Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder; along with its well-known motor symptoms, it leads to cognitive impairment, behavioral symptoms, and autonomic dysfunction. Dementia is highly prevalent in PD (PD-D), especially in the advanced stages of the disease. The cognitive profile of dementia is characterized by predominant deficits in executive, visuospatial functions and attention. Apathy, depressive symptoms, visual hallucinations, and delusions are common behavioral symptoms. The main biochemical deficit is cholinergic, and cholinesterase inhibitors are the first choice treatment for PD-D. This chapter summarizes the epidemiology, clinical features, genetic background, neuropathology, neurochemistry, diagnosis, and management of PD-D.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mild Cognitive Impairment
- Dementia With Lewy Body
- Mattis Dementia Rate Scale
- Movement Disorder Society Task
- Global Outcome Scale
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder commonly presenting with motor symptoms. Current diagnostic criteria are based on motor symptoms and signs. Although the dominant features consist of motor symptoms, neuropathological changes in the nervous system are not confined to motor related areas, but more widespread. As a consequence, nonmotor symptoms are common in the course of the disease. Sleep disturbances, cognitive and behavioral changes, and autonomic dysfunction occur in the majority of the patients.
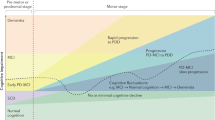
In early descriptions of the condition, cognitive changes and dementia had been largely under-recognized. Due to the advances in the treatment of motor symptoms and the subsequent increase in life expectancy, cognitive impairment and dementia in PD have become increasingly more recognized with substantially increased amount of research in this field. In the last 5 years operational diagnostic criteria have been published for both mild cognitive impairment (PD-MCI) and dementia in PD (PD-D), and more has been understood about the pathological and biochemical changes underlying cognitive changes and first specific treatment for PD-D became available.
In this chapter we will describe the epidemiological features, clinical characteristics, pathology, genetic background, diagnosis, and treatment of PD-D.
Epidemiology and Risk Factors
Both the prevalence and the incidence of cognitive impairment and dementia are substantially higher in patients with PD compared to age-matched controls. In a study in 115 newly diagnosed PD patients, 24 % displayed impaired performance on at least three neuropsychological tests and were classified as cognitively impaired [1]. A pooled analysis of data from eight centers using a standardized evaluation and comprising 1,346 non-demented PD patients showed that 25.8 % of patients had mild cognitive impairment according to the definition used [2]. In a community-based study in the UK, 36 % of newly diagnosed patients were found to have some degree of cognitive impairment at the time of diagnosis, while 57 % of this cohort developed cognitive deficits within 3.5 (±0.7) years. In the same cohort twenty-one incident dementia cases were identified over 5.2 years of follow-up, corresponding to a dementia incidence estimate of 38.7 per 1,000 person-years of observation [3]. At 10 years, cumulative probability of dementia increased to 46 %, and estimated dementia incidence was 54.7 per 1,000 person-years [4].
A systematic review of 12 selected studies on prevalence of PD-D revealed a point prevalence of 24–31 %. The prevalence of PD-D in the general population aged 65 years and over was calculated to be 0.3–0.5 %; 3–4 % of patients with dementia in the general population were estimated to be due to PD-D [5].
Incidence rates are more reliable than the prevalence rates to estimate the risk of dementia in PD, as demented patients are less likely to survive as compared to non-demented patients. The risk of dementia in PD was reported to vary between 1.7 and 5.9 compared to controls [6, 7]. In the longitudinally followed-up Sydney cohort, 48 % of surviving patients had developed dementia 15 years after the diagnosis [8], and the cumulative incidence had risen to 83 % 20 years after the diagnosis [9]. In another longitudinal study, 8-year cumulative prevalence of dementia was 78.2 %, with 26 % of cases being demented already at baseline [10]. The 12-year cumulative probability of dementia in this cohort was 60 % at the end of the follow-up period [11]. In the Rotterdam study, which employed a door-to-door survey, 15 % of the PD patients developed dementia compared to the 4.9 % of the control group, during a mean follow-up time of 4.3 years for the incident and 6.9 years for the prevalent PD group [7]. A recent study conducted in Olmsted County revealed that the incidence of PD-D was 2.5 per 100,000 person-years overall [12].
A number of risk factors have been associated with PD-D, as associated features in cross-sectional studies and as baseline characteristics predictive of dementia in prospective studies. Most consistent risk factors are old age at disease onset or at the time of evaluation, severe motor disability, presence of mild cognitive impairment at the time of diagnosis, and atypical neurological features such as early autonomic symptoms, symmetrical disease at presentation, and poor response to dopaminergic treatment including early development of confusion, hallucinations, or psychosis.
Both cross-sectional and prospective studies indicate advanced age as a prominent risk factor. In the Olmsted County study, the incidence of PD-D increased consistently with age, ranging from 0.6 in persons aged 50–59 years to 47.0 in persons aged 80–99 years [12]. Along with severe disease, patients with old age had a 12-fold increased dementia risk compared to young patients with mild disease demonstrating the combined effect of these risk factors [13].
Presence of mild cognitive impairment at the time of diagnosis appears to be a significant risk factor for dementia. In a community-based study in 182 incident PD patients monitored for 3 years, significantly more patients with MCI at baseline progressed to dementia compared to patients without cognitive impairment [14]. Neuropsychological features such as poor verbal fluency, poor performance on verbal memory, and impairment in executive functions at baseline were significantly associated with incident dementia [15]. In another study, however, impairment in cognitive tests relying on frontal executive functions was associated with a lower risk of dementia, whereas impairment in those tests tapping more posterior cortical functions was associated with a higher risk [3].
Dementia seems to be associated with postural instability and gait disorder phenotype (PIGD type). Tremor-dominant patients seem to have lower risk of developing dementia, unless they convert to PIGD form in their disease course [16]. Similar to constituting risk factors for dementia, PIGD phenotype and longer disease duration were also found to be risk factors for mild cognitive impairment [17].
Sleep-wake cycle disturbances such as excessive daytime sleepiness and rapid eye movement (REM) sleep behavior disorder (RBD) may also be associated with increased risk of dementia. RBD is frequently seen in PD and may be more frequent in patients who eventually develop dementia. In one study, PD patients with RBD had a sixfold higher occurrence of dementia than those without [18]. Similarly RBD was associated with a 2.2-fold increased risk of developing mild cognitive impairment over 4 years [19].
In terms of potential biomarkers, reduced CSF β-amyloid levels, an established CSF biomarker in Alzheimer’s disease (AD), were found to be related with cognitive decline in PD patients [20, 21]. In neuroimaging studies, white matter hyperintensities were associated with cognitive decline in PD patients, regardless of age, sex, education status, duration or severity of PD symptoms, and vascular risk factors [22]; the load of vascular lesions was found to be correlated with impairment in executive functions, memory, and language [23].
Although several environmental factors have been associated with PD, less is known about their role in PD-D. Smoking was associated with a fourfold higher risk for dementia in PD [24]. Another study with a mean follow-up of 3.6 ± 2.2 years found a twofold increase in the risk of dementia in PD patients with a history of smoking. In the same study there was no significant association with head injury, diabetes mellitus, and incident dementia [25]. Risk factors associated with PD-D are summarized in Table 3.1.
Clinical Features
Clinical features of PD-D include cognitive and behavioral changes as well as frequently associated autonomic symptoms and disturbances of sleep-wake cycle. In typical cases, the profile of dementia can be described as a dysexecutive syndrome with prominent impairment of attention, executive and visuospatial functions, moderately impaired memory, and behavioral symptoms including apathy and psychosis (Table 3.2).
Cognitive Features
PD patients may show some degree of cognitive impairment already at the time of diagnosis. Cognitive impairment without dementia is designated as mild cognitive impairment of PD (PD-MCI), where the activities of daily living are largely preserved. Diagnostic criteria for PD-MCI have been recently described by a Movement Disorder Society Task Force [26]. Cognitive deficits in PD-MCI may vary; the most common deficits are in executive functions, visuospatial functions, memory, and attention. Transition from MCI to dementia is gradual, both in terms of symptom severity as well as temporal course.
In PD-D impairment of attentional functions and working memory is an early and prominent feature. Vigilance and reaction time impaired, attention frequently fluctuates similar to what is seen in patients with dementia with Lewy bodies (DLB). Impaired attention is an important determinant of activities of daily living (ADL) in PD-D: the measure of vigilance and focused attention was the strongest cognitive predictor of ADL status, matching the strength of the effect of motor function on ADL [27].
The term executive function designates a set of cognitive skills including the ability to plan, organize, and perform goal-directed behavior (the central executive), as well as higher intellectual skills such as insight and foresight. Impairment in executive functions is one of the core features of PD-D. In the Mattis Dementia Rating scale, PD-D patients were found to have lower initiation, perseverance, and construction, but higher memory subscores [28]. Insight to impaired function, however, is usually preserved in PD-D, in contrast to patients with AD, where cognitive impairment is usually denied.
All types of memory functions such as working memory, explicit (declarative) memory (both verbal and visual modalities), and implicit memory such as procedural learning can be impaired in PD-D. Deficits in working memory are found early on. The relative severity of declarative memory impairment and its profile usually differ from that seen in AD, in general being less severe. In typical cases the memory impairment is characterized by a deficit in free recall with relatively preserved recognition, indicating that information is stored, but not readily accessed; when structured cues or multiple choices are provided, retrieval usually improves. Same pattern of memory impairment is also seen in mild cognitive impairment associated with PD [29]. Memory scores in patients with PD-D were found to correlate with performance in executive function tests. Based on this observation, it was suggested that impairment of memory in PD-D may be partly due to difficulties in developing internally cued search strategies, although this view has been recently challenged [30]. Memory impairment of limbic type, similar to that seen in AD, can also be seen in a subpopulation of PD-D patients [31].
Another early impairment in PD-D is seen in visuospatial functions. Impairment, especially in visual-perceptual abilities, is more severe compared to AD patients with similar dementia severity. Visuospatial abilities such as object assembly are more impaired in PD-D, whereas visuospatial memory tasks are worse in AD. Deficits in visuospatial functions become more evident in more complex tasks, which require planning and sequencing of response or generation of strategies. These deficits may thus be partly due to problems in sequential organization of response, i.e., executive functions.
Core language functions are largely preserved in PD-D, deficits usually consist of word-finding difficulties resulting in pauses in spontaneous speech, and understanding of complex sentences may be difficult, probably due to attentional deficits.
Behavioral Features
A wide range of behavioral symptoms can be seen in PD-D. The most common symptoms include depression, apathy, anxiety, hallucinations, and insomnia, and at least one neuropsychiatric symptom is present in more than 90 % of the patients [32]. Hallucinations are mostly visual, usually well-formed figures of humans or animals; insight is usually preserved, but may become lost causing agitation, especially in more advanced stages. Auditory hallucinations may occur, but are less common. Hallucinations and delusions may emerge following treatment with dopaminergic agents. When minor forms such as feeling of presence are included, hallucinations occur in 70 % of patients with PD-D, as compared to 25 % of those with AD [33]. Delusional misidentification syndromes are found in 17 % of PD-D patients and are associated with hallucinations and more severe memory and language deficits [34]. Apathy is common in the earlier stages, while delusions increase with more severe cognitive dysfunction. In a longitudinal study, patients with PD-D were discriminated by the presence of cognitive fluctuations, visual and auditory hallucinations, depression, and sleep disturbance from patients with AD, whereas these features were identical to those observed in DLB [35].
Motor, Autonomic, and Other Associated Features
In PD-D patients motor symptoms are usually more symmetrical with predominance of bradykinesia, rigidity, and postural instability; tremor is usually a less prominent feature or may disappear all together. These features are also correlated with more rapid cognitive decline. In a cross-sectional study, PIGD subtype was found in 88 % of patients with PD-D in contrast to 38 % in non-demented patients [36]. In another study it was found that in nearly all cases, dementia was preceded by PIGD phenotype or by a transition from tremor dominant to PIGD phenotype [16]. In the CamPaign study, severity of akinetic-rigid syndrome was associated with a higher risk for dementia independent of age [37]. PD patients with falls are more likely to have lower MMSE scores than those without falls and also more likely to have overt dementia.
L-dopa responsiveness of motor symptoms may diminish as cognitive impairment emerges, although this assumption is largely based on retrospective clinical data. Mechanisms underlying relative loss of l-dopa response may include development of alpha-synuclein pathology in striatum and loss of striatal dopamine D2 and D3 receptors. On the other hand, it may also reflect the development or predominance of non-dopaminergic symptoms such as postural instability and dysarthria.
Autonomic symptoms include constipation, urinary incontinence or frequency, orthostatic or postprandial hypotension resulting in syncope and falls, reduced heart rate variability predisposing to ventricular arrhythmias, and sexual dysfunction. In a comparative study, cardiovascular autonomic dysfunction was more frequent in patients with PD-D as compared to those with DLB, vascular dementia, and AD; PD-D patients demonstrated impairment in both parasympathetic and sympathetic function tests as compared to controls [38].
Sleep and sleep-wake cycle disturbances are common in PD-D including insomnia, sleep disruption, and REM sleep behavior disorder (RBD). The latter can also occur in non-demented patients, but is probably more common in demented patients, and in some it can be an early indicator of incipient dementia. Excessive daytime sleepiness (EDS) and poor sleep quality are more common in patients with PD, PD-D, and DLB as compared to AD [39]. A PIGD phenotype predicts more frequent and severe EDS in PD-D patients, although this association may be lost during the course of the disease [39].
Genetic Factors
Genetic factors related to PD-D include susceptibility genes and increased occurrence of dementia in some monogenic forms of PD. Apolipoprotein epsilon4 (ApoE4) has been strongly linked with Alzheimer’s disease and is the most frequent genetic risk factor for AD. The data on the association of ApoE4 with PD-D have been inconsistent: in one study along with ApoE4, ApoE2 was also suggested to be associated with PD-D [40]. A large meta-analysis failed to show an association between ApoE4 and risk for PD-D [41], and a large case-control study which included autopsy-proven patients revealed that ApoE4 increases the likelihood of presenting with dementia in the context of a pure synucleinopathy [42].
In the CamPaIGN cohort the H1/H1 haplotype of the tau (MAPT) gene has been associated with a greater rate of cognitive decline and dementia in PD patients [43, 44], and with a 12.1 odds ratio to predict dementia, it has not been associated with other neurodegenerative diseases such as DLB and AD [45].
A significantly higher frequency of heterozygote mutations in the glucocerebrosidase gene (GBA) is found in patients with PD or DLB compared with control subjects. GBA mutations may exert a large effect on susceptibility for Lewy body disorders at the individual level, but they are associated with a modest (approximately 3 %) population-attributable risk in individuals of European ancestry [46]. In one cohort up to half of the PD patients heterozygous for GBA mutations developed cognitive impairment later in their disease [47]. PD patients with GBA mutations were more likely to demonstrate cognitive dysfunction compared to patients without [48], and in another study hazard ratio for progression to dementia was found to be significantly higher in GBA mutation carriers [49].
Altered expression of or missense mutations in the alpha-synuclein gene have been linked to early-onset familial PD, sometimes associated with dementia. There is an association with cognitive decline and duplication and more so with the triplication of the alpha-synuclein gene [50, 51].
Dementia rates seem to be lower in patients with Parkin, PINK1, and LRRK2 mutations. Frequency of dementia in monogenic forms of PD does not appear to be higher and may be lower than in sporadic PD [52].
Neuropathological and Biochemical Correlates of PD-D
There is a lack of unanimous agreement with regard to the main neuropathological correlate of PD-D. This may be partly due to variation in clinical diagnosis in different cohorts and partly due to methodological differences in assessing pathology. In general PD-D is characterized by variable combination of the three types of pathological changes: degeneration in subcortical nuclei, cortical cell loss combined with AD-type pathology, and Lewy body (LB)-type degeneration [53]. It is probably the topographical and temporal sequence of neuronal loss rather than the type of protein aggregation which ultimately determines the clinical phenotype. In addition, AD-type and LB pathologies do not need to be mutually exclusive; they frequently coexist; there are interactions between different protein aggregations (i.e., accumulation of alpha-synuclein, tau, and beta-amyloid). Beta-amyloid deposits in the cerebral cortex enhance alpha-synuclein-induced damage; beta-amyloid may promote the aggregation of alpha-synuclein [54]. Hence protein aggregation may be synergistic, with one protein promoting the aggregation of the other, although the consequences of these protein aggregations in terms of cellular function are uncertain.
As opposed to earlier studies which utilized ubiquitin staining, more recent studies performed using alpha-synuclein immunohistochemistry (which is more sensitive to detect cortical Lewy bodies) revealed that dementia better correlates with LB pathology. The fact that families with alpha-synuclein gene duplications and more so those with triplications develop dementia more often also supports a primary and possibly a “dose-dependent” role for synuclein-based pathology in development of PD-D. In a clinicopathological study, however, approximately 55 % of subjects with widespread alpha-synuclein pathology lacked clinical signs of dementia or extrapyramidal signs before their demise [55].
AD-type pathology of variable extent (more plaques than tangles) usually coexists in PD-D [56]. In a recent study, combination of LB-type and AD-type pathologies was found to be a better predictor of dementia than the severity of a single pathology [57].
Dementia in PD usually develops later in the disease course and may be related to an ascending order of pathological changes, as suggested by Braak et al. [58], involving the limbic and cortical structures later in the disease course. Some support for this “bottom-up” hypothesis is also provided by other studies [59]: PD patients with relatively longer disease duration prior to dementia onset had lower levels of cortical choline acetyltransferase than those with a shorter disease duration, implying greater loss of ascending cholinergic projections before dementia developed. In contrast, a more “top-down” pathological process, with greater burden of cortical pathology in PD patients with a more malignant disease course and short time before dementia onset, has also been suggested [60]. This clinicopathological study arising from the Sydney cohort suggested that there may be three types of pathological constellations associated with three different clinical phenotypes, in particular with regard to the temporal course of dementia. Younger patients who developed dementia late in the disease seem to have a predominance of alpha-synuclein pathology with little amyloid, whereas those with a later age of onset and rapid progression to dementia seem to have mixed alpha-synuclein and amyloid pathology: these patients constituting 25 % of the sample had severe neocortical LB pathology, more consistent with a “DLB-like” phenotype, but also with a high amyloid burden [60]. It is not known which factors determine this clinical and pathological variability, and age of onset is probably one of them.
Biochemically, degeneration of the subcortical nuclei results in various neurochemical changes including cholinergic, dopaminergic, serotoninergic, and noradrenergic deficits. The most profound is the cholinergic deficit although the others may also contribute to some aspects of mental dysfunction. In PD-D loss of cholinergic cells in the nucleus basalis of Meynert (nbM) is observed, which is greater than that seen in AD, and LBs are also frequently found in nbM cells. Choline acetyltransferase (ChAT) activity is markedly decreased in the frontal cortex of PD and DLB when compared to normal controls and AD [61, 62]. In contrast to AD, PD is also associated with neuronal loss in the pedunculopontine cholinergic nuclei which project to structures such as the thalamus [63]. Using SPECT and vesicular acetylcholine transporter ([123I] iodobenzovesamicol (IBVM)) as a marker of cholinergic innervation, reduction in IBVM binding in parietal and occipital cortices was shown in non-demented PD patients, while demented PD cases had a more extensive decrease in cortical binding [64]. Functional imaging studies with PET demonstrated that compared with controls mean cortical AChE activity was lowest in patients with PD-D, followed by patients with PD without dementia and AD patients with equal severity of dementia [65]. A subsequent study revealed that the degree of cortical cholinergic deficits correlated particularly well with typical cognitive deficits found in PD-D, e.g., impaired performance on tests of attention and executive functions [66].
Some cognitive deficits, in particular in early stages, may be due to dopaminergic dysfunction, whereas dopaminergic stimulation may be detrimental in later stages [67]. In one study, reduced 18F-fluorodopa uptake in the caudate nucleus and frontal cortex correlated with impairment in tests of verbal fluency, working memory, and attention, indicating that ascending dopaminergic projections may be involved in mediating some of the cognitive deficits in PD [68]. In fluorodopa PET, bilateral impairment in the anterior cingulate area, ventral striatum, and right caudate nucleus was shown in PD patients with dementia, as compared to those without [69]. In another study, dopamine levels in neocortical areas were found to be decreased to a greater level in demented than in non-demented PD patients [70], suggesting some role for the degeneration of the mesocortical dopaminergic system. Neuronal loss was observed in ventral tegmental area as well, which provides dopaminergic input to the mesolimbic and prefrontal cortex and medial substantia nigra [71]. However, clinical experience shows that dementia does not improve with dopaminergic treatment; it may even worsen behavioral and cognitive functions, especially in demented patients. In addition, in a multi-tracer PET study, dopaminergic deficits were comparable between demented and non-demented PD patients, whereas cholinergic deficits were significantly more in demented patients [72]. These observations suggest that dopaminergic deficit is unlikely to play a major role in PD-D.
Deficits in other ascending monoaminergic systems were also suggested to be associated with cognitive impairment. Locus coeruleus, main source of noradrenergic input to the forebrain and cortex, shows neuronal loss especially in demented and depressed PD patients [73]. Degeneration in serotoninergic dorsal raphe nucleus was also suggested to be associated with dementia [74]. The association between dementia and noradrenergic or serotoninergic deficits, however, has not been consistently shown.
Neuroimaging in PD-D
For routine diagnostic structural imaging purposes, magnetic resonance imaging (MRI) is superior to computerized tomography to assess both cortical atrophy and white matter changes. Frontal, occipital, and parietal atrophy as well as an increased amount of whole brain atrophy have been described in PD-D patients compared with controls, whereas no significant gray matter loss was found in patients with PD-MCI compared to PD patients without any cognitive impairment [21]. Medial temporal lobe atrophy may be seen in PD-D patients, but it is less common and less prominent as compared to AD. Supratentorial white matter hyperintensities were suggested to be independently associated with cognitive impairment in PD-D patients [22], and an association between the volume of caudate nucleus and cognitive decline was shown in one study [75].
Novel imaging techniques have also been used in PD-D patients. In a diffusion tensor imaging study, PD-D patients showed significant reduction in fractional anisotropy (FA) in the bilateral posterior cingulate bundles compared with non-demented PD patients [76]; both FA and mean diffusivity (MD) values in the cingulate and corpus callosum showed significant correlations with cognitive parameters [76, 77]. Default mode (DM) is a resting state network detected by functional MRI; it is altered in patients with AD. DM network has been found to be preserved, whereas corticostriatal functional correlations were decreased in bilateral prefrontal regions in PD-D patients compared to control subjects [78]. In another study, however, significant decrease of DM connectivity in the right inferior frontal gyrus has been reported [79].
In functional imaging studies using radiotracers, hypometabolism in the parietal and temporal cortex can be seen in metabolic PET, similar to the pattern seen in AD; hypometabolism in the visual association areas and frontal lobe were also described. A review of rCBF SPECT studies in PD-D revealed frontal hypoperfusion or bilateral temporoparietal deficits [80]. Perfusion deficits in the precuneus and inferior lateral parietal regions have also been described in PD-D patients.
PET using N-[11C]-methyl-4-piperidyl acetate (MP4A) is a method to evaluate cholinergic innervation. PD-D patients showed a severe cholinergic deficit in various cortical regions including frontal and temporoparietal cortices [81]. Iodine-123 metaiodobenzylguanidine (123I-MIBG) is a marker of postganglionic sympathetic cardiac innervation and SPECT studies with this compound demonstrate innervation deficits both in PD-D and DLB, but not in AD. The integrity of nigrostriatal dopaminergic terminals can be visualized using SPECT and markers of dopamine transporter, such as (123)I-FP-CIT. Significant reductions were found in (123)I-FP-CIT binding in the caudate and anterior and posterior putamens in subjects with DLB and PD-D compared to those with AD and controls. Both 123I-MIBG and (123)I-FP-CIT SPECT methods can be used to differentiate patients with DLB or PD-D from those with AD and motor symptoms.
In PET studies with the Pittsburgh compound B (PiB), which shows amyloid burden, mean cortical amyloid load in PD-D patients was comparable to controls and non-demented PD patients [82]. In another study 83 % (10/12) of PD-D patients had “normal” PiB uptake, whereas 85 % (11/13) of DLB patients had significantly increased amyloid load in one or more cortical regions [83]. In a further PiB study, an increased cortical amyloid burden was found in DLB, but not in PD-D patients; interestingly striatal PiB retention in the DLB and PD-D groups was associated with less impaired motor function [84]. In line with these findings, only 15 % of MCI patients with PD showed increased cortical PiB binding at levels seen in patients with AD; however, cerebral amyloid load was associated with cognitive scores [85]. These findings suggest that global cortical amyloid burden is low and infrequent in PD-D compared to DLB and AD.
Diagnosis of PD-D
The clinical diagnostic criteria for PD-D have been published by the Movement Disorder Society Task Force [86], along with practical recommendations for diagnostic procedures [87]. Clinical features of PD-D are shown in Table 3.2, and diagnostic criteria for probable and possible PD-D, representing two levels of diagnostic certainty, are given in Table 3.3. Recently diagnostic criteria for mild cognitive impairment in PD (PD-MCI) have been published, also by the Task Force of Movement Disorder Society [26]. These are developed using the same structure as the PD-D criteria, and applying both sets of criteria together helps to differentiate PD patients with cognitive impairment, but no dementia from those with dementia.
The diagnosis of PD-D can be complicated by various factors, such as severe motor symptoms and dysarthria. These symptoms may make it difficult to assess if cognitive deficits are present and to what extent they contribute to impairment in daily function, a prerequisite for the diagnosis of dementia. Comorbid conditions such as depression, acute confusion (delirium) due to systemic abnormalities, or adverse effects of drugs may also mimic symptoms of dementia.
The diagnosis of dementia in PD can be subsumed into two main steps: first, ascertaining the presence of dementia, i.e., differentiating it from conditions mimicking dementia, and second, the differential diagnosis as to the etiology of dementia, i.e., if the dementia is due to the neurodegenerative process associated with PD or if it involves other etiologies such as vascular disease. Along with a detailed history elucidating the onset, course, and pattern of cognitive and behavioral symptoms, the clinical context within which these symptoms occurred and the findings from laboratory and imaging studies can be helpful in differential diagnosis.
Once other factors mimicking dementia are excluded and presence of dementia is ascertained, the second step involves differential diagnosis. In patients with established PD, this is a rather straightforward process and simply requires excluding alternative causes of dementia. If history is unclear, other conditions which can present with dementia and parkinsonism should be considered. These include cerebrovascular (in particular small vessel) disease, normal-pressure hydrocephalus, AD with drug-induced parkinsonism, DLB, and other neurodegenerative diseases such as corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. A detailed history including a search for features known to be associated with PD-D, use of appropriate neuropsychological tests with assessment of all cognitive domains affected in PD-D, and review of current medication and auxiliary examinations are helpful to make the correct diagnosis.
Assessment of cognitive functions can be achieved either using structured scales or an appropriately constituted neuropsychological test battery. Several structured scales, some specifically developed for PD, can be used for screening purposes. Montreal Cognitive Assessment (MoCA) can be used as a screening instrument, and a cutoff score of 21/30 yielded a sensitivity of 90 % in PD-D patients [88]. Both MoCA and cognitive screening instruments specifically developed for PD including Mini-Mental Parkinson [89] and the Parkinson Neuropsychometric Dementia Assessment (PANDA) [90] have been shown to be more sensitive than the Mini-Mental State Examination (MMSE) to detect cognitive impairment in PD. More elaborate cognitive scales include the general purpose scales such as Mattis Dementia Rating Scale and the CAMCOG-R, as well as PD-specific scales such as SCales for Outcomes of PArkinson’s disease-cognition (SCOPA-Cog) [91] and PD-Cognitive Rating Scale (PD-CRS) [92]. The presence, frequency, severity, and caregiver burden of behavioral symptoms can be assessed using a semi-structured interview or scales such as Neuropsychiatric Inventory (NPI) [93].
Management of Patients with PD-D
The first step in management is to exclude that cognitive impairment and behavioral symptoms are not due to other factors such as adverse effects of drugs, concomitant depression, systemic diseases, or metabolic abnormalities. All medication should be reviewed; drugs which can cause mental dysfunction (such as anticholinergics, tricyclic antidepressants, benzodiazepines, dopamine agonists) should be discontinued. Once other causes are excluded, the next step is to take all non-pharmacological measures and then decide if pharmacological treatment is necessary based on symptom frequency, severity, and burden. PD-D patients receive a number of medications to treat motor impairment, cognitive deficits, psychiatric symptoms, and autonomic dysfunction. Before initiating any new drug, the risk/benefit ratio should be carefully considered. In principle, one drug should be introduced at a time with low doses and titrated up as needed. Drugs to treat behavioral symptoms should be tapered and discontinued, once sufficient symptom control is obtained.
Non-pharmacological Management
Non-pharmacological measures may contribute to preserving and improving cognitive abilities. Adequate information should be provided to the patient and the family about the disease; for example, they may be less concerned when they understand that hallucinations can be part of the disease. Sufficient mental and physical activation, avoidance of aggravating factors such as too much sensory stimuli, and inappropriate environmental factors should be recommended. Physiotherapy and exercise programs should be a part of daily activities to decrease risk of falls since they are frequently associated with cognitive deficits in PD [94, 95] and common in PD-D patients.
A recent systematic review of non-pharmacological and noninvasive therapies in PD revealed no studies specifically in PD-D patients [96]. In non-demented patients although five trials showed positive results, only one study of cognitive training achieved sufficient evidence grading. The result of a small randomized controlled study demonstrated a significant benefit for cognitive training in the domains of attention, executive functions, memory, and visuospatial functions [97]. Positive effect of sudoku puzzle and computerized cognitive training was also observed in executive functions [98, 99]. Likewise, passive cycling and physical exercise were associated with improvement in executive functions [100, 101].
Pharmacological Treatment
Pharmacological treatment of patients with PD-D includes “specific” substitution treatment strategies based on neurotransmitter deficits and nonspecific treatment aimed at amelioration of behavioral symptoms.
Cholinesterase Inhibitors
Cholinergic deficits are the most consistent biochemical correlates of cognitive dysfunction in PD-D. All available ChE-Is including donepezil, rivastigmine, and galantamine have been tested in PD-D. Except for two, most of these studies have been open label with small sample sizes. One of the two large, randomized controlled trials is the EXPRESS study in which 541 patients with mild to moderate PD-D either received rivastigmine (up to 12 mg/day) or placebo over 24 weeks [102]. Both primary end points (ADAS-cog and Clinical Global Impression of Change scale) and secondary end points (MMSE, Neuropsychiatric Inventory, the clock drawing test, verbal fluency, and computer-based attention tests) showed statistically significant improvements on rivastigmine. Activities of daily living scores showed a minimal worsening from baseline in rivastigmine group, whereas those on placebo worsened statistically significantly more. Adverse events, mainly nausea and vomiting, were significantly more frequent with rivastigmine. Worsening of tremor was reported in 10 % of the patients on rivastigmine, and UPDRS motor score did not reveal any significant differences between the groups. Based on this study rivastigmine became the first specific treatment approved for the treatment of patients with mild to moderate PD-D. A patch form of rivastigmine also became available and has been approved for PD-D in several countries. Its long-term efficacy and safety as compared to capsules has been described in a large open-label prospective study, revealing no long-term safety concerns, in particular with regard to worsening of motor symptoms [103].
A post hoc analysis of computerized tests from the EXPRESS study revealed that rivastigmine improved all aspects of attention [104], and a subgroup analysis suggested that patients who had visual hallucinations at baseline may draw more benefits from cholinergic treatment, i.e., less worsening in cognitive functions as compared to placebo [105]. The EXPRESS study had a 6-month extension period during which all patients received active treatment [106]. The beneficial effects seen during the first 6 months were largely maintained, although there was some decline. Importantly, there was no evidence of worsening motor function over the course of 1-year treatment [107].
The second large randomized, controlled trial was conducted with donepezil [108], in which 550 patients with mild to moderate PD-D received either placebo, donepezil 5 mg, or donepezil 10 mg/day for 24 weeks. In the primary analysis there were no significant differences between placebo and active groups. When the country interaction was controlled for in a secondary analysis, both primary end points ADAS-cog and CIBIC-plus scores showed statistically significant superiority for 10 mg, but not for 5 mg. Statistically significant differences in favor of donepezil were also found in some secondary measures including MMSE, Brief Test of Attention, and Verbal Fluency Test, whereas there were no significant differences from placebo on the ADL scale and the behavioral scale NPI. UPDRS motor scores did not reveal any significant worsening of motor functions on donepezil. In another open-label study, the effects of donepezil on central processing speed and other attentional measures were tested over 20 weeks. Power of attention, continuity of attention, and reaction time variability were found to be improved as compared to baseline [109].
Open-label studies have suggested that galantamine may have beneficial effects in patients with PD-D [110], and there are however no randomized controlled trials in this population.
In summary, ChE-Is have been found to be efficacious in PD-D, and the strongest evidence from randomized controlled trials exists for rivastigmine. The effect size is modest: in a Cochrane meta-analysis evaluating the efficacy of ChE-Is in PD-D, 5.3 % of patients were concluded to have benefits on outcome scales, whereas 10.1 % patients on placebo demonstrated worsening, suggesting that the effect size is close to 15 % [111]. In a more recent Cochrane meta-analysis, the authors suggested that the use of ChE-Is in PD-D is associated with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living scales [112]. Taking together all the evidence, patients with PD-D should be considered for treatment with a ChE-I taking in account expected benefits and risks. The doses should be slowly titrated up to therapeutic dose levels and then maintained; adverse effects such as nausea, vomiting, loss of appetite, diarrhea, and worsening of tremor should be monitored. At the usual dose range, dropout rates are in 10–31 % of patients. In addition to the relatively more common gastrointestinal adverse effects, muscle cramps, hypersalivation, rhinorrhea, lacrimation, postural hypotension, falls, bradycardia, and syncope can occur in a small proportion of patients [113]. There is no evidence-based guidance on if and when to discontinue treatment, and if such a decision is made because there is no more expectations for clinical benefits, patients should be closely monitored after discontinuation. Abrupt withdrawal of ChE-I can be associated with rapid emergence of neuropsychiatric symptoms and cognitive decline in DLB and PD-D [114]. Although reinstatement of treatment may reverse this deterioration, it is recommended that patients who are assessed as responding to ChE-I are maintained on treatment long term.
NMDA Antagonists
Memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist approved for treatment of moderate to severe Alzheimer’s disease. There is circumstantial evidence for glutamatergic dysfunction in PD-D. In a randomized controlled trial which included both patients with DLB and PD-D, there was a significant difference in favor of memantine in the global outcome scale, whereas MMSE and NPI did not show any differences. Patients with PD-D seem to have more benefits as compared to those with DLB [115]. An opposite finding was obtained in a larger randomized placebo-controlled trial which included 199 patients either with DLB or PD-D. In the total population, there were no statistically significant differences between memantine and placebo groups. The global outcome scale and NPI scores were significantly better under memantine in the DLB subgroup; there were no significant differences between memantine and placebo in the PD-D population, although trends were favoring memantine. The ADCS-ADL scale, Zarit caregiver burden scale, and a number of cognitive tests did not show any consistent or significant differences between active and placebo in the overall or in the either population [116]. Based on these studies it can be concluded that memantine may have mild beneficial effects in patients with Lewy body-related dementias, possibly more so in the DLB population, predominantly on the global status and behavioral symptoms.
Dopaminergic Drugs
The effects of dopaminergic medication on mental dysfunction have been scarcely studied in patients with PD-D; most formal studies have been conducted in non-demented patients. The results have been mixed describing either no effects or improvement in some and worsening in other functions [117]. In one of the few studies which specifically included demented patients, patients with PD, PD-D, or DLB were tested for cognitive functions and behavioral symptoms after acute l-dopa challenge and following 3 months of treatment. After acute challenge patients reported improvement in subjective alertness, but fluctuations increased; reaction time and accuracy remained unchanged in those with PD-D. After 3 months of treatment, neuropsychiatric scores improved both in PD and PD-D, and mean global cognitive score was better, but attention and memory scores were worse in PD patients without dementia. Reaction time became slower in those with PD-D, there were however no patients with marked deterioration, and the authors concluded that levodopa treatment is not detrimental in PD-D patients [118]. The common clinical practice suggests that dopaminergic drugs do not exert any significant benefits on cognitive and behavioral functions in PD-D patients; in particular dopamine agonists can induce or worsen psychosis or confusion.
Pharmacological Treatment of Behavioral Symptoms
Behavioral symptoms may be a source of greater distress to patients and carers than motor dysfunction [119]. Treatment with ChE-Is may exert mild benefits on some neuropsychiatric symptoms, particularly on apathy, anxiety, sleep disturbance, and delusions [110], and these benefits may be at least partly mediated through improvements in attention and cognitive processing. Since cognitive and behavioral symptoms usually coexist, the choice of a ChE-I as first-line treatment is a rationale choice, targeting both symptoms.
Aggression, agitation, and other psychotic behaviors are less likely to improve with ChE-Is. Traditional neuroleptics such as haloperidol can provoke severe neuroleptic sensitivity reactions due to their D2 receptor antagonism and are therefore contraindicated in this patient population. Such reactions are usually acute or subacute, emerging after the first few doses or following a dose increase from a previously tolerated one. These reactions or worsening of parkinsonism can be seen with second-generation (“atypical”) antipsychotics such as risperidone, olanzapine, and aripiprazole. In addition, all neuroleptics can increase the risk of cerebrovascular accidents and death. Hence risk/benefit ratio should be carefully evaluated before initiating neuroleptic treatment. Although evidence base is weak, quetiapine [120, 121] can be tried as first-line treatment because of its lower risk for worsening motor symptoms. Robust evidence from randomized controlled trials to treat psychosis in PD patients, however, exists only for clozapine [122, 123]. It requires weekly blood monitoring for the first 3 months and monthly thereafter, and in addition antimuscarinic properties [124] may increase confusion in patients with dementia.
Although depression or depressive features are frequent in PD and PD-D, there have been few randomized, controlled trials of antidepressants in PD and none in PD-D. A meta-analysis of all studies in PD patients with depression showed large effect sizes, both under active and placebo, without statistically significant differences between them; increasing age and major depression predicted better response [125]. In a systematic review amitriptyline was found to be the only drug with evidence of efficacy in PD depression [126]. Albeit not large several placebo-controlled randomized trials have been conducted in PD patients with depression without dementia, desipramine and citalopram were both significantly better than placebo [127], and nortriptyline was found to be significantly better than placebo, whereas paroxetine was not [128], whereas both paroxetine and venlafaxine were significantly better than placebo in a larger randomized trial [129]. Dopamine agonist pramipexole was shown to be significantly better than placebo in a large randomized placebo-controlled trial on non-demented patients with depressive symptoms; [130] agonists, however, can worsen psychosis and cognition in PD-D patients. It should be kept in mind that all randomized controlled trials were performed in non-demented patients. There is a hint that tricyclics such as amitriptyline and nortriptyline may have larger effect sizes, and they should be, however, avoided in PD-D patients because of their anticholinergic effects and hence their potential to worsen cognition; SSRIs or SNRIs should be preferred in PD-D patients.
Excessive daytime sleepiness (EDS) and REM sleep behavior disorder (RBD) are frequent in PD-D patients [131]. In small studies clonazepam has been reported to be efficacious and well tolerated [132] in suppressing the motor features, but does not restore REM sleep atonia [133]. In a small study melatonin was beneficial, with improvement noted in 10 of 14 patients who had failed to respond to clonazepam or were unable to tolerate it [134]. Possible benefits of ChE-Is as a treatment for RBD need further study [135]. Although not specifically tested in demented PD patients, modafinil, an agent which promotes wakefulness, can be considered to treat EDS. In non-demented PD patients, modafinil was significantly better than placebo in two small randomized, placebo-controlled and one open-label study [136, 137], whereas no difference was found in another small, placebo-controlled trial [138].
Although apathy is very common and seen in the majority of PD-D patients [139], its treatment has not been well studied. In a small study, methylphenidate was reported to be beneficial [140]. In a small randomized, placebo-controlled trial in non-demented, nondepressed patients with apathy, rivastigmine given as patch significantly improved apathy score as compared to placebo [141].
References
Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–45. doi:10.1212/01.wnl.0000180516.69442.95.
Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–9. doi:10.1212/WNL.0b013e3181f39d0e.
Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–69. doi:10.1093/brain/awp245.
Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatr. 2013;84:1258–64. doi:10.1136/jnnp-2013-305277.
Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20:1255–63. doi:10.1002/mds.20527.
Marder K, Tang MX, Cote L, et al. The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol. 1995;52:695–701.
De Lau LML, Schipper CMA, Hofman A, et al. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam study. Arch Neurol. 2005;62:1265–9. doi:10.1001/archneur.62.8.1265.
Hely MA, Morris JGL, Reid WGJ, Trafficante R. Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–9. doi:10.1002/mds.20324.
Hely MA, Reid WGJ, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. doi:10.1002/mds.21956.
Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92.
Buter TC, van den Hout A, Matthews FE, et al. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–22. doi:10.1212/01.wnl.0000306632.43729.24.
Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70:1396–402. doi:10.1001/jamaneurol.2013.3579.
Levy G, Schupf N, Tang M-X, et al. Combined effect of age and severity on the risk of dementia in Parkinson’s disease. Ann Neurol. 2002;51:722–9. doi:10.1002/ana.10219.
Pedersen KF, Larsen JP, Tysnes O-B, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70:580–6. doi:10.1001/jamaneurol.2013.2110.
Woods SP, Tröster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. J Int Neuropsychol Soc. 2003;9:17–24.
Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–30. doi:10.1002/mds.20897.
Sollinger AB, Goldstein FC, Lah JJ, et al. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16:177–80. doi:10.1016/j.parkreldis.2009.11.002.
Marion M-H, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J Neurol. 2008;255:192–6. doi:10.1007/s00415-008-0629-9.
Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71:49–56. doi:10.1002/ana.22655.
Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–61. doi:10.1212/WNL.0b013e3181f39a78.
Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology. 2014;82:308–16. doi:10.1212/WNL.0000000000000066.
Lee S-J, Kim J-S, Yoo J-Y, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord. 2010;24:227–33. doi:10.1097/WAD.0b013e3181d71a13.
Kandiah N, Mak E, Ng A, et al. Cerebral white matter hyperintensity in Parkinson’s disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat Disord. 2013;19:680–3. doi:10.1016/j.parkreldis.2013.03.008.
Ebmeier KP, Calder SA, Crawford JR, et al. Mortality and causes of death in idiopathic Parkinson’s disease: results from the Aberdeen whole population study. Scott Med J. 1990;35:173–5.
Levy G, Tang M-X, Cote LJ, et al. Do risk factors for Alzheimer’s disease predict dementia in Parkinson’s disease? An exploratory study. Mov Disord. 2002;17:250–7.
Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder Society Task Force guidelines. Mov Disord. 2012;27:349–56. doi:10.1002/mds.24893.
Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2006;77:1136–42. doi:10.1136/jnnp.2006.093146.
Aarsland D, Litvan I, Salmon D, et al. Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatr. 2003;74:1215–20.
Costa A, Monaco M, Zabberoni S, et al. Free and cued recall memory in Parkinson’s disease associated with amnestic mild cognitive impairment. PLoS One. 2014;9:e86233. doi:10.1371/journal.pone.0086233.
Brønnick K, Alves G, Aarsland D, et al. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114–24. doi:10.1037/a0020857.
Weintraub D, Moberg PJ, Culbertson WC, et al. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17:195–200.
Aarsland D, Brønnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatr. 2007;78:36–42. doi:10.1136/jnnp.2005.083113.
Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733–45.
Pagonabarraga J, Llebaria G, García-Sánchez C, et al. A prospective study of delusional misidentification syndromes in Parkinson’s disease with dementia. Mov Disord. 2008;23:443–8. doi:10.1002/mds.21864.
Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67:1605–11. doi:10.1212/01.wnl.0000242630.52203.8f.
Burn DJ, Rowan EN, Minett T, et al. Extrapyramidal features in Parkinson’s disease with and without dementia and dementia with Lewy bodies: a cross-sectional comparative study. Mov Disord. 2003;18:884–9. doi:10.1002/mds.10455.
Williams-Gray CH, Foltynie T, Brayne CEG, et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–98. doi:10.1093/brain/awm111.
Allan LM, Ballard CG, Allen J, et al. Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatr. 2007;78:671–7. doi:10.1136/jnnp.2006.102343.
Boddy F, Rowan EN, Lett D, et al. Subjectively reported sleep quality and excessive daytime somnolence in Parkinson’s disease with and without dementia, dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22:529–35. doi:10.1002/gps.1709.
Huang X, Chen PC, Poole C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62:2198–202.
Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256:493–8. doi:10.1007/s00415-009-0119-8.
Tsuang D, Leverenz JB, Lopez OL, et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–8. doi:10.1001/jamaneurol.2013.600.
Goris A, Williams-Gray CH, Clark GR, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann Neurol. 2007;62:145–53. doi:10.1002/ana.21192.
Healy DG, Abou-Sleiman PM, Lees AJ, et al. Tau gene and Parkinson’s disease: a case-control study and meta-analysis. J Neurol Neurosurg Psychiatr. 2004;75:962–5.
Setó-Salvia N, Clarimón J, Pagonabarraga J, et al. Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch Neurol. 2011;68:359–64. doi:10.1001/archneurol.2011.17.
Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–82. doi:10.1001/archneurol.2007.68.
Goker-Alpan O, Lopez G, Vithayathil J, et al. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–7. doi:10.1001/archneur.65.10.1353.
Chahine LM, Qiang J, Ashbridge E, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70:852–8. doi:10.1001/jamaneurol.2013.1274.
Winder-Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136:392–9. doi:10.1093/brain/aws318.
Sironi F, Trotta L, Antonini A, et al. Alpha-Synuclein multiplication analysis in Italian familial Parkinson disease. Parkinsonism Relat Disord. 2010;16:228–31. doi:10.1016/j.parkreldis.2009.09.008.
Farrer M, Kachergus J, Forno L, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–9. doi:10.1002/ana.10846.
Kasten M, Kertelge L, Brüggemann N, et al. Nonmotor symptoms in genetic Parkinson disease. Arch Neurol. 2010;67:670–6. doi:10.1001/archneurol.67.6.670.
Emre M. What causes mental dysfunction in Parkinson’s disease? Mov Disord. 2003;18 Suppl 6:S63–71. doi:10.1002/mds.10565.
Pletnikova O, West N, Lee MK, et al. A beta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26:1183–92. doi:10.1016/j.neurobiolaging.2004.10.006.
Parkkinen L, Pirttilä T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi:10.1007/s00401-008-0346-6.
Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–98. doi:10.1002/ana.23659.
Compta Y, Parkkinen L, O’Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–505. doi:10.1093/brain/awr031.
Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67:1931–4. doi:10.1212/01.wnl.0000249130.63615.cc.
Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409–15. doi:10.1007/s00401-008-0344-8.
Mattila PM, Röyttä M, Lönnberg P, et al. Choline acetyltransferase activity and striatal dopamine receptors in Parkinson’s disease in relation to cognitive impairment. Acta Neuropathol. 2001;102:160–6.
Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology. 2000;54:407–11.
Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci U S A. 1987;84:5976–80.
Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40:399–410. doi:10.1002/ana.410400309.
Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–8. doi:10.1001/archneur.60.12.1745.
Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol. 2006;253:242–7. doi:10.1007/s00415-005-0971-0.
Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging. 2000;16:365–79.
Rinne JO, Portin R, Ruottinen H, et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57:470–5.
Ito K, Nagano-Saito A, Kato T, et al. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125:1358–65.
Scatton B, Javoy-Agid F, Rouquier L, et al. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–8.
Rinne JO, Rummukainen J, Paljärvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50. doi:10.1002/ana.410260107.
Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–92. doi:10.1212/WNL.0b013e3181d55f61.
Jellinger KA. Morphological substrates of mental dysfunction in Lewy body disease: an update. J Neural Transm Suppl. 2000;59:185–212.
Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–97.
Apostolova LG, Beyer M, Green AE, et al. Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord. 2010;25:687–8. doi:10.1002/mds.22799.
Matsui H, Nishinaka K, Oda M, et al. Dementia in Parkinson’s disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177–81. doi:10.1111/j.1600-0404.2007.00838.x.
Wiltshire K, Concha L, Gee M, et al. Corpus callosum and cingulum tractography in Parkinson’s disease. Can J Neurol Sci. 2010;37:595–600.
Seibert TM, Murphy EA, Kaestner EJ, Brewer JB. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology. 2012;263:226–34. doi:10.1148/radiol.12111280.
Rektorova I, Krajcovicova L, Marecek R, Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener Dis. 2012;10:232–7. doi:10.1159/000334765.
Bissessur S, Tissingh G, Wolters EC, Scheltens P. rCBF SPECT in Parkinson’s disease patients with mental dysfunction. J Neural Transm Suppl. 1997;50:25–30.
Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–22. doi:10.1212/01.wnl.0000191154.78131.f6.
Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson’s disease dementia. Neuroimage. 2008;39:1027–33. doi:10.1016/j.neuroimage.2007.09.072.
Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatr. 2008;79:1331–8. doi:10.1136/jnnp.2007.127878.
Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–10. doi:10.1212/01.wnl.0000326146.60732.d6.
Petrou M, Bohnen NI, Müller MLTM, et al. Aβ-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79:1161–7. doi:10.1212/WNL.0b013e3182698d4a.
Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. doi:10.1002/mds.21507; quiz 1837.
Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–24. doi:10.1002/mds.21844.
Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–25. doi:10.1212/WNL.0b013e3181fc29c9.
Mahieux F, Boller F, Fermanian J, Guiallard D. Mini-Mental Parkinson: first validation study of a new bedside test constructed for Parkinson’s disease. Behav Neurology. 1995;8:15–22.
Kalbe E, Calabrese P, Kohn N, et al. Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord. 2008;14:93–101. doi:10.1016/j.parkreldis.2007.06.008.
Marinus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61:1222–8.
Pagonabarraga J, Kulisevsky J, Llebaria G, et al. Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord. 2008;23:998–1005. doi:10.1002/mds.22007.
Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14.
Nocera JR, Price C, Fernandez HH, et al. Tests of dorsolateral frontal function correlate with objective tests of postural stability in early to moderate stage Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:590–4. doi:10.1016/j.parkreldis.2010.08.008.
Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26:2496–503. doi:10.1002/mds.23932.
Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson’s disease: a systematic review. Mov Disord. 2013;28:1034–49. doi:10.1002/mds.25377.
París AP, Saleta HG, de la Cruz Crespo Maraver M, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord. 2011;26:1251–8. doi:10.1002/mds.23688.
Nombela C, Bustillo PJ, Castell PF, et al. Cognitive rehabilitation in Parkinson’s disease: evidence from neuroimaging. Front Neurol. 2011;2:82. doi:10.3389/fneur.2011.00082.
Sammer G, Reuter I, Hullmann K, et al. Training of executive functions in Parkinson’s disease. J Neurol Sci. 2006;248:115–9. doi:10.1016/j.jns.2006.05.028.
Ridgel AL, Kim C-H, Fickes EJ, et al. Changes in executive function after acute bouts of passive cycling in Parkinson’s disease. J Aging Phys Act. 2011;19:87–98.
Cruise KE, Bucks RS, Loftus AM, et al. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123:13–9. doi:10.1111/j.1600-0404.2010.01338.x.
Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509–18. doi:10.1056/NEJMoa041470.
Emre M, Poewe W, De Deyn PP, et al. Long-term safety of rivastigmine in Parkinson disease dementia: an open-label, randomized study. Clin Neuropharmacol. 2014;37:9–16. doi:10.1097/WNF.0000000000000010.
Wesnes KA, McKeith I, Edgar C, et al. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology. 2005;65:1654–6. doi:10.1212/01.wnl.0000184517.69816.e9.
Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21:1899–907. doi:10.1002/mds.21077.
Poewe W, Wolters E, Emre M, et al. Long-term benefits of rivastigmine in dementia associated with Parkinson’s disease: an active treatment extension study. Mov Disord. 2006;21:456–61. doi:10.1002/mds.20700.
Oertel W, Poewe W, Wolters E, et al. Effects of rivastigmine on tremor and other motor symptoms in patients with Parkinson’s disease dementia: a retrospective analysis of a double-blind trial and an open-label extension. Drug Saf. 2008;31:79–94.
Dubois B, Tolosa E, Katzenschlager R, et al. Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27:1230–8. doi:10.1002/mds.25098.
Rowan E, McKeith IG, Saxby BK, et al. Effects of donepezil on central processing speed and attentional measures in Parkinson’s disease with dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2007;23:161–7. doi:10.1159/000098335.
Aarsland D, Mosimann UP, McKeith IG. Role of cholinesterase inhibitors in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2004;17:164–71. doi:10.1177/0891988704267463.
Maidment I, Fox C, Boustani M. Cholinesterase inhibitors for Parkinson’s disease dementia. Cochrane Database Syst Rev. 2006;(1):CD004747. doi:10.1002/14651858.CD004747.pub2.
Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2012;(3):CD006504. doi:10.1002/14651858.CD006504.pub2. CD006504.
Thomas AJ, Burn DJ, Rowan EN, et al. A comparison of the efficacy of donepezil in Parkinson’s disease with dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry. 2005;20:938–44. doi:10.1002/gps.1381.
Minett TSC, Thomas A, Wilkinson LM, et al. What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson’s disease with dementia. Int J Geriatr Psychiatry. 2003;18:988–93. doi:10.1002/gps.995.
Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613–8. doi:10.1016/S1474-4422(09)70146-2.
Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:969–77. doi:10.1016/S1474-4422(10)70194-0.
Barker RA, Barrett J, Mason SL, Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. doi:10.1016/S1474-4422(12)70295-8.
Molloy SA, Rowan EN, O’Brien JT, et al. Effect of levodopa on cognitive function in Parkinson’s disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatr. 2006;77:1323–8. doi:10.1136/jnnp.2006.098079.
Aarsland D, Larsen JP, Karlsen K, et al. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866–74.
Fernandez HH, Okun MS, Rodriguez RL, et al. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci. 2009;119:2196–205. doi:10.3109/00207450903222758.
Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27:153–6.
Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. The Parkinson Study Group. N Engl J Med.1999;340:757–63. doi:10.1056/NEJM199903113401003.
Clozapine in drug-induced psychosis in Parkinson’s disease. The French Clozapine Parkinson Study Group. Lancet. 1999;353:2041–2.
Coward DM, Imperato A, Urwyler S, White TG. Biochemical and behavioural properties of clozapine. Psychopharmacology (Berl). 1989;99(Suppl):S6–12.
Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson’s disease: a review and meta-analysis. Mov Disord. 2005;20:1161–9. doi:10.1002/mds.20555.
Miyasaki JM, Shannon K, Voon V, et al. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:996–1002. doi:10.1212/01.wnl.0000215428.46057.3d.
Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord. 2008;23:850–7. doi:10.1002/mds.21966.
Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72:886–92. doi:10.1212/01.wnl.0000336340.89821.b3.
Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology. 2012;78:1229–36. doi:10.1212/WNL.0b013e3182516244.
Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:573–80. doi:10.1016/S1474-4422(10)70106-X.
Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16:622–30.
Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(Pt 2):331–9.
Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4.
Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–4.
Di Giacopo R, Fasano A, Quaranta D, et al. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord. 2012;27:559–61. doi:10.1002/mds.24909.
Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord. 2003;18:287–93. doi:10.1002/mds.10390.
Nieves AV, Lang AE. Treatment of excessive daytime sleepiness in patients with Parkinson’s disease with modafinil. Clin Neuropharmacol. 2002;25:111–4.
Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatr. 2005;76:1636–9. doi:10.1136/jnnp.2005.065870.
Leroi I, Pantula H, McDonald K, Harbishettar V. Neuropsychiatric symptoms in Parkinson’s disease with mild cognitive impairment and dementia. Parkinsons Dis. 2012;2012:308097. doi:10.1155/2012/308097.
Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14:461–2.
Devos D, Moreau C, Maltête D, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatr. 2013. doi:10.1136/jnnp-2013-306439.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bilgiç, B., Hanağası, H.A., Emre, M. (2015). Parkinson’s Disease Dementia. In: Reichmann, H. (eds) Neuropsychiatric Symptoms of Movement Disorders. Neuropsychiatric Symptoms of Neurological Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-09537-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-09537-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09536-3
Online ISBN: 978-3-319-09537-0
eBook Packages: MedicineMedicine (R0)