Abstract
Organotins such as triphenyltin (TPT) form an important group of bio-environmental tin-substituted aromatic contaminants used in various industries. Anthropogenic activities have led to an increase in TPT concentrations in water, soil, sediments and organisms. Knowledge about the environmental concentrations of any chemical compound is required to understand its effects on the system. Presence of such compounds in the environment is a serious threat and danger for human health and aquatic organisms and these combinations are very resistant against degradation. The reduction of TPT compounds in polluted ecosystems is a function of physical (adsorption to suspended solids and sediments), chemical (chemical and photochemical degradation) and biological (uptake and biological degradation) removal mechanisms.
In the present study, triphenyltin-transforming bacteria were isolated from polluted harbors by enrichment technique and their growth in presence of this substrate was investigated. Genus assignment of the 24 bacterial isolates indicated that the Gram-negative organisms belonged to Enterobacter, Pseudomonas and Vibrio, while Gram-positive organisms were identified as Bacillus, Staphylococcus and Streptococcus. Among all the isolates identified, Pseudomonas spp. were predominant and accounted for 48 % of the total bacterial isolates, followed by Bacillus (24 %), Vibrio (12 %), Staphyolococcus (8 %), Streptococcus (4 %)andEnterobacter (4 %). A non-fluorescent Pseudomonas sp. identified as Pseudomonas stutzeri by 16S rRNA analysis was found to be most potential isolate with the ability to transform ~ 65 % of TPT when grown in BSS-SG medium with 100 mg L−1 TPT. Such bacterial strains having capability of transforming and withstanding high concentration of pollutants will be useful in bioremediation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The earth’s crust consists of nearly 80 elements. The elements, especially the metals, play a pivotal role in human life. Among several metals, tin (Sn) has been used for over 3000 years. This silvery, malleable posttransition metal is not easily oxidized in air and hence its compounds are used to coat other metals to prevent corrosion. The organic derivatives of tin are known as organotins (OTs). The estimated worldwide industrial production of OTs exceeds 50,000 t per annum. About 70 % of the total production is being used as additives in the plastic industry, for the production of polyurethane foams and silicones (Bennett 1996). Some OT compounds are highly toxic and have been used as biocides in antifouling paints and agriculture (Champ and Seligman 1996). Triphenyltin (TPT) is one such OT compound used as a biocide. TPT is also been used as a fungicidal component in agriculture, and for timber preservation (Hoch 2001). However, TPT has a harmful effect on nontarget aquatic organisms when released into the environment, even at trace levels (Harino et al. 1998, 2000) . TPT pollution in aquatic systems may cause various changes in the affected fauna, such as thickening of the shell and failure of spat in oysters (Alzieu 1996), impotence in gastropods and neogastropods (Bryan et al. 1998, CICAD 13 1999), reduction of dogwhelk populations (Gibbs et al. 1991), retardation of growth in mussels (Salazar and Salazar 1991) and immunological dysfunction in fish (Suzuki et al. 1992).
The European Commission now considers OTs such as tributyltins (TBT) and TPT as priority hazardous substances in water and the maximum allowable concentration of OTs is proposed to be fixed at 0.0015 µg L‒1 in inland surface waters (COM 2006). The International Maritime Organisation (IMO) has banned the use of OTs on ship hulls and in aquaculture since 2008. But these compounds are illegally used in many South Asian countries including India. As TPT is cheaper compared to TBT, it has replaced TBT in many industrial applications. This has given rise to a substantial amount of TPT pollution (Kannan et al. 1995; Kannan and Lee 1996) .
10.2 Chemical and Physical Properties of TPT
TPT consists of three phenyl molecules having a covalent bond with the Sn atom (Fig. 10.1a). The covalent bond between Sn and C remains stable in the presence of water, atmospheric O2 and heat. TPT compounds may be characterised by a general formula (C6H5)3Sn-X, where X is an anion or an anionic group, such as chloride, hydroxide and acetate. The physical and chemical properties of TPT compounds vary depending upon the X linked to the Sn molecule (Table 10.1). These compounds are lipophilic in nature and have low water solubility (typically a few mg L‒1 at neutral pH). Ultraviolet radiations, strong acids and electrophile agents may cleave the covalent bond between Sn and the carbon moiety. Diphenyltin (DPT) and monophenyltin (MPT) are degradation products of TPT, together called as phenyltins (PTs). OTs can be synthesized using three different routes, Grignard, Wurtz and aluminium alkyl route (Fig. 10.1b) .
Synthesis of organotin compounds a and structure of TPT b (Blunden and Evans 1990). Where R can be phenyl, butyl, ethyl, methyl, alkyl or aryl group and X can be any halide
Environmental analysis of TPT requires methods that are sensitive enough for an accurate determination at extremely low concentrations (ng Sn L‒1). Species-selective analysis of TPT compounds is performed by coupled techniques based on a combination of a chromatographic separation technique with a sensitive and element-selective detection method. The most common technique is gas chromatography (GC) coupled with element-specific detection methods like atomic absorption spectrometry (GC-AAS; Cai et al. 1993), mass spectroscopy (GC-MS; Jadhav et al. 2009), microwave-induced and inductively coupled plasma atomic emission spectrometry (GC-MIP-AES and GC-ICP-AES, respectively) or flame photometric detection (GC-FPD; Bhosle et al. 2004, 2006) .
10.3 TPT as Source of Environmental Pollution
Any surface immersed in water adsorbs dissolved organic matter thereby leading to conditioning of the surface . Conditioned surfaces are then colonized by microorganisms followed by macroorganisms. Attachment and growth of organisms on a surface is called fouling. Fouling is of economic concern to the shipping industries because it induces frictional drag on the hulls of ships thereby increasing the fuel consumption. In order to reduce economic losses due to fouling, the ship hull is coated with paints containing antifouling agents like OTs (TBT and TPT). These biocides are slowly released from the hull when it comes in contact with water. The release of biocide prevents the settlement of fouling organisms such as barnacles, tubeworms, etc. The OT-based paint protection for 5–7 years is estimated to save the shipping industry some US$ 5.7 billion per annum (Rouhi 1998). This also results in annual fuel saving of 7.2 million tonnes per year (Bennett 1996).
Another major use of TPT lies in the agricultural industry (Kannan et al. 1995). TPT has been used as a fungicide across the globe, to treat a variety of plants such as potatoes, sugar beets, peanuts and rice. There has been considerable increase in the amount of TPT used as a fungicide in some areas over the last three decades as it is an approved pesticide for feedstock. Kannan and Lee (1996) reported a 3-fold increase in the usage of TPT as pesticide all over the world. TPT compounds are also used in the textile and paper industry (Hoch 2001). The PVC polymer becomes unstable under the influence of heat and light resulting in discolouration and embrittlement. Addition of OT compounds prevents this degradation process of the polymer.
PTs enter the aquatic environment directly by leaching through antifouling paints, through river run-off from agriculture and industrial waste, municipal sewage, etc (Fig. 10.2). TPT compounds may also enter the environment by leaching into soil and groundwater from consumer products containing PT compounds disposed off in landfills (Fent 1996; Fent and Hunn 1991). The environmental concentration of TPT varies based on where, when and how the compounds are used .
Industrial applications and sources of PTs in the environment. (Jadhav 2013)
10.4 Fate of TPT Compounds in Environmental Systems
With the wide industrial applications, considerable amounts of TPT compounds have entered various ecosystems. The persistence of TPT compounds in polluted ecosystems is a function of physical (adsorption to suspended solids and sediments), chemical (chemical and photochemical degradation) and biological (uptake and biological degradation) removal mechanisms (Fig. 10.3). Thus, it is important to study the distribution and the degradation processes of TPT compounds under natural conditions. Degradation of TPT in the natural environment can be caused by three processes;
Fate of TPT in the aquatic environment. (Hoch 2001)
-
1.
Photolysis by sunlight appears to be the fastest route of degradation in water. But because of attenuation of sunlight with depth in the water column, photolysis is probably not important at greater depths in water, nor in sediments or soils (Hoch 2001).
-
2.
The Sn–C bond can be attacked by both nucleophile and electrophile reagents. For example, mineral acid, carboxylic acids and alkali metals are agents which are able to cleave Sn–C bonds (Hoch 2001).
-
3.
Barnes et al. (1973) showed stepwise decomposition of TPT acetate in soil to DPT and MPT by bacteria capable of degrading TPT such as Pseudomonas aeruginosa, P. putida and Alcaligenes faecalis. The study demonstrated that bacteria may play an important role in TPT degradation. However, only a limited number of such species have been identified until now and little is known about the conditions required for biological degradation (Dubey and Roy 2003).
10.5 Distribution and Effects of TPT Compounds on Various Ecosystems
Anthropogenic activities have led to an increase in TPT concentrations in water, soil, sediments and organisms. Knowledge about the environmental concentrations of any chemical compound is required to understand its effects on the system. The aquatic system is most susceptible to TPT contamination, as TPT is directly released into the aquatic environment by antifouling coatings, agricultural and municipal waste into coastal areas, marinas, bays and the open sea (Fig. 10.2). Concentrations of PTs in the marine environment can vary among seasons (Lee et al. 2006). Concentrations of TPT in organisms have been found to be greater in summer (Hung et al. 1998, 2001; Lee et al. 2005). Because antifouling paints are not the only source of PTs, this seasonal trend of PTs might be due to their use in aquaculture nets and application of PT-containing biocides in both mariculture and agriculture during the summer (Hung et al. 1998; Meng et al. 2009) .
The observed distribution and variation in the concentrations of PTs are caused by different sources of contaminants. In the aquatic environment, TPT compounds have low solubility and mobility, and they are adsorbed onto suspended particulate matter (SPM). The deposition of SPM leads to TPT scavenging in sediments, where considerable amounts of TPT and its degradation products can be detected. TPT is expected to be present in the upper 2–3 cm of the sedimentary column because of its recent use in aquatic systems. Nevertheless, a core collected (107 ng Sn g‒1) from freshwater marina of Switzerland indicated the presence of TPT upto 11 cm of the sediment core in varying concentration (Fent and Hunn 1991). Dated sediment indicates that anoxic harbour sediments are long-term reservoirs of TPT compounds. The degradation rate of OT in sediments may range from 1.8 to 2.8 years (De Mora and Pelletier 1997). Therefore, the subject of growing concern and debate is persistence of OT, the transformation kinetics and their possible release from sediments. The presence of PT species on suspended matter or sediment makes them available to filter or sediment feeding organisms. Another possible contamination risk is resuspension and remobilisation of contaminants from sediments due to dredging, swirling or desorption due to life activities (Hoch 2001) .
TPT accumulation in marine fauna was first reported by Takami et al. (1988). In general, TPTs are known to bind to amino acids, peptides lipids and proteins and this complexation may influence tissue distribution in organisms (Davies and Smith 1980, Hu et al. 2009). TPT is hazardous to aquatic life and has been proved to be more neurotoxic than TBT (Lee et al. 2006). TPT compounds are found to be potential endocrine disruptors and induce imposex in some prosobranch (snails) species (Barroso and Moreira 2002; Horiguchi et al. 1997; Santos et al. 2009; Schulte-Oehlmann et al. 2000). Laboratory experiments showed that ‘imposex’ is initiated and promoted by TPT at concentrations of 1 ng Sn L‒1 in Japanese rock shell Thais clavigera (Horiguchi et al. 1997). Imposex gives rise to reproductive failures and, as a consequence, population decline. TPT and other OT compounds can have adverse effects on molting, growth and reproduction of crustaceans (Rodriguez et al. 2007). Widdows et al. (1995) reported that threshold values for affecting the growth of mussels was 2 µg g‒1 dw. Individual populations may vary in their susceptibility to TPT exposure due to different genotypes, rate of metabolism, ontogenic development and environmental history. TPT affects algal cells at 5–15 µg L‒1 and cells were totally damaged at 20 µg L‒1 (Rumampuk et al. 2004). This study showed that reproductive cells of algae were more sensitive than somatic cells (Rumampuk et al. 2004). TPT had adverse effects on the freshwater plant Lemna polyrhiza, at concentrations of 2–5 µg L‒1 (Song and Huang 2005). Due to the hydrophobicity of the PT molecules, it can easily solubilise in biological membranes and thus affect the organism. However, individual populations may vary in their susceptibility to TPT exposure due to different genotypes, rate of metabolism, ontogenic development and environmental history. The review of measured TPT concentrations in marine ecosystem can be found in Yi et al. (2012) .
TPTs inhibit a number of microbial processes. TPTs affect energy transduction, solute transport and retention and oxidation of substrates. The mode of action of OT compounds has been described in terms of hydrogen bonding with the active centres of cell constituents resulting in interference with normal processes. Since the OT complexes inhibit the growth of microorganisms, it has been assumed that the production of an enzyme is being affected as a result of which the microbes are less able to metabolize the nutrients resulting in growth cessation. Those enzymes that require free sulfydryl groups (–SH) for activity, appear to be especially susceptible to deactivation by ions of the complexes (Basu Baul 2008) .
In general, OT compounds are more toxic towards Gram-positive bacteria than Gram-negative bacteria, but TPT–Cl is equally toxic towards both. TPTs are known to inhibit methanogens and fermentative bacteria (Harino et al. 1997). Triphenyltin actetate (TPT–Ac) inhibited the nitrification by bacteria and fungi (Wurtz and Cooney 1989). TPT-inhibited light-induced proton uptake in Halobacterium halobium (Mukhohata and Kaji 1981). A good activity minimum inhibitory concentration (MIC) 3.1 µg ml‒1 against Staphylococcus epidermis was noted for DPT-Cl. PTs are known to inhibit several Gram-positive bacteria like Bacillus subtilis , Staphlococcus aureus and several bacilli. The recommended dose for TPT and DPT is given as 3 µg L‒1 for bactericidal action (Basu Baul 2008) .
10.6 Microbial Transformation of TPT
Bacteria have developed very efficient and very different mechanisms for tolerating OTs (Trevors et al. 1985). Often, normal toxic levels of OT have no effect on cell growth of these tolerant organisms. The mechanisms responsible for the tolerance of OT by bacteria could be because of the following reasons :
-
1.
Exclusion of OTs from cell-mediated by a multidrug efflux pump
-
2.
Bioaccumulation into cell without breaking down
-
3.
Degradation/utilization of OTs as a carbon source
-
4.
Transformation of OTs to less toxic compounds (Cruz et al. 2007)
The microbial transformation and degradation of OT compounds with special reference to TBT and TPT are summarized in this section, Barug (1981) reported that P. aeruginosa was able to degrade 40 % TBT–Cl when grown in presence of 2.5 mg L‒1 TBT–Cl. Pseudomonas diminuta isolated from polluted river, Osaka, Japan, was able to degrade 20 µg L‒1 TBT (Kawai et al. 1998). P. aeruginosa capable of utilizing TBT–Cl as a sole source of carbon was isolated from marine water samples (Roy and Bhosle 2006).
Suzuki et al. (1992) found that nearly 90 % of the bacterial flora mostly belonging to Vibrio species of natural seawater was resistant to TBT–Cl. One of the cultures, Alteromonas sp. was found to be tolerant to 250 nm TBT–Cl (Suzuki et al. 1992). According to most of the reports, OT resistance in bacteria is rendered by chromosomal genes (Suzuki et al. 1994, Suzuki and Fukagawa 1995). Pseudomonas stutzeri 5MP1 isolated from sediments of Arcachon harbour (France) resisted 1000 mg L‒1 of TBT which was attributed to the presence of operon TbtABM homologus to resistance-nodulation-division (RND) multidrug efflux pump (Jude et al. 2004). Aeromonas veronii isolated from an estuarine environment of Portugal was able to resist 3 mM TBT (Cruz et al. 2007). However, OT resistance in some bacteria can be plasmid-mediated (Miller et al. 1995) .
A study conducted on TPT acetate degradation in soil revealed that P. aeruginosa, P. putida and A. faecalis were responsible for degradation of TPT to DPT, MPT and inorganic Sn (Barnes et al. 1973). Visoottiviseth et al. (1995) isolated P. putida no.C from soil samples collected from the dockyards of Thailand. P. putida no. C was able to transform 97 % of TPT in minimal medium supplemented with glucose and 7 mg L‒1 TPT. Inoue et al. (2000) isolated Pseudomonas chlororaphis CNR 15 from coastal sediment. The culture was able to transform 47 % of TPT when grown in minimal medium containing 40 mg L‒1 TPT. Later, they identified TPT degrading factor as suc-pyoverdine (succinic acid derivative of siderophore) in P. chlororaphis CNR 15. (Inoue et al. 2003). Immobilized cells of P. chlororaphis with calcium alginate were able to adsorb and transform TPT. The transformation activity was influenced by temperature and pH and showed maximum at 30 °C and pH 8.8 (Osamu et al. 2003). Yet another fluorescent pseudomonad, P. aeruginosa CGMCC 1.860 was able to transform nearly 50 % of TPT when grown in succinate M9 medium with 100 µM of Fe (III) and 77 mg L‒1 TPT (Sun et al. 2006). Sun and Zhong (2006) demonstrated that the presence of TPT–Fe–pyochelin complex was instrumental in TPT degradation by P. aeruginosa. Pyochelin is a secondary siderophore present in the culture along with pyoverdine. So far, only siderophores like pyoverdine and pyochelin have been reported to be instrumental in TPT transformation process of TPT by bacteria .
Other than bacteria, few algal and fungal species have also been reported to carry out OT degradation. Reader and Pelletier (1992) reported that microalgal species Skeletonema costatum is capable of degrading TBT at even 4 °C. Maguire et al. (1984) demonstrated that the green algae Ankistrodesmus falcatus was capable of degrading TBT to dibutyltins (DBT). Unicellular green algae, Chlorella vulgaris and Chlorella sp. degrade TBT–DBT and monobutyltins (MBT) intracellularly with the help of a TBT-metabolizing enzyme (Tsang et al. 1999). Cunninghamella elegans IM 1785/21Gp, a fungus was able to degrade 70 % of TBT (added at 10 mg L‒1) to less toxic derivatives such as DBT and MBT when grown over a period of 7 days in Sabouraud medium and M3 medium involving cytochrome P-450 system (Bernat and Długonski 2002, 2006) .
Bacteria can be used as an effective tool in bioremediation processes as they are able to tolerate and transform OT compounds at higher concentration and the transformation period is considerably reduced with bacteria as compared to fungi and algae. However, the process of TPT transformation by bacteria is poorly understood. The question is whether only fluorescent bacteria are capable of TPT degradation/transformation and whether the transformation is by enzymes or due to chelation process. In view of this, there is a need to search for bacteria which can tolerate and transform/degrade TPT, especially at environmental concentrations present in harbours and marinas. These TPT-tolerant bacteria can be used as an effective tool to transform TPT to nontoxic forms .
10.7 TPT-Transforming Bacteria from Coastal Sediments
Studies were undertaken to isolate TPT-transforming bacterial cultures from marine sediments collected from Sancoale shipyard (South Goa), Betim fish jetty (North Goa), and Visakhapatnam harbour, east coast of India.
10.7.1 Enrichment Technique
Enrichment was carried out by adopting two methods. In the first method, sediment sample was collected from Sancoale shipbuilding industrial area in Goa. Sediment sample (100 g) was transferred to a 500 mL beaker and 500 mg of TPT-chloride, nitrates and phosphates (9:1 ratio) were added and the sample was thoroughly mixed. The beaker was incubated at room temperature (28 ± 2 °C) in the dark. After 30 days, 1 g sediment was appropriately diluted and plated on ZoBell marine agar (ZMA) plates containing 100 mg L‒1 of TPT. Plates were incubated at room temperature (28 ± 2 °C) for 48 h. The individual colonies were collected and restreaked on ZMA plates to check their purity. The cultures thus obtained were maintained on ZMA slants containing TPT (100 mg L‒1) at 4 °C.
In the second method, enrichment was carried out by inoculating 10 g of sediment collected from Betim fish jetty (North Goa) and Visakhapatnam harbour to Basal Salt Solution (BSS) medium supplemented with TPT-chloride (100 mg L‒1; Bhosle 1981). The flask was incubated on a rotary shaker (100 rpm) at room temperature (28 ± 2 °C) in the dark for one week. The culture was subcultured twice using the same but fresh medium. After obtaining consistent growth, 1 mL of the culture was appropriately diluted and plated on ZMA containing 100 mg L‒1 of TPT. These plates were incubated for 48 h at room temperature (28 ± 2 °C). Individual colonies were picked, purified and maintained as described above.
Using enrichment technique, 24 bacterial cultures were isolated from marine sediments collected from Sancoale shipbuilding yard (South Goa), Betim Fish Jetty (North Goa) and Visakhapatnam harbour.
10.7.2 Growth of Bacterial Isolates in BSS with Varying Concentrations of TPT
Bacterial isolates were tested for their ability to grow or transform various concentrations of TPT (0, 25, 50, 75 and 100 mg L‒1) in BSS medium. The growth of bacterial cultures was slow and minimal in BSS medium supplemented with 25 and 50 mg L‒1 of TPT. Only 4 cultures (SG 01, SG 04, SV 04, and SV 14) showed visible turbidity when grown with 75 and 100 mg L‒1 of TPT. Hence, additional carbon sources in the form of sodium succinate and glycerol were used in BSS medium to boost the growth of the bacteria and the medium was designated as BSS-SG. Bacterial cultures were grown by preparing an inoculum in BSS, BSS with 100 mg L‒1 of TPT, BSS with succinate (0.4 %) glycerol (0.1 %) and BSS with succinate (0.4 %), glycerol (0.1 %) and 100 mg L‒1 of TPT, individually. Growth was monitored using UV-VIS spectrophotometer. The successive transfers of bacterial cultures in BSS-SG medium supplemented with TPT produced luxuriant growth; therefore, BSS-SG medium supplemented with 100 mg L‒1 of TPT was used for the screening of potential bacterial cultures.
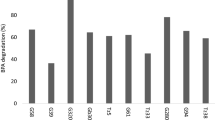
To check for transformation ability, aliquots of culture broth (100 µL) were withdrawn at 0 days and 7 days following inoculation, and transferred to clean stoppered test tubes. The culture broth was then analysed for TPT using GC-MS-EI (70 eV) as described by Jadhav et al. (2009). Out of 24 isolates, 17 were able to grow and transform TPT to varying extents (5–65 %), while the rest of the isolates did not transform TPT but were able to tolerate TPT (Table 10.2).
10.7.3 Characterisation and Identification
The cultures consistently growing in the presence of TPT (100 mg L‒1) were examined for morphological, biochemical and physiological characteristics to identify the isolates upto generic level using the standard techniques (Kreig and Holt 1994). Among Gram-positive organisms, Bacillus sp. were dominant while among Gram-negative isolates it was found that Pseudomonas sp. were predominant and accounted for 48 % of the total bacterial isolates. The permeable architecture of cell membrane in Gram-negative bacteria allows the mobilization of metal ions (Cruz et al. 2007) . Moreover, the presence of multidrug efflux pumps in these bacteria makes them more resistant to metals and pollutants compared to Gram-positive bacteria (Jude et al. 2004).
Most (81 %) of the isolates belonging to Pseudomonas genus were able to transform TPT. One of the Pseudomonas isolate, designated as SG-04 was able to degrade ~ 65 % of TPT and was identified as a strain of P. stutzeri. Further, 16S rDNA molecular sequencing followed by NCBI-BLAST search also confirmed the isolate SG-04 as a strain of P. stutzeri (Fig. 10.4). The 16S rDNA fragment from the strain has been deposited in the GenBank database (Accession no. JF509451.1). P. stutzeri is a ubiquitous bacterium in the environment (both in water and sediment) with a high degree of physiological and genetic adaptability. It is present in different natural environments. P. stutzeri is involved in environmentally important metabolic activities (Lalucat et al. 2006). Members of this species can metabolize a wide range of organic substrates, including environmental contaminants like naphthalene, carbon tetrachloride, etc. (Baggi et al. 1987; Dybas et al. 1995; Rossello-Mora et al. 1994). They can also be used as model denitrifier in marine environments (Lalucat et al. 2006). P. stutzeri strains have been widely studied as a model for natural transformation processes (Sikorski et al. 1998, 2002; Lorenz and Sikorski 2000).
Marine strains of P. stutzeri are located in the water column and in sediment. The most relevant strains studied in detail are ZoBell, AN10, NF13, MT-1 and HTA208 (Lalucat et al. 2006). The ZoBell strain was isolated from the water column of the Pacific Ocean, and studied as a model denitrifier in marine environments. AN10 was isolated from polluted Mediterranean marine sediment and studied as a naphthalene degrader. NF13 isolated from a sample taken at 2500–2600 m depth in the Galapagos rift from near a hydrothermal vent was studied as a strain that oxidizes sulphur chemolithotrophically and strains MT-1 and HTA208 were isolated from deep-sea samples taken at the Mariana Trench at 10,897 m depth (Lalucat et al. 2006).
P. stutzeri strain isolated from black sea was able to oxidize thiosulphate to tetrathionate (Lalucat et al. 2006). P. stutzeri 5MP1 is a tributyltin-resistant strain (MIC 1000 mg L‒1) isolated from the sediment of Arcachon harbour (France). Tributyltin resistance was found to be associated with the presence of the operon tbtABM. It is a member of the resistance-nodulation-cell division efflux pump family (Jude et al. 2004).
10.8 Conclusions and Future Prospects
The review indicates the presence of microbes in the coastal ecosystem which have the potential of transforming TPT and other oganotins. The study undertaken in Goan coastal waters also showed the presence of such bacterial cultures capable of transforming/degrading TPT. A nonfluorescent Pseudomonas spp. identified as P. stutzeri was obtained which transformed ~ 65 % of TPT when grown in BSS–SG medium with 100 mg L‒1 TPT. To the best of our knowledge, this is the first report of the nonfluorescent Pseudomonas carrying out the transformation of TPT. TPT transformations have been reported so far by only fluorescent Pseudomonas spp. with the help of siderophores called pyoverdine and pyochelin.
The major application of TPT-transforming bacteria lies in bioremediation of pollutants from the contaminated sites. The bacterial strains having the capability of transforming and withstanding high concentration of pollutants is a prerequisite for such in field bioremediation experiments. The bacterial strain obtained in the present study showed the ability to survive and transform TPT at environmentally relevant concentrations and could be an ideal vector for carrying out bioremediation at contaminated sites. This study opens new avenues for TPT transformation by nonfluorescent bacteria which might lead to new information on TPT transformation mechanism.
References
Alzieu, C. (1996). Biological effects of tributyltin on marine organisms. In S. J. de Mora (Ed.), Tributyltin, case study of an environmental contaminant (pp. 167–211). Cambridge: Cambridge University Press.
Baggi, G., Barbieri, P., Galli, E., & Tollari, S. (1987). Isolation of a Pseudomonas stutzeri strain that degrades ortho-xylene. Applied Environmental Microbiology, 53, 2129–2132.
Barnes, R., Bull, A., & Poller, R. (1973). Studies on the persistence of the organotin fungicide fentin acetate (triphenyltin-acetate) in the soil and on surfaces exposed to light. Pesticide Science, 4, 305–317.
Barroso, C., & Moreira, M. (2002). Spatial and temporal changes of TBT pollution along the Portuguese coast, inefficacy of the EEC directive 89/677. Marine Pollution Bulletin, 44, 480–486.
Barug, D. (1981). Microbial degradation of bis(tributyltin)oxide. Chemosphere, 10, 1145–1154.
Basu Baul, T. (2008). Antimicrobial activity of organotin(IV) compounds, a review. Applied organometallic chemistry, 22, 195–204.
Bennett, R. (1996). Industrial manufacture and application of tributyltin compounds. In S. J. de Mora (Ed.), Tributyltin, a case study of an environmental contaminant. Cambridge environmental chemistry series (pp. 21-61). Cambridge: Cambridge University Press.
Bernat, P., & Długonski, J. (2002). Degradation of tributyltin by the filamentous fungus Cunninghamella elegans, with involvement of cytochrome P-450. Biotecholgy Letters, 24, 1971–1974.
Bernat, P., & Dlugonski, J. (2006). Tributyltin chloride interactions with fatty acids composition and degradation ability of the filamentous fungus Cunninghamella elegans. International Biodeterioration Biodegradation. doi:10.1016/j.ibiod.2006.12.004.
Bhosle, N. B. (1981). Microbial degradation of petroleum hydrocarbon. PhD thesis. India: University of Mumbai.
Bhosle, N. B., Garg, A., Jadhav, S., Harji, R., Sawant, S., Venkat, K., & Anil, A. C. (2004). Butyltins in water, biofilm and sediments of the west coast of India. Chemosphere, 57, 897–907.
Bhosle, N., Garg, A., Harji, R., Jadhav, S., Sawant, S., Venkat, K., & Anil, A. C. (2006). Butyltins in the sediments of Kochi and Mumbai harbours, west coast of India. Environment International, 32, 252–258.
Blunden, S., & Evans, C. (1990). Organotin compounds. In O. Hutzinger (ed.), The handbook of environmental chemistry Vol. 3, Part E, Anthropogenic compounds (pp. 1–44). Berlin: Springer.
Bryan, G., Gibbs, P., & Burt, G. (1998). A comparison of the effectiveness of tributyltin chloride and five other organotin compounds in promoting development of imposex in dogwhelk, Nucella lapillus. Journal of Marine Biology Association, 68, 733–744.
Cai, Y., Rapsomanikis, S., & Andreae, M. (1993). Determinination of butyltin compounds in sediments using gas chromatography atomic absorption spectrometry; comparison of sodium tertahydroborate and sodium tertaethylborate derivatisation methods. Analytica Chimica Acta, 274, 243–251.
Champ, M., & Seligman, P. (1996). An introduction to organotin compounds and their use in antifouling coatings. In M. A. Champ & P. F. Seligman (Eds.), Organotin environmental fate and effects (pp. 1–25). London: Chapman and Hall.
CICAD 13. (1999). Concise international chemical assessment document on triphenyltin compounds. Geneva: WHO.
COM (2006). Commission of the European communities, 398 final, Sec (2006) 947: Directive of the European parliament and Council of communities on the environmental quality standard in the field of water policy and amending directive 2000/60/EC.
Cruz, A., Caetano, T., Suzuki, S., & Mendo, S. (2007). Aeromonas veronii, a tributyltin (TBT)-degrading bacterium isolated from an estuarine environment, Ria de Aveiro in Portugal. Marine Environment Resources, 64, 639–650.
Davies, R., & Smith, P. (1980). Recent advances in organotin chemistry. Journal of Advance Inorganic Chemistry and Radiochemistry, 23, 1–185.
De Mora, S., & Pelletier, E. (1997). Environmental tributyltin research, past, present and future. Environment Science and Technology, 18, 1169–1177.
Dubey, S., & Roy, U. (2003). Biodegradation of tributyltins (organotins) by marine bacteria. Applied Organometallic Chemistry, 17, 3–8.
Dybas, M., Tatara, G., & Criddle, C. (1995). Localization and characterization of the carbon-tetrachloride transformation activity of Pseudomonas sp. strain KC. Applied Environmental Microbiology, 61, 758–762.
Fent, K. (1996). Ecotoxicology of organotin compounds. Critical Reviews in Toxicolology, 26, 3–117.
Fent, K., & Hunn, J. (1991). Phenyltins in water, sediment, and biota of freshwater marinas. Environment Science and Technology, 25, 956–963.
Gibbs, P., Pascoe, P., & Bryan, G. (1991). Tributyltin-induced imposex in stenoglossan gastropods, pathological effects on the female reproductive system. Comprehensive Biochemistry and Physiology, 100C, 231–235.
Harino, H., Fukushima, M., Kurokawa, Y., & Kawai, S. (1997). Susceptibility of bacterial populations to organotin compounds and microbial degradation of organotin compounds in environmental water. Environment Pollution, 98, 157–162.
Harino, H., Fukushima, M., Yamamoto, Y., Kawai, S., & Miyazaki, N. (1998). Organotin compounds in water, sediment, and biological samples from the Port of Osaka, Japan. Archives of Environmental Contamination and Toxicology, 35, 558–564.
Harino, H., Fukushima, M., & Kawai, S. (2000). Accumulation of butyltin and phenyltin compounds in various fish species. Archives of Environmental Contamination and Toxicology, 39, 13–19.
Hoch, M. (2001). Organotin compounds in the environment-an overview. Applied Geochemistry, 16, 719–743.
Horiguchi, T., Shiraishi, H., Shimizu, M., & Morita, M. (1997). Effects of triphenyltin chloride and five other organotin compounds on the development of imposex in the rock shell, Thais clavigera. Environment Pollution, 95, 85–91.
Hu, J., Zhang, Z., Wei, Q., Zhen, H., Zhao, Y., Peng, H., Wan, Y., Giesy, J., Li, L., & Zhang, B. (2009). Malformations of the endangered Chinese sturgeon, Acipenser sinensis, and its causal agent. Proceedings National Academy Science USA, 106, 9339–9344.
Hung, C., Lee, T., & Liao, T. (1998). Determination of butyltins and phenyltins in oysters and fishes from Taiwan coastal waters. Environment Pollution, 102, 197–203.
Hung, C., Hsu, W., Mang, P., & Chuang, A. (2001). Organotins and imposex in the rock shell, Thais clavigera, from oyster mariculture areas in Taiwan. Environment Pollution, 112, 145–152.
Inoue, H., Takimura, O., Fuse, H., Murakami, K., Kamimura, K., & Yamaoka, Y. (2000). Degradation of triphenyltin by a fluorescent pseudomonad. Applied Environmental Microbiology, 66, 3492–3498.
Inoue, H., Takimura, O., Kawaguchi, K., Nitoda, T., Fuse, H., Murakami, K., & Yamaoka, Y. (2003). Tin-carbon cleavage of organotin compounds by pyoverdine from Pseudomonas chlororaphis. Applied Environmental Microbiology, 69, 878–883.
Jadhav, S., Bhosle, N. B., Massanisso, P., & Morabito, R. (2009). Organotins in the sediments of the Zuari estuary, west coast of India. Journal of Environmental Management, 90, S4–S7.
Jadhav, S. (2013). Biodegradation of triphenyltin by marine bacteria. PhD thesis. India: Goa University.
Jude, F., Arpin, C., Brachet-Castang, C., Capdepuy, M., Caumette, P., & Quentin, C. (2004). TbtABM, a multidrug efflux pump associated with tributyltin resistance in Pseudomonas stutzeri. FEMS Microbiology Letters, 232, 7–14.
Kannan, K., Tanabe, S., Iwata, H., & Tatsukawa, R. (1995). Butyltins in muscle and liver of fish collected from certain Asian and Oceanian countries. Environment Pollution, 90, 279–290.
Kannan, K., & Lee, R. (1996). Triphenyltin and its degradation products in foliage and soils from sprayed pecan orchards and in fish from adjacent ponds. Environment Toxicology and Chemistry, 15, 1492–1499.
Kawai, S., Kurokawa, H., Harino, M., & Fukushima (1998). Degradation of tributyltin by a bacterial strain isolated from polluted river water. Environment Pollution, 102, 259–263.
Krieg, N., & Holt, J. (1984). Bergey’s manual of systematic bacteriology (Vol. 1, pp. 140–309). Baltimore: Williams and Wilkins.
Lalucat, J., Bennasar, A., Bosch, R., Garcia-Valdes, E., & Palleroni, N. J. (2006). Biology of Pseudomonas stutzeri. Microbiological Molecular Biology Reviews, 70, 510–547.
Lee, C., Wang, T., Hsieh, C., & Tien, C. (2005). Organotin contamination in fishes with different living pattern and its implications for human health risk in Taiwan. Environment Pollution, 137, 198–208.
Lee, C., Hsieh, C., & Tien, C. (2006). Factors influencing organotin distribution in different marine environmental compartments and their potential health risk. Chemosphere, 65, 547–559.
Lorenz, G., & Sikorski, J. (2000). The potential for intraspecific horizontal gene exchange by natural genetic transformation, sexual isolation among genomovars of Pseudomonas stutzeri. Microbiology (Reading, England), 146, 3081–3090.
Maguire, R., Wong, P., & Rhamey, J. (1984). Accumulation and metabolism of tri-n-butyltin cation by a green algae, Ankistrodesmus falcutus. Canadian Journal of Aquatic Science, 41, 537–540.
Meng, P., Lin, J., & Liu, L. (2009). Aquatic organotin pollution in Taiwan. Journal of Environmental Management, 90, S8–S15.
Miller, C. E., Wuertz, S., & Cooney, J. (1995). Pfister RM. Plasmids in tributyltin-resistant bacteria from fresh and estuarine waters. Journal of Industrial Microbiology, 14, 337–342.
Mukohata, Y., & Kaji, Y. (1981). Light-induced membranepotential increase, ATP synthesis, and proton uptake in Halobacterium halobium RImR Catalyzed by Halorhodopsin, Effects of N, N'-dicyclohexylcarbodiimide triphenyltin chloride and 3,5-di -tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Archives of Biochemistry and Biophysics, 206, 72–76.
Osamu, T., Hiroyuki, I., Hiroyuki, F., M Katsuji, M., Yukiho, Y., Masato, A (2003). Adsorption and Degradation of Triphenyltin by Pseudomonas chlororaphis Immobilized in Alginate Beads. Journal of Japan Society’s Water Environment, 26, 713–717.
Reader, S., & Pelletier, E. (1992). Biosorption and degradation of butyltin compounds by the marine diatom Skeletonema costatum and the associated bacterial community at low temperature. Bulletin of Environmental Contamination and Toxicology, 48, 599–607.
Rodríguez, E., Medesani, D., & Fingerman, M. (2007). Endocrine disruption in crustaceans due to pollutants, a review. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 146, 661–671.
Rossello-Mora, R., Lalucat, J., & Garcıa-Valdes, E. (1994). Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains. Applied Environmental Microbiolgy, 60, 966–972.
Rouhi, A. (1998). The queeeze of tributyltin. Chemical Engineering News, 27, 41–42.
Roy, U., & Bhosle, S. (2006). Microbial transformation of tributyltin chloride by Pseudomonas aeruginosa strain USS25 NCIM-5224. Applied Organometallic Chemistry, 20, 5–11.
Rumampuk, N., Grevo, G., Rumengan, I., Ohji, M., Arai, T., & Miyazaki, N. (2004). Effects of triphenyltin exposure on red alga Eucheuma denticulatam. Coastal marine science, 29, 81–84.
Salazar, M., & Salazar, S. (1991). Assessing site-specific effects of TBT contamination with mussel growth rates. Marine Environmental Resource, 32, 131–150.
Santos, M., Enes, P., Reis-Henriques, M., Kuballa, J., Castro, L., & Vieira, M. (2009). Organotin levels in seafood from Portuguese markets and the risk for consumers. Chemosphere, 75, 661–666.
Schulte-Oehlmann, U., Tillmann, M., Markert, B., Oehlmann, J., Watermann, B., & Scherf, S. (2000). Effects of endocrine disruptors on prosobranch snails (Mollusca, Gastropoda) in the laboratory. Part II. Triphenyltin as a xeno-androgen Ecotoxicology, 9, 399–412.
Sikorski, J., Graupner, M., Lorenz, G., & Wackernagel, W. (1998). Natural genetic transformation of Pseudomonas stutzeri in a non-sterile soil. Microbiology (Reading, England), 144, 569–576.
Sikorski, J., Mohle, M., & Wackernagel, W. (2002). Identification of complex composition, strong strain diversity and directional selection in local Pseudomonas stutzeri populations from marine sediment and soils. Environmental Microbiology, 4, 465–476.
Song, Z., & Huang, G. (2005). Toxic effect of triphenyltin on Lemna polyrhiza. Applied Organometallic Chemistry, 19, 807–810.
Sun, G., & Zhong, J. (2006). Mechanism of augmentation of organotin decomposition by ferripyochelin, formation of hydroxyl radical and organotin-pyochelin-iron ternary complex. Applied Environmental Microbiology, 72, 7264–7269.
Sun, G., Zhou, W., & Zhong, J. (2006). Organotin decomposition by pyochelin, secreted by Pseudomonas aeruginosa even in an iron-sufficient environment. Applied Environmental Microbiology, 72, 6411–6413.
Suzuki, S., Fukagawa, T., & Takama, K. (1992). Occurrence of tributyltin-tolerant bacteria in tributyltin- or cadmium-containing seawater. Applied Environmental Microbiology, 58, 3410–3412.
Suzuki, S., & Fukagawa, T. (1995). Tributyltin-resistant marine bacteria, a summary of recent work. Journal of Industrial Microbiology, 14, 154–158.
Suzuki, S., Kita-Tsukamoto, K., Fukagawa, T (1994). The 165 rRNA sequence and genome sizing of tributyltin resistant marine bacterium, strain M-1. Microbios, 77, 101–109.
Takami, K., Okumura, H., Yamasaki, H., & Nakamoto, M. (1988). Determination of triphenyltin and tributyltin compounds in fish and shellfish by capillary GC. Bunseki Kagaku, 37, 449–455.
Tomlin, C. (1997). The pesticide manual (11th ed., p. 1606). Cambridge: The Royal Society of Chemistry (Surrey, British Crop Protection Council).
Trevors, J., Oddie, K., & Belliveau, B. (1985). Metal resistance in bacteria (Environmental; transformation; genetics; plasmid; toxic; metal-tolerant; metal-sensitive). FEMS Microbiology, 32, 39–54.
Tsang, C., Lau, P., Tam, N., & Wong, Y. (1999). Biodegradation capacity of tributyltin by two Chlorella species. Environment Pollution, 105, 289–297.
Visoottiviseth, P., Kruawan, K., Bhumiratana, A., & Wilairat, P. (1995). Isolation of bacterial culture capable of degrading triphenyltin pesticides. Applied Organomettallic Chemistry, 9, 1–9.
Widdows, J., Donkin, P., Brinsley, M., Evans, S., Salkeld, P., Franklin, A., Law, R., & Waldock, M. (1995). Scope for growth and contaminant levels in North Sea mussels (Mytilus edulis). Marine Ecological Progress Series, 127, 131–148.
Wuertz, S., & Cooney, J. J. (1989). Toxic effects of tin on microorganisms. Journal of Industrial Microbiology. Biotechnology, 4, 375–402.
Yi, A., Leung, K., Lam, M., Lee, J., & Giesy, J. (2012). Review of measured concentrations of triphenyltin compounds in marine ecosystems and meta-analysis of their risks to humans and the environment. Chemosphere, 89, 1015–1025.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jadhav, S. (2015). Transformation of Triphenyltin by Eubacteria: Fate and Effects in Environmental System. In: Borkar, S. (eds) Bioprospects of Coastal Eubacteria. Springer, Cham. https://doi.org/10.1007/978-3-319-12910-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-12910-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12909-9
Online ISBN: 978-3-319-12910-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)