Abstract
Stroke is the 4th leading cause of death and the leading cause of severe long-term disability worldwide, with no effective treatment for most cases. The development of new effective therapies is needed to improve functional neurological recovery in stroke patients. Researches in experimental stroke in animal models over the past decade demonstrate that ischemic stroke enhances endogenous neural stem cells proliferation in SVZ and SGZ and promotes SVZ NSCs migration to the ischemic infarct site, differentiation into functional mature neurons. Ischemic injury triggers endogenous neural stem cell proliferation by a variety of growth factors, morphogens and neurotransmitters. Neuroblast migration occurs through SDF-1, MCP-1, MMP production and through association with vasculature and endothelial cells. These promising findings in stroke have brought hope to the development of neurorestorative therapy which aims to enhance endogenous neurogenesis after ischemic stroke and thereby contribute to the functional recovery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stroke is the 4th leading cause of death in the United States and the leading cause of severe long-term disability worldwide and the number of patients suffered from stroke is on the increase at present, with no effective treatment for most cases. Currently, thrombolysis with recombinant tissue plasminogen activator (rt-PA) remains the only FDA approved treatment for acute ischemic stroke, but the short therapeutic time window makes this treatment applicable to only a minority of stroke patients (Brott and Bogousslavsky 2000). Therefore, the development of new effective therapies is needed to improve functional neurological recovery in stroke patients.
Endogenous Neural stem cells (NSCs) resident in the brain neurogenic regions raise the possibility of developing neural repair strategies for stroke . Researches in experimental stroke in animal models over the past decade have demonstrated that ischemic stroke enhances endogenous neural stem cells proliferation in SVZ and SGZ of dentate gyrus and promotes SVZ NSC migration to the ischemic infarct regions (Jin et al. 2001; Thored et al. 2006). Moreover, stroke-induced neurogenesis has also been reported in the adult human brain (Jin et al. 2006). These promising findings in stroke have brought hope to develop neurorestorative therapy which aims to enhance endogenous neurogenesis after stroke and thereby contribute to the functional recovery. This chapter aims to provide an understanding the role of endogenous neural stem cells in stroke and to describe current knowledge of how stroke induces endogenous neural stem cells proliferation , migration and differentiation .
2 Proliferation of Endogenous Neural Stem Cells After Stroke
It is well accepted that NSCs are primarily present in the adult SVZ and SGZ of dentate gyrus. Based on the morphology, protein expression, proliferation kinetics, and differentiation potential, three types of SVZ cells have been identified: type A (neuroblasts), type B (GFAP-positive progenitors) and type C (transit amplifying cells) (Zhao et al. 2008). GFAP, Nestin and Sox2 -positive type B cells are considered as the primary NSCs in the SVZ. This population resides in the wall of lateral ventricle and gives rise to actively dividing type C cells. Type C cells, which are negative for GFAP but express Mash1 and EGF receptor, generate type A cells that migrate into the olfactory bulb via the rostral migratory stream (RMS). In the SGZ of dentate gyrus, two types of NSCs, type 1(GFAP+SOX2+) and type 2(GFAP-) NSCs are present, maintaining a reciprocal relationship in the DG of hippocampus (Suh et al. 2007).
Proliferation is the first step of endogenous NSCs’ response to ischemic stroke injury. It is reported that ischemic injury alone is sufficient to promote the proliferation of endogenous NSCs and thereby expands the NSCs pool (Zhang et al. 2007). The expansion of the NSCs pool is an essential factor in the development of endogenous neurorestorative strategies after stroke . Stroke induces early expansion of the NSCs pool by increasing the proportion of proliferating cells and shortening the length of cell cycle (Zhang et al. 2008b). In the adult rat brain, the proportion of actively dividing SVZ NSCs is about 15–21 % and the length of cell cycle of this population is approximately 18–21 h (Zhang et al. 2006). Stroke increases the proportion of proliferating SVZ NSCs, starting 2 days (24 %) and reaches a maximum at 7 days (31 %) after stroke. Two weeks after stroke, the level of proliferation returns to the baseline. Concurrently, stroke changes the cell cycle length of NSCs. The length of cell cycle shortens to 11 h at 2 days after stroke, which is significantly shorter than cell cycle length of 18–21 h in normal brain. Alteration of the G1 phase of SVZ NSCs may contribute to stroke-induced changes of the cell cycle length. Furthermore, studies by Zhang and his colleagues also revealed that stroke transiently switches NSCs division from asymmetric to symmetric (Zhang et al. 2004b). Symmetric division gives rise to two identical daughter cells that stay in the SVZ to maintain the NSCs pool, whereas asymmetric division generates two different daughter cells and the basal daughter becomes a young migratory cell that migrates away. The switching NSCs division from asymmetric to symmetric amplifies the pool of NSCs.
Mediators of Stroke-Induced NSCs Proliferation
Although little is known about the exact molecular mechanisms underlying the regulation of endogenous NSC proliferation after stroke, several potential mediators are beginning to be identified. Here, we review current data on growth factors, morphogens and neurotransmitters that are involved in the regulation of endogenous NSC proliferation after stroke.
2.1 Growth Factors and Neurotrophic Factors
2.1.1 FGF-2
FGF-2 is a well-known growth factor that plays a role in neurogenesis in the adult brain. Studies have reported that FGF-2 expression in the brain increased significantly after ischemic stroke (Naylor et al. 2005). Overexpression of FGF-2 significantly increased the proliferation of progenitor cells after ischemic stroke in both FGF-2 deficient mice and wild-type mice (Yoshimura et al. 2001). Conversely, FGF-2 knockout mice showed a reduction of ischemia-induced progenitor proliferation when compared with wild type mice. Moreover, administration of bone marrow stromal cells engineered to produce FGF-2 (Ikeda et al. 2005) has been shown to decrease infarct size. Taken together, these findings suggest that FGF-2 plays a role in NPC proliferation and neuroprotection after ischemic stroke . Several potential mechanisms underlying the neurogenic effect of FGF-2 in the ischemic injured brain have been presented. These include upregulation of BDNF, induction of GDNF, and downregulation of the NMDA receptor (Mattson et al. 1993; Lenhard et al. 2002; Kiprianova et al. 2004).
2.1.2 EGF
EGF, a known mitogen involved in the proliferation of adult NPCs, has an effect similar to FGF-2 in regulating endogenous NSC proliferation after stroke. Importantly, the expression of EGF-receptor on type C cells or TAPs was found to be increased after ischemic stroke (Ninomiya et al. 2006). Ischemia also causes an up-regulation of Heparin binding EGF (HB-EGF) (Tanaka et al. 2004), which is known to act through the EGF receptor to promote neurogenesis. Previous studies have demonstrated that exogenous EGF administration in ischemic animals rescued 20 % of the interneurons that would have died after ischemia , suggesting the neurogenic role of EGF in the adult brain after ischemic injury (Teramoto et al. 2003). Furthermore, infusion of EGF together with FGF-2 into the brain of adult rats was found to promote DG and SVZ NPC proliferation after focal ischemic stroke (Nakatomi et al. 2002; Tureyen et al. 2005). Administration of HB-EGF enhanced postischemic neurogenesis and contributed to the improvement of functional recovery (Jin et al. 2004). Overexpression of HB-EGF by viral delivery also led to a significant improvement in neurological function after ischemic stroke, which was attributed to increased neurogenesis by HB-EGF (Sugiura et al. 2005).
2.1.3 IGF-1
IGF-1, primarily produced in the liver, plays a major role in brain development. Several studies have demonstrated that focal ischemia significantly increases IGF-1 expression, its receptor and binding proteins (Yan et al. 2006). Blockage of IGF-1 by intracerebroventricular administration of IGF-1 antibodies resulted in a significant inhibition of neural progenitor proliferation induced by ischemic stroke , suggesting that IGF-1 regulates neurogenesis after ischemia . Conversely, administering IGF-1 after ischemic stroke promoted neurogenesis (Dempsey et al. 2003; Zhang et al. 2004a) and reduced neuronal loss (Brywe et al. 2005). In vitro studies demonstrated that IGF-1 stimulated the proliferation of cultured NPCs via phosphorylation of the PI-3-kinase/Akt signaling pathway (Kalluri et al. 2007). Furthermore, IGF-1 also enhanced glycogen synthase kinase phosphorylation, suggesting its involvement in NPC survival (Kalluri et al. 2007).
2.1.4 VEGF
Vascular endothelial growth factor (VEGF) is the major angiogenetic growth factor, which can induce angiogenesis and vasculogenesis through interaction with the VEGF receptor on endothelial cells. Our previous study revealed that VEGF promotes the proliferation of NSCs both in vitro and in the adult rat brain (Jin et al. 2002). Wang et al. showed that VEGF overexpression in transgenic mice greatly promoted ischemia-induced neurogenesis (Wang et al. 2007). Furthermore, intravenous administration of VEGF after stroke promoted angiogenesis in the ischemic penumbra and improved neurological performance. In addition, we previously showed that VEGF ICV administration to rats after focal ischemic stroke reduced infarct volume and resulted in enhanced neurological recovery suggesting that VEGF induces neurogenesis and neuroprotection after ischemic stroke (Sun et al. 2003) .
2.1.5 BDNF
BDNF, one of the most extensively studied neurotrophic factors, is necessary for NSC proliferation and differentiation in the adult brain. Two research groups have revealed that the expression of BDNF and its receptor increased after ischemic stroke (Arai et al. 1996; Kokaia et al. 1998). Intrastriatal infusion of BDNF before ischemia in adult rats increased the survival of neurons in the dorsolateral side of the striatum and resulted in improved functional recovery (Andsberg et al. 2002). Furthermore, infusion of human mesenchymal stem cells expressing the BDNF gene after ischemic stroke greatly reduced the infarct volume. Consistent with these observations, knockout of BDNF in mice resulted in larger infarct volumes after MCAO as the inhibition of endogenous BDNF after ischemic injury may decrease the survival of neurons (Endres et al. 2000; Larsson et al. 2002).
2.2 Notch Signaling
Notch signaling is involved in neurogenesis in the neurogenic regions of the intact adult brain. Recent studies have documented that expression of Notch and Hes1 in SVZ NPCs significantly is increased after stroke (Zhang et al. 2008a). Inhibition of the Notch signaling pathway with siRNA or γ-secretase inhibitor blocked stroke-induced neurogenesis. Moreover, an in vivo study demonstrated that administration of Notch ligand delta-like 4 (Dll4) together with FGF-2 after stroke significantly increased the rate of proliferation of SVZ neural progenitor cells (Androutsellis-Theotokis et al. 2006). The same study also revealed that the Notch pathway interacts with the Shh pathway in regulating NSCs (Balordi and Fishell 2007; Angot et al. 2008).
2.3 Shh and Wnt Signalings
The Shh signaling pathway is involved in the proliferation and maintenance of NSCs in the adult brain. It is also associated with EPO in mediating adult neurogenesis . EPO is known to regulate neurogenesis in both the adult normal and ischemic brains through its receptor EPOR in the adult SVZ. Infusion of EPO significantly increased ischemia-induced neurogenesis whereas blockage of the Shh pathway with cyclopamine or siRNA significantly suppressed EPO-increased neurogenesis (Liu et al. 2007). Wnt signaling promotes proliferation and neuronal differentiation in adult hippocampal progenitor cells in the DG (Lie et al. 2005). Expression of Wnt and BMP family genes in SVZ NSCs of adult rodents were altered after stroke. However, how these genes regulate the proliferation of endogenous NSCs after stroke remains to be determined (Morris et al. 2007).
2.4 Neurotransmitters
Neurotransmitters released from nerve terminals have been demonstrated to promote NPC proliferation in the adult brains. Studies have also shown the involvement of the glutamate signaling pathway in post-ischemic NSC proliferation. Abnormal glutamatergic neurotransmission, in particular disrupted glutamatergic receptor expression, has been reported to play a significant role in neuronal death after ischemic stroke . Several studies have revealed that activation of the NMDA receptor, an ionotropic glutamate receptor, blocks proliferation of NPCs while inhibition of NMDA receptors via antagonists promotes NPC proliferation (Cameron et al. 1995; Nacher et al. 2003). A subsequent study conducted by Kluska et al. also reported that administration of an NMDA antagonist during brain ischemic stroke induced by photothrombosis resulted in enhanced neurogenesis in the hippocampus of rats (Kluska et al. 2005). However, Bernabeu and Sharp reported that administration of NMDA and AMPA receptor antagonists prevented stroke-induced neurogenesis in the SGZ after transient global ischemia (Bernabeu and Sharp 2000). These conflicting results may be attributed to species-specific differences and the different models of ischemic stroke. The mechanism by which glutamatergic signaling pathway mediate stroke-induced neurogenesis is unclear. Several other neurotransmitters such as GABA and dopamine have also been reported to play significant roles in the intact adult brain. However, their roles in mediating ischemic stroke-induced neurogenesis still need further evaluation.
3 Migration of Endogenous Neural Stem Cells After Stroke
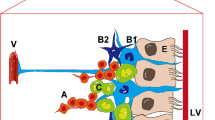
In order for proliferating NSCs to contribute to the functional recovery, it is necessary for these NSCs to migrate from birthplace to the ischemic region. In the normal adult brain, the SVZ neuroblasts are destined to follow rostral migratory stream to migrate to the olfactory bulb. However, many of these SVZ neuroblasts migrate from the SVZ through the brain parenchyma into the ischemic injury region after ischemic stroke, as revealed by several studies (Arvidsson et al. 2002; Jin et al. 2003; Thored et al. 2006). This redirected migration is associated with cellular interactions between immature migrating neuroblasts, astrocytic processes and blood vessels (Yamashita et al. 2006). However, ischemic stroke also up-regulate inhibitory molecules such as chondroitin sulfate proteoglycans (CSPGs), which blocks neuroblast migration (Carmichael 2005). Stroke not only up-regulates attractive factors for neuroblast migration but also forms peri-infarct scar and produces barrier molecule to inhibit neuroblast migration. The net neuroblast migration is dependent on the balance of inhibitory molecules and attractive factors.
The mechanism underlying stroke-induced redirected migration is unclear. Several receptor-ligand signaling pathways and molecular factors involved in the stroke-induced endogenous NSCs migration have been identified. These include: stromal cell-derived factor 1 (SDF-1) and CXC chemokine receptor 4 (CXCR4), monocyte chemoattractant protein-1 (MCP-1) and CC chemokine receptor 2 (CCR2), and matrix metalloproteases (MMP). Further, the neurovascular niche within SVZ and SDG is also reported to be closely associated with post-stroke neuroblasts migration.
3.1 SDF
SDF-1 (CXCL12), a member of the alpha chemokine family, has been demonstrated to play an essential role in the mobilization and homing of stem cells to bone marrow with its receptor CXCR4 (Hattori et al. 2003). A role of SDF-1 and CXCR4 in the directing migration of neuroblasts in brain has also been reported as well. Study by Robin et al. showed that CXCR 4 is expressed in the NPCs and migrating neuroblasts in stroke brain, while blocking SDF-1alpha by a neutralizing antibody against CXCR 4 significantly attenuated stroke-enhanced NPC migration, suggesting that SDF-1alpha generated in the stroke hemisphere may guide NPC migration towards the ischemic boundary via binding to its receptor CXCR 4 in the NPC (Robin et al. 2006). Moreover, Ohab et al. also demonstrated that intraventricular administration of SDF-1 in ischemic mice promote neuroblast migration after stroke and contribute to behavioral recovery (Ohab et al. 2006).
3.2 MCP-1
Monocyte chemoattractant protein-1 (MCP-1), a chemokine of the CC family, was previously shown to increase the migration of neural progenitors in vitro (Widera et al. 2004). It is also reported to plays a critical role in neuroblast migration after focal cerebral ischemia . Study by Yan et al. demonstrated that ischemic stroke in adult rats induces increase of MCP-1 expression in the activated microglia and astrocytes , which lasted for more than 3 days of reperfusion (Yan et al. 2007). This study also found that the migrating neuroblasts in the ischemic brain express the MCP-1 receptor CCR2, and there was a significant decrease in the number of migrating neuroblasts in MCP-1 and CCR2 knockout mice.
3.3 MMP
Matrix metalloproteinases (MMP), a family of proteinases, are known to play a role in extracellular matrix remodeling and cell migration. Recently, MMP have also been implicated in neuroblast migration from the SVZ. MMP-3 and −9 have been shown to express in NPCs, and inhibition of MMPs resulted in reduction in post-stroke neuroblast migration (Barkho et al. 2008). Furthermore, the expression of MMP on the vascular also contributes to the localization of neuroblasts after stroke (Wang et al. 2006). These findings suggest that neuroblasts may “digest” their way through the extracellular matrix as they migrate via secreting MMP. However, the detailed cellular and molecular mechanism underlying is unknown.
3.4 Neurovascular Niche
Neurogenesis is associated with angiogenesis in an environment termed the neurovascular niche within the SGZ and SVZ in the adult brain. In addition, the vasculature also appears to be closely tied to the migration of neuroblasts to ischemic injury regions . The vasculature promotes localization of migrating neuroblasts in the peri-infarct cortex after stroke. Many migrating neuroblasts are found to localize specifically to blood vessels in areas of active vascular sprouting and remodeling in the peri-infarct cortex region (Ohab et al. 2006; Thored et al. 2007). By blocking angiogenesis with endostatin, a direct inhibitor of post-ischemic angiogenesis, Ohab et al. demonstrated that angiogenesis and neurogenesis are causally linked within the post-stroke neurovascular niche (Ohab et al. 2006). One week of endostatin treatment resulted in a significant decrease in the number of new born endothelial cells and overall vascular density, leading to a 10-fold reduction in neuroblasts.
The mechanism by which angiogenic vascular mediate the migration and localization of neuroblasts in the peri-infarct region after stroke remain to be identified. Vasculature may provide a scaffold on which neuroblasts can migrate (Kojima et al. 2010), as the close association of migrating neuroblasts with vascular endothelial cells is observed. Vasculature not only provides a scaffold for the migration of neuroblasts, but also promotes neuroblsts migration via secreting various growth and chemotactic factors, including BDNF, MMPs, angiopoietins, and SDF-1. Another clue demonstrated the role of neurovascular niche in mediating post-stroke neuroblasts migration is that the processes of angiogenesis and neurogenesis share several similar molecular signaling in the peri-infarct neurovascular niche, such as ephrin/EphB signaling and Semaphorin 3α and its receptor neuropilin 1 (Suchting et al. 2006). Taken together, these suggest that neurovascular niche plays an essential role in mediating neuroblasts migration after ischemic stroke.
4 Differentiation and Neuronal Function
What is the fate of endogenous neuroblasts that migrate to peri-infarct region after ischemic stroke? Although a great quantity of neuroblasts reached the ischemic injury region, only few of them survived and differentiated into mature neurons. It is reported that most of neuroblasts which migrate to the peri-infarct region appear to die, perhaps from a failure to integrate or due to the inflammatory milieu (Arvidsson et al. 2002). In the DG of hippocampus , the stroke-induced neuroblasts migrate into GCL. Most of the surviving neuroblasts differentiate into calbindin or NeuN positive mature neurons by 3–4 weeks after ischemic stroke (Jin et al. 2003). Only a small number of the newly generated cells differentiate into GFAP positive astrocytes in the GCL of hippocampus (Zhu et al. 2003). In the SVZ, many of the surviving cells differentiate into neurons, but the precise nature of the neurons in the striatum is controversial. Some research groups reported that most of surviving neuroblasts differentiate into mature striatal neurons in adult rats after stroke (Arvidsson et al. 2002; Parent et al. 2002). However, study by Liu et al. found that the stroke-induced adult-born neurons exclusively differentiated into calretinin-expressing interneurons (Liu et al. 2009). The reasons for these disparate findings are not entirely clear. Further studies using transgenic methods to label adult-born neurons are therefore needed to address this question.
A number of literature supports the concept that increased neurogenesis is related to functional recovery (Kondziolka et al. 2000; Zhang et al. 2003). Whether these adult-born new neurons could replace lost cells by integrating into the surviving brain circuitry and contribute to functional recovery is unclear. Some studies support the functional integration of a small portion of newly generated neurons that migrate to the injured striatum after cerebral ischemia in adult brain (Hou et al. 2008). But whether this limited neurogenesis and functional integration contribute to functional improvement after stroke is uncertain. Arvidsson et al. (2002) doubted on the functional significance of post-stroke neurogenesis, as < 0.2 % of neurons destroyed after stroke were replaced in the infarcted striatum and an even smaller percentage in the infarcted cortex. Although interventions that are aimed at increasing neurogenesis have been shown to improve functional outcome, but these treatments are not specific to neurogenesis (Ohab et al. 2006; Leker et al. 2007). The causal link between neurogenesisand behavioral recovery after stroke remains to be demonstrated. And the approaches to resolve this issue are need in the near future. Using of transgenic technique such as inducible Cre to specifically knockout stroke-induce migrating neuroblasts after stroke could provide insight into the direct effect of neurogenesis on functional improvement.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- DG:
-
Dentate gyrus
- EGF:
-
Epidermal growth factor
- EPO:
-
Erythropoietin
- FGF-2:
-
Fibroblast growth factor-2
- GABA:
-
Gamma-aminobutyric acid
- GFAP:
-
Glial fibrillary acidic protein
- IGF-1:
-
Insulin-like growth factor-1
- MCP-1:
-
Monocyte chemoattractant protein-1
- MMP:
-
Matrix metalloproteinase
- NMDA:
-
N-Methyl-D-aspartate
- NPC:
-
Neural progenitor cells
- NSCs:
-
Neural stem cells
- rt-PA:
-
Recombinant tissue plasminogen activator
- SVZ:
-
Subventricular zone
- SGZ:
-
Subgranular zone
- SDF:
-
Stromal cell-derived factor
- VEGF:
-
Vascular endothelial growth factor
References
Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826
Andsberg G, Kokaia Z, Klein RL, Muzyczka N, Lindvall O, Mandel RJ (2002) Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol Dis 9:187–204
Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E (2008) Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells 26:2311–2320
Arai S, Kinouchi H, Akabane A, Owada Y, Kamii H, Kawase M, Yoshimoto T (1996) Induction of brain-derived neurotrophic factor (BDNF) and the receptor trk B mRNA following middle cerebral artery occlusion in rat. Neurosci Lett 211:57–60
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970
Balordi F, Fishell G (2007) Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci 27:5936–5947
Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X (2008) Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 26:3139–3149
Bernabeu R, Sharp FR (2000) NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab 20:1669–1680
Brott T, Bogousslavsky J (2000) Treatment of acute ischemic stroke. N Engl J Med 343:710–722
Brywe KG, Mallard C, Gustavsson M, Hedtjarn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H (2005) IGF-1 neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci 21:1489–1502
Cameron HA, McEwen BS, Gould E (1995) Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 15:4687–4692
Carmichael ST (2005) Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx 2:396–409
Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R (2003) Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem 87:586–597
Endres M, Fan G, Hirt L, Fujii M, Matsushita K, Liu X, Jaenisch R, Moskowitz MA (2000) Ischemic brain damage in mice after selectively modifying BDNF or NT4 gene expression. J Cereb Blood flow Metab 20:139–144
Hattori K, Heissig B, Rafii S (2003) The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma 44:575–582
Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, Yu Z, Sun FY (2008) Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke 39:2837–2844
Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake S (2005) Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke 36:2725–2730
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A 98:4710–4715
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 99:11946–11950
Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA (2003) Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 24:171–189
Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA (2004) Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 24:399–408
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA (2006) Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A 103:13198–13202
Kalluri HS, Vemuganti R, Dempsey RJ (2007) Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci 25:1041–1048
Kiprianova I, Schindowski K, von Bohlen O, Halbach O, Krause S, Dono R, Schwaninger M, Unsicker K (2004) Enlarged infarct volume and loss of BDNF mRNA induction following brain ischemia in mice lacking FGF-2. Exp Neurol 189:252–260
Kluska MM, Witte OW, Bolz J, Redecker C (2005) Neurogenesis in the adult dentate gyrus after cortical infarcts: effects of infarct location, N-methyl-D-aspartate receptor blockade and anti-inflammatory treatment. Neuroscience 135:723–735
Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K (2010) Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28:545–554
Kokaia Z, Andsberg G, Yan Q, Lindvall O (1998) Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol 154:289–301
Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55:565–569
Larsson E, Mandel RJ, Klein RL, Muzyczka N, Lindvall O, Kokaia Z (2002) Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol 177:1–8
Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD (2007) Long-lasting regeneration after ischemia in the cerebral cortex. Stroke 38:153–161
Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K (2002) Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol Cell Neurosci 20:181–197
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375
Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M (2007) Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab 27:564–574
Liu F, You Y, Li X, Ma T, Nie Y, Wei B, Li T, Lin H, Yang Z (2009) Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci 29:5075–5087
Mattson MP, Kumar KN, Wang H, Cheng B, Michaelis EK (1993) Basic FGF regulates the expression of a functional 71 kDa NMDA receptor protein that mediates calcium influx and neurotoxicity in hippocampal neurons. J Neurosci 13:4575–4588
Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, Liu XS, Chopp M (2007) Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett 418:170–174
Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS (2003) NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging 24:273–284
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429–441
Naylor M, Bowen KK, Sailor KA, Dempsey RJ, Vemuganti R (2005) Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochem Int 47:565–572
Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K (2006) Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett 403:63–67
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26:13007–13016
Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002) Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52:802–813
Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M (2006) Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab 26:125–134
Suchting S, Bicknell R, Eichmann A (2006) Neuronal clues to vascular guidance. Exp Cell Res 312:668–675
Sugiura S, Kitagawa K, Tanaka S, Todo K, Omura-Matsuoka E, Sasaki T, Mabuchi T, Matsushita K, Yagita Y, Hori M (2005) Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke 36:859–864
Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1:515–528
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843–1851
Tanaka R, Yamashiro K, Mochizuki H, Cho N, Onodera M, Mizuno Y, Urabe T (2004) Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke 35:1454–1459
Teramoto T, Qiu J, Plumier JC, Moskowitz MA (2003) EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest 111:1125–1132
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24:739–747
Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38:3032–3039
Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ (2005) EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery 57:1254–1263; discussion 1254–1263
Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M (2006) Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci 26:5996–6003
Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA (2007) VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res 85:740–747
Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C (2004) MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol 83:381–387
Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K (2006) Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci 26:6627–6636
Yan YP, Sailor KA, Vemuganti R, Dempsey RJ (2006) Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci 24:45–54
Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ (2007) Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab 27:1213–1224
Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA (2001) FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A 98:5874–5879
Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Arniego P, Ho KL, Chopp M (2003) Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol 53:259–263
Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M (2004a) Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res 1030:19–27
Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M (2004b) Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci 24:5810–5815
Zhang RL, Zhang ZG, Lu M, Wang Y, Yang JJ, Chopp M (2006) Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J Cereb Blood Flow Metab 26:857–863
Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M (2007) Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci 27:3157–3162
Zhang RL, Zhang ZG, Chopp M (2008a) Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology 55:345–352
Zhang RL, Zhang ZG, Roberts C, LeTourneau Y, Lu M, Zhang L, Wang Y, Chopp M (2008b) Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab 28:602–611
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Zhu DY, Liu SH, Sun HS, Lu YM (2003) Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci 23:223–229
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Conclusions
Conclusions
Ischemic stroke induces endogenous NSC proliferation within the SVZ, migration from the SVZ, and localization in the peri-infarct cortex and striatum. This whole process include three distinct spatiotemporal zones, each of them is associated with distinct molecular and cellular interactions. The mechanisms that trigger augmented endogenous NSC proliferation, migration and their differentiation into specific neural types after stroke remains to be identified. Ischemic injury triggers endogenous NSC proliferation by a variety of growth factors , morphogens and neurotransmitters. Neuroblast migration occurs through SDF-1, MCP-1, MMP production and through association with vasculature and endothelial cells. However, whether these neuroblasts could integrate into the surviving brain circuitry and contribute to functional recovery is controversial. Understanding the fundamental mechanisms underlying stroke-induced adult neurogenesis will thus provide the basis for endogenous NSC therapy for ischemic stroke.
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
ZhuGe, Q., Ruan, L., Jin, K. (2015). The Role of Endogenous Neural Stem Cells in Stroke. In: Zhao, LR., Zhang, J. (eds) Cellular Therapy for Stroke and CNS Injuries. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-11481-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-11481-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11480-4
Online ISBN: 978-3-319-11481-1
eBook Packages: MedicineMedicine (R0)