Abstract
The efficiency of penetration of nanodrugs through cell membranes imposes further complexity due to nanothermodynamic and entropic potentials at interfaces. Action of nanodrugs is effective after cell membrane penetration. Contrary to diffusion of water diluted common molecular drugs, nanosize imposes an increasing transport complexity at boundaries and interfaces (e.g., cell membrane). Indeed, tiny dimensional systems brought the concept of “nanothermodynamic potential,” which is proportional to the number of nanoentities in a macroscopic system, from either the presence of surface and edge effects at the boundaries of nanoentities or the restriction of the translational and rotational degrees of freedom of molecules within them. The core element of nanothermodynamic theory is based on the assumption that the contribution of a nanosize ensemble to the free energy of a macroscopic system has its origin at the excess interaction energy between the nanostructured entities. As the size of a system is increasing, the contribution of the nanothermodynamic potential to the free energy of the system becomes negligible. Furthermore, concentration gradients at boundaries, morphological distribution of nanoentities, and restriction of the translational motion from trapping sites are the source of strong entropic potentials at the interfaces. It is evident therefore that nanothermodynamic and entropic potentials either prevent or allow enhanced concentration very close to interfaces and thus strongly modulate nanoparticle penetration within the intracellular region. In this work, it is shown that nano-sized polynuclear iron (III)-hydroxide in sucrose nanoparticles have a nonuniform concentration around the cell membrane of macrophages in vivo, compared to uniform concentration at hydrophobic prototype surfaces. The difference is attributed to the presence of entropic and nanothermodynamic potentials at interfaces.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Atomic Force Microscopy
- Atomic Force Microscopy Image
- Macroscopic System
- Entropic Potential
- Vibration Isolation Table
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
28.1 Introduction
Nanomedicine is an innovating and a challenging scientific area of interest. It consists of the interdisciplinary combination [1] of nanotechnology and medicine. Nanomedicine includes the development of nanoparticles and techniques for diagnosis and treatment of many diseases at the nanoscale [2–5]. One of its major sectors is nanoparticle drug delivery [1, 3, 6]. Nanotechnology-based drug delivery systems have emerged as promising platforms for targeting and destroying mainly cancer cells [7–9] via photodynamic therapy (PDT) that uses a combination of light sources, photosensitizers such as phthalocyanines, and specific targeting molecules and nano-carriers for localized therapies at the micro/nanoscale. Following light activation of photosensitizers, reactive oxygen species are released and eventually destroy cancer cells [10], after successful intracellular internalization via endocytosis and subsequent accumulation in the endosome/lysosome compartments of the cell. Although that part of nanomedicine is highlighted as one of the most promising field, it faces with two crucial problems. Despite complex design of nano-carriers, such as synthesis of polyion complex micelles via electrostatic interactions between phthalocyanine complexes and various block copolymers [11], uncontrolled nanodrug aggregation and inefficient cell penetration is always present in most nanodrug delivery systems.
Almost all nanoparticles when come in touch with their target start to aggregate [12–15]. For example the aggregate size of SiO2 nanoparticles increases when placed on colloidal solution. The aggregation of nanoparticles at interfaces incommodes their entrance through the cellular membrane and thus into the internal part of the cell that is the aim of many drug delivery treatments. Indeed, the cellular energy unit, the mitochondrion, is the target of many nanoparticles synthesized for the treatment of well-known diseases such as Alzheimer’s disease, cancer, and metabolic disorders [16, 17]. Hence, the thermodynamic aggregation of nanoparticles at the boundaries of the cells could lead to inefficacy arriving on their target (e.g. organelle, metabolic procedure, etc.) despite that they were designed appropriately.
The second constrain in a nanomedicine project is via to the presence of a large amount of electric charge, as most of the nanoparticles used for drug delivery treatment are highly negatively charged. For example, ZnPc is negatively charged [18, 19] as also some categories of dendrimers that could be used as carriers [20]. At this point, it must be mentioned that the cellular membrane is negatively charged because of its structure [21]. Indeed, the head groups of bilayer phospholipids consist of two anionic lipids, phosphatidylserine (PS) and phosphatidylinositol (PI), that are negatively charged [22]. Consequently, at the cellular interface a repulsive force is generated and then the negatively charged nanoparticles do not even reach the cellular membrane.
On the other hand, Tiny dimensional systems introduce the concept of “nanothermodynamic potential” [23] which is proportional to the number of nanoentities in a macroscopic system, from either the presence of surface and edge effects at the interfaces or the restriction of the translational and rotational degrees of freedom of molecules within them that specify an entropic change. In the case of nanothermodynamic potentials, the core element of the thermodynamic potential is based on the assumption that the contribution of a nanosize ensemble to the free energy of a macroscopic system has its origin at the excess interaction energy between the nanostructured entities. As the size of a system is increasing, the contribution of the nanothermodynamic potential to the free energy of the system becomes negligible. Contrary to stronger interactions, which are independent of system’s size, weak physical interactions are dependent on the local morphological features and the various constrains that might restrict the molecular translational motion at the nanoscale. However, the experimental demonstration of nanothermodynamic potentials is difficult to be implemented as sophisticated experimental approaches are required [24].
The nanothermodynamic potential is directly correlated to internal stress, which is responsible for a plurality of morphological structures and shapes at the nano/microscale and therefore it plays a major role in Natural evolution. Indeed, a sound example is a spontaneous generation of complexity in the form of self-assembled structures, the outcome of overstressing during a dynamical transformation of a system attempting to attain a state of minimum energy and maximum entropy [25].
Particularly, the stress–strain response at interfaces is specified, besides the chemical composition and the specific geometrical confrontations, by both the nature of the molecular forces between the boundaries and the difference of the internal, chemical, entropic, and surface components of the free energy of the interface system [26]. The challenging situation in the case of weak interactions within a specific system, e.g., hydrogen polar interactions, where the order of magnitude of free energy difference from physisorption in thin layers is in the pJ range and the associated strained field does not exceed a few Ǻngstroms, is the theoretical description of the energy transfer and the experimental verification of the internal energy differences. Any description should take into account both the molecular interactions and the conformational configuration of the combined specific system, as well as any additional constraint that might be important, such as the size and the concentration of nanocomposites. Finally, besides thermodynamic potential, an additional key theme is the interplay between the combinatorial entropic changes, including any possible restriction of the translational symmetry of molecules at the nanoscale.
In this work, nano-sized particles of polynuclear iron (III)-hydroxide in sucrose are deposited on macrophages’ cells in vivo as also on the surface of hydrophobic silicon wafers and then the size and concentration of the created nano-aggregations on the interfaces between the two substances in the two cases are studied using Atomic Force Microscopy (AFM). The results emphasize the role of thermodynamics in intracellular nanodrug delivery.
28.2 Experimental
Here, THP-1 cell line was used. Cells of that cell line are monocyte-like and they were derived from a 1-year-old boy with leukemia (ATCC®TIB-202). Cell culture was performed in an incubator at a 95 % w/w ambient air, 5 % w/w CO2 gaseous environment, and the temperature is maintained at 37 °C. The medium was RPMI 1640 serum with 2 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10 mM HEPES. Generally, the THP-1 cell line presents a quite quick rate of proliferation and it is suspended. Subsequently, the cells are deposited on silicon wafers coated with Poly-l-Lysine (PLL) to make them adherent. Next, silicon wafers were poured with 5 μL of solution polynuclear iron (III) hydroxide in sucrose (Venofer®, 20 mg Fe/mL in 30 % w/v sucrose solution at a pH of about 10.5–11.1). For the AFM measurements, wafers are left to dry and then fixed with 4 % paraformaldehyde (PFA).
An AFM (Bruker di Innova) was used to evaluate the morphology and roughness of the samples that contain polynuclear iron (III) hydroxide in sucrose diluted on macrophages. The AFM images were acquired in ambient conditions, in tapping mode by a phosphorus-(n)-doped silicon cantilever (Bruker, RTESPA) with a nominal spring constant of 40 N/m at 300 kHz resonance frequency and nominal radius of 8 nm. The AFM images were obtained at different scanning areas at a maximum scanning rate of 0.5 Hz and with an image resolution of 512 × 512 pixels. The AFM was placed on a vibration isolation table and inside an acoustic isolation chamber.
28.3 Results
In the AFM image of Fig. 28.1a spherical nanoparticles of the deposited polynuclear iron (III)-hydroxide are evenly distributed on the Si surface having an average size in the X − Y plane of 70 nm. The size distribution of polynuclear iron (III)-hydroxide nanoparticles in sucrose along the z axis follows the Gaussian distribution as it is shown in the histogram of Fig. 28.1b. It is obvious that there is a uniform spatial distribution of thermodynamic potential gradients between the Si surface and the nanoparticles. Additionally, analyzing with Fast Fourier transform the polynuclear iron (III)-hydroxide particles in sucrose on Si along the x and y axes homogeneous thermodynamic potentials at the interface between the Si surface and the nanoparticles were indicated via the Gaussian and FFT distributions, Fig. 28.2. On the contrary, distribution of nanoparticles among macrophage cells (ΤΗP-1 cell line) is uneven, Fig. 28.3a, suggesting the presence of thermodynamical potential at the interfaces. In Fig. 28.3b the same trend is displayed in an area of the macrophage cell even though the roughness of the aggregated nanodrugs is much lower.
(a) Accumulation of iron (III)-hydroxide nanoparticles in sucrose near the boundaries of macrophage cell in vivo that indicates the presence of nonuniform thermodynamic gradients at the cell’s external wall. (b) A detail (1.5 × 1.5 μm) for a low roughness area of Fig. 28.3a on the macrophage cell
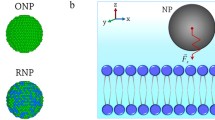
Besides the chemical potential gradient near interfaces that forces particle motion from high to low concentration areas, nanoparticles brought the concept of “nanothermodynamic potential,” which is proportional to the number of nanoentities in a macroscopic system or the restriction of the translational and rotational degrees of freedom of molecules, which is the source of a nanothermodynamic entropic potential. The free energy change δG at the interface between the membrane of a cell and the extra cellular medium, Fig. 28.4, under isobaric isothermal conditions is given by the relation:
Schematic layout of thermodynamic potential gradients near the interface of a cell. The accumulation of nanoparticles at the interface creates an entropic (F s ) and a nanothermodynamic force (F h ) across the interface. Nanoparticles are then forced inwards. A polar force (F p ) from negatively charged nanoparticles diverts them far away from the membrane
where δ(U ) is the internal energy change from polar interactions, δ(S ) is the entropic energy change from trapping of nanoparticles on the external wall of the cell [27], h i and c i are the Hill’s potential and the concentration of nano-sized particles, μ i and c i are the chemical potential and the concentration of macro-sized particles. The spatial variation of free energy \( -\frac{\delta G}{\delta x} \) is the total force F exerted on the nanoparticles at the boundary of membrane and in the absence of macro-sized particles, Fig. 28.4, and is given by the equation,
where F p , F s , F h are the electrostatic force (repulsive or attractive) between the nanoparticles and the membrane, the entropic force due to immobilization of nanoparticles at the interface, and the nanothermodynamic force due to the concentration of nanoparticles at the interface respectively, Fig. 28.4. The index j is omitted from c and h as Eq. (28.2) contains only terms which are related to nano-sized particles. The entropic and nanothermodynamic forces are directed inwards the membrane wall, while the electrostatic force of both negatively charged nanoparticles and the membrane is directed outwards the wall. Therefore both the entropic and nanothermodynamic potentials mediate nanoparticle intracellular transport within living cells.
Conclusions
Nano-sized particles of polynuclear iron (III)-hydroxide in sucrose have an increased concentration at the boundaries of the cell membrane of macrophages in vivo, compared to a uniform concentration on hydrophobic silicon wafers. The difference is attributed to the presence of entropic and nanothermodynamic potentials at interfaces that enhances aggregation of nanoparticles at interfaces.
References
Sanhai WR, Sakamoto JH, Canady R et al (2008) Seven challenges for nanomedicine. Nat Nanotechnol 3:242–244
Sousa AA, Kruhlak MJ (2013) Introduction: nanoimaging techniques in biology. Methods Mol Biol 950:1–10
Riehemann K, Schneider SW, Luger TA et al (2009) Nanomedicine: challenge and perspectives. Angew Chem Int Ed Engl 48(5):872–897
Tasciotti E, Liu X, Bhavane R et al (2008) Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol 3:151–157
Peer D, Karp JM, Hong S et al (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–760
LaVan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21(10):1184–1191
Wu Y, Sefah K, Liu H et al (2010) DNA aptamer–micelle as an efficient detection/delivery vehicle toward cancer cells. P Natl Acad Sci U S A 107:5–10
Dhar S, Gu FX, Langer R et al (2008) Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. P Natl Acad Sci U S A 105:17356–17361
Gu F, Zhang L, Teply BA et al (2008) Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. P Natl Acad Sci U S A 105:2586–2591
Dolmans DE, Fukumura D, Jain RK et al (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3:380–387
Jang WD, Nakagishi Y, Nishiyama N et al (2006) Polyion complex micelles for photodynamic therapy: incorporation of dendritic photosensitizer excitable at long wavelength relevant to improved tissue-penetrating property. J Control Release 113:73–79
Yang Z, Kang S, Zhou R (2014) Nanomedicine: de novo design of nanodrugs (review article). Nanoscale 6:663–677
Jassby D (2011) Impact of the particle aggregation on nanoparticle reactivity, Department of Civil and Environmental Engineering. Dissertation, Duke University
Pranami G (2009) Understanding nanoparticle aggregation. Dissertation, Iowa State University, Ames, Paper 10859
Zhang XF, Xu HJ (1993) Influence of halogenation and aggregation on photosensitizing properties of zinc phthalocyanine (ZnPC). J Chem Soc Faraday Trans 89:3347–3351
Siddiqui MA, Alhadlaq HA, Ahmad J et al (2013) Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PloS One 8(8):e69534
Marrache S, Dhar S (2012) Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. P Natl Acad Sci U S A 109:16288–16293
Mutter AC, Norman JA, Tiedemann MT et al (2014) Rational design of a zinc phthalocyanine binding protein. J Struct Biol 185:178–185
Kažukauskas V, Arlauskas A, Pranaitis M et al (2010) Conductivity, charge carrier mobility and ageing of ZnPc/C60 solar cells. Opt Mater 32(12):1676–1680
Thiagarajan G, Greish K, Ghandehari H (2013) Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur J Pharm Biopharm 84(2):330–334
Magalhaes MAO, Glogauer M (2010) Pivotal advance: phospholipids determine net membrane surface charge resulting in differential localization of active Rac1 and Rac2. J Leukoc Biol 87(4):545–555
Yeung T, Gilbert GE, Shi J et al (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319(5860):210–213
Trepagnier EH, Jarzynski C, Ritort F et al (2004) Experimental test of Hatano and Sasa’s nonequilibrium steady-state equality. P Natl Acad Sci U S A 101:15038–15041
Carberry DM, Reid JC, Wang GM et al (2004) Fluctuations and irreversibility: an experimental demonstration of a second-law-like theorem using a colloidal particle held in an optical trap. Phys Rev Lett 92:140601
Park BJ, Furst EM (2010) Fluid-interface templating of two-dimensional colloidal crystals. Soft Matter 6:485–488
Sarantopoulou E, Kollia Z, Cefalas AC et al (2008) Surface nano/micro functionalization of PMMA thin films by 157 nm irradiation for sensing applications. Appl Surf Sci 254:1710–1719
Cefalas AC, Sarantopoulou E, Kollia Z et al (2012) Entropic nanothermodynamic potential from molecular trapping within photon induced nano-voids in photon processed PDMS layers. Soft Matter 8:5561–5574
Acknowledgments
Partial financial support from the European Union, under the FP7-NMP-2012-LARGE-6 “CosmoPhos-Nano” project (reference number: 310337), and from the Russian Government under the Grand No. 02.A03.21.0002 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Stefi, A.L. et al. (2015). Nanothermodynamics Mediates Drug Delivery. In: Vlamos, P., Alexiou, A. (eds) GeNeDis 2014. Advances in Experimental Medicine and Biology, vol 822. Springer, Cham. https://doi.org/10.1007/978-3-319-08927-0_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-08927-0_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08926-3
Online ISBN: 978-3-319-08927-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)