Abstract
In this chapter, the effects of emotions on the spinal and cerebral processes underlying nociception and pain perception are examined. Throughout the chapter, the effects of emotions will be compared with those of attention, and the potential interactions between emotions and attention will be discussed. The overall portrait that emerges from this literature review is that emotions and attention can exert their effects at multiple levels of pain processing, from the spinal cord to the cerebral cortex. Moreover, because of the highly integrated and dynamic nature of the neural processes underlying pain perception, it is difficult to identify the origins of emotions’ and attention’s effects on pain. Future research should therefore aim at probing the effects of emotions and attention at various levels of pain processing by combining different psychophysiological methods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The primary function of pain is to alert the organism that its corporal integrity has been compromised in order to attend to both the sources of the pain and its potential consequences. Contrary to purely exteroceptive senses (e.g., vision or audition) which function to gather information about the outside world, pain conveys information about the internal condition of the body. It is the last defense against life-threatening injuries, and in this sense, pain can be conceived as both an interoceptive sense, a homeostatic emotion, and a behavioral motivation (Craig 2003). The inherent survival value of pain shapes its processing at all levels of the neuraxis, from the spinal cord to the cerebral cortex. In this chapter, the influence of emotional factors on pain neural processing and subjective perception will be examined, as well as with attentional factors. First, a more general discussion of the relationship between emotional and cognitive sources of pain modulation will be undertaken. The modulatory effects influencing preconscious nociceptive processes versus modulatory effects affecting the cortical generation of the subjective experience of pain will also be considered.
2 Emotional and Cognitive Sources of Pain Modulation
Emotions have been traditionally considered as qualitatively different from, and somewhat inferior to, cognitions (Descartes 1649). However, this view has been challenged within the field of psychology, where there is a long-lasting debate concerning the role of cognitive factors as determinants of emotions (Arnold 1960; Lazarus 1966). As the emerging field of cognitive neuroscience grew, this debate over the relationship between cognition and emotion rapidly spread to the neural systems underlying these two types of processes, with some researchers arguing that the neural processes underlying emotions are computationally inseparable from those underlying cognitions (Ledoux 2000; Pessoa 2008), while others made the case that emotions’ subjective valence requires a separate explanation (Panksepp 2007). Within the field of pain research, a classical distinction is generally made between emotional and attentional sources of pain modulation (Villemure and Bushnell 2002). While redirecting attention away from pain seems to predominantly affect the sensory dimension, emotions appear to influence the affective dimension of pain (Villemure et al. 2003). Moreover, both sources of modulation seem to be associated with different neural systems. Whereas emotions seem to modulate ascending nociceptive signals through descending modulatory mechanisms centered around the periaqueductal gray (PAG) (Villemure and Bushnell 2009; Bushnell et al. 2013), attention appears to bias sensory processing in primary sensory (S1) and insular cortices through the frontoparietal attentional orienting system (Corbetta and Shulman 2011; Bushnell et al. 2013). In agreement with these findings, the effects of emotions on pain ratings have been repeatedly associated with a parallel modulation of spinal nociceptive flexion reflexes (NFRs; Rhudy et al. 2005, 2006; Roy et al. 2011, 2012a), which can be considered as an index of spinal nociception (Sandrini et al. 2005). By contrast, performance of a distractive task (McIntyre et al. 2006; Petersen et al. 2001; Edwards et al. 2007), or simply reorienting attention away from pain (Roy et al. 2011), seems to have facilitatory or null effects on NFRs, suggesting that attention and emotion have different effects on spinal nociception.
However, the boundaries between emotional and attentional sources of pain modulation may not be as clear as first thought. Indeed, decades of research in humans and animals have suggested that distraction also engages descending modulatory controls. Moreover, relatively recent evidence of increased PAG activity during distraction has been interpreted as a sign that distraction engages descending pain inhibitory mechanisms (Tracey et al. 2002), although this interpretation may constitute a case of abusive reverse inference (Poldrack 2006). More compelling evidence comes from earlier physiological studies showing increases in NFR thresholds during performance of a cognitively demanding task (Bathien 1971) or decreased activity in wide dynamic range (WDR) neurons when attention is directed away from pain (Bushnell et al. 1985). Finally, another line of evidence in support of descending modulatory effects comes from electrophysiological studies in humans showing that directing attention away from or toward the stimulated limb affects the earliest component of nociceptive event-related responses (ERPs; Legrain et al. 2002). This suggests an upstream modulation of nociception at the spinal level. While these effects of attention on pain can be described in purely cognitive terms, they also reflect a fundamental motivational function: because pain is an alarm signal, it can be toned down when performance of a concurrent task is to be prioritized.
Thus, although cognition and affect may be ontologically distinct processes, they are in practice very difficult to disentangle at the neural level. Indeed, cognitive processes are ultimately at the service of one’s goals and desires. Moreover, because cognitive systems have limited information-processing capacities, stimuli constantly have to compete for these limited resources as a function of their behavioral relevance. This competition, which is thought to occur at both the perceptual and executive levels (Pessoa 2008), has to be orchestrated by affective valuation systems. The neural architecture underlying this constant competition therefore requires a profound integration of affective/motivational and cognitive systems through parallel and reciprocal connections between the sensory, cognitive, and affective regions of the brain. For instance, in the visual domain, sophisticated visual information stemming from “late” visual areas is conveyed to emotional structures, such as the amygdala and orbitofrontal cortex (OFC), which then bias early visual processing through reciprocal connections with both late and early visual cortices. In parallel, visual inputs also reach components of the frontoparietal attentional network, such as the lateral prefrontal cortex (LPFC), which also projects back to early visual cortices. Therefore, visual cortical responses reflecting an item’s significance will be a result of simultaneous top-down modulation from frontoparietal attentional regions and emotional modulation from the amygdala and OFC (Pessoa 2008). In this manner, the cognitive or affective origin of the modulation is lost, and the item’s impact on behavior is a product of both cognitive and emotional influences.
The same level of integration between emotional, cognitive, and sensory/perceptual processes also applies for pain. As a matter of fact, this integration may even run a little deeper for pain since descending modulatory pathways can influence ascending nociceptive signals as soon as their first synapse in the dorsal horn of the spinal cord. When reaching the cortex, these signals are then integrated with multisensory inputs in order to produce a higher-order representation of the source of the pain in relation with the body-in-space (Haggard et al. 2013), thereby allowing finely coordinated responses to the source of pain. Finally, the whole episode is also evaluated as a function of the immediate context together with long-term goals and beliefs in order to judge the general aversiveness of the experience (Craig 2003; Roy et al. 2012b). In the next sections, the influence of emotions on pain processing at the spinal and supraspinal levels and how these effects may interact with attention will be examined.
3 Spinal Modulation of Nociception by Emotions
The principles governing the effects of emotions on nociceptive processing can be broadly characterized as a process of competition between nociception and other sources of motivation, which can either be congruent or incongruent with pain (Fields 2007). Anything more important than pain should therefore exert inhibitory effects on nociceptive processing. For instance, tolerance to pain induced by the hot plate test is increased in rats for which the plate had been previously associated with delivery of chocolate versus regular food pellets (Dum and Herz 1984). Pain is also strongly inhibited when animals feed on palatable foods (Foo and Mason 2005), while the effects dissipate when the food is rendered aversive through pairing with a toxic substance (Foo and Mason 2009). Extremely aversive stimuli can also have important analgesic effects, such as when animals are subjected to intense and inescapable noxious stimuli or are put in the presence of a predator. In these cases, the importance of responding to the life-threatening situation surpasses the importance of attending to the pain (Butler and Finn 2009). By contrast, stressors of lower intensities, such as air puffs (Wagner et al. 2013) or pairing with aggressive cagemates (Andre et al. 2005), typically produce pain facilitation, probably to facilitate defensive responses in situations where injuries are likely (Gray and Mcnaughton 2000).
These pro- and antinociceptive effects of emotions appear to be implemented by descending pain modulatory mechanisms depending on endogenous mu-opioid (MOP) agonists and originating in the PAG, rostroventral medulla (RVM)/raphe magnus (RM), ventromedial medulla (VMM), and dorsolateral pontine tegmentum (DLPT) (Fields 2004; Mason 2012). These structures contain the following two distinct types of neurons with opposing roles: “OFF” cells are deactivated by noxious stimuli and activated by MOP agonists, while “ON” cells are activated by noxious stimuli and inhibited by MOP agonists. Most importantly, OFF cells can reduce responses to noxious stimulation while ON cells facilitate nociceptive processing. Conditions that produce analgesia should therefore be expected to exert their effects by deactivating ON cells and/or activating OFF cells. Consistent with this hypothesis, opioid antagonists (e.g., naloxone), given either systemically or directly into the PAG or RVM, have been shown to reduce the analgesic effects of appetitive (Dum and Herz 1984) or stressful stimuli (Butler and Finn 2009). Similarly, feeding on a pleasurable food has been shown to inhibit VMM ON cells and to activate VMM OFF cells (Foo and Mason 2005).
Interestingly, these circuits, and in particular those centering around the VMM, RVM, or RM, are also involved in nonemotional forms of pain inhibition, such as the analgesia accompanying sleep or urination (Mason 2001, 2012). The modulatory effects of emotions therefore seem to be exerted through descending systems, which have a general function to coordinate basic homeostatic processes. Whereas some of these modulatory effects may be mediated by relatively closed reflexive loops, such as those recruited during urination, others may involve top-down projections from forebrain structures capable of assessing the behavioral relevance of environmental stimuli. For instance, stress-induced analgesia critically depends on the amygdala (Watkins et al. 1993). By contrast, stress-induced hyperalgesia has recently been associated with the release of cholecystokinin (CCK) in the RVM through hypothalamic-medullary projections (Wagner et al. 2013). CCK is an endogenous peptide, which post-synaptically antagonizes the effects of endogenous opioids (Heinricher and Neubert 2004). It is therefore well positioned to influence the balance of activation of ON/OFF cells in structures mediating stress-induced hyperalgesia, such as the RVM (Wagner et al. 2013) or PAG (Lovick 2008). Interestingly, the same mechanisms also seem to account for pain modulatory phenomena requiring more elaborate forms of cognitive processing, such as placebo analgesia or nocebo hyperalgesia. Indeed, the administration of MOP or CCK antagonists in humans has been shown to counteract the effects of placebo or nocebo instructions (Benedetti et al. 2005). Placebo effects constitute a good example of the close interactions between cognition and emotions during pain modulation because they require elaborate cognitive processing in prefrontal systems capable of processing placebo instructions (Wager et al. 2004; Atlas et al. 2010) before engaging the brainstem’s motivational circuits responsible for descending pain modulation.
In humans, positively and negatively valenced emotions induced by odors (Villemure et al. 2003), tastes (Lewkowski et al. 2003), pictures (Rhudy et al. 2005), films (Weisenberg et al. 1998), music (Roy et al. 2008), hypnotic suggestions (Rainville et al. 2005), or sentences (Zelman et al. 1991) have also been shown to modulate pain. Pleasant emotions generally reduce pain ratings and increase pain perception thresholds, while unpleasant emotions increase pain ratings and decrease pain perception thresholds. These valence-dependent effects of emotion on pain ratings seem to be mediated by descending modulatory circuits, as evidenced by parallel modulations of the lower limb NFR (Rhudy et al. 2005; Roy et al. 2011, 2012a; Bartolo et al. 2013). This polysynaptic heterosegmental reflex, which is characterized by a flexion of the stimulated limb, occurs in a time window (approximately 90–180 ms) consistent with the conduction velocity of A∂ nociceptive afferents (Sandrini et al. 2005). Moreover, the threshold of the reflex also coincides with pain perception thresholds, and the amplitude of the reflex increases with perceived pain, suggesting that modulation of NFR amplitude by emotions may reflect spinal nociceptive processes (Sandrini et al. 2005). Additional indices of spinal modulation of pain by emotions come from measures reflecting processes occurring immediately downstream of spinal projections. For instance, emotions were shown to modulate the amplitude of the N150 component of nociceptive ERPs (Kenntner-Mabiala and Pauli 2005; Kenntner-Mabiala et al. 2008), the timing of which coincides with the NFR’s temporal window. Moreover, sympathetic responses to nociceptive inputs, such as heart rate accelerations and skin conductance responses (Rhudy et al. 2005), which are controlled by homeostatic control systems receiving direct nociceptive inputs in the brainstem, are also modulated by emotions.
It thus appears that the same descending modulatory mechanisms mediating the effects of emotions on pain in animals are also involved in humans, though human advanced cognitive capacities may broaden the range of emotional stimuli influencing pain. However, evidence in favor of the involvement of endogenous MOP and CCK agonists in human models of pleasure-induced analgesia or stress-induced hyperalgesia remains equivocal. Indeed, only two studies have examined the effects of MOP antagonists on the analgesia induced by pleasurable pictures (Kut et al. 2011) or tastes (Lewkowski et al. 2003), and both failed to observe any effects of opioid receptor blockade on pain modulation. However, it is difficult to draw strong conclusions from these negative findings, especially since both studies employed measures of pain tolerance that may only weakly reflect basic nociceptive processes. Therefore, a pressing concern for future research would be to examine the influence of MOP and CCK antagonists on the effects of emotions on pain ratings and NFR in humans. Alternatively, the lack of MOP receptor blockade effects could indicate the involvement of opioid-insensitive descending modulatory systems (Amanzio and Benedetti 1999; Flor et al. 2002).
Extremely aversive stimuli should also be expected to produce stress-induced analgesia in humans. However, because it is ethically difficult to expose human subjects to the same levels of fear used when experimenting with animals, the experimental paradigms employed in human studies of stress-induced analgesia complicate the interpretation of the results in terms of purely emotional processes. For instance, Rhudy and Meagher (2000) showed that the fear associated with the administration of painful electric shocks increases pain thresholds, whereas the anxiety associated with threats of electric shocks lowers pain thresholds. However, using pain to induce fear raises the possibility that the observed effects may be caused by the engagement of diffuse noxious inhibitory controls (DNIC; Millan 2002) rather than fear per se. Similarly, using a cognitively demanding task to induce stress (Yilmaz et al. 2010) introduces distraction as a confounding factor if pain is tested during the task and cognitive fatigue if pain is tested after the task. While it could be argued that counterirritation, stress, and distraction analgesia all share a common motivational basis in the sense that they all reflect a competition between pain and other sources of motivation, most experimental paradigms used in humans do not match the purely emotional forms of fear-induced analgesia observed in animals, such as that instigated by exposure to a predator.
One exception is found in studies of patients with post-traumatic stress disorder (PTSD), for whom traumatic reexposure produces an important, naloxone-reversible analgesia (Pitman et al. 1990). Another exception comes from studies using a classic protocol in human stress research in which participants are subjected to a public speaking task to induce social stress. Using this type of protocol, al’Absi and Petersen (2003) showed that public speaking produced a state of hypoalgesia that appeared to be mediated by task-induced increases in systolic blood pressure (SBP). Indeed, it has been shown that activation of arterial baroreceptors has widespread inhibitory influences on central nervous system activity, including pain perception, and these effects could mediate some of emotions’ effects on pain. Interestingly, a recent study showed that in addition to abolishing the analgesia associated with a stressful cognitive task, naloxone also prevented stress-related increases in blood pressure and baroreflex sensitivity, suggesting that endogenous opioids modulate both nociceptive inputs and the interplay between stress, pain, and vegetative responses (Fechir et al. 2012). Finally, besides fear, anger constitutes another negative emotional state associated with an opioid-dependent hypoalgesic state (Frew and Drummond 2007), suggesting that anger-related hypoalgesia may be considered as another form of stress-induced analgesia. This is particularly interesting considering that the main purpose of stress-induced pain inhibition is to prioritize fight or flight reactions in the face of important threats or obstacles.
The fact that negative emotions can produce either hyper- or hypoalgesia also raises the possibility that both effects can sometimes compete with one another. For instance, al’Absi and Petersen (2003) observed that public speaking also induced increases in self-reported levels of distress that predicted increases in pain ratings independently from SBP-mediated analgesia. The resulting net analgesic effects of their public speaking task therefore appeared to have resulted from a competition between SBP-mediated hypoalgesia and negative mood-induced hyperalgesia. Although the net balance of hypo- and hyperalgesic effects resulted in a net hypoalgesia in al’Absi and Petersen’s study, it is easy to imagine factors that could disrupt this balance in favor of hyperalgesic effects. While this could explain some of the discrepancies in the literature, it could also account for the important interindividual variability in the effects of negative emotions on pain. For instance, Rhudy and Meagher (2003) observed that participants who mostly reacted with fear to aversive electric shocks showed subsequent increases in pain thresholds, whereas those who responded with a mix of fear and humor showed no analgesic effects. Similarly, participants who have a heightened propensity to experience anger are more likely to experience hyperalgesia than hypoalgesia following acute anger induction, an effect which appears to be related to an unmasking of anger’s proalgesic effects by reduced opioid-dependent analgesia (Bruehl et al. 2012).
A related phenomenon is the sudden transition from hypo- to hyperalgesia that can take place when the source of stress is abruptly removed. For instance, Cornélio et al. (2012) demonstrated that stressful exposure to open spaces (elevated plus maze task) had immediate antinociceptive effects that rapidly transitioned to hyperalgesia once the rats were removed from the stressful condition. Moreover, RVM lesions had no effects on the initial stress-induced analgesia but completely abolished the ensuing hyperalgesia, allowing the hypoalgesia to persist in time. Again, this confirms that the antinociceptive and hyperalgesic effects of stress are mediated by distinct and competing systems. Similarly, the results of a recent study showed that the stress-related release of endogenous opioids secondarily induces a long-lasting and latent pain hypersensitivity mediated by NMDA receptors (Le Roy et al. 2011). Thus, sustained stress may predispose individuals to develop chronic pain when exposed to injury, which could partially explain the important comorbidity between stress, chronic pain, and anxio-depressive symptoms observed in humans.
Emotions thus appear to have profound influences on spinal nociceptive processing. However, it is still unclear whether attentional and emotional sources of pain modulation can be differentiated on the basis of their effects on spinal nociceptive processing. First, distraction induced by performance of a difficult concurrent task also seems to have antinociceptive effects at the spinal level, as evidenced by lowered NFR amplitudes or heightened thresholds (Bathien 1971; Sandrini et al. 2005). However, as noted previously, these effects could also be caused by the stress induced by the difficult cognitive task. Using predictive cues to direct attention toward upcoming visual or nociceptive stimulations, Dowman (2001) observed a dissociation between pain ratings, which were reduced by invalid cues, and NFRs and somatosensory evoked potentials (SEPs), which were either unaffected or increased by invalid cues. This apparently paradoxical increase in SEPs was interpreted as reflecting non-pain-specific processes, such as reorientation toward the source of pain. More recently, Roy et al. (2011) observed a similar dissociation between pain ratings and NFRs during the presentation of neutral pictures versus a fixation point, which reduced pain ratings but increased NFRs. By contrast with these paradoxical effects of attention, comparison of neutral pictures with pleasant or unpleasant pictures replicated the previously observed parallel modulation of pain and NFRs as a function of valence. These findings suggest that driving attention away from pain may produce increases in NFR amplitudes that are independent from pain perception and which could reflect the need to tune up defensive reflexes when attention is directed away from the source of pain. Although this may suggest separate mechanisms for attentional and emotional effects, there is also evidence of interactions between attentional and emotional effects that contradicts strong claims about their dissociability. Indeed, cueing shocks abolishes the effects of emotions on NFR but not on pain ratings (Rhudy et al. 2006), suggesting that the effects of emotions on NFRs may rather reflect a facilitation of defensive responses, which becomes unnecessary when pain is fully predictable. By contrast, modulation of pain ratings may reflect supraspinal processes that are relatively independent from spinal reflexes. Alternatively, the dissociation between NFRs and pain ratings during distraction may reflect a facilitation of spinal motor-neuron responses coinciding with an inhibition of nociceptive transmission in the dorsal horn, a proposition which is compatible with observations of modulation of early SEP components by emotions (Kenntner-Mabiala et al. 2008).
Finally, findings of differential effects of attention and emotions’ effects on the sensory and affective dimensions of pain also raise interesting questions regarding the engagement of descending modulatory processes in these two forms of pain modulation. Using a 2 × 2 crossover design during which emotions were manipulated by pleasant or unpleasant odors and attention was manipulated by an odor or pain discrimination task, Villemure and Bushnell (Villemure et al. 2003; Villemure and Bushnell 2009) found that effects of attention were stronger on intensity ratings while emotions had a greater effect on unpleasantness ratings. This dissociation is difficult to explain through descending modulatory mechanisms, which in all likelihood should indiscriminately affect ascending nociceptive signals as a whole. However, upon closer examination of the reported effects, the relatively low statistical power of these studies does not allow strong conclusions about the absence of effects on intensity or unpleasantness ratings. Therefore, another possible interpretation of these effects could be that both attention and emotion influence nociception through common descending modulatory mechanisms but that in addition they have different effects at the supraspinal level, which could explain their preferential effects on the sensory and affective dimensions of pain.
4 Supraspinal Modulation of Pain by Emotions
So far, the focus of the discussion has been on the effects of emotions on spinal nociceptive processes. However, there is an important distinction to be made between nociception, which refers to the biological processes associated with tissue damage, and pain, defined as the “unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Merskey and Spear 1967). According to this largely consensual definition, activation of peripheral nociceptors is neither sufficient nor necessary for pain to be experienced. Indeed, when entering the cerebral cortex, ascending nociceptive signals undergo an important multisensory integration process through which a higher-order representation of the source of the pain in relation with the body-in-space is generated (Haggard et al. 2013). At this stage, nociceptive signals from WDR and nociceptive-specific (NS) neurons are integrated with thermal, tactile, and proprioceptive information, as well as with visual representations of the body and of objects in peripersonal space. This early multisensory integrative process, which is implemented through interactions between somatosensory, parietal, and posterior insular cortices, constitutes a first perceptual stage of pain modulation where external stimuli can influence perception of pain localization and intensity (Haggard et al. 2013). It is also the stage where manipulations of attentional focus are mainly thought to exert their effects on the sensory dimension of pain (Villemure and Bushnell 2009; Bushnell et al. 2013).
The purpose of this integrative process is to provide motivational systems with sufficient information to evaluate the threat level of the situation and respond to it in a coordinated fashion. This initial emotional appraisal process seems to be implemented through projections from parietal and somatosensory cortices to anterior midcingulate and insular cortices (aMCC and aINS), which are involved in processing the affective salience of stimuli that are relevant to the organism. Lesion of these projections, or of their cortical targets, generally produces a state of pain asymbolia characterized by a selective loss of emotional reactions to otherwise preserved painful sensations. Interestingly, these patients also seem to be unreactive to all sorts of threats to their corporal integrity, such as a needle approaching their eye or a hammer menacing to crush their hand (Danziger 2006). By contrast, multisensory cues that are only suggestive of injury, such as a nail passing through one’s boot without causing any actual injury, can sometimes generate an aversive experience that has all the characteristics of pain (Fisher et al. 1995). This suggests that the presence of actual nociceptive inputs is not necessary for a pain’s primary affective dimension if multisensory inputs are sufficiently convincing. Therefore, if one accepts that what makes pain really “painful” is its intrinsic unpleasantness (Bushnell et al. 2013), then pain could be conceived as the specific emotion for which the “core relational theme” (Lazarus 1966) is “actual or potential tissue damage” (but see Fields 1999).
Consistent with this idea, manipulating the threat value of nociceptive stimuli by suggesting that they may cause injury increases pain through preactivation of the aMCC and aINS during anticipation of the nociceptive stimulation and of the aMCC during the actual stimulation (Wiech et al. 2010). Similarly, hypnotic suggestions to reappraise painful thermal stimuli as more or less unpleasant specifically affect ratings of pain unpleasantness, an effect which was linked to an up- or down-regulation of aMCC activity (Rainville et al. 1997). It therefore seems that the same reappraisal strategies proven to be efficient in reducing negative emotions (Gross 2002) also generalize to successful pain regulation. In support of that hypothesis, Lapate et al. (2012) recently found that interindividual differences in successful regulation of pain and negative emotions were correlated with one another and with reductions in amygdala activations to pain and unpleasant pictures. This is particularly interesting since the amygdala is considered part of the brain’s early appraisal system (Ledoux 2000) and is also involved in pain’s affective dimension through nociceptive projections from the spinoparabrachial-amygdala pathway (Neugebauer et al. 2009). The similarity between the effects of reappraisal on pain and negative emotions therefore suggests that the two may rely upon the same lateral prefrontal- (LPFC) and medial prefrontal- (MPFC) subcortical pathways (Atlas et al. 2010; Leknes et al. 2013; Roy et al. 2012b; Salomons et al. 2007; Wager et al. 2004).
Pain can also be the object of other emotions, which can be considered as representing pain’s secondary affect (Price 2000). For instance, pain may cause anger if it is considered unfair, whereas it may cause anxiety or even depression if it is perceived as recurring and inevitable. These secondary emotions may be particularly problematic in patients suffering from chronic pain. Fortunately, the same cognitive strategies proven to be effective for regulation of negative emotions also seem to have positive effects on pain-induced emotions. For instance, Jensen et al. (2012) recently showed that 12 weeks of cognitive-behavioral therapy in patients with fibromyalgia significantly reduced the anxio-depressive symptoms and self-reported levels of disability associated with the disorder. Interestingly, these therapeutic effects of CBT were associated with increases in LPFC activity during painful mechanical stimulation, confirming that reappraisal of secondary pain affect shares some of the same neuroanatomical substrate as reappraisal of negative emotions in general (Buhle et al. 2014). Finally, another therapeutic technique that is being increasingly used to ameliorate pain is mindfulness-based meditation (Ludwig and Kabat-zinn 2014). Contrary to reappraisal, mindfulness-based meditation encourages adoption of a non-elaborative stance, which has been shown to decrease pain unpleasantness in experienced practitioners (Grant et al. 2011; Gard et al. 2012; Lutz et al. 2013). Surprisingly, these decreases in unpleasantness ratings were associated with increases in the activity of structures processing pain’s sensory and primary affective dimension (INS, aMCC), combined with decreases in prefrontal structures responsible for secondary appraisals (Grant et al. 2011; Gard et al. 2012). Although the inverse correlation between pain ratings and INS and aMCC activity may seem paradoxical, these findings are in striking correspondence with the psychological construct of mindfulness, which entails a nonjudgmental awareness of the present moment.
However, these results also raise questions about the sequential organization of primary and secondary pain affect. For example, how can downregulation of secondary appraisals during meditation decrease pain’s primary unpleasantness? One possibility is that primary and secondary pain affect are subjectively difficult to separate, resulting in misattributions of secondary pain affect modulation to primary pain unpleasantness. Another possibility is that secondary emotions can have reciprocal effects on primary pain unpleasantness through various channels. This later possibility would be consistent with recent models of emotional processing stressing the high level of integration between various levels of appraisal during the dynamic unfolding of emotional experiences (Sander et al. 2005). Within this framework, it becomes easier to understand how higher-order appraisals can impact pain perception, such as when the cause of pain is attributed to someone else’s intentions (Gray and Wegner 2008), or when the same level of pain is judged as the worst versus best outcome in comparison with the alternative (Leknes et al. 2013).
This framework also provides a way to explain the supraspinal effects of emotions unrelated to pain, which could presumably also affect pain through increases in the activity of regions involved in secondary appraisals and primary and secondary pain affect. For instance, Berna et al. (2010) recently showed that induction of a sad mood increased pain unpleasantness ratings through increases in catastrophic thinking about pain accompanied by augmented pain-related activations in the insula, thalamus, hippocampus, amygdala, inferior frontal gyrus (IFG), dorsolateral prefrontal cortex (DLPFC), and subgenual ACC (sACC). Similarly, Yoshino et al. (2010) found that painful electric shocks administered during the presentation of sad versus happy or neutral faces were associated with increases in pain-related ACC activations and enhanced ACC-amygdala connectivity in the context of sad faces. Finally, using yet another sadness induction technique, Yang and Symonds (2012) largely replicated these results by showing that the simultaneous presentation of pain and sad pictures produced higher activity in the bilateral subgenual anterior cingulate cortex (sACC), contralateral pINS/S2, contralateral PAG, and bilateral amygdala.
While these results are consistent with top-down effects of emotions on the cerebral processes by which nociceptive signals are interpreted as “pain,” we previously saw that emotions can also have powerful effects on descending pain modulatory mechanisms. Therefore, brain imaging results of emotional modulation of pain paradigms may reflect a combination of both spinal and supraspinal modulation of pain. In order to disentangle these effects, Roy et al. (2009) performed a brain imaging study during which spinal nociceptive reflexes to painful electric shocks were recorded while participants observed pleasant, unpleasant, or neutral pictures. The effects of emotions on NFR amplitude correlated with pain-evoked activity in structures receiving direct or indirect nociceptive inputs, such as the brainstem, thalamus, cerebellum, amygdala, and MPFC. By contrast, the effects of emotions on pain ratings correlated with activity in the anterior insula. This was particularly interesting since recent theories of pain, emotion, and interception postulate that the anterior insula acts as an integrator of ascending interoceptive signals with the broader emotional/motivational context (Craig 2003). In support of this hypothesis, increases in anterior insula activity during the viewing of unpleasant versus pleasant pictures correlated with activity in visual and orbitofrontal cortices, suggesting that it reflected the perception of pain in the context of affective pictures (Roy et al. 2009). Finally, consistent with these brain imaging results, Godinho et al. (2006) also observed that presentation of unpleasant emotional pictures had predominantly late effects (270–500 ms) on shock-induced SEPs in the lateral prefrontal cortex/anterior insula, temporo-occipital junction, and right temporal pole, confirming that some of the effects of emotion on pain may reflect late integrative processes involving the binding of pain to the emotional/sensory context in which it occurs.
Finally, one last question concerns the potential interactions between emotional and attentional processes during modulation of pain by emotions. Indeed, recent cognitive theories of emotions (Sander et al. 2005; Lindquist et al. 2012) suggest that executive attention may be important for performing some of the higher-order appraisals influencing the affective dimensions of pain. Consequently, distraction induced by a concurrent task could decrease the primary and secondary dimensions of pain. By contrast, negative/positive emotions could also increase/decrease attention directed toward or away from pain (Salovey 1992) and thereby influence task performance in addition to pain ratings. Two studies directly addressed this question using 2 × 2 factorial designs where emotion and attention were manipulated independently from one another (Villemure and Bushnell 2009; Ploner et al. 2011). Villemure and Bushnell (2009) showed that the effects of attention and emotions on pain ratings were independent from one another. However, they reported an interactive effect on task performance whereby negative emotions interfered with redirection of attention away from pain. Using a substantially different protocol, Ploner et al. (2011) also observed independent effects of attention and emotions on pain ratings but did not report any interactive effects on task performance. Overall, these results suggest that it is possible to experimentally separate the effects of attention and emotions on pain perception.
However, these two studies diverge in the pain-processing structures identified as the targets of attention or emotion effects on pain. Whereas Ploner et al. (2011) identified the aINS as the common locus of modulation for both emotional and attentional effects, Villemure and Bushnell (2009) found that aINS was only affected by attention, but not emotions. In contrast, emotions influenced activity in a number of pain-processing somatosensory and affective structures, including the medial thalamus, S1, S2, and aMCC. Reports of emotions impacting on somatosensory activity were surprising because emotions did not significantly influence pain intensity ratings in that study. One explanation for the widespread cerebral effects of emotions could be that emotions directly affected ascending nociceptive signals, as discussed previously. Nevertheless, the results of these two studies, as well as those of Roy et al.’s (2009) study, overwhelmingly point toward the aINS as a hub for pain perception and supraspinal modulation. Most interestingly, Ploner et al. (2011) observed that what differentiated attentional and emotional effects were the patterns of aINS functional connectivity during attentional and emotional conditions: attentional manipulations increased aINS connectivity with the brain’s attentional network (intraparietal sulcus, superior parietal lobule, frontal eye fields, frontal pole, aINS, MCC), while emotional manipulations increased aINS connectivity with emotional structures (medial temporal lobe, aINS). Hence, the flexible connectivity of regions associated with the affective dimension of pain, and in particular the aINS, seems to underlie the supraspinal effects of emotions and attention on pain processing.
5 Conclusion
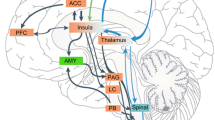
The physiological and psychological mechanisms underlying the effects of attention and emotions on pain are summarized in Fig. 3.1 within a model of pain processing largely inspired from Price (2000) and described throughout this chapter. This model attaches considerable importance to the interactions between sensory, cognitive, and affective/motivational systems in the cerebral construction of the subjective experience of pain. As argued, interactions between attention and emotions during visual processing (Pessoa 2008) and the highly parallel and reciprocal nature of the neural architecture underlying pain perception make it difficult to differentiate the unique effects of attention and emotion on pain-processing structures (Ploner et al. 2011). Indeed, both attention and emotions can influence ascending nociceptive signals through descending modulatory pathways, which would cause largely indifferentiable patterns of modulation at the cerebral level. However, one potential difference between the effects of attention and emotion at the spinal level is the paradoxical increase in NFRs observed during distraction analgesia, which could reflect a disinhibition of spinal motor neurons when attention is directed away from pain. Still, this hypothesis remains to be confirmed with more specific measures of motor neurons’ excitability in order to fully exclude alternative explanations.
Model of emotions’ and attention’s effects on nociception, pain sensation, and pain’s primary and secondary affect. Neural structures likely to have a role in these dimensions are shown by abbreviations in adjacent parentheses. PC parietal cortex, S1–S2 primary and secondary somatosensory cortices, pINS posterior insula, ACC anterior cingulate cortex, aMCC anterior midcingulate cortex, aINS anterior insula, amy amygdala, PFC prefrontal cortex, Nacc nucleus accumbens, PAG periaqueductal gray, hyp hypothalamus, LPFC lateral prefrontal cortex, MPFC medial prefrontal cortex
Although the supraspinal mechanisms engaged by attention and emotions may substantially differ, their effects on pain-evoked activations appear to be difficult to disentangle due to the highly integrated nature of the pain-processing system. It has been suggested that one potential point of separation could be that attention preferentially affects the cerebral structures underlying the sensory dimension of pain, but recent neuroimaging findings are not perfectly congruent with this hypothesis (Villemure and Bushnell 2009; Ploner et al. 2011). It is also important to note that because of the partially sequential relationship between pain sensation and affect (Price 2000), modulation of pain sensation should also indirectly impact the affective dimension of pain, thereby blurring the boundaries between attentional and emotional effects. Another potential point of separation between attentional and emotional effects could be that emotions preferentially modulate pain affect, either directly or indirectly through a modulation of pain-related appraisals. However, distraction away from pain could also impact the affective dimension by interfering with the appraisal processes underlying pain’s primary and secondary affect, which would be consistent with recent reports of selective effects of attention on aINS activity (Ploner et al. 2011). Finally, emotions could also influence pain perception through modulation of autonomic activity, which could be misattributed to pain (Schachter and Wheeler 1962), although this possibility has yet to be formally tested.
Moreover, emotional states can also alter the direction of attention (Salovey 1992) and be associated with different spinal and supraspinal effects that can either work synergistically or antagonistically. Therefore, in order to identify the origins of emotional effects on pain-related brain activity, it is necessary to try to probe as much as possible the various levels of pain processing by combining several methodologies, including NFR recordings, measures of autonomic activity, fMRI, EEG, etc. One interesting avenue for future imaging studies could be the use of cross-validated multivariate pain “signatures” in order to further characterize the nature of the modulatory effects of various interventions (Wager et al. 2013). Hopefully, a better understanding of the psychological factors that influence pain will lead to a better understanding of pain itself, including how it may become dysregulated in chronic pain syndromes.
References
al’Absi M, Petersen KL (2003) Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain 106:285–295
Amanzio M, Benedetti F (1999) Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 19:484–494
Andre J, Zeau B, Pohl M et al (2005) Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J Neurosci 25:7896–7904
Arnold MB (1960) Emotion and personality psychological aspects, vol 1. Columbia University Press, New York
Atlas LY, Bolger N, Lindquist M, Wager TD (2010) Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977
Bartolo M, Serrao M, Gamgebeli Z et al (2013) Modulation of the human nociceptive flexion reflex by pleasant and unpleasant odors. Pain 154:2054–2059
Bathien N (1971) Human spinal reflexes and attention levels. Electroencephalogr Clin Neurophysiol 30:32–37
Benedetti F, Mayberg HS, Wager TD et al (2005) Neurobiological mechanisms of the placebo effect. J Neurosci 25:10390–10402
Berna C, Leknes S, Holmes E et al (2010) Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 67:1083–1090
Bruehl S, Burns JW, Chung OY, Chont M (2012) Naloxone inhibits not only stress-induced analgesia but also sympathetic activation and baroreceptor-reflex sensitivity. Psychosom Med 73:612–619
Buhle JT, Silvers JA, Wager TD et al (2014) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24(11):2981–2990
Bushnell MC, Duncan G, Dubner R et al (1985) Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J Neurophysiol 5:1103–1110
Bushnell MC, Ceko M, Low L (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511
Butler RK, Finn DP (2009) Stress-induced analgesia. Prog Neurobiol 88:184–202
Corbetta M, Shulman GL (2011) Spatial neglect and attention networks. Annu Rev Neurosci 34:569–599
Cornélio AM, Nunes-de-Souza RL, Morgan MM (2012) Contribution of the rostral ventromedial medulla to post-anxiety induced hyperalgesia. Brain Res 1450:80–86
Craig AD (2003) A new view of pain as a homeostatic emotion. Trends Neurosci 26:303–307
Danziger N (2006) Bases neurologiques de l’ affect douloureux. Rev Neurol 162:395–399
Descartes R (1649) Les passions de l’âme. Henry Le Gras, Paris
Dowman R (2001) Attentional set effects on spinal and supraspinal responses to pain. Psychophysiology 38:451–464
Dum J, Herz A (1984) Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol Biochem Behav 21:259–266
Edwards L, Ring C, France CR et al (2007) Nociceptive flexion reflex thresholds and pain during rest and computer game play in patients with hypertension and individuals at risk for hypertension. Biol Psychol 76:72–82
Fechir M, Breimhorst M, Kritzmann S et al (2012) Naloxone inhibits not only stress-induced analgesia but also sympathetic activation and baroreceptor-reflex sensitivity. Eur J Pain 16:82–92
Fields HL (1999) Pain: an unpleasant topic. Pain Suppl 6:S61–S69
Fields HL (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575
Fields HL (2007) Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med 32:242–246
Fisher JP, Hassan DT, O’Connor N (1995) Minerva. Br Med J 310:70
Flor H, Birbaumer N, Schulz R et al (2002) Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. Eur J Pain 6:395–402
Foo H, Mason P (2005) Sensory suppression during feeding. Proc Natl Acad Sci USA 102:16865–16869
Foo H, Mason P (2009) Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci 29:13053–13062
Frew AK, Drummond PD (2007) Negative affect, pain and sex: the role of endogenous opioids. Pain 132(Suppl):S77–S85
Gard T, Hölzel BK, Sack AT et al (2012) Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex 22:2692–2702
Godinho F, Magnin M, Frot M et al (2006) Emotional modulation of pain: is it the sensation or what we recall? J Neurosci 26:11454–11461
Grant J, Courtemanche J, Rainville P (2011) A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 152:150–156
Gray JA, Mcnaughton N (2000) The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system, 2nd edn. Oxford University Press, Oxford, p 443
Gray K, Wegner DM (2008) The sting of intentional pain. Psychol Sci 19:1260–1262
Gross JJ (2002) Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39:281–291
Haggard P, Iannetti GD, Longo MR (2013) Spatial sensory organization and body representation in pain perception. Curr Biol 23:R164–R176
Heinricher MM, Neubert MJ (2004) Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol 92:1982–1989
Jensen KB, Kosek E, Wicksell R et al (2012) Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153:1495–1503
Kenntner-Mabiala R, Pauli P (2005) Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology 42:559–567
Kenntner-Mabiala R, Andreatta M, Wieser MJ et al (2008) Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biol Psychol 78:114–122
Kut E, Candia V, von Overbeck J et al (2011) Pleasure-related analgesia activates opioid-insensitive circuits. J Neurosci 31:4148–4153
Lapate RC, Lee H, Salomons TV et al (2012) Amygdalar function reflects common individual differences in emotion and pain regulation success. J Cogn Neurosci 24:148–158
Lazarus RS (1966) Psychological stress and the coping process. McGraw-Hill, New York
Le Roy C, Laboureyras E, Gavello-Baudy S et al (2011) Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization via a NMDA-dependent process. J Pain 12:1069–1079
Ledoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Legrain V, Guérit J-M, Bruyer R, Plaghki L (2002) Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain 99:21–39
Leknes S, Berna C, Lee MC et al (2013) The importance of context: when relative relief renders pain pleasant. Pain 154:402–410
Lewkowski MD, Ditto B, Roussos M, Young SN (2003) Sweet taste and blood pressure-related analgesia. Pain 106:181–186
Lindquist K, Wager TD, Kober H et al (2012) The brain basis of emotion: a meta-analytic review. Behav Brain Sci 35:121–143
Lovick T (2008) Pro-nociceptive action of cholecystokinin in the periaqueductal grey: a role in neuropathic and anxiety-induced hyperalgesic states. Neurosci Biobehav Rev 32:852–862
Ludwig DS, Kabat-zinn J (2014) Mindfulness in medicine. JAMA 300:1350–1351
Lutz A, McFarlin DR, Perlman DM et al (2013) Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64:538–546
Mason P (2001) Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci 24:737–777
Mason P (2012) Medullary circuits for nociceptive modulation. Curr Opin Neurobiol 22:640–645
McIntyre D, Edwards L, Ring C et al (2006) Systolic inhibition of nociceptive responding is moderated by arousal. Psychophysiology 43:314–319
Merskey H, Spear FG (1967) The concept of pain. J Psychosom Res 11:59–67
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Neugebauer V, Galhardo V, Maione S, Mackey SC (2009) Forebrain pain mechanisms. Brain Res Rev 60:226–242
Panksepp J (2007) Neurologizing the psychology of affects: how appraisal-based constructivism and basic emotion theory can coexist. Perspect Psychol Sci 2:281–296
Pessoa L (2008) On the relationship between emotion and cognition. Nat Rev Neurosci 9:148–158
Petersen KL, Al’Absi M, France C, Wittmers LE (2001) Acute mental challenge reduces nociceptive flexion reflex in men and women. Psychophysiology 38:S76
Pitman RK, van der Kolk B, Orr SP, Greenberg MS (1990) Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry 47:541–544
Ploner M, Lee MC, Wiech K et al (2011) Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex 21:719–726
Poldrack R (2006) Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci 10:59–63
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288:1769–1772
Rainville P, Duncan GH, Price DD et al (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971
Rainville P, Bao QVH, Chrétien P (2005) Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 118:306–318
Rhudy JL, Meagher MW (2000) Fear and anxiety: divergent effects on human pain thresholds. Pain 84:65–75
Rhudy JL, Meagher MW (2003) Individual differences in the emotional reaction to shock determine whether hypoalgesia is observed. Pain Med 4:244–256
Rhudy JL, Williams AE, McCabe KM et al (2005) Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 42:579–587
Rhudy JL, Williams AE, McCabe KM et al (2006) Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain 126:221–233
Roy M, Peretz I, Rainville P (2008) Emotional valence contributes to music-induced analgesia. Pain 134:140–147
Roy M, Piché M, Chen J-I et al (2009) Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci U S A 106:20900–20905
Roy M, Lebuis A, Peretz I, Rainville P (2011) The modulation of pain by attention and emotion: a dissociation of perceptual and spinal nociceptive processes. Eur J Pain 15:641.e1–641.e10
Roy M, Lebuis A, Hugueville L et al (2012a) Spinal modulation of nociception by music. Eur J Pain 16:870–877
Roy M, Shohamy D, Wager TD (2012b) Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156
Salomons TV, Johnstone T, Backonja M-M et al (2007) Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci 19:993–1003
Salovey P (1992) Mood-induced self-focused attention. J Pers Soc Psychol 62:699–707
Sander D, Grandjean D, Scherer KR (2005) A systems approach to appraisal mechanisms in emotion. Neural Netw 18:317–352
Sandrini G, Serrao M, Rossi P et al (2005) The lower limb flexion reflex in humans. Prog Neurobiol 77:353–395
Schachter S, Wheeler L (1962) Epinephrine, chlorpromazine, and amusement. J Abnorm Soc Psychol 65:121–128
Tracey I, Ploghaus A, Gati JS et al (2002) Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22:2748–2752
Villemure C, Bushnell MC (2002) Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95:195–199
Villemure C, Bushnell MC (2009) Mood influences supraspinal pain processing separately from attention. J Neurosci 29:705–715
Villemure C, Slotnick BM, Bushnell MC (2003) Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain 106:101–108
Wager TD, Rilling JK, Smith EE et al (2004) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303:1162–1167
Wager TD, Atlas LY, Lindquist M et al (2013) An fMRI-based neurologic signature of physical pain. N Engl J Med 368:1388–1397
Wagner KM, Roeder Z, Desrochers K et al (2013) The dorsomedial hypothalamus mediates stress-induced hyperalgesia and is the source of the pronociceptive peptide cholecystokinin in the rostral ventromedial medulla. Neuroscience 238:29–38
Watkins LR, Wiertelak EP, Maier SF (1993) The amygdala is necessary for the expression of conditioned but not unconditioned analgesia indicate danger or stimuli that produce fear can produce. Behav Neurosci 107:402–405
Weisenberg M, Raz T, Hener T (1998) The influence of film-induced mood on pain perception. Pain 76:365–375
Wiech K, Lin C, Brodersen KH et al (2010) Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331
Yang L, Symonds LL (2012) Neural substrate for facilitation of pain processing during sadness. Neuroreport 23:911–915
Yilmaz P, Diers M, Diener S et al (2010) Brain correlates of stress-induced analgesia. Pain 151:522–529
Yoshino A, Okamoto Y, Onoda K et al (2010) Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: an fMRI study. Neuroimage 50:1194–1201
Zelman DC, Howland EW, Nichols SN, Cleeland CS (1991) The effects of induced mood on laboratory pain. Pain 46:105–111
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Roy, M. (2015). Cerebral and Spinal Modulation of Pain by Emotions and Attention. In: Pickering, G., Gibson, S. (eds) Pain, Emotion and Cognition. Springer, Cham. https://doi.org/10.1007/978-3-319-12033-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-12033-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12032-4
Online ISBN: 978-3-319-12033-1
eBook Packages: MedicineMedicine (R0)