Abstract
The quantification of the soil-to-plant transfer by means of transfer factor or concentration ratios values presents high range of variation, about 4–5 orders of magnitude for radiocesium. This range can be partially explained by the different association of radionuclides to soil particles, which can be assessed by speciation procedures. The fact that there are a lot of speciation procedures in the literature may, in some occasions, make its interpretation difficult. The source of radionuclides is a major factor influencing the speciation. The anthropogenic radionuclides associated with fuel particles present usually low mobility, although they can be weathered with time. Regarding the radionuclides released in the global fallout, the 90Sr is the most bioavailable. Plutonium is usually associated with organic matter. The mobility and bioavailability of radiocesium depends on the soil clay content, and is also time dependent. The naturally occurring radionuclides in soil are mainly associated with fractions strongly fixed, because they mainly occur in minerals forming part of soil particles. The speciation of soil can also be modified by agricultural procedures, such as the addition of fertilizers or phosphogypsum. The fertilization can be used to reduce the soil-to-plant transfer of radiocesium and radiostrontium by supplying stable elements, potassium, and calcium, respectively, so that their content in soil solution decreased. The phosphate-based fertilizers have also naturally occurring radionuclides, which can be transferred to plants. Phosphogypsum, which can contain high levels of radium, is used as soil amendment. However, its radium content is mainly associated with immobile fractions, and its transfer to plants is of the same order of magnitude than without phosphogypsum amendment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As consequence of the atom bomb blasts during World War II, anthropogenic radionuclides were first released into the environment. Later, during the 1950s and 1960s, there were a great number of atmospheric nuclear weapons tests, which released huge amounts of radionuclides into the atmosphere. Due to atmospheric circulation, they became distributed worldwide and were ultimately deposited onto the soil (UNSCEAR 2000). Once they were on the surface of soil, they began to interact with the different soil components by different processes, and also to migrate downwards as consequence of weathering and percolation. These processes depended on the chemical properties of the radionuclide, the physicochemical properties of the soil on which they were deposited, climate, latitude, among others. As result, the radionuclides were associated with different degrees to soil particles, and partially able to be transferred to plants, and from them to animals and humans.
Naturally occurring radionuclides are also present in the environment, mainly associated with different minerals, and forming part of soil particles. Uranium, thorium, their descendants in the natural decay series, and 40K are such radionuclides. As they are present in the soil, they also are susceptible to be transferred to plants and enter the food chain. The concentration of these naturally occurring radionuclides in the soil depends mainly on geological reasons. There are minerals with high content of these radionuclides, which serve as raw materials for industrial processes. These industries are usually denominated naturally occurring radioactive material (NORM) industries. The production of fertilizers from phosphate rock, coal-fired power plants, and oil and gas extractions are some examples of NORM industries (IAEA 2003). There are other naturally occurring radionuclides of cosmogenic origin, such as 7Be, and 22Na, but their transfer to plants is more limited.
2 Quantification of Radionuclide Transfer to Plants
The first approach to quantify the transfer of radionuclides to plants is based on the so-called transfer factors, TF, transfer coefficients, TC, or concentration ratios, CR, which are usually defined as the ratio between the radionuclide content in plant and the radionuclide content in soil (see Eq. 1). Other way of quantification, the aggregated transfer factors, TFagg, has also been used when a nuclear accident occurred. The TFagg can be defined as the ratio between the radionuclide content in plant and the radionuclide deposition on soil (see Eq. 2).
Radiocesium is the long-lived anthropogenic radionuclide most widely studied, because its release was the highest due to global fallout and other nuclear accidents (UNSCEAR 2000). Table 1 shows the range of TF values reported in the literature for radiocesium to different plant groups worldwide. These values varied within five orders of magnitude, which suggested that it is affected by many variables, such as the type of soil in which they were grown (Nisbet and Shaw 1994; IAEA 2010) and the kind of plant considered (Nisbet and Shaw 1994; Korobova et al. 1998; IAEA 2010). It can also be observed that the radiocesium transfer to different biological compartments presented variations. The maximum value of transfer to stems and shoots was higher than to grain for cereals. It also occurred for root crops, in which the transfer to root was higher than to leaves. The radiocesium transfer also depended on the nutrients available in the soil and the nutritional requirements of each plant. The TFagg values varied within the range 0.0006–0.47 m2 kg−1 (Drissner et al. 1998; Strandberg 1994; Bunzl et al. 1999; Fesenko et al. 2001), which showed a variation of about four orders of magnitude.
However, there are other anthropogenic and naturally occurring radionuclides that are in the environment and able to be up taken by plants. Table 2 shows their range of variation for grass, as an example of reference animal and plant (RAP) (ICRP 2009). The different chemical properties of radionuclides also influenced their transfer from soil to plant, depending on the nutritional requirements. The soil-to-plant transfer to plant decreased in the following order:
The quantification of the process of radionuclide transfer from soil to plant using these transfer factors has some limitations. This approach assumes that the transfer process is steady, without any changes with time, which can be assumed for boreal ecosystems. However, in Mediterranean ecosystems, in which dry and wet seasons alternate, the transfer of radionuclides showed a seasonal dependence reflecting the availability of nutrients (Baeza et al. 2001; Schuller et al. 2005). Another problem with these definitions is the concept of the radionuclide content of soil. It is not well specified, and it can raise some doubts about the depth to be considered: surface soil (0–5 cm), the layer of soil in which roots are located, etc.
In these definitions, the total fraction of radionuclides in soil is usually considered. But, once the radionuclides were deposited on soil, they began to react with soil particles and a process of immobilization occurred, depending on the physicochemical properties of radionuclides and soil, and the chemical form in which they were released into the environment. Therefore, not all radionuclides, but a fraction of them, would be able to be transferred to plant.
3 Speciation Schemes of Radionuclides in Soil
The association of radionuclides to soil particles is usually assessed by the use of speciation schemes or sequential extraction procedures. They can be classified as either methods based on a sequential extraction of soil with selective reagents or those in which the soil is sequentially treated with chemical solutions of increasing replacement/dissolving power (Alexakhin and Krouglov 2001). One of the major disadvantages of this technique is that there is no unified procedure to carry out. In the literature, there are a great variety of procedures, many of them with similar reagents and/or concentrations, but used in different order. Table 3 shows the reagents and steps used in different speciation schemes reported in the literature. Most of them are based on that proposed by Tessier et al. (1979) for metal speciation. As it can be observed in Table 3, there is a great variation on the number of steps and reagents involved in each speciation scheme.
The use of speciation schemes gives extremely useful information about the association of radionuclides to the different components of the soil. However, one of its major problems is the interpretation of the data obtained. Although the reagents are usually designed to attack a single geochemical phase, they are not completely specific (Schultz et al. 1998). The use of denominations for some fractions can sometime be confusing or misleading. This can lead to the fact that the application of different speciation schemes in soils gave different results (Blanco et al. 2004).
The soil solution is one of the easiest fractions to extract in speciation schemes. It consists of water present within pores between soil particles, which contain a great variety of chemical compounds, colloids, and suspended particles. Therefore, it is considered to be an important medium for the transfer of radionuclides and nutrients from soil to plants. The extraction of soil solution from the soil can be carried out by different techniques: using porous ceramic cup samplers (Nisbet et al. 1993a), by addition of distilled water and subsequent centrifugation (Agapkina and Tikhomirov 1994; Agapkina et al. 1995; Amano et al. 1999), or by use of a disk impregnated with a specific resin in direct contact with the soil (Jouve et al. 1999). As one method for its extraction is the water addition, the soil solution can be expected to be similar to the water-soluble fraction defined in some sequential speciation procedures. The association of radionuclides to different compounds detected in soil solution has also been reported. Size and charge fractionation techniques are used to separate the chemical species present according to their nominal molecular mass. High-molecular weight (HMW) fraction (MW > 10 kDa) consist of nanoparticles, colloids, polymers, pseudocolloids, etc., which are assumed to be mobile in water due to mutual repulsion and Brownian movement. Low-molecular weight (LMW) fraction (MW < 10 kDa, and Ø < 1 nm) consist of single compounds, inorganic and organic ions, complexes, molecules, etc. The LMW species are considered to be mobile and potentially bioavailable depending on their charge properties and lipophilic characteristics. Radiostrontium and radiocesium were mainly associated with the LMW fraction, although in different ranges, 90Sr in the fraction about 400 Da and 137Cs in the 800–1,100 Da (Nisbet et al. 1993a; Agapkina and Tikhomirov 1994; Agapkina et al. 1995; Amano et al. 1999). Plutonium and americium were present mainly in the HMW fraction (Agapkina et al. 1995). The content of each radionuclide in the soil solution was not the same, and decreased in the following order: 90Sr > 137Cs > 239,240Pu ≥ 241Am (Agapkina et al. 1995).
In some schemes, the water-soluble fraction can be omitted because it may be considered that the following fraction, usually exchangeable, is also able to extract the radionuclides associated with the water-soluble fraction. The radionuclide content of the exchangeable fraction is associated with ionic exchange sites in soil, which are supposed to the readily available. The extractants frequently used for this fraction are NH4AcO, MgCl2, CaCl2, EDTA, DTPA, NH4NO3, KCl, or NaNO3 (Kennedy et al. 1997; Komosa 2002; Rigol et al. 2002). The K+ and NH4 + ions are considered to be competitive with Cs+ in soils, and therefore able to desorb them effectively. The NH4OAc 1 M at pH 7 is considered to be a robust extractant for acidic or neutral soils but unsuitable for alkaline soils (Kennedy et al. 1997). However, it can be buffered into the soil pH to extend the range of pH in which it can be used. In aquatic sediments, it has the drawback that may also attack the carbonates present. MgCl2 1 M at pH 7 was found to extract less carbonates than NH4AcO (Kennedy et al. 1997). Divalent ions are also able to desorb Cs+ from nonspecific sites, but are considered to be less effective in desorbing Cs+ from clay interlayers (Rigol et al. 2002).
In the Tessier scheme (Tessier et al. 1979), there are also other fractions considered. The carbonated fraction can be extracted with sodium acetate in acetic acid (Tessier et al. 1979). The reducible fraction, also named in some procedures bound to Fe and Mn oxides, are obtained by the application of a reducing extractant, generally NH2OH·HCl (Tessier et al. 1979; Riise et al. 1990; Schultz et al. 1998; Komosa 2002).
The association of radionuclides with organic matter present in the soil can be assessed by using different reagents. The oxidable organic matter is usually extracted with H2SO4, H2O2, and NaClO (Tessier et al. 1979; Riise et al. 1990; Fawaris and Johanson 1995). The hydrolysis with H2SO4 can break down the cellulose and hemicellulose complexes present in the soil. The NaClO was able to extract more radiocesium than H2O2, and it was also observed that the soil minerals were less destructed (Vinichuk et al. 2005). On the other hand, the organic acids present in soil, mainly humic and fulvic acids, can be extracted using Na4P2O7 and NaOH (Kononova 1982; Lee and Lee 2000). These reagents are able to precipitate or form soluble complexes with Ca, Fe, Al, and other polyvalent cations to which humic substances are bound (Kononova 1982). Humic and fulvic acids are extracted together, and they can be separated by adjusting the solution pH to 2. Humic acids precipitate while fulvic acids remain soluble (Lee et al. 2000). Organic extractants can also be used to extract radionuclides from humic and fulvic acids present in soils, such as trimethylchlorosilane and triethylchlorosilane in the presence of dimethylformamide (Szabo et al. 1991). It was based on the modification of the solubility of humic substances by the silylation of the carboxylic, phenolic, alcoholic, and amino moieties of humic and fulvic acids (Szabo et al. 1991).
Some speciation schemes use inorganic acids in advanced steps. The use of dilute inorganic acids, as HCl 1 M, can remove cations from exchange complexes in the soil, and also dissolve oxides, hydroxides, carbonates, and some alkaline earth compounds. This fraction can be considered as potentially available, whereas the use of concentrated mineral acids (HCl 6 M, HNO3 7 M or 8 M) are able to extract cations from interlayer or structural positions not readily accessed by exchange reactions (Krouglov et al. 1998). Finally, the residual fraction represents the radionuclides strongly attached to soil particles, which have the lowest probability to be available.
4 Factors Affecting the Speciation of Radionuclides in Soil
The speciation of radionuclides in soil can be influenced by different factors, such as the chemical form and time elapsed since the deposition occurred, the physicochemical properties of soil, its clay and organic matter content, the climate, etc. The chemical form in which the radionuclides were deposited is of crucial importance for their speciation. If the anthropogenic radionuclides released were associated with refractory particles of fuel, its presence in more mobile and bioavailable fractions is expected to be limited. As a way of example, in Fig. 1 shows the speciation of 239+240Pu in the fractions exchangeable, extracted with MgCl2, associated with organic matter, extracted with NaClO, and residual in two ecosystems affected by different fallout: global fallout and Palomares accident in 1966. The 239+240Pu in Palomares ecosystem was almost exclusively associated with the residual fraction; while in the ecosystem affected by global fallout it was mainly associated with the organic matter fraction followed by residual and exchangeable (Baeza et al. 2004). The association of plutonium with organic matter has also been reported by other authors using other reagents, Na4P2O7 and oxalic acid/ammonium oxalate, and H2O2 (Cook et al. 1984; Komosa 2002). The humic substances in soils, mainly composed of organic acids, were also able to bound plutonium and americium efficiently (Amano et al. 1999; Lee and Lee 2000). The plutonium associated with humic acids was greater than that associated with fulvic acids (Lee and Lee 2000).
Speciation of 239+240Pu in two ecosystems affected by different contamination events: global fallout and Palomares accident. The fractions considered were exchangeable, extracted with MgCl2, associated with organic matter, extracted with NaClO, and residual. Data from Baeza et al. (2004)
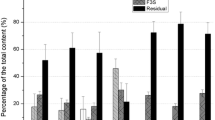
Figure 2 shows the speciation of anthropogenic (137Cs, 90Sr, 239+240Pu, 241Am) and naturally occurring radionuclides (40K, 238U, 226Ra, 210Po, 232Th) in an ecosystem whose source term was mainly global fallout. The scheme used was a modification of that of Pavlotskaya (1974). The fractions considered was exchangeable (extracted with NH4AcO), diluted acid (extracted with HCl 1 M), concentrated acid (extracted with HCl 6 M), and residual. The distribution pattern for anthropogenic and naturally occurring radionuclides was different. The latter were mainly associated with the residual fraction, as consequence of forming part of minerals in soil particles. Only 210Po, presented values higher than 20 % in the diluted-acid and concentrated-acid fractions. The distribution pattern of anthropogenic radionuclides depended on which one was considered. The 90Sr was mainly associated with the exchangeable fraction followed by diluted- and concentrated-acid fractions and residual. The association of radiostrontium with exchangeable fraction, which is usually considered as mobile and able to be transferred to plants, can explain its greater downward migration in soils than other anthropogenic radionuclides (Kagan and Kadatsky 1996; Forsberg et al. 2000). In areas close to Chernobyl Nuclear Power Plant (NPP), 90Sr was observed to be associated with fuel particles, thus being associated with acid-digestible and residual fractions (Krouglov et al. 1998). However, the weathering of the fuel particles released 90Sr into the environment, depending on the soil acidity, and was observed an increase of the exchangeable fraction (Krouglov et al. 1998).
The 137Cs was mainly associated with concentrated-acid and residual fractions, being the exchangeable fraction lower than that of 90Sr. This distribution pattern was also observed in other ecosystems (Riise et al. 1990; Forsberg et al. 2001). The radiocesium associated with exchangeable fraction (extracted with NH4OAc) in soils was found to be within the range 1.8–29 % of the total content of soil (Riise et al. 1990; Bunzl et al. 1997; Lee and Lee 2000; Forsberg et al. 2001; Vinichuk et al. 2005). The exchangeable fraction was observed to decrease while increasing the lapsus of time since the deposition of radiocesium occurred (Cheshire and Shand 1991; Krouglov et al. 1998; Baeza et al. 1999; Forsberg et al. 2001). This is usually known as aging effect, and is associated with the irreversible sorption of cesium in clay at frayed edge sites (FES). The selective adsorption of FES can be attributed to the small hydratation energy of cesium ions. The energy for desorption was found to be so large that desorption was energetically unfavorable (Stauton et al. 2002). The FES selectiveness for monovalent cations decrease in the order: Cs+ > NH4 + > Rb+ > K+ > Na+ > Li+ (Rigol et al. 2002; Stauton et al. 2002). The K+ content in soil can cause the collapse of the expanded interlayers, tapping the cesium binded inside (Rigol et al. 2002).
The distribution pattern of plutonium and americium in this soil (Fig. 2) was similar. They were mainly associated with the concentrated-acid fraction, followed by diluted-acid, residual and exchangeable fractions.
5 Bioavailability of Radionuclides
The data obtained from the speciation schemes can be used to analyze the soil-to-plant transfer of radionuclides. The bioavailable fraction of radionuclides in soil may be defined as the fraction of them able to be transferred to plants. Positive correlations between the transfer factor and bioavailable fraction have been observed for radiocesium (Fesenko et al. 2001). The water soluble and exchangeable fractions are usually considered to be very mobile in soil and, therefore, readily available to be transferred. Other approach is to consider that only the fractions not strongly attached to soil particles would be able to be transferred. The diluted-acid fraction would be included in the latter, as it is considered potentially available or mobile (Fesenko et al. 2001; Baeza et al. 2006). The diluted-acid fraction (HCl 1 M) is able to dissolve oxides, hydroxides, carbonates, and some alkaline earth compounds present in the soil. The carbonated fraction in the Tessier procedure, (Tessier et al. 1979), extracted with sodium acetate in acetic acid, may also be considered similar to diluted-acid fraction, and potentially available (Baeza et al. 2014). The bioavailable fractions, either readily or potentially available, for the anthropogenic and naturally occurring radionuclides in an ecosystem affected mainly by global fallout (see Fig. 2) are usually as follows:
-
Readily available:
90Sr \(\gg\) 137Cs, 226Ra, 239+240Pu > 238U, 241Am > 40K > 210Pb > 232Th
-
Potentially available:
90Sr \(\gg\) 241Am > 210Pb, 239+240Pu > 226Ra > 137Cs > 238U > 40K > 232Th
6 Modification of the Speciation of Radionuclides in Soil
The speciation of radionuclides in soil is not constant with time, due to weathering and the interaction with different soil components (Cheshire and Shand 1991; Krouglov et al. 1998; Baeza et al. 1999; Forsberg et al. 2001). It can also be modified by the addition of soil amendments and/or fertilizers, which can contain high content of naturally occurring radionuclides and other stable elements that can modify the soil equilibrium. In fact, fertilizers have been used as agricultural countermeasures to inhibit or at least decrease the transfer of anthropogenic radionuclides into produces. Their use is based on the saturation of the soil solution with the additional supply of nutrients chemically analogous to the released radionuclides from the fertilizers (Nisbet et al. 1993b); i.e., potassium for radiocesium and calcium for radiostrontium.
The application of potassium-based fertilizers decreases the 137Cs:K ratio in the soil solution (Nisbet et al. 1993b; Zhu and Shaw 2000). At lower concentrations of potassium in soil solution (<10−2–10−1 µM), the root uptake mechanism is unable to distinguish between Cs+, Rb+, and K+ (Nisbet et al. 1993b; Shaw 1993; Zhu et al. 2000). However, at higher concentrations a critical threshold was observed, above which the root uptakes K+ preferentially to Cs+, 20 μM for wheat (Shaw et al. 1993). The application of these fertilizers reduced the soil-to-plant transfer of radiocesium about 40–60 % (Jacob et al. 2009; Rosén et al. 2011), and it was also observed during long periods of time, 10–34 year after fertilization (Kaunisto et al. 2002; Robison et al. 2009; Rosén et al. 2011). The addition of fertilizers that supply NH4 + can also modify the content of 137Cs in the soil solution, increasing it by a factor of 3–4 (Nisbet et al. 1993b). The application of NH4 + and manure can also reduce the uptake of 137Cs, due probably to the release of potassium and other ions from the manure when NH4 + is applied (Fuhrmann et al. 2003). The addition of phosphate stimulates root growth and may also increase the cesium uptake (Shaw 1993). The application of calcium to soils in order to reduce the radiostrontium transfer is usually by liming. Although the reduction is more limited than that of radiocesium, about 20 % of 90Sr (Lembrechts 1993), because in some occasions the application of 1.6–15.6 ton Ca ha−1 was not able to modify significantly the calcium content in the soil solution (Vidal et al. 2001; Camps et al. 2004). The highest reductions were obtained in soils with low-calcium content (Shaw 1993).
Fertilizers, in particular those based on phosphate, can also have high contents of naturally occurring radionuclides, due to the initial content of phosphate rock and the industrial processes carried out in their production. Their worldwide range in NPK fertilizers are within (66–1,710) Bq kg−1 for 238U, (1.5–451) Bq kg−1 for 226Ra, (78–741) Bq kg−1 for 210Pb, (28–307) Bq kg−1 for 232Th, and (25–4,100) Bq kg−1 for 40K (Mustonen 1985; Barišić et al. 1992; Righi et al. 2005; Saueia et al. 2005; Chandrajith et al. 2010; Chauhan et al. 2013). After the fertilizer application, interactions between it and the different soil components occur. There are many methods for analyzing the association of nutrients to fertilizers (CEE 1997), which can also be used to analyze the naturally occurring radionuclides in those fractions. The one used for extraction of water-soluble phosphorus in fertilizers consists in adding 5 g dw of fertilizer to 450 mL of distilled water at room temperature (20–25 °C), and stirred for 30 min (CEE 1977). The water-soluble fraction of a NPK fertilizer (NPK S (MgS) 6–10–18 S(3–36) with potassium from sulfates) showed that content of 238U in that fraction was the highest, about 10 %, followed by 210Po (8 %), and 226Ra (1.6 %), being the 232Th below detection limit (Baeza et al. 2011). However, this fraction can be leached by runoff water into surface and groundwater resources. Other chemical species, such as phosphates, nitrates, and sulfates, also showed increased levels in water bodies contaminated by use of fertilizers, leading to the eutrophication of water bodies (Zielinski et al. 1997; Badruzzaman et al. 2012). Although the application of phosphate-based fertilizers for long periods of time can increase the naturally occurring radionuclide content in soil, the uranium, radium, and thorium content in corn, leaves, grain, and wheat grown in lands using NPK fertilizer for years have been reported similar to those grown in non-fertilized (IAEA 2003).
Other by-products of the phosphate fertilizers industry (NORM), such as phosphogypsum, are also used as soil amendment. The content of radium, 210Po, and 210Pb in phosphogypsum is enriched due to the industrial processes usually carried out (Rutherford et al. 1994). They are usually accumulated in stacks, where are subject to weathering. The stack fluids might be considered as the fraction which has more similar to water-soluble fraction. The 226Ra content in those fluids was lower than other naturally occurring radionuclides, such as uranium and 210Pb (Burnett et al. 1996). The leaching of 226Ra by rainwater has also been found to be a slow process (Haridasan et al. 2002). The 226Ra was mainly associated with fine grains of phosphogypsum and very immobile (Rutherford et al. 1994; Hull and Burnett 1996; Hull and Burnett 1996). The TF values of 226Ra from soil amended with phosphogypsum to rice was of the same order of magnitude that the TF without phosphogypsum (Papastefanou et al. 2006).
7 Conclusion
The quantification of the soil-to-plant transfer of radionuclides, either anthropogenic or naturally occurring, by means of transfer factors or concentration ratios has some limitations. This is especially important to the ratios between what is in the plant and the soil consider the total content of radionuclides. Thus, their range of variation is about four orders of magnitude for the same plant groups. Soil dependence on the TF values has also been reported, which is correlated with the association of radionuclides to soil particles, and therefore their availability to be transferred.
The assessment of this association is usually carried out by speciation procedures, in which the soil is sequentially attacked by reagents with more replacement power. Water-soluble fraction and exchangeable fractions are usually the first fractions to be considered. The reagents used in later fractions depend on the characteristics of the study to be carried out. There a lot speciation schemes, some of them variations of the Tessier method (Tessier et al. 1979), which can make difficult the interpretation of the data. The results obtained by these procedures depend also on the radionuclide source, i.e., on the chemical form in which they were deposited. When they are attached to refractory particles, they are strongly attached to them, but this situation can vary with time. In the case of global fallout origin, the 90Sr is the radionuclide which content is highest in the majority of the soil. The radiocesium is usually attached to clay minerals, which govern its bioavailability, lower than that of 90Sr. Plutonium is frequently associated with organic matter in the soil. Naturally occurring radionuclides are mainly associated with fractions strongly fixed to the soil matrix, because they are found in minerals forming part of soil particles.
The use of fertilizers and soil amendments in agricultural practices can also modify the speciation of radionuclides in soil. This has been used in remediation actions by supplying stable elements chemically analogous to anthropogenic radionuclides, i.e., potassium to reduce the radiocesium transfer and calcium to reduce that of radiostrontium. Fertilizers, especially those produced from phosphate rock, can contain significant levels of naturally occurring radionuclides, which can also be transferred to plants. The radionuclides associated with the water-soluble fraction of the fertilizers can be leached out by runoff water and into water bodies. Phosphogypsum, a by-product of the phosphate fertilizer industry, can also be used as soil amendment, and usually presents a high level of radium. Its use did not increase the transfer of radium to plants.
References
Alexakhin RM, Krouglov SV (2001) Soil as the main compartment for radioactive substances in terrestrial ecosystems. In: Bréchignac F, Howard BJ (eds) Radioactive pollutants. Impact on the environment. EDP Sciences, Les Ulis, France
Agapkina GI, Tikhomirov FA (1994) Radionuclides in the liquid phase of the forest soils at the Chernobyl accident zone. Sci Total Environ 157:267–273
Agapkina GI, Tikhomorov FA, Shcheglov AI (1995) Association of Chernobyl-derived 239+240Pu, 241Am, 90Sr and 137Cs with organic matter in the soil solution. J Environ Radioactiv 29:257–269
Amano H, Matsunaga T, Nagao S, Hanzawa Y, Watanabe M, Ueno T, Onuma Y (1999) The transfer capability of long-lived Chernobyl radionuclides from surface soil to river water in dissolved forms. J Org Chem 30:437–442
Badruzzaman M, Pinzon J, Oppenhaimer J, Jacabgeli JG (2012) Sources of nutrients impacting surface waters in Florida: a review. J Environ Manag 109:80–92
Baeza A, Paniagua JM, Rufo M, Sterling A, Barandica J (1999) Radiocesium and radiostrontium uptake by turnips and broad beans via leaf and root absorption. Appli Radiat Isot 50:467–474
Baeza A, Paniagua J, Rufo M, Guillén J, Sterling A (2001) Seasonal variations in radionuclide transfer in a mediterranean grazing-land ecosystem. J Environ Radioactiv 55:283–302
Baeza A, Guillén J, Espinosa A, Aragón A, Gutiérrez J (2004) A study of the bioavailability of 90Sr, 137Cs, and 239+240Pu in soils at two locations of Spain affected by different radionuclide contamination events. Radioprotection 40(S1):S61–S65
Baeza A, Guillén J, Bernedo JM (2005) Soil-fungi transfer coefficients: importance of the location of mycelium in the soil and of the differential availability of radionuclides in soil fractions. J Environ Radioactiv 81:89–106
Baeza A, Guillén J (2006) Influence of the soil bioavailability of radionuclides on the transfer of uranium and thorium to mushrooms. Appli Radiat Isot 64:1020–1026
Baeza A, Guillén J, Mietelski JW, Gaca P (2006) Soil-to-fungi transfer of 90Sr, 239+240Pu, and 241Am. Radiochim Acta 94:75–80
Baeza A, Corbacho JA, Guillen J, Salas A, Mora JC (2011) Analysis of the different source terms of natural radionuclides in a river affected by naturally occurring radioactive materials (NORM) activities. Chemosphere 83:933–940
Baeza A, Salas A, Guillén J, Muñoz-Serrano A (2014) Association of naturally occurring radionuclides in sludges from drinking water treatment plants previously optimized for their removal. Chemosphere 97:108–114
Barišić D, Lulic S, Miletic P (1992) Radium and their uranium in phosphate fertilizers impact on the radioactivity of waters. Wat Res 26:607–611
Barnett CL, Beresford NA, Walker LA, Baxter M, Wells C, Copplestone D (2013) Transfer parameters for ICRP reference animals and plants collected from a forest ecosystem. Radiat Environ Biophys (on-line publication)
Blanco P, Vera Tomé F, Lozano JC (2004) Sequential extraction for radionuclide fractionation in soil samples: a comparative study. Appli Radiat Isot 61:350–354
Bunzl K, Schimmack W, Belli M, Riccardi M (1997) Sequential extraction of fallout radiocesium from the soil: small scale and large scale spatial variability. J Radioanal Nucl Chem 226:47–53
Bunzl K, Albers BP, Shimmack W, Rissanen K, Suomela M, Puhakainen M (1999) Soil to plant uptake of fallout 137Cs by plants from boreal areas polluted by industrial emissions from smelters. Sci Total Environ 234:213–221
Burnett WC, Schultz MK, Hull CD (1996) Radionuclide flow during the conversion of phosphogypsum to ammonium sulfate. J Environ Radioactiv 32:33–51
Camps M, Rigol A, Hillier S, Vidal M, Rauret G (2004) Quantitative assessment of the effects of agricultural practices designed to reduce 137Cs and 90Sr soil–plant transfer in meadows. Sci Total Environ 332:23–38
CEE (1997) Directive de la Comisión concernant de rapprochement des législations des États membres relatives aux méthodes d’échantillonnage et d’analyse des engrais du, nº L213. (In French) 22 Aug 1977
Chandrajith R, Seneviratna S, Wickramaarachchi K, Attanayake T, Aturaliya TNC, Dissanayake CB (2010) Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: study of a chronic kidney disease area in Sri Lanka. Environ Earth Sci 60:193–201
Chauhan P, Chauhan RP, Gupta M (2013) Estimation of naturally occurring radionuclides in fertilizers using gamma spectrometry and elemental analysis by XRF and XRD techniques. Microchem J 106:73–78
Cheshire MV, Shand C (1991) Translocation and plant availability of radio cesium in an organic soil. Plant Soil 134:287–296
Cook GT, Baxter MS, Duncan HJ, Toole J, Malcolmson R (1984) Geochemical association of plutonium in the Caithness environment. Nucl Inst Nucl Meth Phys Res 223:517–522
Copplestone D, Johnson MS, Jones SR (2001) Behaviour and transport of radionuclides in soil and vegetation of a sand dune ecosystem. J Environ Radioactiv 555:93–108
Drissner J, Bürmann W, Enslin F, Heider R, Klemt E, Miller R (1998) Availability of cesium radionuclides to plants—classification of soils and role of mycorrhiza. J Environ Radioactiv 41:19–32
Fawaris BH, Johanson JK (1995) Fractionation of caesium (137Cs) in a coniferous forest soil in central Sweden. Sci Total Environ 170:221–228
Fesenko SV, Soukhova NV, Sanzharova NI, Avila R, Spiridonov SI, Klein D, Badot PM (2001) 137Cs availability for soil to understorey transfer in different types of forest ecosystems. Sci Total Environ 269:87–103
Forsberg S, Rosén K, Fernandez V, Juhan H (2000) Migration of 137Cs and 90Sr in undisturbed soil profiles under controlled and close-to-real conditions. J Environ Radioactiv 50:235–252
Forsberg S, Rosén K, Bréchignac F (2001) Chemical availability of 137Cs and 90Sr in undisturbed lysimeter soils maintained under controlled and close-to-real conditions. J Environ Radioactiv 54:253–265
Fuhrmann M, Lasat M, Ebbs S, Cornish J, Kochian L (2003) Uptake and release of Cesium-137 by five plants species as influenced by soil amendments in field experiments. J Environ Qual 32:2272–2279
Guillén J, Baeza A, Ontalba MA, Míguez MP (2009) 210Pb and stable lead content in fungi: its transfer from soil. Sci Total Environ 407:4320–4326
Haridasan PP, Maniyan CG, Pillai PMB, Khan AH (2002) Dissolution characteristics of 226Ra from phosphogypsum. J Environ Radioactiv 62:287–294
Hull CD, Burnett WC (1996) Radiochemistry of Florida phosphogypsum. J Environ Radioactiv 32:213–238
IAEA (2003) Extent of environmental contamination by naturally occurring radioactive material (NORM) and technological options for mitigation. IAEA technical reports series nº 419, Vienna
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. International Atomic Energy Agency, Vienna
ICRP (2009) Environmental protection: transfer parameters for reference animals and plants. ICRP Publication 114, Ann. ICRP 39(6)
Jacob P, Fesenko S, Bogdevitch I, Kashparv V, Sanzharova N, Grebenshikova N, Isamov N, Lazarev N, Panov A, Ulanovky A, Zhuchenko Y, Zhurba M (2009) Rural areas affected by the Chernobyl accident: radiation exposure and remediation strategies. Sci Total Environ 408:14–25
Jouve A, Lejeune M, Rey J (1999) A new method for determining the bioavailability of radionuclides in the soil solution. J Environ Radioactiv 43:277–289
Kagan LM, Kadatsky VB (1996) Depth migration of Chernobyl originated 137Cs and 90Sr in soils of Belarus. J Environ Radioactiv 33:27–39
Kaunisto S, Aro L, Rantavaara A (2002) Effect of fertilisation on the potassium and radiocaesium distribution in tree stands (Pinus sylvestris L.) and peat on a pine mire. Environ Pollut 117:111–119
Kennedy VH, Sanchez AL, Oughton DH, Rowland AP (1997) Use of single and sequential chemical extractants to assess radionuclide and heavy metal availability from soils for root uptake. Analyst 122:89R–100R
Komosa A (2002) Study on geochemical association of plutonium in soil using sequential extraction procedure. J Radioanal Nucl Chem 252:121–128
Kononova MM (1982) Materia orgánica del suelo. Ediciones Oikos-Tau, Barcelona. (In Spanish)
Korobova E, Ermakov A, Linnik V (1998) 137Cs and 90Sr mobility in soils and transfer in soil-plant systems in the Novozybkov district affected by the Chernobyl accident. Appl Geochem 13:803–814
Krouglov SV, Kurinov AD, Alexakhin RM (1998) Chemical fractionation of 90Sr, 106Ru, 137Cs and 144Ce in Chernobyl-contaminated soils: an evolution in the course of time. J Environ Radioacitiv 38:59–76
Lee MH, Lee CW (2000) Association of fallout-derived 137Cs, 90Sr and 239,240Pu with natural organic substances in soils. J Environ Radioactiv 47:253–262
Lembrechts J (1993) A review of literature on the effectiveness of chemical amendments in reducing the soil-to-plant transfer of radiostrontium and radiocesium. Sci Total Environ 137:81–98
Mustonen R (1985) Radioactivity of fertilizers in Finland. Sci Total Environ 45:127–134
Nisbet AF, Salbu B, Shaw S (1993a) Association of radionuclides with different molecular size fractions in soil solution: Implications for plant uptake. J Environ Radioactiv 18:71–84
Nisbet AF, Konoplev AV, Shaw G, Lembrechts JF, Merckx R, Smolders E, Vandecasteele CM, Lönsjö H, Carin F, Burton O (1993b) Application of fertilisers and ameliorants to reduce soil to plant transfer of radiocesium and radiostrontium in the medium to long term—a summary. Sci Total Environ 137:173–182
Nisbet AF, Shaw S (1994) Summary of a 5-year lysimeter study on the time- dependent transfer of 137Cs, 90Sr, 239, 240Pu and 241Am to crops from three contrasting soil types: 1. transfer to the edible portion. J Environ Radioact 23:1–17
Pavlotskaya FI (1974) Migration of radioactive products from global fallout in soils. Moskow, Atomizdat. (In Russian)
Papastefanou C, Stoulos S, Ioannidou A, Manolopoulou M (2006) The application of phosphogypsum in agriculture and the radiological impact. J Environ Radioactiv 89:188–198
Righi S, Lucialli P, Bruzzi L (2005) Health and environmental impacts of a fertilizer plant Part I: assessment of radioactive pollution. J Environ Radioactiv 82:167–182
Rigol A, Vidal M, Rauret G (2002) An overview of the effect of organic matter on soil-radiocaesium interaction: implications in root uptake. J Environ Radioactiv 58:191–216
Riise G, Bjørnstad HE, Lien HN, Oughton DH, Salbu B (1990) A study on radionuclide association with soil components using a sequential extraction procedure. J Radioanal Nucl Chem 142:531–538
Robison WL, Brown PH, Stone EL, Hamilton TF, Conrado CL, Kehl S (2009) Distribution and ratios of 137Cs and K in control and K-treated coconut trees at Bikini Island where nuclear test fallout occurred: effects and implications. J Environ Radioactiv 100:76–83
Rosén K, Vinichuk M, Nikolova I, Johanson K (2011) Long-term effects of single potassium fertilization on 137Cs levels in plants and fungi in a boreal forest ecosystem. J Environ Radioactiv 102:178–184
Rutherford PM, Dudas MJ, Samek RA (1994) Environmental impacts of phosphogypsum. Sci Total Environ 149:1–38
Saueia CH, Mazzilli BP, Fávaro DIT (2005) Natural radioactivity in phosphate rock, phosphogypsum and phosphate fertilizers in Brazil. J Radioanal Nucl Chem 264:445–448
Shaw G (1993) Blockade by fertilizers of caesium and strontium uptake into crops: effects on the root uptake process. Sci Total Environ 137:119–133
Schuller P, Bunzl K, Voigt G, Krarup A, Castillo A (2005) Seasonal variation of the radiocaesium transfer soil-to-swiss chard (Beta vulgaris var. cicla L.) in allophanic soils from the lake region. Chile J Environ Radioactiv 78:21–33
Schultz MK, Inn KGW, Lin ZC, Burnett WC, Smith G, Biegalski SR, Filliben J (1998) Identification of radionuclide partitioning in soils and sediments: determination of optimum conditions for the exchangeable fraction of the NIST standard sequential extraction protocol. Appl Radiat Isot 49:1289–1293
Stauton S, Dumat C, Zsolnay A (2002) Possible role of organic matter in radiocesium adsorption in soils. J Environ Radioactiv 58:163–173
Strandberg M (1994) Radiocesium in a danish pine forest ecosystem. Sci Total Environ 157:125–132
Szabo G, Wedgwood AJ, Bulman RA (1991) Comparison and development of new extraction procedures for 239Pu, Ca, Fe and Cu organic complexes in soil. J Enrivon Radioactiv 13:181–189
Tessier A, Campbell PGC, Visón M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 57:844–851
Tsukada H, Nakamura Y (1999) Transfer of 137Cs and stable Cs from soil to potato in agricultural fields. Sci Total Environ 228:111–129
UNSCEAR (2000) United Nations scientific committee on the effects of atomic radiation. UNSCEAR 2000 report to the general assembly, with scientific annexes, New York
Vidal M, Camps M, Grebenshikova N, Sanzharova N, Ivanov Y, Vandecasteele C, Shand C, Rigol A, Firsakova S, Fesenko S, Levchuk S, Cheshire M, Sauras T, Rauret G (2001) Soil- and plant-based countermeasures to reduce 137Cs and 90Sr uptake by grasses in natural meadows: the REDUP project. J Environ Radioactiv 56:139–156
Vinichuk MM, Johanson KJ, Rosén K, Nilsson I (2005) Role of the fungal mycelium in the retention of radiocaesium in forest soil. J Environ Radioactiv 78:77–92
Wang J, Wang C, Huang C, Lin Y (1998) Transfer factors of 90Sr and 137CS from paddy soil to the rice plant in Taiwan. J Environ Radioactiv 39:23–34
Zhu YG, Shaw G (2000) Soil contamination with radionuclides and potential remediation. Chemosphere 41:121–128
Zhu YG, Shaw G, Nisbet AF, Wilkins BT (2000) Effect of potassium (K) supply on the uptake of 137Cs by spring wheat (Triticum aestivum cv. Tonic): A lysimeter study. Radiat Environ Biophys 39:283–290
Zielinski RA, Asher-Bolinder S, Meier AL, Johnson CA, Szabo BJ (1997) Natural or fertilizer-derived uranium in irrigation drainage: a case study in southwestern Colorado, USA. Appli Geochem 12:9–21
Acknowledgments
This work was made possible by the financing provided by the Spanish Ministry of Science and Innovation to the project nº FIS2011-29788. We are also grateful to the Autonomous Government of Extremadura (Junta de Extremadura) for financial support granted to the LARUEX research group (FQM001).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Guillén, J., Baeza, A., Salas, A., Muñoz-Muñoz, J.G., Muñoz-Serrano, A. (2014). Transfer of Radionuclides to Plants: Influence on the Speciation of Radionuclides in Soil. In: Gupta, D., Walther, C. (eds) Radionuclide Contamination and Remediation Through Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-07665-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-07665-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07664-5
Online ISBN: 978-3-319-07665-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)