasive phenotype, leading to increased bone metastasis (Rose et al. 2007). A high level of expression correlated with negative estrogen receptor status and higher tumor grade. Moreover, tumors that express GPNMB have high endothelial cell density compared to those without its expression, suggesting GPNMB recruit endothelial cells to promote tumor growth and enhance the metastatic process. The quantification of apoptotic cells revealed fewer cells in GPNMB-expressing tumors was undergoing apoptosis assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), which further elucidates its functional role in tumor progression (Rose et al. 2010a).
The interaction between the tumor and the microenvironment is emerging as an important component in metastasis, and GPNMB has also been implicated in this relationship. Ogawa et al. found that GPNMB can activate fibroblasts by inducing upregulation of pro-invasive metalloproteases such as matrix metalloproteinase-3 (MMP-3) and myelofibrosis with myeloid metaplasia (MMM-9) in mouse models (Ogawa et al. 2005).
3 Glembatumumab Vedotin
GPNMB has the ideal components required for targeted therapy . The selectively extracellular domain in tumor cells (not normal cells) enables the availability to antibody targets. Glembatumumab vedotin (or CDX-011, CR011-vcMMAE) is a fully humanized monoclonal ADC . The first component of the ADC is the tubulin destabilizing cytotoxin monomethylauristatin E (MMAE). It was originally derived from peptides found in the marine shell-less mollusk Dolabella auricularia known as dolastatins (Bai et al. 1990). This antimitotic agent has potency of up to 200 times compared to that of vinblastine and it was found to block the growth of GPNMB-expressing melanoma cells with half-maximal inhibitory concentration (IC50) of 216–300 ng/mL (Vaklavas and Forero 2014). Therefore, the toxicity profile prevents its antineoplastic usage alone, but the high potency proves to be advantageous when MMAE is linked to an antibody, at which point it is referred to as vedotin. Vedotin is also the cytotoxic agent used with brentuximab targeting CD30 in Hodgkin’s lymphoma and anaplastic large-cell lymphoma (Francisco et al. 2003).
Vedotin is conjugated through a cathepsin B-sensitive valine–citrulline linker to the antibody glembatumumab, which is directed against the extracellular domain of GPNMB (Fig. 13.2). On average, four or five MMAE molecules are bound to a single antibody (Vaklavas and Forero 2014). The ADC is highly stable in serum, and is released and internalized upon binding to GPNMB. After endocytosis, the synthetic dolastin analogue MMAE is released through enzymatic cleavage into the tumor cell cytoplasm where it binds to tubulin and inhibits tubulin polymerization. This result in G2/M phase arrest and apoptosis of the tumor cells (Tse et al. 2006).Footnote 1
Structure of glembatumumab vedotin. MMAE, monomethylauristatin E, PABA p-aminobenzoic acid spacer. (Vaklavas and Forero 2014)
4 Preclinical Models
Glembatumumab vedotin was first demonstrated to be efficacious in metastatic melanoma cells in culture and in xenograft assays. Pollack et al. used the human SK-MEL-2 and SK-MEL-5 melanoma xenografts in athymic mice to assess the antitumor efficacy of glembatumumab vedotin (Pollack et al. 2007). They found that it induced complete tumor regression in 100 % of GPNMB -expressing xenografted melanoma cells. This was achieved at concentrations as low as 2.5 mg/kg, administered intravenously every 4 days for four treatments. Interestingly, tumors did not regrow during the nontreatment observation period of 200 days. In the breast cancer cell line MDA-MB-468, a single dose of 20 mg/kg glembatumumab vedotin was able to induce sustained tumor regression in vivo (Rose et al. 2010a).
5 GPNMB and Triple-Negative Breast Cancer
Rose et al. first investigated GPNMB as a potential therapeutic target in breast cancer by analyzing its expression in several breast cancer gene expression data sets and in primary human breast tumors. They found that GPNMB may serve as an important target, especially for TNBC patients whose clinical courses tend to be more aggressive with increased potential for metastasis. Since TNBC patients have limited therapeutic options, there is a tremendous need for the identification and development of precision therapy for these patients. In the Rose et al. study, the investigators first showed that GPNMB messenger RNA (mRNA) expression in 295 human breast tumors is associated with reduced metastasis-free and overall survival. This was accomplished through comparing GPNMB mRNA levels with clinical outcomes in three published data sets. GPNMB protein expression in tumor epithelium was also found to have significantly inferior outcome in human breast cancer evaluated by immunohistochemical (IHC) staining for GPNMB using breast tissue microarray data. Intriguingly, GPNMB expression correlated with recurrence within the TNBC patients. An independent cohort of breast cancer tissue microarrays enriched for TNBC samples showed that 29 % of triple negative tumors are GPNMB-epithelial positive, compared with only 3.6 % in luminal and 11.6 % in HER2 tumors. The multivariate Cox regression survival analysis revealed that GPNMB was an independent prognostic indicator of distant metastasis in TN tumors (p = 0.0024). Therefore, GPNMB-epithelial expression is more common in TNBC, and within this subtype, its expression is associated with an increased risk of recurrence (Rose et al. 2010).
6 Clinical Trials of Glembatumumab Vedotin
Glembatumumab vedotin was first investigated in two multicenter phase I/II clinical trials for patients with unresectable melanoma and advanced stage or metastatic breast cancer (Hamid et al. 2010; Saleh et al. 2010; Burris et al. 2009). In the melanoma study, tumor shrinkage was achieved in up to 56 % of patients when glembatumumab vedotin was given intravenously at 1.88 mg/kg in the phase II maximum tolerated dose (MTD) expansion at dose frequencies ranging from weekly to q3 weeks. The median progression-free survival (PFS) was highest at 3.9 months in patients treated at the q3 week dose. Importantly, the subset of patients with strong GPNMB expression, defined as 3 + by IHC with 90 % staining, had higher tumor shrinkage at 86 % and longer median PFS of 4.9 months. Of note, 57 % of all patients developed varying degrees of skin rash, which also correlated with greater median PFS of 4.8 versus 1.2 months in those without rash. This may be related to the presence of GPNMB in skin, and act as a predictor of drug efficacy (Hamid et al. 2010).
Similarly, glembatumumab vedotin was used in heavily pretreated advanced breast cancer patients in phase I standard dose escalation followed by a phase II expansion at the MTD to assess a 12-week PFS. Eligible patients for this study had at least two prior chemotherapy regimens that included taxanes, anthracycline, and capecitabine. A total of 42 patients were enrolled, and 71 % of the tumor specimens were positive for GPNMB defined as > 5 % of malignant epithelial or stromal cells with GPNMB expression. IHC with goat polyclonal antibody to GPNMB was performed on 25 % of the patient biopsies that were available for the evaluation (done as a post hoc analysis). This study demonstrated median PFS of 9.1 weeks in all patients enrolled. In GPNMB-expressing patients at the MTD/phase II dose, PFS was doubled at 18.3 weeks. The objective response rate was also the highest amongst this subgroup at 29 % (Burris et al. 2009). This suggestion that efficacy might be enhanced in tumors with higher GPNMB expression led to the design of future studies of glembatumumab vedotin in tumors that had expression of the target.
6.1 Toxicity Profile
Glembatumumab vedotin was generally well tolerated in the phase I/II study in advanced breast cancer patients. The most common adverse reactions (AEs) were rash (61 %), fatigue (50 %), and alopecia (50 %). The most common grade 3 or 4 AEs are neutropenia (17 %) and neuropathy (11 %). Patients with baseline neuropathy worse than grade 1 were excluded after first two patients at 1.34 mg/kg dose experienced worsening of neuropathy (Burris et al. 2009).
6.2 Clinical Trials on the Horizon
The phase I/II studies demonstrated both efficacy and manageable safety profile of glembatumumab vedotin. The encouraging results led to the initiation of the electronic medical records and genomics (EMERGE) study, a phase II, open-label, randomized study designed to evaluate the anticancer activity of glembatumumab vedotin in advanced GPNMB -expressing breast cancer. Patients were stratified by GPNMB expression pattern (any tumor, low stromal, or high stromal) and were randomized in 2:1 fashion to glembatumumab vedotin (1.88 mg/kg IV every 3 weeks) or investigator’s choice (IC) single-agent chemotherapy. Patients were treated until progression of disease (PD) or intolerance to therapy. In this study, patients on the IC arm were allowed to crossover at PD. The primary endpoint is overall response rate (ORR) and secondary endpoints are PFS, toxicity profile, and pharmacokinetics. Preliminary analysis of the study was presented at San Antonio breast symposium in 2012, and the results were promising in the enhanced activity in TNBC and high GPNMB-expressing breast cancer tumors. The study has been completed and the final results are expected to be published at the end of 2014 (Yardley et al. 2012).
In order to understand the activity of the drug in TNBC patients, the randomized phase II METRIC study has also been initiated to assess the efficacy in women with metastatic GPNNB-overexpressing TNBC. Patients with GPNMB -overexpressing TNBC (expression > 25 %) will be randomized to receive glembatumumab vedotin or capecitabine in a 2:1 ratio. The primary endpoints are ORR and PFS. This study is currently enrolling patients from multiple centers.Footnote 2
Studies of glembatumumab vedotin in other GPNMB positive cancers such as osteosarcoma, squamous lung cancer, and melanomas are also under consideration (Table 13.1).
7 Conclusion
GPNMB is a relevant target in breast cancer, and the ADC glembatumumab vedotin has been demonstrated in clinical trials to be efficacious in patients with advanced and metastatic disease. Triple-negative breast cancers may have higher GPNMB expression, which provides the rare opportunity of targeted therapy for this subtype. Further confirmatory clinical trials are underway and we eagerly await the results of the randomized phase II EMERGE and METRIC trials. Glembatumumab vedotin will continue to be explored in other GPNMB-expressing malignancies.
Notes
- 1.
- 2.
Clinicaltrials.gov. Study of glembatumumab vedotin (CDX-011) in patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (METRIC); 2014. http://clinicaltrials.gov/ct2/show/NCT01997333.
References
Bai R, Pettit GR, Hamel E (1990) Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol 15;39(12):1941–1949
Bray F, Ren JS, Masuyer E, Ferlay J (2013) Global estimates of cancer prevalence for 27 sites in the adult population 2008. Int J Cancer 132:1133–1145
Burris H, Saleh MN, Bendell J et al (2009) A Phase (Ph) I/II Study of CR011-VcMMAE, an antibody-drug conjugate, in patients (Pts) with locally advanced or metastatic breast cancer (MBC). Cancer Res 69(24s3):6096
Clinicaltrials.gov. Study of Glembatumumab Vedotin (CDX-011) in Patients With Metastatic, gpNMB Over-Expressing, Triple Negative Breast Cancer (METRIC); 2014. http://clinicaltrials.gov/ct2/show/NCT01997333
Eldai Hl, Periyasamy S, Al Qarni S, Al Rodayyan M, Muhammed Mustafa S et al (2013) Novel genes associated with colorectal cancer are revealed by high resolution cytogenetic analysis in a patient specific manner. PLoS ONE 8(10):e76251
Fiorentini C, Bodei S, Sigala S et al (2014) GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res 323:100–111
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, Deblanc R, Toki BE, Law CL, Doronina SO, Siegall CB, Senter PD, Wahl AF (2003) CAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102(4):1458–1465
Hamid O, Snzol M, Pavlick AC et al (2010) Frequent dosing and GPNMB expression with CDX-011 (CR011-vcMMAE), an antibody-drug conjugate (ADC), in patients with advanced melanoma. J Clin Oncol (Meeting Abstracts) 28(15 s):8525
Haralanova-Ilieva B, Ramadori G, Armbrust T (2005) Expression of osteoactivin in rat and human liver and isolated rat liver cells. J Hepatol 42(4):565–572
Kuan CT, Wakiya K, Dowell JM et al (2006) Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res 12(7 Pt 1):1970–1982
Maric G, Rose AA, Annis MG, Siegel PM (2013) Glycoprotein non-metastatic b (GPNMB): a metastatic mediator and emerging therapeutic target in cancer. Onco Targets Ther 6:839–852
Muller V, Witzel I, Stickeler E (2009) Immunological approaches in the treatment of metastasized breast cancer. Breast Care 4(6):359–366
Ogawa T, Nikawa T, Furochi H et al (2005) Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol 289(3):C697–C707
Pollack VA, Alvarez E, Tse KF et al (2007) Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol 60(3):423–435
Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA (2007) Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol 178(10):6557–6566
Rose AA, Annis MG, Dong Z et al (2010a) ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One 5(8):e12093
Rose AA, Grosset AA, Dong Z et al (2010b) Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res 16(7):2147–2156
Rose AA, Pepin F, Russo C, Abou Khalil JE, Hallett M, Siegel PM (2007) Osteoactivin promotes breast cancer metastasis to bone. Mol Cancer Res 5(10):1001–1014
Saitoh O, Wang WC, Lotan R, Fukuda M (1992) Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 267(8):5700–5711
Saleh MN, Bendell JC, Rose A et al (2010) Correlation of GPNMB expression with outcome in breast cancer (BC) patients treated with the antibody-drug conjugate (ADC), CDX-011 (CR011-vcMMAE. J Clin Oncol 28(15 s):1095
Shuen AY, Foulkes WD (2012) Basal-like breast cancer-characteristics, risks, and associations. Oncol Hematol Rev 8(1):26–29
Tomihari M, Hwang SH, Chung JS, Cruz PD Jr, Ariizumi K (2009) Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp Dermatol 18(7):586–595
Tse KF, Jeffers M, Pollack VA et al (2006) CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res 12(4):1373–1382
Vaklavas C, Forero A (2014) Management of metastatic breast cancer with second-generation antibody-drug conjugates: focus on glembatumumab vedotin. BioDrugs 28:253–263
Weterman MA, Ajubi N, van Dinter IM et al. (1995) nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer 60(1):73–81
Yamaguchi Y, Hearing VJ (2009) Physiological factors that regulate skin pigmentation. Biofactors 35(2):193–199
Yardley DA, Weaver R, Milisko ME et al (2012) A randomized phase 2 study of the antibody-drug conjugate CDX-011 in advanced GPNMB-overexpressing breast cancer: the EMERGE STUDY. Cancer Res 72(24, Supplement 3); (Thirty-fifth annual CTRC-AACR San Antonio breast cancer symposium, San Antonio; 2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Vahdat, L., Chan, N. (2015). The Antibody-Drug Conjugate Glembatumumab Vedotin (CDX-011) and Its Use in Treatment of Breast Cancer. In: Wang, J., Shen, WC., Zaro, J. (eds) Antibody-Drug Conjugates. AAPS Advances in the Pharmaceutical Sciences Series, vol 17. Springer, Cham. https://doi.org/10.1007/978-3-319-13081-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-13081-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13080-4
Online ISBN: 978-3-319-13081-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)