Abstract
Groundwater recharge is a critical hydrological parameter. Identification of recharge zones and recharge estimates are essential to water resources management (Scanlon and Cook, 2002; Raju et al., 2006). In recent years, the overgrowing population and climate change are putting water resources under pressure all over the world. Sustainable management of aquifers to meet human and ecosystem needs will require protection of recharge areas and their augmentation (Reddy et al., 2000).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Groundwater recharge is a critical hydrological parameter. Identification of recharge zones and recharge estimates are essential to water resources management (Scanlon and Cook, 2002; Raju et al., 2006). In recent years, the overgrowing population and climate change are putting water resources under pressure all over the world. Sustainable management of aquifers to meet human and ecosystem needs will require protection of recharge areas and their augmentation (Reddy et al., 2000).
A precise knowledge in distribution of isotopic composition about the local precipitation and its relationship to the local environmental condition is essential for hydrological studies on a regional scale yielding vital information on the mean elevation of recharge area for aquifers, hydrological flow, etc. (Longinelli and Selmo, 2003). Precipitation and stable isotope values are primarily a function of prevailing atmospheric conditions such as relative humidity, temperature etc. during rainfall. Environmental isotopes have been used to understand the moisture source of precipitation, precipitation recharge to ground water and also to understand the relative contribution of precipitation in different seasons (Chidambaram et al., 2009). Many investigators studied the 2H and 18O variations in precipitation to characterize the precipitation isotopically (Dansgaard, 1964; Yurtsever and Gat, 1981; Rozanski, 1982; Nativ and Riggio, 1990; Gedzelman and Lawrence, 1990; Krishnamurthy and Bhattacharya, 1991; Gat, 1996; Araguas-Araguas et al., 1998; Despande et al., 2003; Saravana Kumar et al., 2009).
Groundwater tracers have been widely used to identify the areas contributing to groundwater recharge. Specifically, stable isotopes of hydrogen (2H) and oxygen (18O) have been used as conservative groundwater tracers because values remain constant as long as there are no phase changes or fractionation along the flow path (Clark and Fritz, 1997). The interest for the hydrological framework of the coastal aquifers of Nagapattinam region can be explained by the extensive exploitation of these aquifers for irrigation and growing population water needs. Both shallow and deep groundwaters are being harnessed in this region leading to undesirable effects such as seawater intrusion and up coning of deep seated saline waters. There are cases of sea water intrusion in parts of this region (Chidambaram et al., 2010). The present study involves hydrogeochemical and stable isotopic characters of deep and shallow aquifers during premonsoon and postmonsoon. It also aims to investigate the δD-δ18O relationships and to understand the hydrogeochemical variation of ground water with respect to depth.
Study Area
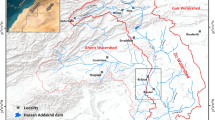
The area chosen for study falls along the coastal stretch of Pondicherry to Nagapattinam region, which is situated on the eastern margin of Tamil Nadu. It includes the taluks of Cuddalore, Chidambaram, Sirkazhi, Mayiladuthurai, Tharangambadi, Nagapattinam, Kilvelur, Thirukkuvalai and Vedaranyam. The area has also been extended into the Karaikkal Union Territory so as to get a complete overview along the coast. The subsurface water in the study area are being tapped by the industries along the coastal regions. Apart from the industrial threat, paddy cultivation is also practiced in many parts of this region extracting sufficient amount of water from the aquifers. Moreover this region has salt pans and several distributary channels, which serve as back-water during non-monsoonal periods. The normal annual rainfall of the study area is 1230 mm. The major part of the study area is covered by black clay soils (matured) as per the classification of soil survey and land use shows an isolated patch of brown clay loam soil in the area bordering the north-western boundary of the Karaikal Region. The study area forms part of Cauvery river basin and is drained by a network of rivers like the Cauvery, the Kollidam, the Arasalar, the Vettar and the Ponnaiyar. All the rivers are almost ephemeral in nature. At the estuaries, all the rivers are filled with the back-waters. The general descriptions of the various geological formations occurring in the study area are briefed in Table 1. Location map of the study area is shown in Fig. 1.
Sampling and Methodology
Stable isotopic studies (δ18O and δD) were carried out for 62 samples from different locations of the study area during PRM in June month and POM in January month by adapting standard procedures (IAEA) for isotopes and the samples were analysed for major cation and anions by using standard procedures (APHA, 1998). Sixteen samples from shallow groundwater representing pre-monsoon (SPRM) and 16 representing post-monsoon seasons (SPOM) were collected and analysed. Similarly, 15 samples from deep groundwater during pre-monsoon (DPRM) and 15 from post-monsoon (DPOM) seasons were collected. Samples of shallow and deep groundwater locations are shown in Table 2. The shallow and deep aquifers were considered based on the intermittent clay layers approximately at an average depth of 15-20 m. Environmental stable isotopes and radioisotopes (2H and 18O) have also been measured for hydrogen and oxygen-18 isotope analyses. The results are expressed in δ values (IAEA), which are in per mil (‰) and are computed as:
The precision of measurement of δ2H was ± 0.5‰ and that of δ18O was ± 0.1‰.
Results and Discussion
The δ2H-δ18O Relationship in Ground Water
The stable δD and δ18O isotopes are ideal tracers for determining the recharge areas and flow path of ground water, because they are from the water molecules and susceptible to physical processes such as mixing and evaporation (Coplen, 1993). Groundwater samples of shallow zone isotopes enrichment of δ18O and δ2H values ranges between –6.4 and –1.4‰ and –43.1 to –10.3‰ during PRM respectively and in POM δ18O ranges from –5.9 to –0.3‰ and δ2H from –41.1 to –11.3‰ (Table 3). In the case of deep samples the δ18O and δ2H during PRM ranges between –6.4 to –2.3‰ and –41.5 to –16.2‰, and during POM the range is from –7.6 to +0.5‰ and –55.6 to –1.8‰ respectively. The slopes and intercepts of the various regression lines are lower than the GMWL and the LMWLs for which the precipitation data are available. The δ18O and δD of the groundwater samples of the region (Fig. 2) was plotted to correlate with the SM (Summer Monsoon), NEM (North East Monsoon) and SWM (South West Monsoon) local meteoric water lines (LMWL).
A wide variation is noted in shallow groundwater samples of both POM and PRM. Few of the samples are found to be contributed from the GMWL and SM (LMWL); further few samples were observed to fall in line with SM. The stable isotope signatures of δ18O and δD values obtained here were also correlated with derived local meteoric water line of SWM precipitation (Chidambaram et al., 2009).
Various studies have also developed LMWLs for several Indian regions (Deshpande et al., 2003; Unnikrishnan Warrier et al., 2010). The LMWL provides a baseline for groundwater investigations of a region, which differs from the global line owing to the variations in climatic and geographic parameters. The isotopic composition of ground water has resulted due to the recharge by local precipitation from both SWM and SM.
Some deep groundwater samples in POM clearly reflect a recharge due to the NEM monsoon without any significant influence of the SM and SWM. Few samples reflect the influence of a saturated soil that limited rainwater percolation during the second monsoon stage (Negrel et al., 2011). On the contrary, the few shallow PRM sample points also show more depleted values when compared to SM and SWM, suggesting that they are more influenced by the NEM monsoon. Groundwater samples were divided into three groups as: (group A) the samples are depleted in heavy isotopes, and that these waters are isotopically not altered during PRM and POM, these sample plots near to the Global meteoric water line and LMWL; (group B) the samples are lighter during PRM and become heavier during the POM; and (group C) show samples with lighter isotopes during POM and heavier during PRM.
Most of the shallow groundwater samples are observed in group A and followed by group B. The samples of group B are represented at Karaikadu, Nagapattinam, Madhanam, Thamarakulam and Thiruvenkadu due to the fact that ground water may be recharged by seepage from surface water, such as rivers. Most of the recharge is from seepage, the ground water should reflect the mean isotopic composition of the river or the lake instead of local precipitation (Aggarwal et al., 2000). In this case samples with heavier isotopes during POM is nearby the river and water may be considerably enriched in heavy isotopes due to the impact of evaporation (Fig. 3) in the surface water.
The sample of group C reflects the fact that enrichment is limited by direct isotopic exchange with atmospheric moisture. Thus this enrichment is higher here and evaporation is more intense with respect to the total volume of water, that is enclosed by rivers in this area (Aggarwal et al., 2000) and the samples like Tharangapadi, Palayar, Karuvi, Kozhaiyar, Vadagal, B. Mutlur belong to the group C which have the lighter isotopes during POM that became heavier during PRM. Due to evaporation the concentration of dissolved salts increases in water as a function of its initial composition, the intensity of evaporation and atmospheric humidity. The direct meteoritic water recharge dilutes total ionic concentration and EC of the samples which are reduced after monsoon reflecting the same. This phenomenon occurs at the surface and during water infiltration to deep saturated zones (Clark and Fritz, 1997) and also may be due to seawater intrusion. Shallow groundwater samples during POM at B. Mutlur, Thirukadaiyur, Palayar and Nallur during PRM fall right below the LMWL line indicate the lighter isotopes may also have contributions from anthropogenic activities like aquaculture and salt pan activities in the study area (Aggarwal et al., 2000).
In deep waters most of the PRM and POM season samples show a clear contribution from SM source indicated by their alignment along the LMWL of SM and few of the PRM samples fall along the SWM (LMWL) which is recharged from the local regional precipitation of SWM (Fig. 4). Few of the POM samples correlate with NEM (LMWL) which indicates that isotopes are derived from the local recharge by NEM monsoon. Most of the samples in deep groundwater in group A followed by Kudikadu, Capper quary, Vanur and Taikalthonithurai belong to the group B and Surnavur, Kaduvanur and Thamaraikulam samples belong to the group C. Groundwater in Kanduvanur and Capper quary during POM observed below the NEM may be due to the anthropogenic factor; heavier isotopes are noted in Thirunallar, Nendukadu, Kudikadu and Nagapattinam in POM, which along the coastal region may be due to saltwater intrusion or dissolution of salts precipitation along the pore spaces.
The δ18O-Deutrium – Excess Relationship
The d-excess value may also be impacted by evaporation of the precipitation, either as it falls through the air (Gat, 1996) or as it sits in the rain collector. D-excess is a useful parameter to distinguish the vapour sources. It is calculated by the following equation:
The d-excess values of the shallow groundwater during PRM and POM are varying from –10.50‰ to +10.594‰ and –14.85‰ to 7.4‰ respectively. In deep groundwater majority of the samples during PRM and POM show d-excess variation between 2.5 and 13.0‰ and from –11.70 to 8.1‰ respectively. Values of the d-excess factor (d − excess = δ2H – 8 × δ18O) around +5‰ indicates that ground water has undergone evaporation prior to infiltration (Caro et al., 2009). In these regions, in PRM deep and shallow groundwater samples are enriched with respect to the precipitation in δ18O and show (Dalai, 2002; Gupta, 2003) lower values of d-excess. This indicates that there is little significance to kinetic evaporation of the precipitated water before groundwater recharge.
Increase of d-excess for ground water in PRM and POM season suggested a new source whose d-excess (>10.5%) was characterized by precipitation or storm runoff (Liu et al., 2010) and the decline in d-excess in POM indicated the recharge of severe-evaporated sources either by lateral recharge or by surface runoff retained by silt arresters. High d-excess values in PRM shallow and deep samples indicate that more evaporated moisture has been added to the atmosphere (Gat and Matsui, 1991), and low values are associated with samples fractionated by evaporation. In Nallur, samples show the negative d-excess values during POM; most of the samples are showing same trend as PRM (Fig. 5). Shallow groundwater from Mutlur, Thirukadaiyur, Palayar, Madhanam and Thamarakulam, and deep groundwater samples from Kaduvanur and Capperquary show the negative d-excess values with depleted δ18O, where ‘d-excess’ values of less than 5, however, suggest significant eva-poration of rainwater, leaving the residual groundwater (Negrel et al., 2011). The deep groundwater samples of Kudikadu show the positive δ18O with negative d-excess value.
Stable Isotopes (δ18O, δD) vs. Chloride
A comparison of the deuterium and chloride data provides greater understanding of ground water-surface water interaction processes in the study area. There are few significant variation of isotopic characters with respect to Cl. The chloride-δ18O plot (Fig. 6) which indicates that major mechanism functioning in the study area are (i) recharge from precipitation, (ii) recharge may be from tank/river (evaporated water) and (iii) recharge of the mixed sea water and the saline back waters. The chloride-deuterium plot suggests that some of the groundwater samples from SPRM and SPOM along the coast have high chloride and enriched in δD (Fig. 7) indicating that these areas rarely receive recharge from meteoric water and recharge of the evaporated waters from a different source nearby or due to the evaporation taking place along the coast. Higher concentration of chloride was attributed to the leaching of secondary salts from the formation (Chidambaram et al., 2007) or due to sea water intrusion. It has been reported that few samples from DPOM are characterized by low chloride and relatively enriched δD signatures, indicating frequent recharge by river waters (Prasanna et al., 2008). Certain groundwater samples of both the depths during PRM and POM show very high Cl- and high deuterium is due to the dominance of seawater/brackish water and some samples from shallow PRM are represented with low Cl- with low δD signatures shows the recharge of fresh water from less evaporated source.
These processes can also be easily discerned by Cl- versus δ18O. The distribution of Cl- variation with respect to δ18O in samples (Fig. 6) shows the dominance in some of the SPRM and SPOM of seawater/brackish water. Few of the samples from DPOM, SPRM and SPOM show the dominance of river/tank water recharge. Some samples show high Cl- and moderate enrichment in δ18O indicating contribution of saline and evaporated surface water bodies such as backwater. Most of the samples are dominated by the meteoric water in both the seasons of the deep aquifers.
Compositional Variations of Major Ions
The maximum/minimum and average is shown in Table 3. The resultant diagram is exhibited in (Fig. 8): Field 1 (Recharging water); Field 2 (Reverse ion-exchange); Field 3 (Na+-Cl-) waters are typical sea water mixing and are mostly constrained to the coastal areas or water with higher residence time; and Field 4 (Na+-HCO3 -) waters possibly represent Base Exchange reactions (Chadha, 1999). The plot shows that majority of samples from deep groundwater during PRM and POM fall in Field 1 (Fig. 8) indicating recharging waters which is evidenced from the δ18O and δD shows that the POM samples are influenced by the LMWL and the PRM samples follows the SM water line SPRM and SPOM samples are represented in Field 3 indicating the dominance of Na+ and Cl- for sea water intrusion. This is also clearly evidenced by stable isotopes followed by these very few representations which are also noted in Field 2, indicating the characteristics of reverse ion exchange.
Stoichiometry of the Waters
Stoichiometry of these waters shows that a considerable amount of [Ca2+ + Mg2+] is balanced by SO4 2-. Assuming that SO4 2- is balanced only by Ca2+ and Mg2+, the balance [Ca2+ + Mg2+]*, which is given by subtracting SO4 2- valences from the total [Ca2+ + Mg2+] equivalences, is accounted for by weathering of carbonate and/or silicate rocks by carbonic acid. Covariation of [Na+ + K+]*/[HCO3 -] versus [Ca2+ + Mg2+]*/[HCO3 -] in these waters indicate (Fig. 9) the relative contributions of carbonate and silicate weathering process in waters. In Fig. 9, most of the waters plot near the intersection of these two lines. The ground water from the study area of SPOM, SPRM, DPRM and DPOM seasons plots in the (Field 1) first quadrant. This suggests that weathering of silicate minerals or saline intrusions are the significant contributors for the water chemistry of this region. On the other hand, in Karuvi during SPRM and Nallur during SPOM (Field 2) second quadrant, showing excess of both [Ca2+ + Mg2+] and excess [Na+ + K+] over [HCO3 -], which may not be ascribed to weathering either of silicate, or of carbonate and of evaporate minerals, but probably to anthropogenic inputs, are responsible for the excess of ions in these waters. Few shallow samples and few deep samples during both seasons are in Field 4 representing Ca–Mg–HCO3 water type, with low Mg + Ca with the increasing Na + K.
Conclusions
The study on the stable isotopes of oxygen and hydrogen of groundwater samples collected during PRM and POM shows that POM samples are generally more depleted than PRM samples. In deep aquifers of both PRM and POM, clear contribution from SM and SWM local meteoric source represents the recharging sources. Shallow groundwater samples during PRM and POM results in lighter isotopes along with the contributions from anthropogenic activities and from salt water intrusion in the study area. High d-excess values in PRM shallow and deep samples indicate that more evaporated recharge and low d-excess values in POM represent the fractionation. Shallow samples in both the seasons show the saline water contribution and deep samples show the recharge by local precipitation and by seepage from surface water. The depth-wise and seasonal variations of the hydrogeochemical process are reflected in the isotopes signature.
References
Aggarwal, P.K. et al. (2000). A report on isotope hydrology of groundwater in Bangladesh: Implications for characterization and mitigation of arsenic in ground water. IAEA–TC Project (BGD/8/016), IAEA, Vienna.
APHA (1998). Standard methods for the examination of water and wastewater. 19th edn. APHA, Washington, DC.
Araguas-Araguas, L., Froehlich, K. and Rozanski, K. (1998). Stable isotope composition of precipitation over Southeast Asia. Journal of Geophysical Research, 103, D22: 28,721-28,742.
Chadha, D.K. (1999). A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Journal of Hydrogeology, 7(5): 431-439.
Chidambaram, S., Prasanna, M.V., Ramanathan, AL., Vasu, K., Hameed, S., Warrier, U.K., Srinivasamoorthy, K., Manivannan, R., Tirumalesh, K., Anandhan., P. and Johnsonbabu, G. (2009). A study on the factors affecting the stable isotopic composition in precipitation of Tamil Nadu, India. Journal of Hydrological Processes, 23(12): 1792-1800.
Chidambaram, S., Vijayakumar, V., Srinivasamoorthy, K., Anandhan, P., Prasanna, M.V. and Vasudeven, S. (2007). A Study on variation in Ionic Composition of Aqueous System in Different Lithounits Around Perambalur Region, Tamil Nadu. Journal Geological Society of India, 70: 1061-1069.
Chidambaram, S., Ramanathan, AL., Prasanna, M.V., Karmegam, U., Dheivanayagi, V., Ramesh, R., Johnsonbabu, G., Premchander, B. and Manikandan, S. (2010). Study on the hydrogeochemical characteristics in groundwater, post- and pre-tsunami scenario, from Portnova to Pumpuhar, southeast coast of India. Environmental Monitoring Assessment, 169: 553-568.
Clark, I.D. and Fritz, P. (1997). Environmental isotopes in hydrogeology. Lewis Publishers, New York.
Coplen, T.B. (1993). Uses of environmental isotopes. In: Alley, W.M. (ed.), Regional ground-water quality. Van Nostrand Reinhold, New York.
Dalai, T.K., Bhattacharya, S.K. and Krishnaswami, S. (2002). Stable isotopes in the source water of the Yamuna and its tributaries: Seasonal and altitudinal variations and relation to major cations. Hydrological Process, 271: 3345-3364.
Dansgaard, W. (1964). Stable isotopes in precipitation. Tellus, 16(4): 436-468.
Deshpande, R.D., Bhattacharya, S.K., Jani, R.A. and Gupta, S.K. (2003). Distribution of oxygen and hydrogen isotopes in shallow groundwaters from Southern India: Influence of a dual monsoon system. Journal of Hydrology, 271: 226-239.
Gat, J.R. (1996). Oxygen and hydrogen isotopes in the hydrological cycle. Annual Review of Earth and Planetary Sciences. 24: 225-262.
Gat, J.R. (1980). The isotopes of hydrogen and oxygen in precipitation. In: P. Fritz and J. Ch. Fontes (eds), Handbook of Environmental Isotope Geochemistry. Elsevier Scientific Pub. Co., Amsterdam.
Gat, J.R. and Matsui, E. (1991). Atmospheric water balance in the Amazon Basin: An isotopic evapotranspiration model. Journal of Geophysical Research, 96: 13179-13188.
Gedzelman, S.D. and Lawrence, J.R. (1989). The isotopic composition of precipitation from two extra tropical cyclones. American Meteorological Society.
Gupta, S.K., Deshpande, R.D., Bhattacharya, S.K. and Jani, R.A. (2003). Groundwater δ18O and δD from Central Indian Peninsula: Influence of Arabian Sea and Bay of Bengal branches of summer monsoon. 303(1-4): 38-55.
IAEA (International Atomic Energy Agency) (1992). Statistical Treatment of data on environmental isotopes in precipitation. Technical reports series 331.
Krishnamurthy, R.V. and Bhattacharya, S.K. (1991). Stable oxygen and hydrogen isotope ratio in shallow ground waters from Northern India and a study of the role of evapotranspiration in the Indian monsoon. In: Taylor, H.P., O’Neil, J.R. and Kaplan, I.R. (eds), Stable Isotope Geochemistry: A Tribute to Samuel Epstein. Spec. Publ. No. 3, The Geochem. Society, San Antonio, Texas.
Longinelli, A. and Selmo, E. (2003). Isotopic composition of precipitation in Italy: A first overall map. Journal of Hydrology, 270: 75-88.
Nativ, R. and Riggio, R. (1990). Precipitation in the southern high plains: Meteorological and isotopic features. Journal of Geophyisical Research, 95: 22,559-22,564.
Negrel, Ph., Pauwels, H., Dewandel, B.J., Gandolfi, M., Mascré, C. and Ahmed, S. (2011). Understanding Groundwater Systems and Their Functioning through the Study of Stable Water Isotopes in a Hard-Rock Aquifer (Maheshwaram Watershed, India). Journal of Hydrology, 397(1–2): 55-70.
Prasanna, M.V., Chidambaram, S., Pethaperumal, S., Srinivasamoorthy, K., John Peter, A., Anandhan, P. and Vasanthavigar, M. (2008). Integrated geophysical and chemical study in the lower sub-basin of Gadilam River, Tamilnadu, India. Environmental Geosciences, 15(4): 145-152.
Raju, N.J., Reddy, T.V.K. and Muniratnam, P. (2006). Subsurface dams to harvest rainwater – A case of Swarnamukhi River basin, Southern India. Hydrogeology Journal, 14: 526-531.
Reddy, M.R., Raju, N.J., Reddy, Y.V. and Reddy, T.V.K. (2000). Water resources development and management in the Cuddapah district, Andhra Pradesh, India. Environmental Geology, 39: 342-352.
Rozanski, K., Araguas, A.L. and Gonfiantini, R. (1993). Isotopic patterns in modern global precipitation. Geophysical Monograph., 78: 1-37.
Rozanski, K., Sonntag, G. and Munnich, K.O. (1982). Factors controlling the stable isotopic composition of European precipitation. Tellus, 34: 142-150.
Saravana Kumar, U., Suman, S., Navada, S.V. and Deodhar, A.S. (2009). Environmental isotopes investigation on recharge processes and hydrodynamics of the coastal sedimentary aquifers of Tiruvadanai, Tamil Nadu State, India. Journal Hydrology, 364: 23-39.
Scanlon, B.R. and Cook, P.G. (2002). Theme issue on groundwater recharge. Hydrogeology Journal, 10: 3-4.
Unnikrishnan Warrier, C., Praveen Babu, M., Manjula, P., Velayudhan, K.T., Shahul Hameed, A. and Vasu, K. (2010). Isotopic characterisation of dual monsoon precipitation evidence from Kerala, India. Current. Science, 98: 1487-1495.
Xin Liu, Xianfang Song, Yinghua Zhang, Jun Xia, Xuecheng Zhang, Jingjie Yu, Di Long, Fadong Li and Bing Zhang (2010). Spatio-temporal variations of δ2H and δ18O in precipitation and shallow groundwater in the Hilly Loess Region of the Loess Plateau, China. Environmental Earth Sciences. DOI 10.1007/s12665-010-0785-y.
Yurtsever, Y. and Gat, J.R. (1981). Atmospheric waters. In: Stable isotope hydrology: Deuterium and oxygen-18 in the water cycle. Technical Report Series, 210: 103-142.
Acknowledgement
The authors wish to express thanks to Board of Research in Nuclear Sciences (BRNS) for providing necessary financial support to carry out this study and author R.T. wishes to express thanks to DST for providing the Inspire fellowship (DST/Inspire Fellowship/2010/[220] 18 March, 2011).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Capital Publishing Company
About this chapter
Cite this chapter
Chidambaram, S. et al. (2015). Stable Isotopic Signatures for Hydrogeochemical Characterisation of Ground Water from Pondicherry to Nagapattinam, Tamil Nadu. In: Raju, N., Gossel, W., Ramanathan, A., Sudhakar, M. (eds) Management of Water, Energy and Bio-resources in the Era of Climate Change: Emerging Issues and Challenges. Springer, Cham. https://doi.org/10.1007/978-3-319-05969-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-05969-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05968-6
Online ISBN: 978-3-319-05969-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)