Abstract
Idiopathic oligoasthenoteratozoospermia (iOAT) affects approximately 30 % of all infertile men. This chapter discusses recent data in this field. Age, noninflammatory functional alterations in post-testicular organs, alterations in gamete genome, mitochondrial alterations, environmental pollutants, and “subtle” hormonal alterations are all considered possible causes of iOAT. Increases in reactive oxygen species in tubules and seminal plasma, as well as apoptosis, are reputed to affect sperm concentration, motility, and morphology. iOAT is commonly diagnosed by exclusion; nevertheless, spectral traces of the main testicular artery may be used as a diagnostic tool for iOAT. The following can be considered reasonably efficient therapies for iOAT: (1) aromatase inhibitors when testosterone/17-β2-estradiol ratio is <10; (2) l-carnitine (2 g/day) in combination with acetyl-l-carnitine (1 g/day) and one 30-mg cinnoxicam tablet every 4 days; (3) recombinant follicle-stimulating hormone 75–100 UI/day when serum follicle-stimulating hormone is <2 mIU/mL.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Definition
Male idiopathic (oligo) ± (astheno) ± (terato)-spermia (iOAT) is defined as a defective spermatogenesis of obscure etiology and is regarded as undetectable using common laboratory methods [1]. iOAT can be classified from a clinical point of view as isolated astheno ± teratospermia (no alteration in sperm concentration), moderate iOAT (sperm concentration <20 × 106/mL), or severe iOAT (sperm concentration <5 × 106/mL) [2].
9.3 Etiology
Descriptions of reputed causes of iOAT have at least two biases. Two patterns whose alterations are linked to male infertility with normal sperm parameters have been described: DNA damage and alterations of polymerase mitochondrial gamma gene (POLG) [4–6] (see Chap. 10). The sum of the percentages of patients with different causes of iOAT gave a result much higher than 100 %. This finding implies that the causes overlap, that the primary cause (if any) of iOAT is still unknown, and/or that more than one cause is needed to affect sperm patterns. The most likely hypothesis is the first; it has been demonstrated that iOAT sufferers comprise at least two different populations of infertile men [7].
9.3.1 Age
There is evidence that sperm motility declines progressively after age 30 years, although there is less evidence that a similar decline in sperm volume and concentration may also occur in typical presentations [8, 9].
9.3.2 Noninflammatory Functional Alteration in Post-testicular Organs
Low seminal concentration of prostate-specific antigen, zinc, fructose, and prostatic acid phosphatase [10], and low seminal activity of neutral α-glycosidase are linked to isolated asthenospermia in addition to increased viscoelasticity [11] and osmolarity of seminal plasma [12]. Alterations of epididymal methylation of spermatogenesis-specific genes have been suspected to be involved in the etiology of iOAT [13, 14]. Demethylation is critical for gene transcription.
9.3.3 Infective Agents
Chlamydia trachomatis (CT) and adenovirus (AV) infections have been regarded as being associated with iOAT; however, proof regarding the role of asymptomatic CT and/or AV infection in infertility is inconclusive [15, 16].
9.3.4 Genetic Factors
Approximately 10 % of rat genomes are specifically linked to spermatogenesis, and about 200 genes are regarded as critical for germ cell development [17]; this means that several genes might be involved in iOAT etiology. To be considered a key factor for iOAT, a gene must display all of the following characteristics: (1) it should be specifically expressed in the germ cell line, (2) its altered expression should be associated with iOAT; and (3) it should have an essential role in spermatogenesis [18]. Despite this restriction, several genes have been identified as causes of iOAT [19, 20]. (Diaginic) heredity and de novo mutations are the theoretical causes of the bad gene expression [1].
9.3.5 Mitochondrial Alterations
In asthenospermia, both mitochondrial membrane potential [21, 22] and DNA mitochondrial content [23, 24] are impaired.
9.3.6 Subtle Hormonal Alterations
A decreased luteinizing hormone (LH) pulse frequency has been found to occur in iOAT men whose amplitude parallels the severity of the disorder [25].
Molecular variants of LH have been associated with iOAT [26].
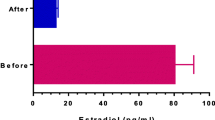
IOAT displays a shift toward lower testosterone (T) serum levels, lower calculated T index, and lower T/LH ratio, and a shift toward higher serum LH levels, higher 17-β2-estradiol (E2), and higher E2/T levels [27]. Increased E2 levels are postulated to contribute to the central suppression of gonadotropin production which, in turn, may decrease both T production and spermatogenesis [28]. E2 is derived mainly from the intratesticular and peripheral aromatization of androstenedione and T by aromatase, a product of the CYP19 gene. CYP 19A1 is a single-copy gene located on chromosome15q21.2. Aromatase polymorphisms have been shown to affect various estrogen-dependent diseases in men and women. The most commonly studied aromatase polymorphism is the tetranucleotide Tyrosine-Tyrosine-Tyrosine-Adenine [TTTA] repeat polymorphism [TTTAn] present in intron 4 of the CYP 19A1 gene. This polymorphism is associated with the activity of the aromatase enzyme both in vivo and in vitro [29]. Higher numbers of TTTA repeats (>7 repeats) in the aromatase gene are associated with a negative relationship between obesity and sperm count. The effect of obesity on E2 and sperm count appears to be absent in men with fewer (≤7) repeats [30].
9.3.7 Environmental Pollutants
Environmental pollutants are regarded as capable of deteriorating semen quality. Chapter 16 is specifically dedicated to this aspect.
9.4 Pathogenesis
The aforementioned causes affect spermatogenesis. Impaired spermatogenesis leads to increased reactive oxygen species (ROS) and unbalanced germ cell apoptosis.
9.4.1 Increased ROS
ROS originate from the cellular physiologic metabolism of O2 in aerobic conditions, and are mainly produced by leukocytes and immature gametes. Immature gametes are common findings in iOAT. ROS are short-lived chemical intermediates containing one or more electrons with unpaired spins. All spermatozoa structures can be attacked and denatured by ROS [1, 31], ultimately resulting in death and/or irreversible damage. Physiologic (low) levels of ROS exert critical function in normal sperm physiology, such as fertilizing ability (acrosome reaction, hyperactivation, capacitation, and chemotaxis) and sperm motility; whereas increased ROS generation and/or decreased antioxidant capacity leads to the imbalance between oxidation and reduction in living systems, which is called sperm oxidative stress. This condition was widely considered to be a significant contributory factor to sperm DNA damage/apoptosis, lipid peroxidation, and reduced motility, which, in turn, increased the risk of male factor infertility/subfertility and birth defects [31].
9.4.2 Modified Apoptosis
Apoptosis (programmed cell death) is a physiologic mechanism aimed at achieving optimal Sertoli cell/gamete ratio and removing damaged gametes [32]. The range of stimuli that triggers this activity is impressively broad and includes various forms of electromagnetic radiation, environmental toxicants, heavy metals, and chemotherapeutic agents [33–37]. In addition, genetic perturbation of the germ cell line occurs through, for example, overexpression of SPATA17 [38] or androgen-binding protein [39], or deletion of key genes involved in the regulation of spermatogenesis [40–42]. The impression given is that if spermatogenesis is disrupted in any way, the germ cells tend to default to an apoptotic state. The stage of spermatogenesis when apoptosis is induced appears to be predominantly pachytene spermatocytes, and the Fas (fibroblast-associated death receptor)/Fas ligand and caspase systems seems to be the major mediators of this process [34].
9.6 Therapy
Therapy for iOAT is commonly regarded as empiric, because it is not possible in the current outpatient clinical setting to define the exact etiology of the spermatogenetic disorder of each iOAT patient. A number of therapies have been proposed, the most effective of which, according to author’s experience and literature review, are reported here. Obviously these therapies might improve the sperm count in the majority of patients but not in all, and these therapies should be intended as symptomatic therapies: i.e., sperm count is improved as long as these therapies are administered, and decrease immediately after their suspension. Therapies should be administered for at least 3 months, because a stem cell requires about 61 days to achieve the final status of mature spermatozoon [43]. A rough therapeutic classification can be compiled on the basis of sperm analysis results.
9.6.1 Isolated (Astheno) ± (Terato)-Spermia
Coenzyme Q10 100 mg twice daily for at least 3 months. Coenzyme Q10 is a lipophilic antioxidant agent and should be administered after meals. Galenic preparations should use lipophilic excipients (e.g., cocoa butter) [44].
9.6.2 Oligo-Astheno-Teratospermia with Sperm Concentration >5 × 106/mL
l-Carnitine 1 g twice daily; acetyl-l-carnitine 500 mg twice daily; cinnoxicam 30 mg, one tablet every 4 days after the main meal. These drugs are antioxidant agents [45, 46].
9.6.3 All Degrees of Dyspermia with Serum Follicle-Stimulating Hormone <2 mIU/mL
Intramuscular recombinant Follicle-Stimulating Hormone (FSH) 100–300 IU every 2 days. FSH stimulates Sertoli cell function and spermatogenesis [47, 48].
9.6.4 All Degrees of Dyspermia with a low (<10) T/E2 Ratio
These dyspermias have exhibited an increased sperm count after letrozole (2.5 mg/day) and/or anastrozole (1 mg/day) treatment. Nonobstructive azoospermic patients with T/E2 ratio <10 also had their sperm count increased with letrozole and/or anastrozole treatment. Letrozole and anastrozole are members of a novel class of nonsteroidal, hormone-targeting agents used for breast cancer therapy. They reversibly inhibit the aromatase enzyme, which converts the androgen precursors in adipose tissue to E2. Blocking of estrogen production has been shown to provoke increased gonadotropin and androgen levels in the blood and a parallel E2 decrease, resulting in spermatogenesis stimulation [49, 50].
9.7 Prognosis
Prognosis is difficult to define in these patients, mainly because of the empiric nature of the therapies. However, antioxidant drugs and aromatase inhibitors significantly lower the number of couples that might require treatment with assisted reproduction to achieve a pregnancy [51].
References
Cavallini G (2006) Male idiopathic oligoasthenoteratospermia. Asian J Androl 8:143–157
World Health Organization (2010) WHO manual for the examination and processing of human semen, 5th edn. Cambridge University Press, Cambridge
Burton A (2013) Study suggests long-term decline in French sperm quality. Environ Health Perspect 121:46–47
Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, Thomas AJ Jr, Sharma RK (2002) Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril 78:313–318
Jensen M, Leffers H, Petersen JH, Nyboe Andersen A, Jørgensen N, Carlsen E, Jensen TK, Skakkebaek NE, Rajpert-De Meyts E (2004) Frequent polymorphism of the mitochondrial DNA polymerase gamma gene [POLG] in patients with normal spermiograms and unexplained subfertility. Hum Reprod 19:65–70
Esteves SC (2013) A clinical appraisal of the genetic basis in unexplained male infertility. J Hum Reprod Sci 6:176–182
Cavallini G, Crippa A, Magli MC, Cavallini N, Ferraretti AP, Gianaroli L (2008) A study to sustain the hypothesis of the multiple genesis of oligoasthenoteratospermia in human idiopathic infertile males. Biol Reprod 79:667–673
Kidd SA, Eskenazi B, Wyrobek AJ (2001) Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 75:237–248
Zhu QX, Meads C, Lu ML, Wu JQ, Zhou WJ, Gao ES (2011) Turning point of age for semen quality: a population-based study in Chinese men. Fertil Steril 96:572–576
Carpino A, Sisci D, Aquila S, Salerno M, Siciliano L, Sessa M, Andò S (1994) Adnexal gland secretion markers in unexplained asthenozoospermia. Arch Androl 32:37–43
ELzanaty S, Malm J, Giwercman A (2004) Visco-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. Int J Androl 27:94–100
Rossato M, Balercia G, Lucarelli G, Foresta C, Mantero F (2002) Role of seminal osmolarity in the reduction of human sperm motility. Int J Androl 25:230–235
Ariel M, Robinson E, McCarrey JR, Cedar H (1995) Gamete-specific methylation correlates with imprinting of the murine Xist gene. Nat Genet 9:312–315
Rotondo JC, Selvatici R, Di Domenico M, Marci R, Vesce F, Tognon M, Martini F (2013) Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics 8:990–997
Eggert-Kruse W, Rohr G, Demirakca T, Rusu R, Näher H, Petzoldt D, Runnebaum B (1997) Chlamydial serology in 1303 asymptomatic subfertile couples. Hum Reprod 12:1464–1475
Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W (2012) Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod 27:770–778
Schlecht U, Demougin P, Koch R, Hermida L, Wiederkehr C, Descombes P, Pineau C, Jégou B, Primig M (2004) Expression profiling of mammalian male meiosis and gametogenesis identifies novel candidate genes for roles in the regulation of fertility. Mol Biol Cell 15:1031–1043
Mäkelä S, Eklund R, Lähdetie J, Mikkola M, Hovatta O, Kere J (2005) Mutational analysis of the human SLC26A8 gene: exclusion as a candidate for male infertility due to primary spermatogenic failure. Mol Hum Reprod 11:129–132
O’Flynn O’Brien KL, Varghese AC, Agarwal A (2010) The genetic causes of male factor infertility: a review. Fertil Steril 93:1–12
Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C (2007) Male infertility: role of genetic background. Reprod Biomed Online 14:734–745
Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, Fujie M, Suzuki K, Hirata S, Hoshi K (2002) Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl 4:97–103
Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P (2004) Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod 19:2267–2276
Kao SH, Chao HT, Liu HW, Liao TL, Wei YH (2004) Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil Steril 82:66–73
Song GJ, Lewis V (2008) Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil Steril 90:2238–2244
Odabas O, Atilla MK, Yilmaz Y, Sekeroglu MR, Sengul E, Aydin S (2002) Luteinizing hormone pulse frequency and amplitude in azoospermic, oligozoospermic and normal fertile men in Turkey. Asian J Androl 4:156–158
Ramanujam LN, Liao WX, Roy AC, Ng SC (2000) Association of molecular variants of luteinizing hormone with male infertility. Hum Reprod 15:925–928
Andersson AM, Jørgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE (2004) Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab 89:3161–3167
Zhang Q, Bai Q, Yuan Y, Liu P, Qiao J (2010) Assessment of seminal estradiol and testosterone levels as predictors of human spermatogenesis. J Androl 31:215–220
Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A (2004) A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 89:2803–2810
Hammoud AO, Griffin J, Meikle AW, Gibson M, Peterson CM (2010) Association of aromatase [TTTAn] repeat polymorphism length and the relationship between obesity and decreased sperm concentration. Hum Reprod 25:3146–3151
Chen SJ, Allam JP, Duan YG, Haidl G (2013) Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288:191–199
Aitken RJ, Baker MA (2013) Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol 57:265–272
Alam MS, Ohsako S, Tay TW, Tsunekawa N, Kanai Y, Kurohmaru M (2010) Di[n-butyl] phthalate induces vimentin filaments disruption in rat Sertoli cells: a possible relation with spermatogenic cell apoptosis. Anat Histol Embryol 39:186–193
Li YJ, Song TB, Cai YY, Zhou JS, Song X, Zhao X, Wu XL (2009) Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci 108:427–436
Shaha C, Tripathi R, Mishra DP (2010) Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci 365:1501–1515
Wang C, Cui YG, Wang XH, Jia Y, Sinha Hikim A, Lue YH, Tong JS, Qian LX, Sha JH, Zhou ZM, Hull L, Leung A, Swerdloff RS (2007) Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab 92:3292–3304
Xu G, Zhou G, Jin T, Zhou T, Hammarström S, Bergh A, Nordberg G (1999) Apoptosis and p53 gene expression in male reproductive tissues of cadmium exposed rats. Biometals 12:131–139
Nie DS, Liu Y, Juan H, Yang X (2013) Overexpression of human SPATA17 protein induces germ cell apoptosis in transgenic male mice. Mol Biol Rep 40:1905–1910
Jeyaraj DA, Grossman G, Petrusz P (2003) Dynamics of testicular germ cell apoptosis in normal mice and transgenic mice overexpressing rat androgen-binding protein. Reprod Biol Endocrinol 12:48–49
Gutti RK, Tsai-Morris CH, Dufau ML (2008) Gonadotropin-regulated testicular helicase [DDX25], an essential regulator of spermatogenesis, prevents testicular germ cell apoptosis. J Biol Chem 283:17055–17064
Kosir R, Juvan P, Perse M, Budefeld T, Majdic G, Fink M, Sassone-Corsi P, Rozman D (2012) Novel insights into the downstream pathways and targets controlled by transcription factors CREM in the testis. PLoS One 7(2):e31798. doi:10.1371/journal.pone.0031798
Liu Z, Zhou S, Liao L, Chen X, Meistrich M, Xu J (2010) Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J Biol Chem 285:2758–2770
Anawalt BD (2013) Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab 98:3532–3542
Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S (2012) Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol 188:526–531
Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G (2004) Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl 25:761–770
Cavallini G, Magli MC, Crippa A, Ferraretti AP, Gianaroli L (2012) Reduction in sperm aneuploidy levels in severe oligoasthenoteratospermic patients after medical therapy: a preliminary report. Asian J Androl 14:591–598
Foresta C, Bettella A, Merico M, Garolla A, Ferlin A, Rossato M (2002) Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil Steril 77(2):238–244
Paradisi R, Natali F, Fabbri R, Battaglia C, Seracchioli R, Venturoli S (2013) Evidence for a stimulatory role of high doses of recombinant human follicle-stimulating hormone in the treatment of male-factor infertility. Andrologia. doi:10.1111/and.12194. [Epub ahead of print]
Schlegel PN (2012) Aromatase inhibitors for male infertility. Fertil Steril 98:1359–1362
Cavallini G, Biagiotti G, Bolzon E (2013) Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl 15:806–811
Comhaire F, Decleer W (2012) Comparing the effectiveness of infertility treatments by numbers needed to treat (NNT). Andrologia 44:401–404
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cavallini, G. (2015). Male Idiopathic (Oligo) ± (Astheno) ± (Terato)-Spermia. In: Cavallini, G., Beretta, G. (eds) Clinical Management of Male Infertility. Springer, Cham. https://doi.org/10.1007/978-3-319-08503-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-08503-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08502-9
Online ISBN: 978-3-319-08503-6
eBook Packages: MedicineMedicine (R0)