Abstract
There have been remarkable advancements in treating acute coronary syndrome with different angioplasty techniques, novel antithrombotic and antiplatelet agents, and heart failure therapies using mechanical assist devices. However, most of these interventions are done in patients with complex comorbidities, which lead to an increased risk of bleeding. Anemia is one of the most prevalent coexisting conditions in patients with heart failure and acute coronary syndrome. There is growing evidence that anemia in these patient populations is an independent predictor of mortality and adverse outcomes. Increasing the hemoglobin through blood transfusion should in theory increase oxygen delivery and reduce myocardial ischemia. However, there are several risks associated with transfusion. Randomized trials in some patient populations have demonstrated that restrictive use of blood transfusion, using a hemoglobin trigger of <7 g/dL, is associated with similar or even better outcomes compared with a liberal transfusion strategy using 10 g/dL as a transfusion trigger. However, it is not clear which strategy is safest for patients with ischemic heart disease or heart failure. The aim of this chapter is to describe and attempt to understand the pathophysiology of anemia in heart failure and ischemic heart disease and summarize recent advances and evidence behind using blood transfusion to treat anemia in patients with heart disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Congestive Heart Failure
- Blood Transfusion
- Acute Coronary Syndrome
- Transfusion Strategy
- Transfusion Trigger
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Advanced congestive heart failure (CHF) and coronary artery disease (CAD) are commonly associated with anemia. Approximately 4–61 % [1–16] of patients with CHF and 10–20 % [17–19] of patients with CAD have anemia. Variability in prevalence of anemia is attributable to varying and inconsistent definition of anemia reported in each study. There is ample evidence that anemia in heart disease is associated with adverse clinical outcomes like worsening of symptoms, decreased exercise tolerance and quality of life, as well as increased hospitalization and mortality rates [20–23].
Different strategies have been tried for treating anemia in patients with heart disease, including intravenous iron, erythropoiesis-stimulating agents, and red blood cell (RBC) transfusion. The aim of this chapter is to describe and understand the pathophysiology of anemia in heart diseases and to summarize recent advances and evidence of using RBC transfusion for treating anemia in patients with heart disease, including potential risks and benefits.
4.2 Cardiac Oxygen Consumption
The heart has the highest resting oxygen consumption per tissue mass compared to other organs in our body. The resting coronary blood flow is 250 ml/min, which represents approximately 5 % of cardiac output. Also oxygen extraction, defined as the difference between arterial and venous concentrations in oxygen (CaO2–CvO2), is high in the heart, with 70–80 % compared to 25 % for the rest of the body. In addition, there is an observed fivefold increase in the oxygen consumption during any exertion like exercise. Hence, increase in oxygen consumption must be met by an increase in coronary blood flow, which is impaired in the setting of anemia due to low oxygen content.
4.3 Pathophysiology of Anemia in Heart Disease
Deficiency in new erythrocyte production relative to the rate of removal of old erythrocytes causes anemia. Erythropoietin, a glycoprotein hormone produced primarily by the kidney, plays a pivotal role in tissue oxygen delivery and red blood cell homeostasis by preventing apoptosis of progenitor red blood cells [24, 25]. Any abnormality in renal production or decreased bone marrow response to erythropoietin can result in anemia.
Many factors probably contribute to the development of anemia in heart disease, including comorbid chronic kidney disease, blunted erythropoietin production, hemodilution, advanced age, aspirin-induced gastrointestinal blood loss, the use of renin–angiotensin–aldosterone system blockers, cytokine-mediated inflammation, gut malabsorption, and iron deficiency [16, 19]. Anemia is seen commonly in patients with more severe symptoms (30–61 %) when compared with less symptomatic ambulatory populations (4–23 %) [16], but some reports indicate that anemia is also prevalent in patients with CHF and preserved ejection fraction [26–28]. Iron deficiency is reported only in <30 % of patients with heart disease, and hence most of the anemia is normocytic. Cardiorenal anemia syndrome is an important concept in CHF pathophysiology. This entity is a complex vicious cycle of congestive heart failure, chronic kidney disease, and anemia, each entity compounding the severity of the others via numerous mechanisms, some long understood, and others newly realized as explained in Fig. 4.1.
Cardiorenal anemia syndrome in congestive heart failure (Tang and Katz [16])
Anemia in CHF has multiple causes and effects. Ventricular dysfunction causes backward failure and venous congestion, producing hypervolemia with hemodilution, but also forward failure with hypoperfusion and ischemic damage to critical organs including the kidney. Advancing renal failure produces not only uremia and accelerated atherosclerosis but also decreases erythropoietin production and may be aggravated by angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. These drugs also may suppress erythropoiesis, thus aggravating similar effects of inflammatory cytokines, which are typically elevated in CHF. Uremia produces platelet dysfunction, which may aggravate aspirin-induced gastric bleeding. Bowel edema and the general debility of CHF lead to malnutrition and poor iron and vitamin absorption. This multifactorial anemia reduces capacity and, if severe enough, further stresses the compromised heart for which cardiac work is increased as part of the physiological response to anemia. It is at this arc of the vicious cycle that clinicians commonly believe that erythropoietin therapy or RBC transfusion may improve cardiac function and patient status.
4.4 Hemoglobin Triggers for Transfusion in Patients with Heart Disease
Patients with coexisting heart disease tolerate moderate normovolemic hemodilution or acute anemia well, provided that normovolemia is maintained [29–32]. However, an aggressive hemodilution, including normovolemic hemodilution, can cause myocardial ischemia that is reversible with a blood transfusion [33]. Among patients refusing any blood transfusions for religious reasons who have coexisting cardiovascular disease, postoperative hemoglobin levels below 6.0 g/dL were associated with an increased mortality and morbidity, and an increasingly greater difference in mortality and morbidity was observed between patients with and without coexisting cardiovascular diseases [34]. The question of when to transfuse an individual patient with a coexisting cardiac disease thus remains unanswered except at extremely low hemoglobin levels (e.g., <6.0 g/dL). Blood transfusions may be indicated in some anemic patients with coexisting cardiac disease [34–37].
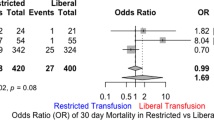
Pooled data from randomized controlled trials in heterogeneous patient populations show that restricting blood transfusions to patients whose hemoglobin drops below 7 g/dL results in a significant reduction in total mortality, acute coronary syndrome, pulmonary edema, rebleeding, and bacterial infection, compared to a more liberal transfusion strategy [38]. The number needed to treat to save one life was 33. This strategy resulted in a 40 % reduction in the number of patients receiving a blood transfusion, with an average of 2 units less per person; however, over one-half of patients were still transfused.
Observational studies have consistently shown that transfusions are associated with an increased risk for adverse events after controlling for potential confounding variables, even when using a restrictive transfusion strategy [39–41]. It has been the traditional teaching that patients with cardiac ischemia should have a more liberal transfusion strategy to maintain oxygenation, but pooled observational studies show that transfusions are associated with especially high risk when given during an acute coronary syndrome [42, 43]. For patients with non-acute cardiac disease, subgroup analysis of data from a trial in critically ill patients showed that the restrictive strategy was not associated with worse outcomes for critically ill patients with cardiovascular disease [44].
It remains impossible to determine the optimum hemoglobin/hematocrit number at which a transfusion would be indicated generally and hence guidelines published in 2006 by the American Society of Anesthesiologists which state that “the decision of red blood cell transfusions should be based on the patient’s risk of developing complications of inadequate oxygenation” is valid even for patients with coexisting cardiovascular disease [36]. It is therefore important to recognize signs of inadequate oxygenation in patients with coexisting heart diseases. Inadequate oxygenation may become manifest locally in the form of myocardial ischemia or globally in the form of a general hemodynamic instability with a tendency to hypotension and tachycardia despite normovolemia [33]. Myocardial ischemia may be detected by continuous electrocardiogram (ECG) monitoring and by transesophageal echocardiography. New ST-segment depressions of greater than 0.1 mV or new ST-segment elevations of greater than 0.2 mV for more than 1 min are generally regarded as a marker of myocardial ischemia (Table 4.1) [33]. During progressive hemodilution, one observes mostly ST-segment depression, suggesting subendocardial ischemia. In controlled studies such anemia-related ischemia is reversible by decreasing the heart rate, if elevated, and by minimal transfusion to increase the hemoglobin by 1–2 g/dL [45]. Also, new wall motion abnormalities clinically detected by transesophageal echocardiography are suggestive of myocardial ischemia and can be treated by an increase in the hemoglobin of only 1–2 g/dL.
Early signs of an inadequate circulation are a general hemodynamic instability characterized by a relative tachycardia and hypotension, an oxygen extraction rate of greater than 50 %, a low mixed-venous oxygen partial pressure (PvO2), and a decrease in oxygen consumption [33]. In a position paper of the College of American Pathologists, an oxygen extraction rate of greater than 50 %, a PvO2 less than 25 mmHg, and a reduction in oxygen consumption to less than 50 % of baseline are described as threshold values above which a blood transfusion would be indicated [35]. An oxygen extraction of greater than 50 % has been found to indicate exhaustion of compensatory mechanism in several studies and thus represents a clear transfusion indication [46, 47]. In contrast, a threshold of 25 mmHg for PvO2 appears very low, since the PvO2 decreases below the threshold of 25 mmHg only after circulatory collapse. A PvO2 threshold of 32 mmHg appears more reasonable, because oxygen consumption started to decrease at a PvO2 of 32 mmHg during progressive normovolemic hemodilution in pigs [33]. A decrease in oxygen consumption by greater than 50 % at normovolemia is certainly a transfusion indication; however, such a large reduction usually is observed only after hemodynamic collapse. Indeed, oxygen consumption decreases very late. Therefore, any decrease of greater than 10 % in oxygen consumption at low hemoglobin levels should be viewed as a potential sign of a compromised oxygenation of the organism, and a blood transfusion should be considered, provided that normovolemia has been achieved [48].
The main goal of blood transfusions is to increase oxygen-carrying capacity and mitigate myocardial ischemia, but experimental studies indicate no increase in tissue oxygenation and improvement in clinical outcomes with transfusion in any setting or with any nadir hemoglobin level [39, 49, 50]. This inability to improve oxygen uptake in vital organs is due to the hemodynamic response to increased blood viscosity as well as to chemical changes in red cells during preservation and storage, such as depletion of 2,3 diphosphoglycerate and nitric oxide, that diminish the ability of transfusion to deliver oxygen [51–55]. With millions of blood transfusions given yearly over the past century, it would be hard to calculate how many deaths may have been contributed to by transfusions. The adverse effects seen with blood transfusions, including bacterial infections, acute respiratory distress syndrome, multiorgan failure, rebleeding, and total mortality, may be due to an inflammatory response to the transfused blood product. Very little mechanistic explanation is known for no benefit or increased risk with transfusion using liberal strategy among patients with anemia and ACS. One such recent work by Silvain et al. found that blood transfusion was associated with modest but significant increase in measures of platelet reactivity and was more robust in patients previously on P2Y12 inhibitors [56]. At present, there is no randomized trial evidence that blood transfusions improve oxygen delivery or clinical outcomes in any setting, which underscores the urgent need for a randomized control trial of transfusion strategies especially in patients with ACS and CHF. There are two randomized trials, the CRIT study [57] and the MINT trial [58] examining liberal vs restrictive transfusion strategy in patients with ACS and had contrasting results. However, they had small sample sizes and were grossly underpowered to make any relevant conclusions regarding clinically important intervention effects and essentially showed divergent results on clinical outcomes such as mortality.
There remains an urgent and unmet need, as noted in recent guidelines [59], for more studies to help guide clinicians in finding optimal treatment threshold and options in the setting of anemia and bleeding in patients with ACS and CHF.
4.5 Conclusions
With the limited available evidence, we conclude that a restrictive transfusion strategy with a hemoglobin transfusion trigger of <7 g/dL might be safely practiced in patients with ischemic heart disease, including stable coronary artery disease and acute coronary syndrome unless they are symptomatic from anemia. We believe that at this threshold, benefits of transfusion probably exceed the risks. For patients who are symptomatic even at rest, hemoglobin transfusion trigger for these patients could be <8 g/dL. Also, other individual factors like severity of myocardial ischemia, plans for coronary artery revascularization, and rate of blood loss should be considered. Our conclusions are similar to European practice guidelines published recently where transfusion is recommended for hemoglobin of less than 8 g/dL or symptomatic from anemia in patients with unstable angina or non-ST-segment elevation MI [59].
References
Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–44.
Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–62.
Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–9.
Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–5.
Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol. 2003;41:1933–9.
Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–6.
Cromie N, Lee C, Struthers AD. Anaemia in chronic heart failure: what is its frequency in the UK and its underlying causes? Heart. 2002;87:377–8.
Kosiborod M, Smith GL, Radford MJ, Foody JM, Krumholz HM. The prognostic importance of anemia in patients with heart failure. Am J Med. 2003;114:112–9.
McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002;13:1928–36.
Tanner H, Moschovitis G, Kuster GM, Hullin R, Pfiiffner D, Hess OM, Mohacsi P. The prevalence of anemia in chronic heart failure. Int J Cardiol. 2002;86:115–21.
Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, O’Connor CM. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92:625–8.
Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, Chiang YT, Aknay N, Maggioni AP, Opasich C, Latini R, Cohn JN. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation. 2005;112:1121–7.
Szachniewicz J, Petruk-Kowalczyk J, Majda J, Kaczmarek A, Reczuch K, Kalra PR, Piepoli MF, Anker SD, Banasiak W, Ponikowski P. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90:303–8.
Wexler D, Silverberg D, Sheps D, Blum M, Keren G, Iaina A, Schwartz D. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96:79–87.
Maggioni AP, Opasich C, Anand I, Barlera S, Carbonieri E, Gonzini L, Tavazzi L, Latini R, Cohn J. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11:91–8.
Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–61.
Malyszko J, Bachorzewska-Gajewska H, Malyszko J, Levin-Iaina N, Iaina A, Dobrzycki S. Prevalence of chronic kidney disease and anemia in patients with coronary artery disease with normal serum creatinine undergoing percutaneous coronary interventions: relation to New York Heart Association class. Isr Med Assoc J. 2010;12:489–93.
Boyd CM, Leff B, Wolff JL, Yu Q, Zhou J, Rand C, et al. Informing clinical practice guideline development and implementation: prevalence of coexisting conditions among adults with coronary heart disease. J Am Geriatr Soc. 2011;59:797–805.
Kansagara D, Dyer E, Englander H, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159(11):746–57.
O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, et al. CHARM Committees and Investigators. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–94.
Komajda M, Anker SD, Charlesworth A, Okonko D, Metra M, Di Lenarda A, et al. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: results from COMET. Eur Heart J. 2006;27:1440–6.
da Silveira AD, Ribeiro RA, Rossini AP, Stella SF, Ritta HA, Stein R, et al. Association of anemia with clinical outcomes in stable coronary artery disease. Coron Artery Dis. 2008;19:21–6.
Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–9.
Katz SD. Mechanisms and treatment of anemia in chronic heart failure. Congest Heart Fail. 2004;10:243–7.
Bauer C, Kurtz A. Oxygen sensing in the kidney and its relation to erythropoietin production. Annu Rev Physiol. 1989;51:845–56.
Berry C, Hogg K, Norrie J, Stevenson K, Brett M, McMurray J. Heart failure with preserved left ventricular systolic function: a hospital cohort study. Heart. 2005;91:907–13.
Brucks S, Little WC, Chao T, Rideman RL, Upadhya B, Wesley-Farrington D, Sane DC. Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol. 2004;93:1055–7.
Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown Jr EJ, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8.
Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg. 1996;82:687–94.
Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93:242–55.
Herregods L, Foubert L, Moerman A, Francois K, Rolly G. Comparative study of limited intentional normovolaemic haemodilution in patients with left main coronary artery stenosis. Anaesthesia. 1995;50:950–3.
Spahn DR, Seifert B, Pasch T, Schmid ER. Effects of chronic beta-blockade on compensatory mechanisms during isovolaemic haemodilution in patients with coronary artery disease. Br J Anaesth. 1997;78:381–5.
Spahn DR, Dettori N, Kocian R, Chassot PG. Transfusion in the cardiac patient. Crit Care Clin. 2004;20(2):269–79.
Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–8.
Simon TL, Alverson DC, AuBuchon J, Cooper ES, DeChristopher PJ, Glenn GC, et al. Practice parameter for the use of red blood cell transfusions: developed by the Red Blood Cell Administration Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:130–8.
Nuttall GA, Brost BC, Connis RT, Gessner JS, Harrison CR, Miller RD, et al. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208.
Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86.
Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2013. doi:10.1016/j.amjmed.2013.09.017. pii: S0002-9343(13)00841-3.
Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27.
Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol. 2008;23(6):607–12.
Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908–14.
Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292(13):1555–62.
Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. Arch Intern Med. 2012;24:1–8.
Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29(2):227–34.
Spahn DR, Smith RL, Veronee CD, McRae RL, Hu W, Menius AJ, et al. Acute isovolemic hemodilution and blood transfusion: effects on regional function and metabolism in myocardium with compromised coronary blood flow. J Thorac Cardiovasc Surg. 1993;105:694–704.
Spahn DR, Leone BJ, Reves JG, Pasch T. Cardiovascular and coronary physiology of acute isovolemic hemodilution: a review of nonoxygen-carrying and oxygen-carrying solutions. Anesth Analg. 1994;78:1000–21.
Wilkerson DK, Rosen AL, Gould SA, Sehgal LR, Sehgal HL, Moss GS. Oxygen extraction ratio: a valid indicator of myocardial metabolism in anemia. J Surg Res. 1987;42:629–34.
Spahn DR, Schanz U, Pasch T. Perioperative transfusionskriterien. Anaesthesist. 1998;47:1011–20.
Hebert PC, McDonald BJ, Tinmouth A. Clinical consequences of anemia and red cell transfusion in the critically ill. Crit Care Clin. 2004;20(2):225–35.
Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20(2):255–68.
Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269(23):3024–9.
Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102(1):6–12.
McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102(41):14801–6.
Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8.
Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–62.
Silvain J, Abtan J, Kerneis M, Martin R, Finzi J, Vignalou JB, Barthelemy O, O’Connor SA, Luyt CE, Brechot N, Mercadier A, Brugier D, Galier S, Collet JP, Chastre J, Montalescot G. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and non-coronary patients the TRANSFUSION-2 study. J Am CollCardiol. 2014;63(13):1289–96.
Aronson D, Dann EJ, Bonstein L, et al. Impact of red blood cell transfusion on clinical outcomes in patients with acute myocardial infarction. Am J Cardiol. 2008;102(2):115–9.
Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165(6):964–971.e1.
Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B, Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Krishnamoorthy, P., Mukherjee, D., Chatterjee, S. (2015). Red Blood Cell Transfusion Trigger in Cardiac Disease. In: Juffermans, N., Walsh, T. (eds) Transfusion in the Intensive Care Unit. Springer, Cham. https://doi.org/10.1007/978-3-319-08735-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-08735-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08734-4
Online ISBN: 978-3-319-08735-1
eBook Packages: MedicineMedicine (R0)