Abstract
Stroke is a major cause of death globally, which induces irreversible neuronal and endothelial cell death. Endothelial progenitor cell (EPC) based therapeutics result in neovascularization and the improvement of vascular perfusion, which benefits clinical stroke patients. Although EPC transplantation in experimental stroke models shows functional improvement, EPC therapy in clinical stroke patients continues to face an arduous task. In this chapter, we give a brief introduction of EPCs including the source of EPCs, methods of isolation and identification of EPC, the therapeutic potential for stroke, and signaling in modulating EPC function. Furthermore, we summarize the molecular mechanisms of EPCs action after transplantation either through differentiating into mature endothelial cells to replace damaged cells or by enhancing trophic/regenerative support for endogenous repair processes. We discuss the routes of transplantation and the modifying methods for EPCs safety and efficacy in vivo. Finally, we discuss the pros and cons for the application of EPCs for transplantation in clinical patients. Though EPC-based therapy is a potential treatment for stroke and holds promise for vascular regeneration, this field needs more study to uncover and resolve unsolved problems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Stroke
Stroke is a multifactorial disease, and ischemic and hemorrhagic stroke are the leading causes of death globally (Hassan and Markus 2000; Rubattu et al. 2000). A variety of risk factors have been illustrated to relate with stroke incidence including cerebrovascular diseases, aging, smoking, hypertension, diabetes, hypercholesterolemia, and lack of exercise. (Hankey 2006; Allen and Bayraktutan 2008; Flynn et al. 2008; Karam et al. 2008). The brain is very vulnerable to ischemic insult because it is sensitive to a lack of oxygen and glucose. Neurological dysfunction usually occurs within minutes after stroke onset. However, the deterioration of the brain may continue in the following minutes, hours or even days.
Ischemic stroke (> 70 % of strokes) is the most common type of stroke in clinical stroke patients. After ischemic stroke onset, a process of pathophysiological events are triggered, including energy failure, loss of cell ion homeostasis, the release of excitatory amino acid and reactive oxygen species, increase of intracellular calcium, disruption of the blood-brain barrier (BBB), activation of glial cells, and the infiltration of leukocytes (Bliss et al. 2007; Moskowitz et al. 2010). These interrelated and coordinated events result in ischemic cell necrosis, which exhibits non-selective damage of all cells including neurons, astrocytes, oliogdendrocytes, microglia and endothelial cells (Broughton et al. 2009). The size and location of these infarcts are determinants of the long-term functional deficits (Sims and Muyderman 2010). The ischemic penumbra area represents the region in which there is a chance for recovery via post-stroke therapy (Ginsberg 1997).
The only effective treatment for ischemic stroke patients is to administer recombinant tissue plasminogen activator (tPA). However, very few patients are lucky enough to receive tPA treatment because tPA has a very narrow time window (< 4.5 h). Stem cell therapy has been proposed as a potential treatment for ischemic stroke in recent years, especially after putative progenitor endothelial cells have been isolated from bone marrow (BM) and identified as CD34 positive (Asahara et al. 1997). This kind of cells is named endothelial progenitor cells (EPCs) and is capable of contributing to the formation of new vessels by differentiating into mature endothelial cells (ECs) or supporting/promoting the endogenous repair process. EPCs can also serve as a marker during stroke occurrence and prognosis (Chu et al. 2008), and preclinical studies have shown EPC transplantation improves functional recovery by promoting neurogenesis and angiogenesis or provide trophic/protective factors through paracrine effects . Several clinical studies are currently investigating the safety and efficacy of EPC transplantation. EPC transplantation in stroke represents a promising therapeutic approach, although it is still in its infancy.
2 Endothelial Progenitor Cells (EPCs)
2.1 Discovery of EPCs
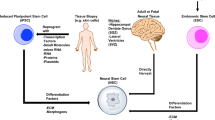
EPCs are BM mononuclear cells (MNCs) , which were first isolated from peripheral blood (PB) by Asahara in 1997 (Asahara et al. 1997). This novel technique opened a new field for the treatment of vascular disease. Increasing evidence showed that EPCs could be mobilized to the PB after ischemic stroke and restore the damaged vessels via vasculogenesis (Asahara et al. 1999; Takahashi et al. 1999; Kalka et al. 2000a; Shintani et al. 2001; Murayama et al. 2002; Asahara and Kawamoto 2004; Zhan et al. 2013). Considering these cells’ lack of unique markers, and that they share similar surface antigens with some hematopoietic lineages and mature ECs, such as CD31/KDR (VEGFR-2)/CD34/VE-cadherin/E-selectin (Rafii 2000; Khakoo and Finkel 2005) , it is difficult to precisely define EPCs. However, it has been generally accepted that EPCs exist in circulating blood and possess angiogenic capability and the potential to differentiate into ECs, which contribute to the process of vasculogenesis and the maintenance of homeostasis in vascular ECs (Asahara et al. 1997; Shi et al. 1998; Asahara et al. 1999; Lin et al. 2000; Rafii 2000; Cesari et al. 2012). EPCs may be mixed with the circulating ECs in peripheral circulation, which may partially differentiate into mature ECs. EPCs play a more important role in promoting postnatal vasculogenesis compared with circulating ECs (Kalka et al. 2000a). Therefore, EPCs and circulating ECs are two different cells .
2.2 Source of EPCs
EPCs can be divided into two types based on their origin: hematopoietic and non-hematopoietic EPCs. Hematopoietic EPCs originating from BM are considered a subtype of hematopoietic stem cells (HSCs) . Non-hematopoietic cells could be isolated from PB, umbilical cord blood (UCB), and tissue samples (Asahara et al. 2011). Although the origin of non-hematopoietic cells is unclear, this type of cell is likely derived from organ blood vessels and tissue stem cells (Alev et al. 2011). In addition, increased studies have reported additional sources of non-hematopoietic cells. For example, the myogenic-EPCs in the interstitial spaces of skeletal muscle contribute to skeletal muscle growth (Tamaki et al. 2002) ; EPCs could also exist in the boundary between smooth muscle and the adventitial layer of human vascular walls (Zengin et al. 2006). Other sources of EPCs include the liver and intestine (Aicher et al. 2007), dental pulp-derived iPS cells (Yoo et al. 2013), the kidney (Sirker et al. 2009) and adipose tissue (Planat-Benard et al. 2004). Therefore, it is plausible that EPCs could be found in other sources, further study is needed to investigate the mysterious origin of EPCs.
2.3 Methods for the Isolation and Culturing of EPCs
Actually, it is a challenge, and controversial work, to isolate and identify EPCs from the PB, because of these cells’ lack of unique and specific surface markers. Currently three methods are mainly used to isolate EPCs from the PB . The first and perhaps simplest method is to collect low density MNCs via density barrier centrifugation, and then plate these cells on fibronectin coated dishes with culture medium containing a variety of growth factors and fetal serum. After 4–5 days, remove non-adherent cells (Asahara et al. 2000; Vasa et al. 2001; Tepper et al. 2002). The remaining adherent cells present the early EPCs with spindle shape (Fig. 7.1). The second method is based on cell surface antigens, using a technology known as fluorescence activated cell (FACS) analysis to distinguish EPCs from other cells in PB (Yoder 2009; Kirton and Xu 2010; Basile and Yoder 2014). Although there are no specific antigens to isolate and identify EPCs, some have been accepted as fundamental elements representing the EPC phenotype, which are CD34, CD133 (AC133), and KDR (VEGFR−2) (Peichev et al. 2000). Subsequently, different combinations of these antigens have been used to isolate EPCs from PB, UCB and fetal liver (Timmermans et al. 2009). However, recent studies provide opposing evidence that cells expressing that the three antigens mentioned above should not represent EPCs, but stand for hematopoietic progenitors, because no observed vessel structure formed in vivo (Timmermans et al. 2007). Other surface antigens have also been used to identify EPCs, such as CXCR4, CD31, CD144, CD105, CD106, CD117, and CD45 (Basile and Yoder 2014). However, all of these surface antigens, including CD34, CD133 and KDR, do not only emerge on EPCs, but are also expressed on other cells, for example HSCs (Hirschi et al. 2008; Alaiti et al. 2010; Fadini et al. 2012; Yoder 2012), leading to unpersuasive results when isolating and identifying EPCs. Therefore, novel specific markers need to be found to identify true EPCs. The last method includes two colony-forming assays in vitro, which are based on cluster formation. One is called colony forming unit-Hill (CFU-Hil) assay. Briefly, the MNCs isolated from the PB are plated on the fibronectin coated dishes for 48 h, and the non-adherent cells are collected to culture again, clusters occur ring after 4–9 days, which are named CFU-Hil EPCs (Hill et al. 2003) . These cells have similar characteristics to early EPCs (Fadini et al. 2012), express endothelial and hematopoietic cell markers, and fail to form vessels in vivo. The other method is endothelial colony forming cells (ECFCs) assay. Plate the isolated MNCs on collagen I coated dishes, and adherent cells form colonies 2–3 weeks later. These cells are named ECFCs, which are known as late EPCs (Ingram et al. 2004; Kirton and Xu 2010). ECFCs express antigens like primary ECs, have a huge potential to form colonies, and are able to form vessels in vivo and in vitro (Yoder 2009) (Fig. 7.1) .
2.4 Classification of EPCs
According to their culture characteristics and functions, circulation EPCs can be classified into two different populations: early EPCs, which are also called circulating angiogenic cells (CACs), and late EPCs, which are also known as ECFCs (Hur et al. 2004) (Table 7.1) . The early EPCs emerge 5–7 days after isolation of MNCs from the PB and disappear at 4 weeks. They have spindle shape, can be stained with Ulex europaeus agglutinin 1 (UEA−1) and take up acetylated low-density lipoprotein (acLDL) (Hur et al. 2004; Hirschi et al. 2008). They express EC markers and keep hematopoietic antigen expression (Kirton and Xu 2010). Early EPCs cannot form vessels in vivo, but contribute to angiogenesis by secreting angiogenic cytokines (Gehling et al. 2000; Lin et al. 2000; Vaughan and O’Brien 2012). Late EPCs form a monolayer of cobblestone shaped cells 2–4 weeks after plating, have huge potential to proliferate, and can be maintained for up to 12 weeks . Similarly, these cells can also be stained with UEA−1, take up acLDL and express the same markers as early EPCs, such as CD34/KDR (VEGFR−2)/CD31, but they lack the expression of antigens like CD14, CD133, CD45 and CD115 (Hur et al. 2004; Ingram et al. 2004; Kirton and Xu 2010). More importantly, late EPCs are able to form vessels in vitro and in vivo (Lin et al. 2000; Grant et al. 2002). Late EPCs are thought to be the true EPCs and show greater sensitivity to vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and placental growth factor (PlGF) (Bompais et al. 2004; Pasquier 2010). Therefore, these two types of EPCs have different morphologies, proliferative abilities and survival rates but both of them display vasculogenic capacity in vivo (Hur et al. 2004) (Fig. 7.1) .
3 Therapeutic Potential of EPCs for Stroke
3.1 A Biomarker of Diseases
There is no doubt that EPCs exist in adult PB (Asahara et al. 1997), promote vascular repair after ischemia , and attenuate the progression of arteriosclerosis (Medina et al. 2012). In the past years, a lot of studies have demonstrated that the number and functional stage of circulation EPCs are associated with arteriosclerosis, hypertension, diabetes, and metabolic syndrome (Vasa et al. 2001; Hill et al. 2003; Werner et al. 2005; Liao et al. 2010; Mandraffino et al. 2011; Devaraj and Jialal 2012; Flammer et al. 2012). Based on this evidence, levels of circulation EPCs can be used as novel biomarkers. More importantly, the levels of EPCs also have a close relationship with ischemic stroke, studies have shown that lower levels of circulation EPCs indicate poor outcomes among ischemic stroke patients (Ghani et al. 2005; Sobrino et al. 2007; Chu et al. 2008; Yip et al. 2008; Tsai et al. 2014) (Table 7.2).
3.2 Protection of Blood Brain Barrier (BBB)
As we all know, the BBB is comprised of brain microvascular ECs, basement membrane, astrocytes and pericytes, all of these parts are now called the neurovascular unit (Wong et al. 2013). The integrity of the BBB plays an important role in maintaining the homeostasis of the brain. Once destroyed, the balance of the brain’s microenvironment is disrupted, leading to a series of pathological processes, including the swelling of endothelial cells, an increase in vascular permeability, inflammatory cell infiltration and tissue edema. As mentioned above, EPCs have the potential to differentiate into ECs and promote vascular repair (Ponio et al. 2014), and to support the integrity and function of the BBB (Kaneko et al. 2012). However, how EPCs beneficially influence the BBB is still a mystery. Therefore, more work is needed to elucidate the protective mechanism of EPCs on the BBB after stroke.
3.3 Promotion of Neovascularization After Stroke
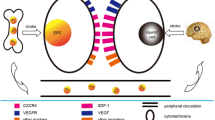
It has been widely accepted that neovascularization after stroke is essential and critical for tissue repair and neurological function recovery. Animal and human studies have proved that EPCs participate in neovascularization (Zhang et al. 2002; Fan et al. 2010; Paczkowska et al. 2013), mainly via two approaches: by directly differentiating into ECs and incorporating into the damaged vessels, which is called vasculogenesis; and by indirectly promoting migration and proliferation of pre-existing ECs, which is called angiogenesis, through releasing a variety of angiogenic cytokines (Masuda and Asahara 2003; Tepper et al. 2005; Urbich et al. 2005; Chen et al. 2013b). In addition, these cytokines also enhance EC and neuron survival, and recruit endogenous progenitor cells (Chen et al. 2013b). Because of the unique characteristic and advantage of angiogenic ability, EPCs may be an important agent for the treatment of stroke .
3.4 Factors Influence EPCs In Vivo
In the past decade, it had been demonstrated that tissue ischemia and exogenous cytokines could mobilize endogenous circulating EPCs and thereby contribute to neovascularization (Asahara et al. 1999; Takahashi et al. 1999). Subsequently, more and more studies have proven that the levels and functional stages of EPCs are correlated with many diseases and are considered as a biomarker (Table 7.2). Moreover, transplantation of EPCs as a therapeutic strategy is beneficial to the hindlimb and cerebral ischemia (Kalka et al. 2000a; Fan et al. 2010; Moubarik et al. 2011). Recently, several studies showed that a variety of factors could influence the number and function of circulating EPCs in vivo. For example, statin treatment for 4 days may increase circulating EPCs levels in acute ischemic stroke patients, probably by nitric oxide (NO)-related mechanisms (Sobrino et al. 2012a). VEGF and stromal derived factor−1α (SDF−1α) are independent factors for the increment of circulating EPCs (Sobrino et al. 2012b). In addition, factors like homocysteine, haptoglobin 1–1, citicoline, cilostazol, systolic blood pressure, total cholesterol, erythropoietin (EPO), high-mobility group box 1 (HMGB−1), and matrix metalloproteinase (MMP−9) are also proven to influence the number of circulating EPCs in humans and animals (Table 7.3). The therapeutic effects of transplantation of EPCs for stroke can be improved by modulating these factors (Morancho et al. 2013).
4 Signaling in Regulating EPC Functions
Studies are investigating a variety of factors that influence EPC proliferation, migration and maturation (Table 7.3). Additional researchers are trying to discover the signaling pathways activated by these factors to influence EPCs. Early EPCs secrete a large number of factors, including VEGF, brain-derived neurotrophic factor (BDNF), bFGF, insulin-like growth factor 1 (IGF−1), and interleukin−8 (IL−8). (He et al. 2005; Moubarik et al. 2011; Rosell et al. 2013), which are pro-angiogenic factors that increase endothelial proliferation, tube formation, migration and MMP secretion in ECs to enhance the invasiveness of EPCs (Carmeliet 2003; Li et al. 2003). MMP−9 is essential for ischemia-induced neovascularization, which modulates the neovascularization of EPCs by increasing the release of cytokines (Huang et al. 2009; Morancho et al. 2013). Integrin-linked kinase is upregulated in ECs and associated with increased intercellular adhesion molecule 1 (ICAM−1) and SDF−1 under hypoxic stress, which recruits EPCs to ischemic tissue (Lee et al. 2006). CD18 and its ligand ICAM−1 also play an essential role in mediating EPC recruitment in infracted hearts (Wu et al. 2006). Activated AKT signals promoted the expression of ICAM−1 on ECs and closely associated with EPC entrapment, which might be important in regulating the process of neovascularization through enhancing EPC migration and trans-endothelial migration (Yoon et al. 2006; Hur et al. 2007).
IL−10 increases EPC survival and mobilization through the activation of STAT3/VEGF signaling cascades (Krishnamurthy et al. 2011). SDF−1 released from the ischemic tissue form a concentration gradient to promote EPC homing through interaction with its receptor CXCR4 (Fan et al. 2010). Deltalike−4 gene modified EPCs show enhanced functional neovascularization in ischemic tissue due to the activation of Notch/Hey1/mTOR/p70S6K signaling pathways (Huang et al. 2013a). Wnt1 is a pro-angiogenic molecule and enhances EPC function in a hepatocyte growth factor (HGF)-dependent manner (Gherghe et al. 2011). HMGB1 secreted by astrocytes after ischemic stroke increases EPC homing involved in neurovascular remodeling and functional recovery (Hayakawa et al. 2012). Other factors have also been reported to influence EPC functions, such as E-selectin, estrogen and β2-adrenergic receptor (Oh et al. 2007; Tan et al. 2012; Galasso et al. 2013). Fully understanding the mechanisms underlying EPC function will help improve the safety and efficiency of EPC transplantation.
5 Action Mechanism of EPCs
5.1 Cell Replacement
EPCs derived from BM or other tissues have an intrinsic capacity for differentiating into ECs (Asahara et al. 1997; Beltrami et al. 2003; Planat-Benard et al. 2004; Chen et al. 2008; Chen et al. 2012; Nih et al. 2012; Iskander et al. 2013; Pellegrini et al. 2013). The injured ECs in the brain can be replaced by transplanted EPCs. Granulocyte colony-stimulating factor (G-CSF) mobilizes circulating EPCs to engage 39 % of the total luminal length of the neoendothelium (Takamiya et al. 2006). LacZ-transduced CD34+EPC transplantation leads to about 60 % reendothelialization of balloon-injured rabbit carotid arteries costained with CD31 as early as 4 days after transplantation and this increases to about 70 % at 30 days after transplantation (Griese et al. 2003b). Fluorescence-labeled EPCs are found in the neointima and costaining with vWF is found after 4 weeks in the injured carotid artery of balloon injured New Zealand white rabbits (Hu et al. 2013). Hence, cell replacement is one of the mechanisms of vascular repair by progenitor cells. BM derived EPCs contribute to the microvascular structure of the choroid plexus by differentiating into ECs during cerebral ischemia in adult mice (Zhang et al. 2002). 14 days after the transplantation of EPCs in the cerebral ischemia rabbit model, a decrease in the number of apoptotic cells and an increase in the microvessel density in the ischemic boundary area has been witnessed, and most of EPCs capable of binding to UEA−1 lectin are incorporated into capillaries (Chen et al. 2008).
However, the extent of incorporation of BM derived cells in cerebral vessels after stroke has varied in previous studies (Hess et al. 2002; Zhang et al. 2002; Machein et al. 2003; Chen et al. 2008; Moubarik et al. 2011). Whereas positive vessels had an average of 34 % endothelial marker expressing BM derived cells (Hess et al. 2002; Zhang et al. 2002), others could not detect endothelial marker expressing cells (Machein et al. 2003; Moubarik et al. 2011).
5.2 Enhanced Trophic/Regenerative Support for Endogenous Repair Processes
Neovascularization is not solely the result of the incorporation of EPCs in newly formed vessels; the release of trophic factors in a paracrine manner may also influence neovascularization. Cultured PB MNCs secrete high levels of VEGF, HGF, G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Rehman et al. 2003). More and more researchers are paying close attention to the trophic effects of EPCs. In vitro, early EPCs cultivated from different sources have shown marked expression and the release of angiogenic cytokines including G-CSF, GM-CSF, VEGF, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), FGF, HGF, IL−8, transforming growth factor β2 (TGF-β2), IGF−1 and etc (He et al. 2004; Hur et al. 2004; Yoon et al. 2005). The release of these growth factors in turn may influence the classical process of angiogenesis, particularly the proliferation and migration as well as the survival of mature ECs (Folkman 1995; Urbich and Dimmeler 2004). EPCs can also exert a strong mitogenic effect on mature ECs and enhance the angiogenic capacity of outgrowth of ECs via secretion of IL−8 with/without VEGF (He et al. 2005; Yoon et al. 2005).
In cerebral arteries, the paracrine effect of EPCs promotes vasoprotection by increasing prostacyclin production and the intracellular concentration of cAMP (Santhanam et al. 2007). EPCs from stroke patients present higher levels of pro-angiogenic factors at early stages, which decrease in mature ECs when they become more similar to mature microvascular ECs (Navarro-Sobrino et al. 2013). 24 h after the administration of EPCs expressing GFP, they are found to express endothelial NO-synthase (eNOS) and distribute in the brain parenchyma and around the endothelial layer of pial arteries in the ischemic lesions (Ohta et al. 2006). EPC transplantation can also induce humoral effects, which are sustained by host tissues, decrease apoptosis and augment proliferation of cells. Transplantation of EPCs enhances the mobilization of endogenous EPCs and HSCs mainly by upregulation of humoral VEGF, FGF−2, IGF, HGF, angiopoietin−1 and SDF−1 (Cho et al. 2007).
Studies have shown that vascular niche can support neurogenesis in the subventricular zone and the dentate gyrus by secreting growth factors associated with neurogenesis, such as VEGF or BDNF (Leventhal et al. 1999; Palmer et al. 2000). In an experimental stroke study, neovascularization related to neurogenesis, and also to the migration of neural progenitor cells (NPCs), along the newly formed vessels (Thored et al. 2007). Thus, administered EPCs may enhance the proliferation of endogenous NPCs in the brain (Rouhl et al. 2008). EPCs injected 24 h after MCAO were found in the injured area and improved functional recovery, which was linked to a reduction in ischemia-induced apoptosis and a stimulation of ischemia-induced angiogenesis and neurogenesis (Moubarik et al. 2011). Transplantation of BM-derived EPCs exerts potent neuroprotective functions against cerebral ischemia/reperfusion injury in rats, and the protective effects may be associated with decreased expression of Bax, caspase−3 and p67phox and the increasing expression of Bcl−2 and manganese superoxide dismutase (MnSOD), which promotes anti-oxidative and anti-apoptotic properties (Qiu et al. 2013).
6 Transplantation of EPCs in Ischemic Stroke Animals
6.1 Transplantation Routes for EPCs
The optimal transplantation route for EPCs following ischemic stroke may be important for the therapeutic efficacy . The two routes mostly used for the transplantation of EPCs in stroke are intracerebral and intravascular injections. They each have their own advantages and disadvantages. EPCs intracerebrally injected into the peri-infarct area may be immediately involved in incorporating newly formed vessels or secreting trophic factors to support endogenous repair processes, especially in permanent ischemic stroke to bypass the occlusion of blood vessels. However, invasive injury to the brain raises safety issues.
Intravascular injection either through veins or arteries has minimal invasive injury potential for systematical cell distribution, as well as the far-flung secretion of neuroprotective, pro-angiogenic and immunomodulatory factors (Misra et al. 2012). Intravenously grafted cells can follow a chemokine generated gradient formed by the injured brain and penetrate through the BBB (Guzman et al. 2008), and grafted cells do not have to be near the lesion to be effective (Borlongan et al. 2004) . However, very few cells have been found to integrate into the infarct area. The majority of cells became stuck in the lung, liver, and spleen after intravenous administration. Intra-arterial delivery, in contrast, overpasses the peripheral filtering organs, leading to higher cell engraftment to the brain (Li et al. 2010; Zhang et al. 2012), and greater efficacy (Kamiya et al. 2008; Pendharkar et al. 2010). There is a concern that intra-artery transplanted cells can stick together and cause microemboli, including lethal pulmonary emboli or a reduction in cerebral blood flow, which is associated with microstrokes (Walczak et al. 2008).
In preclinical experimental stroke, intravascular injections are usually used, and they are applied through the tail vein (Zhang et al. 2002; Chen et al. 2012; Nih et al. 2012; Chen et al. 2013d; Decano et al. 2013; Qiu et al. 2013), femoral vein (Moubarik et al. 2011; Pellegrini et al. 2013), jugular vein (Fan et al. 2010; Li et al. 2013), and internal carotid artery (Ohta et al. 2006) (Table 7.4) . Despite the different routes used for EPC transplantation, decreased infarct volume, improved neurobehavioral outcomes, increased angiogenesis and neurogenesis, attenuation of endothelial dysfunction, even anti-apoptosis effects have been observed during study. These studies may benefit from both functions of EPCs during cell replacement and enhanced trophic/regenerative support for endogenous repair processes.
However, when considering application in clinical trials , the routes of transplantation of EPCs should be standardized to ease administration. Several clinical studies have been carried out as illustrated in Table 7.5. These studies look into the safety and efficiency of routes for EPC transplantation in human patients, and there is still a lot of work to do in this field .
6.2 Modification of EPCs
6.2.1 Gene Modification
Considering the paracrine-mediated mechanisms of EPCs, the enhancement of their secretion of trophic factors capacity by the overexpression of related genes would be valuable to magnify the efficacy of EPC therapies in stroke treatment (Chen et al. 2013a). EPCs have been modified by a variety of genes before transplantation and have been reported to enhance functional recovery, these genes include VEGF (Asahara 2007; Gou et al. 2011; Yang et al. 2012), HGF (Song et al. 2009), IGF−1 (Sen et al. 2010), paraoxonase−1 (Wang et al. 2010), CXCR4 (Chen et al. 2012), SDF−1 (Schuh et al. 2012), NO (Chen et al. 2013c), home oxygenase−1 (Long et al. 2013), hypoxia-inducible factor−1α (HIF−1α) (Jiang et al. 2008) and Deltalike−4 (Huang et al. 2013a). There are two major methods for gene transfer systems, viral and nonviral. The most widely used viral vectors for gene transfer are adenovirus and retrovirus. Nonviral methods include the introduction of naked DNA into the target cells and the use of liposomes (Vale et al. 2001).
EPCs modified by VEGF gene show significantly enhanced neovascularization, even when ten times fewer cells were infused (Asahara 2007), and promote vascular regeneration of ischemic flaps (Yi et al. 2006). In ischemic hindlimb model, transfection of VEGF or heme oxygenase−1genes into EPCs significantly increased the number of differentiated ECs, blood perfusion levels and neovascularization compared to the bare EPCs (Yang et al. 2012; Long et al. 2013). Transfection of EPCs with other genes, such as IGF−1 (Sen et al. 2010), SDF−1 (Schuh et al. 2012), NO (Chen et al. 2013c) and Deltalike−4 (Huang et al. 2013a) genes, to treat ischemic myocardial injury show cell protective and myocardial regeneration effects and functional neovascularization recovery. EPCs modified by paraoxonase−1 genes are potentially valuable in the treatment of atherosclerosis (Wang et al. 2010). Transfection of HGF genes enhances EPC function and improves EPC transplantation efficiency by decreasing neointima formation and increasing reendothelialization for balloon-induced arterial injury (Song et al. 2009).
Some investigators tried to transfect multiple genes into EPCs by using retroviruses to encode both tPA and heparin. Local transplantation of engineered EPCs in a balloon-injured carotid artery model attenuates reendothelialization of angioplasty-injured arteries, but fails to inhibit neointima proliferation (Griese et al. 2003a). In experimental stroke models, only one study found that the transplantation of CXCR4 gene-modified EPCs reduces cerebral ischemic damage and promotes repair in diabetic mice, and that modified EPCs show better therapeutic effects for ischemic stroke than unmodified EPCs (Chen et al. 2012).
Until now, there have been no clinical trials using gene-modified EPC therapy for the treatment of stroke. It is important to confirm the safety and efficacy of delivering exogenous genes into patients by modifying EPCs. The main concern is the possibility of tumorigenesis after gene delivery. Although exogenous genes are transferred into EPCs rather than to host cells, viral vectors may increase the risk of genotoxicity by insertional mutagenesis and the activation of adjacent oncogenes. To avoid malignant transformation in clinical patients, the vector should be designed for self-inactivation and only contain nonviral, physiologic promoter/enhancer elements (Payen and Leboulch 2012). Second, the therapeutic genes may serve different functions during different pathological stages. For example, SDF−1 plays a key role in promoting angiogenesis and neurogenesis during development (Mithal et al. 2012; Virgintino et al. 2013) and can recruit EPCs towards ischemic lesions for reendovascularization (Fan et al. 2010). Blocking SDF−1/CXCR4 interaction suppresses inflammatory responses and reduces brain infarction in the acute phase of ischemic stroke (Huang et al. 2013b; Ruscher et al. 2013), which indicates that SDF−1 is an inflammation initiator and exaggerates the BBB leakage and ischemic lesions. Whether SDF−1-overexpressing cells could exhibit a similar deterioration effect is unknown, but such studies are fundamental in calling attention to the administration paradigm of EPC gene modified therapy. Third, most completed and ongoing clinical trials employ autologous EPCs for transplantation ; the exogenous gene expression in EPCs is time consuming and unavoidably delays cell transplantation. Further studies should be carried out on the effects of delivering gene-modified EPCs in a later period after stroke or the transplantation ex vivo of expanded EPCs from allogenic sources, which allows for transformation and in vitro expansion of EPCs before transplantation (Chen et al. 2013a).
6.2.2 Preconditioning EPCs
In addition to the exogenous gene modification of EPCs, investigators have been trying to manipulate endogenous mechanisms for optimizing the therapeutic potential of cell-based stroke therapy by pre-treating EPCs before transplantation. Various factors seem to influence the number of EPCs and their functions, both in experimental stroke models and in clinical patients (Table 7.3). The hypoxia induced by HIF−1α and trophic growth factors such as BDNF, glial cell line-derived neurotrophic factor (GDNF), VEGF and its receptor FIK−1, EPO and its receptor EPOR, SDF−1 and its receptor CXCR4, enhance EPC proliferation, mobilization and the homing to ischemic lesions involved in the repairing process (Kalka et al. 2000b; Vale et al. 2001; Yamaguchi et al. 2003; Bennis et al. 2012). Increased HIF−1α and its downstream genes play central roles in hypoxia-induced defense responses (Ogle et al. 2012). Ischemic preconditioning increases EPC numbers to attenuate partial nephrectomy-induced ischemia/reperfusion injury (Liu et al. 2013a).
VEGF is an important humoral factor for EPC mobilization/differentiation, which is supported by the correlation between the increase in VEGF serum concentration and the increase in circulating EPCs (Sobrino et al. 2012a). EPO stimulates normal EPC-mediated endothelial turnover and improves cardiac microvascularization and function in the presence of ischemia (Westenbrink et al. 2008). Pretreatment of EPCs with EPO before transplantation enhances their angiogenic potential (Bennis et al. 2012). SDF−1 pretreatment during EPC expansion stimulates the adhesion of EPCs to ECs and augments the efficiency of EPC-based cell therapy for ischemic diseases (Zemani et al. 2008). The hormone melatonin stimulates the protective effect of EPCs in acute ischemic kidney injury (Patschan et al. 2012). Exposure to sub-lethal hypoxia can significantly increase the tolerance and regenerative properties of stem/progenitor cells in vitro and after transplantation for other cell types (Francis and Wei 2010; Wei et al. 2013; Yu et al. 2013).
Estradiol preserves the integrity of ischemic tissue by augmenting the mobilization and incorporation of EPCs into sites of neovascularization by the eNOS-mediated augmentation of MMP−9 expression in the BM (Iwakura et al. 2006). Angiotensin-converting enzyme 2 (ACE2) improves EPC functions, by regulating eNOS and Nox pathways, enhancing the efficacy of EPC-based therapy for ischemic stroke (Chen et al. 2013b). Other methods, such as the pretreatment of EPCs with extracorporeal shock waves (Lee and Kou 2012) or magnetic bionanoparticles (Li et al. 2013) to enhance the homing and functions of EPCs may also be promising and novel strategies. It is expected that the preconditioning strategy will be further explored due to its potential to enhance the benefits of EPC-based transplantation therapies in stroke therapy (Liu et al. 2013b).
7 Pros and Cons in the Application of EPCs in Clinical Trials
EPC transplantation in stroke has pros and cons. As mentioned above, EPCs have shown much potential for stroke therapy either through directly differentiating into mature ECs to replace damaged tissue or by secreting trophic factors to enhance the endogenous repairing processes. Additionally, EPCs can be derived from a variety of sources including PB (Medina et al. 2010), BM (Kwon et al. 2010), cord blood (Li et al. 2013), spleen (Wassmann et al. 2006), adipose tissue (Planat-Benard et al. 2004), and the liver or intestines (Aicher et al. 2007). Ethical limitations are avoided because fetal or embryonic tissues are not necessary sources. A lot of experience in administration of HSCs in clinical treatment of patients with leukemia (Rouhl et al. 2008) shows it is not necessary for autogenous transplanted cells. It allows plenty of time for ex vivo expanded EPCs to be cultured, pretreated or even gene modified, so as to enhance therapeutic capacity when transplanted in vivo. However, there are still difficulties that need to be resolved. As EPCs can be cultured by many methods and derived from different sources, and they bear both the characteristic of hematopoietic and endothelial cells, there are no specific markers to identify them and they may also be contaminated by other cell lines like lymphocytes, macrophages or other dendritic cells (Ishikawa and Asahara 2004). Gene expression profiles may also change during EPC culturing (Gremmels et al. 2011). Whether the exogenous gene modified EPCs increase malignant transformation in clinical patients still needs to be further explored.
8 Problems Need to be Clarified for the Treatment of Patients
8.1 Evaluation of Clinical Safety
Preclinical studies have shown that EPC transplantation is beneficial for functional outcomes without showing side effects, such as enhancing inflammatory responses or forming teratoma. However, to fully ensure the safety of transplanting EPCs in clinical patients, clinical studies have been carried out. A small pilot study suggested that intravenous infusion of autologous EPCs was safe and improved exercise capacity in children with idiopathic pulmonary arterial hypertension (Zhu et al. 2008). EPC transplantation in 20 patients with acute myocardial infarction showed no incensement in the levels of inflammatory markers or troponin T (a marker for cardiac ischemia) (Assmus et al. 2002). Thus, in this small number of patients, EPCs neither seem to stimulate the inflammatory response nor increase ischemia. A variety of clinical studies has also shown that autologous BM stem cell and mesenchymal stem cell transplantation in stroke patients showed nothing related to abnormal cell growth or tumorigenesis, deteriorated functional outcomes or venous thrombuses (Suarez-Monteagudo et al. 2009; Lee et al. 2010; Honmou et al. 2011; Friedrich et al. 2012; Moniche et al. 2012).
EPCs can secret inflammatory factors such as IL−8 and monocyte chemoattractant protein−1 (MCP−1) (Hur et al. 2004; van der Strate et al. 2007), which might recruit monocytes and macrophages to aggravate ischemia. Currently, several clinical trials (clinicaltrials.gov identifier: NCT00950521; NCT01468064; NCT00535197) are trying to evaluate the safety and efficacy of autologous EPC transplantation in ischemic stroke. EPC transplantation cannot be routinely performed on patients for the treatment of stroke before larger clinical trials further ensure their clinical safety.
8.2 Identifying Acceptable Patients for EPC Transplantation
No treatment is appropriate for all stroke patients therapy. Therefore, establishing a criterion for choosing suitable patients for EPC transplantation is vitally important. Stroke patients range in age from 30–80 years old; age should be something to consider because elder patients tend to be suffering from other diseases such as hypertension, diabetes mellitus and dyslipidemic syndromes. Patients with these syndromes show endothelial dysfunction and decreased EPC numbers (Rouhl et al. 2012). It might be difficult for this kind of patient to receive autologous EPCs , which may lose their functional therapeutic effects after transplantation. Methods of modifying EPCs to increase their vasculogenic potential or allogenic EPCs from healthy people may provide options.
Studies have shown that the pathology of stroke in young and aged rats are not identical. For example, after intracerebral hemorrhage , aged rats showed a wider spread of activated microglia/macrophages around the parenchyma and higher astrocyte activity than young rats (Wasserman et al. 2008). Another study showed that aging mice had significantly less edema formation after stoke (Liu et al. 2009). In addition, EPCs are critical components of tumor angiogenesis (Nolan et al. 2007); therefore, EPC transfusion to patients with tumors should be avoided. Considering estrogen has the capacity to promote EPC proliferation (Tan et al. 2012), men and women may respond differently to EPC-based treatments. Whether EPC treatment would have the same efficacy in males and females needs to be considered further.
Infarct location and volume are other factors for determining a patient’s suitability for cell transfusion. Preclinical studies in EPC transplantation in stroke (Table 7.4) and different animal models may result in different infarct location and volume in striatum, cortex or both. EPC transplantation shows improvement in functional recovery; however, we cannot exclude the possibility that it may not be as effective in clinical patients. Scoring patients with different lesions, which are usually determined by magnetic resonance imaging (MRI), choosing suitable patients and accordingly giving the appropriate EPC treatment is necessary.
8.3 Time, Dose, Route and Type of EPCs for Transplantation
8.3.1 Time of EPC Transplantation
Preclinical studies provide various time points to deliver EPCs (Table 7.4). However, the optimal time for transplantation after a stroke is still unclear . After stroke onset, the microenvironment in the brain changes dramatically (Moskowitz et al. 2010). The optimal timing of delivery depends on EPC mechanisms of action, which could replace the damaged cells and promote the endogenous repair process by paracrine effects . If the treatment strategy focuses on cell survival and later cells integrate into the damaged tissue to replace the dead cells, cell survival is extremely important and transplanting during the recovery phase of stroke to avoid inflammation could be beneficial. Otherwise, if the treatment acts to enhance the endogenous repair process or protective mechanisms by paracrine effects, acute phase delivery is critical (Hayashi et al. 2003; Carmichael 2006). Preclinical studies of the delivery of EPCs were done either immediately after stroke or from 1 h to 1 day after stroke, which showed functional recovery in animals. However, a systematic analysis of transplantation timing and its effect on functional recovery has not been done .
8.3.2 Dose Injection of EPCs
In addition, as we move towards clinical trials , cell dosage becomes an important question to consider. Different cell dosages have been applied during preclinical trials (Table 7.4). Cell dosages influence cell viability after transplantation; fewer cells may not be enough to function as a therapeutic treatment, while an excessive amount of cells may result in side effects such as inflammation , teratoma or microembolus. Ongoing clinical studies are designed to use 2–8 × 106 EPCs to treat stroke patients (clinical trials.gov identifier: NCT01468064; NCT01438593; NCT00950521), and the safety and efficacy of EPC therapies are not yet clear.
8.3.3 Routes of Administration
Studies have reported functional recovery using the intravenous and intracerebral delivery of EPCs. All routes resulted in cells targeting the lesion, but more cells were found at the lesion with intracerebral delivery than with intravenous delivery (Jin et al. 2005). Preclinical studies choosing intravenous or intra-artery delivery of EPCs have shown functional recovery. Clinical studies plan to apply either intravenous or intracerebral delivery routes. In regards to EPC transplantation in clinical trials , intravenous infusion should be the optimal route because intracerebral injection is invasive and inconvenient, and intra-artery delivery may cause embolisms (Borlongan et al. 2004).
8.3.4 Types of Used EPCs
Different types of EPCs play specific roles, with early EPCs protecting damaged tissue by secreting amounts of pro-angiogenic factors, and late EPCs integrate in to host vessels to replace damaged ECs. However, it is still difficult to define EPCs, because they have multiple markers. Investigators are trying to identify, isolate and expand EPCs using their normal markers, such as CD34, KDR, and CD133, but related cell types might bear the same markers. All the ongoing clinical trials use CD34 to identify EPCs, which might not be sufficient. Optimizing the isolation and identification of EPCs from patients is still a critical problem.
8.4 Bio-Distribution and Persistence of EPCs
When EPCs are transplanted into ischemic animals or patients, it is crucial to monitor where the EPCs travel and into what cell types they differentiate. This helps us to understand how these cells mediate functional recovery. Therefore, dynamic noninvasive tracking of grafted EPCs in vivo is necessary. Optical imaging, MRI and nuclear imaging are potential imaging strategies and MRI is most often used for the dynamic tracking of grafted EPCs in vivo. For the tracking of exogenous EPCs in vivo, the grafted cells must be labeled with contrast agents in vitro before transplantation so that they are distinguishable from the host tissue. Molecular probes such as transferrin have successfully been used to tag putative stem cells followed by high-resolution MRI to track the homing of cells (Weissleder et al. 2000). Currently, gadolinium rhodamine dextran (GRID) and superparamagnetic iron oxide (SPIO) are two groups of commonly used contrast agents. Some studies have been successful in long-term monitoring EPCs using MRI in a rat hindlimb ischemic model (Agudelo et al. 2011; Agudelo et al. 2012). However, little work has been done on the dynamic tracking of EPCs in ischemic stroke and it calls for much attention in order to provide fundamental data for its application in clinical trials .
Abbreviations
- acLDL:
-
Acetylated low density lipoprotein
- ACE2:
-
Angiotensin-converting enzyme 2
- BBB:
-
Blood-brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- bFGF:
-
Basic fibroblast growth factor
- BM:
-
Bone marrow
- CACs:
-
Circulating angiogenic cells
- CFU-Hil:
-
Colony forming unit-Hill
- ECFC:
-
Endothelial colony forming cell
- ECs:
-
Endothelial cells
- EGF:
-
Epidermal growth factor
- eNOS:
-
Endothelial NO synthase
- EPCs:
-
Endothelial progenitor cells
- EPO:
-
Erythropoietin
- FACS:
-
Fluorescence activated cell
- G-CSF:
-
Granulocyte colony-stimulating factor
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HGF:
-
Hepatocyte growth factor
- HIF−Iα:
-
Hypoxia-inducible factor−1α
- HMGB−1:
-
High-mobility group box 1
- HSCs:
-
Hematopoietic stem cells
- ICAM−1:
-
Intercellular adhesion molecule 1
- IGF−1:
-
Insulin-like growth factor−1
- IL−8:
-
Interleukin−8
- MCP−1:
-
Monocyte chemoattractant protein−1
- MMP−9:
-
Matrix metalloproteinase 9
- MNCs:
-
Mononuclear cells
- MRI:
-
Magnetic resonance imaging
- NPCs:
-
Neural progenitor cells
- PB:
-
Periblood blood
- PDGF:
-
Platelet-derived growth factor
- PlGF:
-
Placental growth factor
- SDF−1α:
-
Stromal derived factor−1α
- TGF-b2:
-
Transforming growth factor b2
- tPA:
-
Recombinant tissue plasminogen activator
- UCB:
-
Umbilical cord blood
- UEA−E:
-
Ulex europaeus agglutinin 1
- VEGF:
-
Vascular endothelial growth factor
References
Agudelo CA, Tachibana Y, Noboru T, Iida H, Yamaoka T (2011) Long-term in vivo magnetic resonance imaging tracking of endothelial progenitor cells transplanted in rat ischemic limbs and their angiogenic potential. Tissue Eng Part A 17:2079–2089
Agudelo CA, Tachibana Y, Hurtado AF, Ose T, Iida H, Yamaoka T (2012) The use of magnetic resonance cell tracking to monitor endothelial progenitor cells in a rat hindlimb ischemic model. Biomaterials 33:2439–2448
Ahrens I, Domeij H, Topcic D, Haviv I, Merivirta RM, Agrotis A, Leitner E, Jowett JB, Bode C, Lappas M, Peter K (2011) Successful in vitro expansion and differentiation of cord blood derived CD34 + cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS ONE 6:e23210
Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C (2007) Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 100:581–589
Alaiti MA, Ishikawa M, Costa MA (2010) Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res 156:112–129
Alam MM, Mohammad AA, Shuaib U, Wang C, Ghani U, Schwindt B, Todd KG, Shuaib A (2009) Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J Cereb Blood Flow Metab 29:157–165
Alev C, Ii M, Asahara T (2011) Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal 15:949–965
Allen CL, Bayraktutan U (2008) Risk factors for ischaemic stroke. Int J Stroke 3:105–116
Asahara T (2007) Cell therapy and gene therapy using endothelial progenitor cells for vascular regeneration. Handb Exp Pharmacol 180:181–194
Asahara T, Kawamoto A (2004) Endothelial progenitor cells for postnatal vasculogenesis. Am J physiol Cell Physiol 287:C572–579
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Asahara T, Kalka C, Isner JM (2000) Stem cell therapy and gene transfer for regeneration. Gene Ther 7:451–457
Asahara T, Kawamoto A, Masuda H (2011) Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 29:1650–1655
Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM (2002) Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 106:3009–3017
Basile DP, Yoder MC (2014) Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229:10–16
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776
Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, Blot-Chabaud M, Dignat-Georges F, Sabatier F, Pisano P (2012) Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost 10:1914–1928
Bliss T, Guzman R, Daadi M, Steinberg GK (2007) Cell transplantation therapy for stroke. Stroke 38:817–826
Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M, Frank J, Warach S (2010) Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 75:2059–2062
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J, Warach S (2011a) Circulating CD133 + CD34 + progenitor cells inversely correlate with soluble ICAM−1 in early ischemic stroke patients. J Transl Med 9:145
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J, Warach S (2011b) Stromal-derived factor-1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42:618–625
Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G (2004) Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 103:2577–2584
Borlongan CV, Hadman M, Sanberg CD, Sanberg PR (2004) Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35:2385–2389
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Carmichael ST (2006) Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol 59:735–742
Cesari F, Gori AM, Romagnuolo I, Abbate R (2012) [Endothelial progenitor cells and vascular health: effects of lifestyle’s modifications]. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace/ Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo, 78:66–72
Chen ZZ, Jiang XD, Zhang LL, Shang JH, Du MX, Xu G, Xu RX (2008) Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett 445:36–41
Chen J, Chen S, Zhang C, Zhang L, Xiao X, Das A, Zhao Y, Yuan B, Morris M, Zhao B, Chen Y (2012) Transfusion of CXCR4-primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice. PLoS ONE 7:e50105
Chen C, Wang Y, Yang GY (2013a) Stem cell-mediated gene delivering for the treatment of cerebral ischemia: progress and prospectives. Curr Drug Targets 14:81–89
Chen J, Xiao X, Chen S, Zhang C, Yi D, Shenoy V, Raizada MK, Zhao B, Chen Y (2013b) Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension 61:681–689
Chen X, Gu M, Zhao X, Zheng X, Qin Y, You X (2013c) Deterioration of cardiac function after acute myocardial infarction is prevented by transplantation of modified endothelial progenitor cells overexpressing endothelial NO synthases. Cell Physiol Biochem 31:355–365
Chen X, Yin J, Wu X, Li R, Fang J, Chen R, Zhang B, Zhang W (2013d) Effects of magnetically labeled exogenous endothelial progenitor cells on cerebral blood perfusion and microvasculature alterations after traumatic brain injury in rat model. Acta Radiol 54:313–323
Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS (2007) Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 204:3257–3269
Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, Kim M, Lee SK, Roh JK (2008) Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 39:1441–1447
Decano JL, Moran AM, Giordano N, Ruiz-Opazo N, Herrera VL (2013) Analysis of CD45- [CD34 +/KDR +] endothelial progenitor cells as juvenile protective factors in a rat model of ischemic-hemorrhagic stroke. PLoS ONE 8:e55222
Devaraj S, Jialal I (2012) Dysfunctional endothelial progenitor cells in metabolic syndrome. Exp Diabetes Res 2012:585018
Fadini GP, Losordo D, Dimmeler S (2012) Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110:624–637
Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, Chen Y, Su H, Young WL, Yang GY (2010) Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol 67:488–497
Flammer AJ, Gossl M, Widmer RJ, Reriani M, Lennon R, Loeffler D, Shonyo S, Simari RD, Lerman LO, Khosla S, Lerman A (2012) Osteocalcin positive CD133 +/CD34-/KDR + progenitor cells as an independent marker for unstable atherosclerosis. Eur Heart J 33:2963–2969
Flynn RW, MacWalter RS, Doney AS (2008) The cost of cerebral ischaemia. Neuropharmacology 55:250–256
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Francis KR, Wei L (2010) Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis 1:e22
Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, Raupp EF, Sartori EAmmarJ, Machado DC, Costa JC, Nogueira RG, Rosado-de-Castro PH, Mendez-Otero R, Freitas GR (2012) Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant 21(Suppl 1):S13–S21
Galasso G, De Rosa R, Ciccarelli M, Sorriento D, Del Giudice C, Strisciuglio T, De Biase C, Luciano R, Piccolo R, Pierri A, Di Gioia G, Prevete N, Trimarco B, Piscione F, Iaccarino G (2013) Beta2-adrenergic receptor stimulation improves endothelial progenitor cell-mediated ischemic neoangiogenesis. Circ Res 112:1026–1034
Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W (2000) In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 95:3106–3112
Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K (2005) Endothelial progenitor cells during cerebrovascular disease. Stroke 36:151–153
Gherghe CM, Duan J, Gong J, Rojas M, Klauber-Demore N, Majesky M, Deb A (2011) Wnt1 is a proangiogenic molecule, enhances human endothelial progenitor function, and increases blood flow to ischemic limbs in a HGF-dependent manner. FASEB J 25:1836–1843
Ginsberg MD (1997) The new language of cerebral ischemia. AJNR Am J Neuroradiol 18:1435–1445
Gou X, He WY, Xiao MZ, Qiu M, Wang M, Deng YZ, Liu CD, Tang ZB, Li J, Chen Y (2011) Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl 13:332–338
Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW (2002) Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8:607–612
Gremmels H, Fledderus JO, van Balkom BW, Verhaar MC (2011) Transcriptome analysis in endothelial progenitor cell biology. Antioxid Redox Signal 15:1029–1042
Griese DP, Achatz S, Batzlsperger CA, Strauch UG, Grumbeck B, Weil J, Riegger GA (2003a) Vascular gene delivery of anticoagulants by transplantation of retrovirally-transduced endothelial progenitor cells. Cardiovasc Res 58:469–477
Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ (2003b) Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation 108:2710–2715
Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK (2008) Intravascular cell replacement therapy for stroke. Neurosurg Focus 24:E15
Gyan B, Goka BQ, Adjei GO, Tetteh JK, Kusi KA, Aikins A, Dodoo D, Lesser ML, Sison CP, Das S, Howard ME, Milbank E, Fischer K, Rafii S, Jin D, Golightly LM (2009) Cerebral malaria is associated with low levels of circulating endothelial progenitor cells in African children. Am J Trop Med Hyg 80:541–546
Hankey GJ (2006) Potential new risk factors for ischemic stroke: what is their potential? Stroke 37:2181–2188
Hassan A, Markus HS (2000) Genetics and ischaemic stroke. Brain 123(Pt 9):1784–1812
Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH (2012) Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A 109:7505–7510
Hayashi T, Noshita N, Sugawara T, Chan PH (2003) Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23:166–180
He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS (2004) Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 35:2378–2384
He T, Peterson TE, Katusic ZS (2005) Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin−8. Am J Physiol Heart Circ Physiol 289:H968–972
Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J (2002) Bone marrow as a source of endothelial cells and NeuN-expressing cells aAfter stroke. Stroke 33:1362–1368
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Hirschi KK, Ingram DA, Yoder MC (2008) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28:1584–1595
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134:1790–1807
Hu CH, Ke X, Chen K, Yang DY, Du ZM, Wu GF (2013) Transplantation of human umbilical cord-derived endothelial progenitor cells promotes re-endothelialization of the injured carotid artery after balloon injury in New Zealand white rabbits. Chin Med J (Engl) 126:1480–1485
Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, Lo WY, Wu TC, Sata M, Chen JW, Lin SJ (2009) Matrix metalloproteinase−9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 29:1179–1184
Huang H, Huang F, Huang JP (2013a) Transplantation of bone marrowderived endothelial progenitor cells overexpressing Deltalike4 enhances functional neovascularization in ischemic myocardium. Mol Med Rep 8:1556–1562
Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y (2013b) CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke 44:190–197
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293
Hur J, Yoon CH, Lee CS, Kim TY, Oh IY, Park KW, Kim JH, Lee HS, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS (2007) Akt is a key modulator of endothelial progenitor cell trafficking in ischemic muscle. Stem Cells 25:1769–1778
Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760
Ishikawa M, Asahara T (2004) Endothelial progenitor cell culture for vascular regeneration. Stem Cells Dev 13:344–349
Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NR, Bagher-Ebadian H, Ali MM, Arbab AS, Janic B (2013) Intravenous administration of human umbilical cord blood-derived AC133 + endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl Med 2:703–714
Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW (2006) Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase−9. Circulation 113:1605–1614
Jiang M, Wang B, Wang C, He B, Fan H, Shao Q, Gao L, Liu Y, Yan G, Pu J (2008) In vivo enhancement of angiogenesis by adenoviral transfer of HIF−1alpha-modified endothelial progenitor cells (Ad-HIF−1alpha-modified EPC for angiogenesis). Int J Biochem Cell Biol 40:2284–2295
Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, Logvinova A, Banwait S, Greenberg DA (2005) Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis 18:366–374
Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T (2000a) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 97:3422–3427
Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T (2000b) Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86:1198–1202
Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, Katayama Y (2008) Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci 83:433–437
Kaneko Y, Tajiri N, Shinozuka K, Glover LE, Weinbren NL, Cortes L, Borlongan CV (2012) Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr Pharm Des 18:3731–3734
Karam JG, Loney-Hutchinson L, McFarlane SI (2008) High-dose atorvastatin after stroke or transient ischemic attack: tThe sStroke pPrevention by aAggressive rReduction in cCholesterol lLevels (SPARCL) iInvestigators. J Cardiometab Syndr 3:68–69
Khakoo AY, Finkel T (2005) Endothelial progenitor cells. Annu Rev Med 56:79–101
Kirton JP, Xu Q (2010) Endothelial precursors in vascular repair. Microvasc Res 79:193–199
Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R (2011) Interleukin−10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res 109:1280–1289
Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T (2010) Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion. J Histochem Cytochem 58:687–694
Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau CP, Kwong YL, Tse HF (2007) Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens 21:445–451
Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, Chung JW, Hur J, Yoon CH, Park KW, Oh BH, Park YB, Kim HS (2006) Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation 114:150–159
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28:1099–1106
Lee TS, Kou YR (2012) Enhancing endothelial progenitor cell therapy for critical limb ischemia by extracorporeal shock wave. Crit Care Med 40:332–333
Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA (1999) Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 13:450–464
Li A, Dubey S, Varney ML, Dave BJ, Singh RK (2003) IL−8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170:3369–3376
Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Lu M, Ewing JR, Chopp M (2010) Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab 30:653–662
Li Q, Tang G, Xue S, He X, Miao P, Li Y, Wang J, Xiong L, Wang Y, Zhang C, Yang GY (2013) Silica-coated superparamagnetic iron oxide nanoparticles targeting of EPCs in ischemic brain injury. Biomaterials 34:4982–4992
Liao YF, Chen LL, Zeng TS, Li YM, Fan Y, Hu LJ, Ling Y (2010) Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med 15:279–285
Lin Y, Weisdorf DJ, Solovey A, Hebbel RP (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105:71–77
Liu F, Yuan R, Benashski SE, McCullough LD (2009) Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 29:792–802
Liu H, Wu R, Jia RP, Zhong B, Zhu JG, Yu P, Zhao Y, Ge YZ, Wu JP (2013a) Ischemic preconditioning increases endothelial progenitor cell number to attenuate partial nephrectomy-induced ischemia/reperfusion injury. PLoS ONE 8:e55389
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Chen J (2013b) Cell based therapies for ischemic stroke: fFrom basic science to bedside. Progress Neurobiol 115:92–115ogy.
Liu Z, Ding X, Fang F, Wang R, Chen Y, Ma Y, Zhang G, Kang X (2013c) Higher numbers of circulating endothelial progenitor cells in stroke patients with intracranial arterial stenosis. BMC Neurol 13:161
Long J, Wang S, Zhang Y, Liu X, Zhang H (2013) The therapeutic effect of vascular endothelial growth factor gene- or heme oxygenase−1 gene-modified endothelial progenitor cells on neovascularization of rat hindlimb ischemia model. J Vasc Surg 58(756–765):e752
Machein MR, Renninger S, de Lima-Hahn E, Plate KH (2003) Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol 13:582–597
Mandraffino G, Sardo MA, Riggio S, Loddo S, Imbalzano E, Alibrandi A, Saitta C, Cinquegrani M, Mormina EM, Saitta A (2011) Circulating progenitor cells are increased in newly diagnosed untreated hypertensive patients with arterial stiffening but normal carotid intima-media thickness. Hypertens Res 34:876–883
Marti-Fabregas J, Crespo J, Delgado-Mederos R, Martinez-Ramirez S, Pena E, Marin R, Dinia L, Jimenez-Xarrie E, Fernandez-Arcos A, Perez-Perez J, Querol L, Suarez-Calvet M, Badimon L (2013) Endothelial progenitor cells in acute ischemic stroke. Brain Behav 3:649–655
Masuda H, Asahara T (2003) Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res 58:390–398
Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW (2010) Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 3:18
Medina RJ, O’Neill CL, O’Doherty TM, Wilson SE, Stitt AW (2012) Endothelial progenitors as tools to study vascular disease. Stem Cells Int 2012:346735
Misra V, Ritchie MM, Stone LL, Low WC, Janardhan V (2012) Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology 79:S207–212
Mithal DS, Banisadr G, Miller RJ (2012) CXCL12 signaling in the development of the nervous system. J Neuroimmune Pharmacol 7:820–834
Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, Garcia-Solis D, Cayuela A, Montaner J, Boada C, Rosell A, Jimenez MD, Mayol A, Gil-Peralta A (2012) Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke 43:2242–2244
Morancho A, Hernandez-Guillamon M, Boada C, Barcelo V, Giralt D, Ortega L, Montaner J, Rosell A (2013) Cerebral ischaemia and matrix metalloproteinase−9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells. J Cell Mol Med 17(12):1543–1553
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67:181–198
Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L, Foucault-Bertaud A, Velly L, Dignat-George F, Pisano P (2011) Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev 7:208–220
Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C (2002) Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 30:967–972
Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribo M, Alvarez-Sabin J, Montaner J (2010) Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res 80:317–323
Navarro-Sobrino M, Hernandez-Guillamon M, Fernandez-Cadenas I, Ribo M, Romero IA, Couraud PO, Weksler BB, Montaner J, Rosell A (2013) The angiogenic gene profile of circulating endothelial progenitor cells from ischemic stroke patients. Vasc Cell 5:3
Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova-Rainon T, Pocard M, Margaill I, Kubis N (2012) Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur J Neurosci 35:1208–1217
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V (2007) Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 21:1546–1558
Ogle ME, Gu X, Espinera AR, Wei L (2012) Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor−1alpha. Neurobiol Dis 45:733–742
Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, Kim HS (2007) Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 110:3891–3899
Ohta T, Kikuta K, Imamura H, Takagi Y, Nishimura M, Arakawa Y, Hashimoto N, Nozaki K (2006) Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery 59:679-686 (discussion 679–686)
Paczkowska E, Golab-Janowska M, Bajer-Czajkowska A, Machalinska A, Ustianowski P, Rybicka M, Klos P, Dziedziejko V, Safranow K, Nowacki P, Machalinski B (2013) Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin−1. J Neurol Sci 325:90–99
Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494
Pasquier E, Dias S (2010) Endothelial progenitor cells: hope beyond controversy. Curr Cancer Drug Targets 10:914–921
Patschan D, Hildebrandt A, Rinneburger J, Wessels JT, Patschan S, Becker JU, Henze E, Kruger A, Muller GA (2012) The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am J Physiol Renal Physiol 302:F1305–1312
Payen E, Leboulch P (2012) Advances in stem cell transplantation and gene therapy in the beta-hemoglobinopathies. Hematology Am Soc Hematol Educ Program 2012:276–283
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR−2 and AC133 by circulating human CD34( + ) cells identifies a population of functional endothelial precursors. Blood 95:952–958
Pellegrini L, Bennis Y, Guillet B, Velly L, Garrigue P, Sabatier F, Dignat-George F, Bruder N, Pisano P (2013) Therapeutic benefit of a combined strategy using erythropoietin and endothelial progenitor cells after transient focal cerebral ischemia in rats. Neurol Res 35:937–947
Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, De A, Choi R, Chen S, Rutt BK, Gambhir SS, Guzman R (2010) Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke 41:2064–2070
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109:656–663
Ponio JB, El-Ayoubi F, Glacial F, Ganeshamoorthy K, Driancourt C, Godet M, Perriere N, Guillevic O, Couraud PO, Uzan G (2014) Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS ONE 9:e84179
Qiu J, Li W, Feng S, Wang M, He Z (2013) Transplantation of bone marrow-derived endothelial progenitor cells attenuates cerebral ischemia and reperfusion injury by inhibiting neuronal apoptosis, oxidative stress and nuclear factor-kappaB expression. Int J Mol Med 31:91–98
Rafii S (2000) Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest 105:17–19
Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169
Rosell A, Morancho A, Navarro-Sobrino M, Martinez-Saez E, Hernandez-Guillamon M, Lope-Piedrafita S, Barcelo V, Borras F, Penalba A, Garcia-Bonilla L, Montaner J (2013) Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS ONE 8:e73244
Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, Tervaert JW, Lodder J (2008) Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke 39:2158–2165
Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Theunissen RO, Knottnerus IL, Staals JE, Delanghe JR, Tervaert JW, Lodder J (2009) Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res 6:32–41
Rouhl RP, Mertens AE, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Henskens LH, Kroon AA, de Leeuw PW, Lodder J, Tervaert JW (2012) Angiogenic T-cells and putative endothelial progenitor cells in hypertension-related cerebral small vessel disease. Stroke 43:256–258
Rubattu S, Giliberti R, Volpe M (2000) Etiology and pathophysiology of stroke as a complex trait. Am J Hypertens 13:1139–1148
Ruscher K, Kuric E, Liu Y, Walter HL, Issazadeh-Navikas S, Englund E, Wieloch T (2013) Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab 33:1225–1234
Santhanam AV, Smith LA, He T, Nath KA, Katusic ZS (2007) Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase−2. Circ Res 100:1379–1388
Schuh A, Kroh A, Konschalla S, Liehn EA, Sobota RM, Biessen EA, Bot I, Tolga Taha S, Schober A, Marx N, Weber C, Sasse A (2012) Myocardial regeneration by transplantation of modified endothelial progenitor cells expressing SDF−1 in a rat model. J Cell Mol Med 16:2311–2320
Sen S, Merchan J, Dean J, Ii M, Gavin M, Silver M, Tkebuchava T, Yoon YS, Rasko JE, Aikawa R (2010) Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor−1 gene after myocardial infarction. Hum Gene Ther 21:1327–1334
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92:362–367
Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103:2776–2779
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802:80–91
Sirker AA, Astroulakis ZM, Hill JM (2009) Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci (Lond) 116:283–299
Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Davalos A, Lizasoain I, Castillo J (2007) The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 38:2759–2764
Sobrino T, Rodriguez-Gonzalez R, Blanco M, Brea D, Perez-Mato M, Rodriguez-Yanez M, Leira R, Castillo J (2011) CDP-choline treatment increases circulating endothelial progenitor cells in acute ischemic stroke. Neurol Res 33:572–577
Sobrino T, Blanco M, Perez-Mato M, Rodriguez-Yanez M, Castillo J (2012a) Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. Eur J Neurol 19:1539–1546
Sobrino T, Perez-Mato M, Brea D, Rodriguez-Yanez M, Blanco M, Castillo J (2012b) Temporal profile of molecular signatures associated with circulating endothelial progenitor cells in human ischemic stroke. J Neurosci Res 90:1788–1793
Song MB, Yu XJ, Zhu GX, Chen JF, Zhao G, Huang L (2009) Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol 51:205–213
Suarez-Monteagudo C et al (2009) Autologous bone marrow stem cell neurotransplantation in stroke patients. an open study. Restor Neurol Neurosci 27:151–161
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438
Takamiya M, Okigaki M, Jin D, Takai S, Nozawa Y, Adachi Y, Urao N, Tateishi K, Nomura T, Zen K, Ashihara E, Miyazaki M, Tatsumi T, Takahashi T, Matsubara H (2006) Granulocyte colony-stimulating factor-mobilized circulating c-Kit + /Flk−1 + progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol 26:751–757
Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR (2002) Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157:571–577
Tan Z, Zhou LJ, Li Y, Cui YH, Xiang QL, Lin GP, Wang TH (2012) E(2)-BSA activates caveolin−1 via PI(3)K/ERK1/2 and lysosomal degradation pathway and contributes to EPC proliferation. Int J Cardiol 158:46–53
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC (2002) Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106:2781–2786
Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC (2005) Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105:1068–1077
Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38:3032–3039
Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B (2007) Endothelial outgrowth cells are not derived from CD133 + cells or CD45 + hematopoietic precursors. Arterioscler Thromb Vasc Biol 27:1572–1579
Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J (2009) Endothelial progenitor cells: identity defined? J Cell Mol Med 13:87–102
Tsai NW, Hung SH, Huang CR, Chang HW, Chang WN, Lee LH, Wang HC, Lin YJ, Lin WC, Cheng BC, Chiang YF, Su YJ, Tsai TR, Lu CH (2014) The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta 427:6–10
Ueno H, Koyama H, Mima Y, Fukumoto S, Tanaka S, Shoji T, Emoto M, Nishizawa Y, Inaba M (2011) Comparison of the effect of cilostazol with aspirin on circulating endothelial progenitor cells and small-dense LDL cholesterol in diabetic patients with cerebral ischemia: a randomized controlled pilot trial. J Atheroscler Thromb 18:883–890
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S (2005) Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39:733–742
Vale PR, Isner JM, Rosenfield K (2001) Therapeutic angiogenesis in critical limb and myocardial ischemia. J Interv Cardiol 14:511–528
van der Strate BW, Popa ER, Schipper M, Brouwer LA, Hendriks M, Harmsen MC, van Luyn MJ (2007) Circulating human CD34 + progenitor cells modulate neovascularization and inflammation in a nude mouse model. J Mol Cell Cardiol 42:1086–1097
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Vaughan EE, O’Brien T (2012) Isolation of circulating angiogenic cells. Methods Mol Biol 916:351–356
Virgintino D, Errede M, Rizzi M, Girolamo F, Strippoli M, Walchli T, Robertson D, Frei K, Roncali L (2013) The CXCL12/CXCR4/CXCR7 ligand-receptor system regulates neuro-glio-vascular interactions and vessel growth during human brain development. J Inherit Metab Dis 36:455–466
Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW (2008) Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39:1569–1574
Wang F, Xue J, Wang D, Wang X, Lu S, Tan M (2010) Treatment of atherosclerosis by transplantation of bone endothelial progenitor cells over-expressed paraoxonase−1 gene by recombinant adeno-associated virus in rat. Biol Pharm Bull 33:1806–1813
Wasserman JK, Yang H, Schlichter LC (2008) Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci 28:1316–1328
Wassmann S, Werner N, Czech T, Nickenig G (2006) Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 99:e74–e83
Wei H, Mao Q, Liu L, Xu Y, Chen J, Jiang R, Yin L, Fan Y, Chopp M, Dong J, Zhang J (2011a) Changes and function of circulating endothelial progenitor cells in patients with cerebral aneurysm. J Neurosci Res 89:1822–1828
Wei HJ, Wang D, Chen JL, Xu Y, Lei P, Jiang RC, Liu L, Dong JF, Zhang JN (2011b) Mobilization of circulating endothelial progenitor cells after endovascular therapy for ruptured cerebral aneurysms. Neurosci Lett 498:114–118
Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L (2013) Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 22:977–991
Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, Basilion JP (2000) In vivo magnetic resonance imaging of transgene expression. Nat Med 6:351–355
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999–1007
Westenbrink BD, Oeseburg H, Kleijn L, van der Harst P, Belonje AM, Voors AA, Schoemaker RG, de Boer RA, van Veldhuisen DJ, van Gilst WH (2008) Erythropoietin stimulates normal endothelial progenitor cell-mediated endothelial turnover, but attributes to neovascularization only in the presence of local ischemia. Cardiovasc Drugs Ther 22:265–274
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC (2013) The blood-brain barrier: an engineering perspective. Front Neuroeng 6:7.
Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ (2006) Essential role of ICAM−1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res 99:315–322
Xu Y, Tian Y, Wei HJ, Chen J, Dong JF, Zacharek A, Zhang JN (2011) Erythropoietin increases circulating endothelial progenitor cells and reduces the formation and progression of cerebral aneurysm in rats. Neuroscience 181:292–299
Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell-derived factor−1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107:1322–1328
Yang HN, Park JS, Woo DG, Jeon SY, Park KH (2012) Transfection of VEGF(165) genes into endothelial progenitor cells and in vivo imaging using quantum dots in an ischemia hind limb model. Biomaterials 33:8670–8684
Yi C, Xia W, Zheng Y, Zhang L, Shu M, Liang J, Han Y, Guo S (2006) Transplantation of endothelial progenitor cells transferred by vascular endothelial growth factor gene for vascular regeneration of ischemic flaps. J Surg Res 135:100–106
Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW (2008) Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke 39:69–74
Yip HK, Tsai TH, Lin HS, Chen SF, Sun CK, Leu S, Yuen CM, Tan TY, Lan MY, Liou CW, Lu CH, Chang WN (2011) Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care 15:R40
Yoder MC (2009) Defining human endothelial progenitor cells. J Thromb Haemost 7(Suppl 1):49–52
Yoder MC (2012) Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2(7):a006692
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809
Yoo CH, Na HJ, Lee DS, Heo SC, An Y, Cha J, Choi C, Kim JH, Park JC, Cho YS (2013) Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials 34:8149–8160
Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS (2005) Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 112:1618–1627
Yoon CH, Hur J, Oh IY, Park KW, Kim TY, Shin JH, Kim JH, Lee CS, Chung JK, Park YB, Kim HS (2006) Intercellular adhesion molecule−1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 26:1066–1072
Yu SP, Wei Z, Wei L (2013) Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 4:76–88
Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C (2008) Ex vivo priming of endothelial progenitor cells with SDF−1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 28:644–650
Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S (2006) Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133:1543–1551
Zhan K, Bai L, Xu J (2013) Role of vascular endothelial progenitor cells in construction of new vascular loop. Microvasc Res 90:1–11
Zhang ZG, Zhang L, Jiang Q, Chopp M (2002) Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res 90:284–288
Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K (2012) Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res 1489:104–112
Zhao YH, Yuan B, Chen J, Feng DH, Zhao B, Qin C, Chen YF (2013) Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther 19:67–75
Zhou WJ, Zhu DL, Yang GY, Zhang Y, Wang HY, Ji KD, Lu YM, Gao PJ (2009) Circulating endothelial progenitor cells in Chinese patients with acute stroke. Hypertens Res 32:306–310
Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Chen JZ (2008) Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant 12:650–655
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Conclusions
Conclusions
Preclinical studies have shown great promise for EPC transplantation as a therapy for stroke. Beneficial effects from EPC transplantation have been observed, including functional improvement, increased neovascularization and decreased apoptotic cells. However, there are many fundamental unsolved problems, mentioned above, and relevant clinical trials are needed. A guideline called Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) in 2009 (The STEPS Participants, 2009) has been drawn up. To some extent, this guideline can help scientists in all fields collaborate to accelerate the use of EPC transplantation in clinical patients for those who might benefit from EPC therapy. Currently, EPC transplantation for stroke treatment in clinic is only a vision, preclinical studies and clinical research are needed to maximize the therapeutic benefit and minimize the risks of EPCs transplantation in stroke.
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Li, Y., Ma, Y., Wang, Y., Yang, GY. (2015). Endothelial Progenitor Cell Therapy in Stroke. In: Zhao, LR., Zhang, J. (eds) Cellular Therapy for Stroke and CNS Injuries. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-11481-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-11481-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11480-4
Online ISBN: 978-3-319-11481-1
eBook Packages: MedicineMedicine (R0)