Abstract
“She had her first collapse in August. This was following a heavy dinner and she also recall that on that day she has had few coffees and no water intake. The collapse was described as follows: she was sitting on the sofa, she felt palpitation and she lost consciousness apparently only for a few seconds and then she did come back and she felt a little bit confused. She did feel herself going. There has been no infection prior to the episode. Following the collapse episode, she started experiencing exercise intolerance, and she also reported postprandial fatigue in the past which has now resolved.”
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
3.1 Diagnostic Approaches to Cardiovascular Autonomic Diseases
3.1.1 History, Neurological and General Evaluation, and ECG Assessment

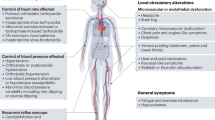
Adapted from Benarroch [1]
Cardiovascular autonomic dysfunction and disease may affect only one organ or system but may be an important feature of underlying neurological disorder [2]. Cardiovascular autonomic dysfunction is a common part of many neurological disorders and is often the most disabling part of the disorder.
This chapter outlines the diagnostic approach to the most common cardiovascular autonomic dysfunctions, hypotension, autonomically mediated (or reflex) syncope, postural tachycardia syndrome (PoTS), and autonomic failure, and how to manage these by the use of both non-pharmacological measurements and pharmacological measurements.
The clinical picture of autonomic failure is usually dominated by disabling orthostatic hypotension (OH). Severely affected patients are able to stand only for a few seconds because of dramatic blood pressure (BP) falls produced by impaired cardiovascular adaption to upright posture [3].
Patients with autonomic failure share a similar clinical presentation; they are unable to tolerate upright posture because of severe orthostatic hypotension. It is however important to distinguish the different syndromes associated with autonomic failure because they differ in their disease pathophysiology, response to pharmacological treatment, and prognosis.
Virtually any disease that affects peripheral nerve function can produce autonomic failure.
Disorders associated with autonomic failure can be classified according to the type and severity of autonomic manifestations, associated neurological symptoms, and temporal profile [1].
Orthostatic hypotension (OH) is a prominent feature of autonomic failure, and it is often the symptom that leads the patient to seek medical advice. OH, also called postural hypotension, is a temporary lowering of blood pressure (hypotension), usually due to standing up suddenly (orthostatic). The change in position causes a temporary reduction in blood flow and oxygen to the brain. Upon standing gravity promotes the pooling of blood in the lower extremities, which decreases venous return of blood circulating back to the heart. Normally, cardiac and carotid sinus baroreceptors sense the decrease in blood volume and initiate increased heart rate and peripheral vasoconstriction.
In individuals with OH, there is an impaired efferent sympathetic signal to the arterioles and a failure to release norepinephrine appropriately upon standing. The consequent vasoconstrictor insufficiency results in blood pooling in the lower extremities, with subsequent decreased venous return to the heart and brain.
OH can be confirmed by measuring blood pressures and heart rate in supine and upright positions. BP and heart rate should be measured after symptoms develop or after 3 min of standing. If the patient is unable to stand, testing for orthostatic hypotension may be done after the patient has risen to a sitting position with the feet dangling over the edge of the bed [4].
3.1.2 What Clinical Signs Hint to a Cardiovascular Autonomic Disease?
-
Severe orthostatic hypotension
-
Postprandial hypotension
-
Supine hypertension
-
High blood pressure variability
-
Blunted heart rate variability
-
Often a “non-dipping” or “reverse dipping” pattern on 24-h ambulatory blood pressure monitoring
-
Medications influencing the cardiovascular autonomic nervous system/polypharmacy
3.2 What Can I Differentiate Already at Bedside and How Do I Manage the Patient?
3.2.1 Hypotension
Hypotension is the most common symptom of all cardiovascular autonomic dysfunctions. It can be the only symptom, it can occur together with syncope and postural tachycardia syndrome (PoTS), or it may be the initial sign of autonomic failure in both primary and secondary disorders of the autonomic nervous system (ANS): pure autonomic failure (PAF), multiple system atrophy (MSA), Parkinson’s disease (PD), dementia with Lewy bodies, autoimmune autonomic ganglionopathy, amyloidosis, and diabetic autonomic neuropathy [5]. The prevalence of OH increases with age [6].
Common secondary causes are spinal cord injury (SCI), stroke, multiple sclerosis (MS), Guillain–Barré syndrome, motor neuron disease, adrenal insufficiency, and vitamin deficiencies (e.g. B1, B12).
Non-neurogenic causes are volume depletion, pump failure, drugs, mitral valve prolapse, electrolyte disturbance, prolonged bed rest, pregnancy, and alcohol [2].
3.2.1.1 Orthostatic Hypotension
3.2.1.1.1 Definition
Orthostatic hypotension (classic) (OH) is defined as a sustained drop in blood pressure (BP) of greater than 20 mmHg systolic or 10 mmHg diastolic 3 min after rising to a standing position from a supine position [4]. OH is an inability to maintain sufficient BP and adequate cerebral perfusion against gravity.
In neurogenic OH the heart rate response to changes in blood pressure is minimal, although there may be a mild compensatory increase, i.e. below 30 beats per minute.
Immediately after standing, there is gravitationally mediated redistribution of the blood volume and a pooling of 300–800 ml of blood in the lower extremities and splanchnic venous system [7]. This results in decreased stroke volume, as well as decreased systolic pressure and increased diastolic pressure.
“Initial OH” is characterised by a BP decrease immediately on standing of >40 mmHg systolic and/or >20 mmHg diastolic. BP then spontaneously and rapidly returns to normal, so the period of hypotension and symptoms is short (<30 s) [8, 9]. To confirm the presence of initial OH, BP must be recorded continuously (beat to beat) ideally in an autonomic laboratory. Bedside tests are not available.
“Delayed (progressive) OH” is not uncommon in elderly persons [10] and in patients with spinal cord injuries (SCI) [11]. Recent studies from Gibbons and Freeman [12] and Pavy-Le Traon [13] found a 10-year conversion rate to OH and higher prevalence of delayed OH in possible vs. probable MSA, respectively.
It is characterised by a slow progressive decrease in systolic BP on assuming erect posture. The absence of a bradycardiac reflex (vagal) differentiates delayed OH from reflex syncope. Delayed OH may be followed by reflex bradycardia [9].
In recently injured tetraplegics (2–13 days post injury), the basal supine level of blood pressure usually is lower than normal (mean arterial pressure, 57 mmHg in tetraplegics and 82 mmHg in normal subjects) [14]. Also in recently injured tetraplegics, the basal heart rate is usually <100 beats/min [15]. Frankel et al. found an inverse correlation between level of lesions and both systolic and diastolic blood pressure [16].
For physiology see Sect. 1.2.1, for history taking see Sect. 2.2.1.1
3.2.1.1.2 Epidemiology
In an unselected population >65 years, the prevalence of OH was reported to be 5–30% [17].
The prevalence of symptomatic OH increased from 14.8% in persons aged 65–69 years to 26% in persons >85 years, signifying the association between symptomatic OH and ageing [18]. In other populations, such as in Parkinson’s disease, the prevalence of OH may be as high as 60% [19]. The presence of OH increases risk for falls and all-cause mortality in middle-aged and elderly persons [20]. OH is an independent risk factor for cardiovascular morbidity and mortality from stroke, coronary heart disease, and chronic kidney disease [20].
Although symptoms of OH may include dizziness and syncope, asymptomatic OH is far more common and represents an independent risk factor for mortality and cardiovascular disease. In a prospective study of more than 33,000 individuals, asymptomatic OH was present in over 6% and was associated with age, female sex, hypertension, antihypertension treatment, increased heart rate, diabetes, low body mass index, and recurrent smoking [21]. Those with OH had significantly greater risk for all-cause mortality, especially those younger than 42 years, and higher risk for coronary events. OH has a prognostic role on cognitive and cardio- and cerebrovascular outcome in α-synucleinopathies.
3.2.1.1.3 Non-neurogenic Causes of OH
Drugs are the main non-neurogenic cause of orthostatic hypotension. Reducing or changing medications may lead to significant improvement. The α-blockers attenuate the α-adrenergic response, which increases vascular resistance and therefore should be avoided. The prevalence of orthostatic hypotension with the use of calcium antagonists ranges from 1% to 7%. The rate is low with thiazide diuretics and β-blockers, and some β-blockers with intrinsic sympathomimetic activity may even improve orthostatic hypotension. The co-administration of loop diuretics with other antihypertensives increases orthostatic hypotension [20].
Drugs that may cause or aggravate orthostatic hypotension and syncope [22]:
-
α-Adrenoceptor agonists (α-blockers)
-
Antipsychotics
-
β-blockers
-
Nitrates
-
Hypnotics
-
ACE inhibitors
-
Anaesthetics
-
Angiotensin II antagonists
-
Barbiturates
-
Calcium antagonists
-
Clonidine
-
Diuretics
-
Levodopa and dopamine agonists
-
Methyldopa
-
Nitrates
-
Phenothiazines
-
Sildenafil
-
Tricyclic and MAOI antidepressants
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; MAOI, monoamine oxidase inhibitor
Other non-neurogenic causes are low intravascular volume (blood or plasma loss, fluid or electrolyte loss), impaired cardiac function due to structural heart disease, and vasodilatation due to drugs, alcohol, heat, and bed rest [2, 23].
OH and tachycardia may occur after prolonged bed rest or following exposure to microgravity, such as in spaceflights [24].
3.2.1.1.4 Examination
The history is of particular importance and has a high diagnostic value (including pre-existing conditions, a detailed description of the order of symptoms, and exhaustive drug history including over-the-counter drugs) [5].
The European Federation of Neurological Societies (EFNS) guidelines on the diagnosis and management of OH [23] recommend the following actions:
-
Structured history taking see Sect. 2.2.1.1
-
Detailed physical examination
-
12-lead ECG recording
-
Laboratory testing [25]:
-
Blood tests (HbA1C), oral glucose tolerance test, urea and electrolytes, thyroid-stimulating hormone, HIV serology, hepatitis C serology/viral load, ACE level, ANA, anti-Ro/La antibodies, rheumatoid factor/anti-cyclic citrullinated peptide antibodies, anti-tissue transglutaminase antibody, serum electrophoresis, vitamin B12 levels, leucocyte α-galactosidase A activity (Fabry’s disease), lipid profile, erythrocyte sedimentation rate, and SAP (serum amyloid P component)
-
Eventually additional tests are needed:
-
Genetic testing: SCN9A/SCN10A mutations and transthyretin mutations (familial amyloid)
-
Imaging: if malignancy or sarcoidosis suspected, chest X-ray/CT with contrast and SAP (Serum Amyloid P component) scan (amyloid)
-
Tissue biopsy: abdominal fat biopsy (amyloid), small bowel biopsy (coeliac disease), biopsy of suspicious lesion to confirm malignancy, lip biopsy (Sjögren’s syndrome), and nerve biopsy (generally not performed unless there is large fibre involvement)
-
-
BP measurements while supine and upright
-
Cardiologic referral, if heart disease or abnormal ECG is present or suspected
-
Active standing or head-up tilt (HUT), ideally with continuous assessment of BP and HR for 3 min
-
Further ANS screening tests, with other appropriate investigations, depending on the possible aetiology of the underlying disorder
Non-neurogenic causes of OH must be considered, as they can exacerbate neurogenic OH [5].
3.2.1.1.5 Treatment of OH
3.2.1.1.5.1 General Management [5, 26]
Longitudinal studies have suggested that OH can increase the risk for stroke, myocardial ischaemia, and mortality. The therapeutic goal is to attenuate or eliminate symptoms rather than restore normotension.
3.2.1.1.5.2 Non-pharmacological Treatment
Non-pharmacological measurements are the basis for all interventions. Commonly before starting on any medication, the patient should have boosted the blood pressure by non-pharmacologic measurements first for a couple of months, i.e. venous compression, use of physical counter manoeuvres, and intermittent water bolus treatment. Treatment can be difficult, and the development of supine hypertension should be minimised, especially in patients with diabetes, heart failure, or cardiac ischaemia [20].
Standing upright results in translocation of between 500 and 700 mL of blood from central compartments to the lower limbs; this causes marked pressure differentials, with a substantial rise in pressure below and a fall in pressure above heart level [27]. It is essential to use adaptive mechanisms to ensure the maintenance of arterial blood pressure and in providing an adequate perfusion pressure to organs, to avoid malfunction especially while standing upright.
-
Avoidance of factors that may induce OH, like elevated environmental temperatures (hot bath, hot shower, and sauna) which may cause venous pooling.
-
Avoid prolonged recumbence during daytime.
-
Avoid sudden head-up postural change (especially on waking when BP may be lowered by nocturnal polyuria) [28]. In the morning, move to head-up position slowly, sit on the edge of the bed for some minutes after recumbence, and activate calf muscles while supine.
-
Two glasses of water on the bed table, slowly getting out of bed [29].
-
Elevation of the bed head (20–30 cm) to avoid supine hypertension.
-
High salt diet (6–10 g/day).
-
High fluid intake (six to eight cups of water each day) [29].
-
A small amount of coffee or tea is beneficial, coffee (one or two cups) after meals.
-
Avoid alcohol.
-
Avoid large meals (especially with refined carbohydrate).
-
Maintain postural stimuli.
-
Move slowly when sitting up or standing after lying down.
-
Avoid standing for long periods of time.
-
Physical counter manoeuvres (e.g. leg crossing on standing, gentle marching on the spot instead of standing still) (Fig. 3.1).
-
Squatting to reduce blood pooling will effectively reduce OH temporarily [30, 31].
-
Elastic stockings and abdominal compression bands reduce venous pooling [32].
-
Avoid straining during micturition and defaecation. For males: moving to a sitting position for micturition. The use of prokinetic drugs to avoid constipation.
-
Resting in morning and postprandial.
-
Staged standing.
-
Physical activity: carefully controlled and individualised exercise training (swimming, aerobics, cycling, and walking if possible) often improves OH.
-
Correction of anaemia.
-
Education of patients and carers on the mechanisms of OH.
-
Black liquorice (3 g/day) (should not be used for a longer period due to potential hormonal side effects) [33].
3.2.1.1.5.3 Pharmacologic Treatment
Non-pharmacological treatment should always be optimised before starting on drugs. Strategies in pharmacological treatment are volume expansion, vasoconstriction, or combination of the two [20].

Printed with permission Schroeder et al. [34]
Volume expansion
-
Desmopressin nasal spray (5–40 μg), orally (100–800 μg) or intramuscularly (2–4 μg) [37]
Vasoconstriction
-
Ephedrine (25–50 mg tds) [23]
-
Pseudoephedrine (30 mg qid) [23]
-
Octreotide (12.5–25 μg, subcutaneous) – for postprandial hypotension – contraindicated in diabetic patients [19, 39, 40]
-
Droxidopa (L-DOPS) (100–600 mg tds) – presently only licenced in the USA and Japan [41,42,43]
Combination therapy
-
Fludrocortisone (0.1–0.3 mg/day) and Midodrine (5–10 mg tds)
-
Midodrine (5–10 mg) or Pseudoephedrine (30 mg) and water bolus
Other agents previously tried are:
3.2.1.2 Postprandial Hypotension (PPH)
3.2.1.2.1 Definition
Postprandial hypotension (PPH) was first described by Seyer-Hansen in a patient with severe Parkinson’s disease in 1977 [46]. PPH is by definition a decrease in systolic blood pressure of ≥20 mm Hg or a decrease below 90 mm Hg from a pressure of ≥100 mm Hg within 2 h after a meal [39]. PPH can be detected by either a 24-h blood pressure profile or testing in the autonomic laboratory.
Studies have shown a prevalence of PPH in institutionalised elderly persons ranging from 25% to 67% [20, 47]. Patients with PAF, Parkinson’s disease, MSA, postprandial syncope, diabetes, and SCI are also prone to PPH [39].
3.2.1.2.2 Risk Factors for Postprandial Hypotension [47]
-
Medications
-
Polypharmacy (>3 medications)
-
Diuretics
-
-
Meals
-
Carbohydrate-rich meals
-
Breakfast
-
Hot meals
-
Alcohol
-
-
Comorbid conditions
-
Diabetes mellitus
-
Autonomic dysfunction
-
Parkinson’s disease
-
Hypertension
-
End-stage renal disease on haemodialysis
-
Fragile X mutation
-
3.2.1.2.3 Treatment of PPH
3.2.1.2.3.1 Non-pharmacological Management [48]
-
Drink water before meals.
-
Eat frequently, smaller meals.
-
Assume a recumbent or sitting position after a meal.
-
Avoid large meals.
-
Avoid alcohol before and after meal.
-
Dietary modification, reduce refined carbohydrates.
-
Water with the meal.
-
Wear abdominal binders.
3.2.1.2.3.2 Pharmacological Management [26, 49]
-
Caffeine 250 mg (two cups) either 30 min before the meal or by the end of the meal.
-
Midodrine 10 mg with meal.
-
Octreotide 25–50 μg subcutaneous 30 min before meal.
-
α-Glucosidase inhibitor
-
Guar gum 9 g [40].
3.2.1.3 Exercise-Induced Hypotension (EIH) and Post-exercise Hypotension (PEH)
3.2.1.3.1 Definition
Exercise-induced hypotension was first reported in autonomic failure patients cycling in supine position in 1961 by Shepherd and colleagues in a group of patients who performed supine cycling exercise [51].
Exercise-induced hypotension (EIH) is defined as a ≥10 mmHg fall in systolic blood pressure during exercise due to fall in total peripheral resistance [52]. Impairment of sympathetic vasoconstriction in autonomic failure has been documented as systemic vascular resistance falls substantially in the patients during exercise [53]. EIH can be a significant symptom in patients with PAF, MSA, and SCI. The severity of EIH seems to be higher during dynamic relative to static exercise [52].
Post-exercise hypotension (PEH) is defined as a reduction in systolic and/or diastolic arterial blood pressure (i.e. reduction in mean arterial pressure) below control levels after a single bout of exercise for approximately 1–3 h [54]. PEH has been well documented in humans with both borderline hypertension and hypertension [55], with diabetes [56], in healthy endurance training athletes [57], and in SCI [58]. Data suggest that PEH may also occur after resistance exercise [55]. Studies have suggested that a reflex similar to the Bezold–Jarisch reflex is the final pathway triggering the vasovagal reaction in exercise-induced neurally mediated syncope [53]. The Bezold–Jarisch reflex is a triad of responses (apnoea, bradycardia, and hypotension) and depends on intact vagal nerves and is mediated through cranial part of medullary centres controlling respiration, heart rate, and vasomotor tone [59].
3.2.1.3.2 Treatment of EIH and PEH
3.2.1.3.2.1 Non-pharmacological Measurements
-
Adequate hydration prior to exercise.
-
Avoiding food intake for several hours before exercise to prevent postprandial hypotension.
-
Increased daily salt intake [60].
-
Prevention of dehydration after exercise by oral water intake during exercise [61].
-
Muscle tensing [60].
-
Use of abdominal compression/binders and lower limb elastic stockings [60].
-
Mild physical exercise/activity [60].
-
Reducing risk factors by exercise training in the supine position (swimming, rowing) [22, 52].
3.2.1.3.2.2 Pharmacological Measurements
Unfortunately there are limitations to pharmacological treatment in exercise-induced hypotension [52]. Both octreotide and midodrine did not show any effect on exercise-induced hypotension in studies; however, the increase in blood pressure overall may be beneficial in reducing fatigue [62].
In a small study with four patients with SCI (C6–C8), treatment with 10 mg Midodrine was associated with elevated systolic blood pressure during peak exercise in three participants. Two participants showed a concurrent decrease in perceived exertion and increase in oxygen consumption, suggestive of some benefit from Midodrine on EIH in SCI [63].
3.2.1.4 Supine Hypertension
Supine hypertension is a common finding in autonomic failure and complicates the treatment of the OH [64]. Supine hypertension can worsen OH and predispose to end-organ damage [65], resulting from medication and/or being part of the disease, especially in PAF.
Supine hypertension, defined as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, is present in one half of patients with severe autonomic failure, despite normal seated and low upright blood pressures [64, 66].
Nocturnal hypertension is defined as night-time BP means ≥120/70 mm Hg (fixed cut-off limits) [67]. A 24-h ambulatory BP monitor is needed to screen for nocturnal hypertension and missing dipping and may be very useful before and if needed after starting a new therapy [68].
3.2.1.4.1 Ways to Counteract Supine Hypertension [65]
-
Avoid supine position during daytime.
-
Patients can sit in a reclining chair with feet on the floor when rest is needed.
-
Avoid pressor medications after 16.00 h.
-
Elevate the bed head (20–30 cm).
-
Have a snack just before going to bed (inducing postprandial hypotension).
-
If Fludrocortisone is used, this may worsen the supine hypertension. Changing to a short-acting pressor agent (e.g. Midodrine, Droxidopa, etc.) may sometimes be of help.
-
Avoid over-the-counter medications that increase blood pressure such as nasal decongestants or eye drops containing sympathomimetics and non-steroidal anti-inflammatory agents (indomethacin and ibuprofen).
3.2.1.4.1.1 Pharmacological Interventions (Single Dose Given at Bedtime)
-
Losartan (angiotensin I receptor blocker), 50 mg at bedtime [70].
-
Transdermal nitroglycerin, 0.05–0.2 mg/h only during night [3].
-
Hydralazine, 50 mg [3].
-
Short-acting nifedipine, 30 mg [3].
-
Clonidine 0.1 mg early in the evening – Clonidine has a long half-life which may exacerbate morning OH [3].
-
Sildenafil, 25 mg [3].
-
Minoxidil, 2.5 mg [3].
3.2.2 Syncope
Syncope is defined as transient loss of consciousness due to global cerebral hypoperfusion. It is characterised by rapid onset, brief duration of loss of consciousness with spontaneous, and full recovery [9].
Syncope is a common medical problem, with a frequency between 15% and 39% [70]. In the general population, the annual number episodes are 18.1–39.7 per 1000 patients, with similar incidence between genders. The first report of the incidence of syncope is 6.2 per 1000 person-years [9], with a significant increased incidence after 70 years of age [70]. Syncope is responsible for 3–5% of emergency department visits [70].
The prognosis depends on the diagnosis [71]. If the patient has a structural heart disease or primary cardiac disease, there is an increased incidence of sudden death and overall mortality. If the syncope is caused by orthostatic hypotension, it is associated with a twofold increase in mortality, while young patients with neurally mediated syncope have a very good prognosis [71].
3.2.2.1 Classification
3.2.2.1.1 Causes of Syncope [72, 73]
Neurally mediated (vasovagal/reflex) syncope
-
(a)
Vasodepressive
-
(b)
Cardioinhibitory
-
(c)
Mixed
Situational syncope (vasovagal in nature)
-
Cough and sneeze
-
Micturition (post-micturition)
-
Post-exercise
-
Postprandial
Gastrointestinal stimulation (swallow, defaecation, visceral pain)
Other (laughter, brass instrument playing, weightlifting)
-
Post-ejaculation
Carotid sinus syncope
Orthostatic/postural syncope
-
(a)
Autonomic failure
-
Primary autonomic failure syndromes (i.e. pure autonomic failure, multiple system atrophy, Parkinson’s disease with autonomic failure, dementia with Lewy bodies)
-
Secondary autonomic failure syndromes (i.e. diabetic neuropathy, amyloid neuropathy)
-
Post-exercise
-
Postprandial
-
-
(b)
Volume depletion
-
Haemorrhage, diarrhoea, and Addison’s disease
-
Cardiac arrhythmias as primary cause
-
Sinus node dysfunction (including bradycardia/tachycardia syndrome)
-
Atrioventricular conduction system disease
-
Paroxysmal supraventricular and ventricular tachycardias
-
Inherited syndromes (i.e. long QT syndrome, Brugada syndrome)
-
Implanted device (pacemaker, implantable cardioverter defibrillator) malfunction
-
Drug-induced arrhythmias
Structural cardiac or cardiopulmonary disease
-
Obstructive cardiac valvular disease
-
Acute myocardial infarction/ischaemia
-
Obstructive cardiomyopathy
-
Atrial myxoma
-
Acute aortic dissection
-
Pericardial disease/tamponade
-
Pulmonary embolus/pulmonary hypertension
Cerebrovascular
-
Vascular steal syndromes
-
Epilepsy with bradyarrhythmias/asystole (ictal asystole)
Non-cardiovascular/nonsyncopal causes of transient loss of consciousness
-
Epileptic
-
Non-epileptic (“sleep attacks”)
-
Metabolic disorders (hypoglycaemia)
-
Neuroendocrine disorders (pheochromocytoma, carcinoid)
-
Drugs
-
Psychogenic pseudo-syncope and psychogenic non-epileptic seizure
-
Traumatic
3.2.2.2 Neurally Mediated Syncope (Vasovagal/Reflex)
Vasovagal syncope was first used by William Gowers in 1907, and Thomas Lewin described the mechanism in 1932 [70]. The neurally mediated syncope, known as neurocardiogenic or vasovagal syncope, is the most frequent, accounting for one third of the causes and reaching 66% of cases of syncope in emergency units [70].
Neurally mediated syncope is characterised by periodic syncopal episodes with normal autonomic function between episodes. Symptoms that trigger syncope include orthostatic stress, prolonged standing, hot temperature, emotional stress, pain, or sight of blood. During neurally mediated syncope, vasodilatation and bradycardia occur simultaneously. The bradycardia is due to increased parasympathetic (vagal) outflow to the sinus node of the heart. The decrease in blood pressure is due to vasodilatation most likely by brainstem shut down of vascular sympathetic outflow, but the mechanism is not clear.
These reflex bradycardia and hypotension are similar to the response evoked by the Bezold–Jarisch reflex. The characteristic pre-syncopal symptoms are weakness, light-headedness, feelings of warmth or cold, and eventual brief loss of consciousness. Potential cardiac causes of syncope must be considered before making the diagnosis. Spontaneous syncope is common, and in the absence of any underlying cardiovascular, neurologic, or other disease, an isolated vasovagal syncope may represent a variation of normal. The persons are usually normotensive with normal blood pressure regulation.
The diagnosis may sometimes be difficult to make. Situational syncope must be excluded, as well as phobia syndromes or other organic causes. Tilt table testing has good specificity but uncertain sensitivity in diagnosis and is not always reproducible. Implantable loop recorders, which store 45 min of retrospective electrocardiographic data, may also be used and can be activated by patients after each syncopal event.
Treatment of neurocardiogenic syncope is aimed at avoiding possible triggers. Information and education of the patients with special emphasis on potential predisposing factors and the recognition of prodromal symptoms are important. Education about possible pre-syncopal symptoms can help avert a syncopal episode by assuming a seated or supine position when possible. Increased fluid and salt intake may also help in avoiding the development of syncope and should always be tried first. Other non-pharmacological measurements include lower limb exercise, leg crossing, use of stockings, avoidance of triggers like heat, standing still for a long period, etc. Exercise and tilt training have been suggested.
In patient with the cardioinhibitory form, a dual-chamber pacemaker may be of value; however, the pacemaker will not improve the vasodepressor component of the syncope [74].
Pharmacotherapy includes β-blockers, which inhibit the activation of mechanoreceptors; Fludrocortisone, which expands central fluid volume via retention of sodium; and vasoconstrictors and selective serotonin reuptake inhibitors (SSRI) which may have a role in regulating sympathetic nervous system activity.
3.2.2.3 Carotid Sinus Syncope
Carotid sinus syndrome is associated with episodes of brief loss of consciousness, drop attacks, and unexplained falls without prodromal symptoms, especially in older males who often have cardiovascular disease [75]. It often occurs after neck movements, such as looking up or going down the stairs.
Carotid sinus syndrome is defined by a symptomatic 3 s asystole and/or a 50 mmHg reduction in systolic blood pressure (SBP) in response to carotid sinus massage (CSM) [76]. The syncope is caused by carotid sinus hypersensitivity resulting from stimulation of carotid sinus baroreceptors located in the internal carotid artery above the bifurcation of the common carotid artery. The presence of asymptomatic asystole or drop in BP during the CSM is not a diagnosis of carotid sinus hypersensitivity. CSM should be performed in the supine position and, if negative, repeated with the patient in the upright position [77]. The sensitivity of the CSM is increased by 31–52%, if it’s performed while the patient is tilted 60–70° upright [77].
Carotid sinus syndrome is present in 8.8% of patients with cardiac syncope of all types. It represents 10% of unexplained syncope by the initial evaluation and 5% after the final diagnosis [77], with a rightsided preponderance [77], with a right-sided preponderance. A study found that >70% of all the patients had a positive CSM on the right [75].
3.2.2.3.1 Treatment
Most patients can be treated with education, lifestyle changes, expectancy, and routine follow-up. If there are recurrent symptoms, the patient may need treatment.
Therapy can be divided into medical, surgical (carotid denervation), and pacing.
Medical treatment includes anticholinergics which blunt the bradycardia, Fludrocortisone, Ergotamine, Ephedrine to increase the blood pressure, and β-blockers and serotonin reuptake inhibitors (SSRI) which decrease both peripheral elements of the carotid sinus reflex [78].
Surgical denervation of the carotid sinus was previously used as treatment for carotid sinus syncope since the 1950s but is now abandoned [78].
Cardiac pacing has emerged as the primary therapy for patients with cardioinhibitory syncope, but may have an effect in patients with carotid sinus syncope as well [78, 79].
3.2.2.4 Situational Syncope
Situational syncope is diagnosed if syncope occurs during or immediately after urination, defaecation, cough, or swallowing [73, 80]. The precipitating causes are not always clear. Many of the incidents are related to the loss of intrathoracic pressure leading to syncope or Valsalva-like manoeuvres. A typical profile of the cough syncope patient based on the literature is that of a middle-aged, large-framed, or overweight male with obstructive airways disease [81].
3.2.2.4.1 Treatment
Management includes reducing or preventing exposure to precipitating causes, although these may be unclear. In some, especially those with phobias, behavioural therapy is needed.
The defaecation syncope is due to raised intra-abdominal pressures and Valsalva-type responses, and the use of abdominal binders may counteract this response [82].
3.2.3 Postural Tachycardia Syndrome (PoTS)
Postural tachycardia syndrome (PoTS) is primarily characterised by the development of tachycardia and orthostatic symptoms, with postural changes in the absence of significant hypotension.
PoTS as a condition was recognised by Rosen and Cryer in 1982 [62, 83] and later in 1993 by Schondorf and Low [84]. The most important symptom is orthostatic intolerance due to intermittent cardiovascular autonomic dysfunction [85].
The symptoms include palpitations, dizziness, and, in some patients, syncope, usually upon standing, and may be exacerbated by modest exertion, food ingestion, and heat.
PoTS affects predominantly young females, 5:1 ratio over males, and most patients are between 20 and 40 years of age [85]. There is a strong link to joint hypermobility syndrome, also known as Ehlers–Danlos syndrome (EDS) type III or Joint Hypermobility Syndrome. Symptoms may be brought on by infection, trauma, surgery, or stress. Possible pathophysiological mechanisms include alterations in neural control, humoral factors, vascular properties, and intravascular volume, as well as physical deconditioning [85].
PoTS is not a unique entity but the common presentation of a number of different causes. In addition, PoTS may be more subclassified as presented here. However, for the purpose of this book to present autonomic diagnostics at bedside, this would lead too far.
3.2.3.1 Frequent Reported Symptoms of PoTS [85]
-
Dizziness and light-headedness
-
Palpitations
-
Visual disturbances
-
Clumsiness
-
Loss of consciousness
-
Nausea
-
Headache
-
Pain (chest or upper abdomen)
-
Shortness of breath
-
Fatigue and lethargy
-
Difficulty thinking or concentrating
-
Psychiatric symptoms such as anxiety, panic attacks, and solitude
The symptoms are often relieved by lying flat.
Factors which may induce or worsen PoTS symptoms [85]
-
Time of day (may be worse in the morning, especially on initial rising after wakening)
-
Speed of positional change
-
Raised temperature (hot weather, hot bath)
-
Dehydration
-
Food ingestion
-
Alcohol
-
Physical exertion
-
Menstrual period
-
Deconditioning or prolonged recumbence
-
Drugs that cause vasodilatation
For further remarks on history taking see Sects. 2.2.1.1 and 2.3.2
3.2.3.2 Diagnostic Criteria for PoTS
PoTS is defined by a symptomatic heart rate increment of >30 beats/min or more within 10 minutes of standing or head-up tilt (HUT) in the absence of orthostatic hypotension; the standing heart rate is often 120 beats/min or higher [86]. For individuals aged 12–19 years, the required increment is ≥40 beats/min [86].
The excessive tachycardia during orthostatic stress seen in patient with PoTS is a physiologic response that helps maintain arterial pressure during venous pooling. Patients with PoTS also demonstrate excessive tachycardia during exercise, which does not appear to be secondary to abnormal baroreflex regulation of the heart rate. Orthostatic intolerance is potentiated after deconditioning (spaceflight or prolonged bed rest).
3.2.3.2.1 Treatment
A multifactorial treatment strategy that includes pharmacological agents as well as non-pharmacological measures and interventions is often required. The approach should be pragmatic and holistic and focus on self-management. The patient should be taught to recognise triggers and manage and prevent symptoms. Exercise training and improved physical conditioning is an important strategy for PoTS as well as other deconditioned patients.
Non-pharmacological measurements to prevent hypotension and counteract tachycardia are increased fluid and salt intake, lower limb exercise, counterpressure manoeuvres, and the use of stockings. Gentle and gradual increasing core strengthening exercises like Pilates and swimming may be useful, especially for those with a diagnosis of Ehlers–Danlos syndrome (EDS) type III.
Pharmacological treatment approaches for PoTS are similar to those used for orthostatic hypotension, including Fludrocortisone, Ephedrine, and Midodrine, and low-dose β-blockers or Ivabradine. Associated disorders, such as the joint hypermobility syndrome and pain, need to be addressed. With time many patients get less symptomatic.
3.3 How to Manage Cardiovascular Autonomic Dysfunction and Disease?
Management depends on the underlying cause. After a review of a patient’s medications for drugs that may contribute to syncope, rehydration, correction of any metabolic abnormality, and blood transfusion are appropriate first steps. Many medications are implicated, and stopping these should be considered. Further management includes non-pharmacological and pharmacological treatments. Although other medications have been studied, Fludrocortisone and Midodrine have accrued the best evidence for efficacy.
3.3.1 Overview of Non-pharmacological Measures
3.3.1.1 Water and Salt
Water drinking elicits a large, acute, pressor response in patients with autonomic failure who experience severe orthostatic hypotension [87]. Drinking water and increasing salt intake increase plasma volume, which helps maintain blood pressure upon standing.
The increase in blood pressure is evident within 5 min after drinking water, reaches a maximum after approximately 20–30 min, and is sustained for more than 60 min [87]. The recommended daily intake of water is 1.5–2.0 L/day and of sodium chloride is 6–10 g either incorporated into meals or taken as supplement tablets [88].
3.3.1.2 Orthostatic Training
The prescription of progressively prolonged periods of enforced upright posture may reduce the recurrence of neurally mediated syncope [88, 89]. However the patients need to be highly motivated when participating [90].
3.3.1.3 Counterpressure Manoeuvres
Counterpressure manoeuvres are simple ways to induce a significant blood pressure increase to counteract orthostatic hypotension, neurally mediated syncope, and PoTS [90, 88].
Countermeasures which are able to induce significant blood pressure increase include:
-
Clenching the teeth
-
Squeezing the buttocks
-
Isometric hand grip and arm tensing
-
Leg crossing on standing
-
Gentle marching on the spot instead of standing still
-
Squatting
3.3.1.4 Raising the Head of the Bed
Raising the head of the bed 10–20° activates the renin–angiotensin–aldosterone system [88, 91] and decreases the nocturnal diuresis. Raising the head of the bed may also reduce the supine hypertension that is prevalent in patients suffering from cardiovascular autonomic dysfunction, either as a consequence of baroreceptor denervation or as a side effect of treatment [92].
3.3.1.5 Compression Stockings
The rationale for compression therapy is to reduce venous pooling in the lower extremities to promote venous return and cardiac output. The categories of compression stockings include knee-length, thigh-length, full-length, and abdominal compression. The current literature reports the use of waist-high stockings that afford abdominal compression is needed to affect cardiovascular dynamics at the onset of head-up tilt and may prevent OH [93].
Although compression stockings provide orthostatic relief, there may be difficulties with compliance. Full-length compression stockings are uncomfortable and may be a burden to put on and wear. If the patient is able to comply, compression stockings should be incorporated in the treatment regimen for orthostatic hypotension. Ideally, abdominal compression should also be included because there is often considerable pooling in the splanchnic circulation. Fanciulli et al. found an elastic abdominal binder significantly reduced blood pressure fall upon tilting in OH associated with Parkinson’s disease (PD) [94]. In our experience, patients prefer compression stockings that do not cover the feet.
3.3.2 Overview of Pharmacological Measurements
Pharmacological treatment should only be initiated when sufficient control can’t be achieved by non-pharmacological measurements.
3.3.2.1 Fludrocortisone
Fludrocortisone acts as a systemic corticosteroid, increasing sensitivity to circulating catecholamines. Fludrocortisone is the first-line pharmacological treatment of OH, and it promotes a positive response in 40–75% of patients. It is also used as a first-line treatment in PoTS. It has central adrenergic effects and increases arteriolar sensitivity to catecholamine and angiotensin. The starting dose is 50 μg once daily, which is increased by 25–50 μg every 1–2 weeks to alleviate symptoms without incurring side effects.
The recommended dose is 100–300 μg/day, and it can take up to 5 days to see the full effects.
Higher doses that elevate circulating epinephrine can cause hypokalaemia and supine hypertension. Fludrocortisone is not recommended for patients with congestive heart failure or chronic renal failure.
Side effects include hypertension, oedema, hypokalaemia, depression, and headache. Electrolyte levels should be checked 1 week after starting Fludrocortisone and a week after a dose change.
3.3.2.2 Midodrine
Midodrine, a peripheral selective alpha-1-adrenergic agonist, significantly increases standing systolic blood pressure and improves symptoms in patients with neurogenic OH [95] and is used as second-line therapy in OH and in selected PoTS patients. Standing systolic blood pressure is elevated by approximately 15–30 mm Hg at 1 h after 10 mg dose, with some effect persisting for 2–3 h. Midodrine has no clinically significant effect on standing or supine pulse rates in patients with autonomic failure. It can be used as a fludrocortisone-sparing agent.
Midodrine acts as a pressor agent both on venous and arterial constrictions and is effective 1 h after ingestion. The recommended dose (typically given in the morning, noon, and afternoon to avoid supine hypertension in the evening) is up to 10 mg three times daily; each dose typically lasts for 4 h, consistent with blood levels of the active metabolite desglymidodrine. The initial usual dose is 2.5 mg three times daily, with a slow and progressive increment as needed.
The major adverse effect is supine hypertension. This adverse reaction can be minimised by taking it half an hour before standing up in the morning and avoiding becoming supine 4 h after each dose. Other reported adverse effects include piloerection, scalp pruritus, tingling, and urinary retention/urgency [96].
Midodrine is contraindicated during pregnancy and breastfeeding. Women in fertile age should be explained this contraindication prior to be started on it.
Increased central serotoninergic activity has been suggested to play a role in sudden inhibition of sympathetic activity, potentially precipitating neurally mediated syncope, especially in patients resistant to or intolerant of previous traditional therapies [97].
3.3.2.3 Ephedrine
Ephedrine has adrenergic effects on the circulation and increases the mean heart rate as well as the systolic and, slightly, diastolic arterial blood pressure [98]. Adverse effects are hypertension and thrombosis [96]. Recommended dosage is 15 mg three times daily.
3.3.2.4 Octreotide
Octreotide is a somatostatin analogue which attenuates the pancreatic and gastrointestinal hormone response to food ingestion [92].
It reduces the postprandial blood pressure fall and the orthostatic hypotension in patients with autonomic failure by local effect on splanchnic vasculature via inhibiting the release of vasoactive gastrointestinal peptides [92]. Adverse effects are diarrhoea, nausea, and abdominal cramps which limit the use [96]. Subcutaneous doses of octreotide range from 25 to 200 μg. It has been used in PoTS patients who are refractory to other treatments with some success.
Octreotide is contraindicated in pregnancy.
3.3.2.5 Erythropoietin
Erythropoietin increases standing blood pressure and improves orthostatic tolerance in patients with OH. It also corrects the normochromic normocytic anaemia that frequently accompanies patients with autonomic failure [92]. Erythropoietin is not effective in the treatment of PoTS [99]. The mechanism of the pressor effect of this agent is unknown.
Adverse effects are supine hypertension and thrombosis [92, 96].
3.3.2.6 Droxidopa
Droxidopa (L-threo-dihydroxyphenylserine (DOPS)) is a synthetic prodrug that is converted into norepinephrine by the ubiquitous enzyme dopa decarboxylase. Droxidopa decreases postural drop in patients with orthostatic hypotension and reduces orthostatic symptoms [100].
Reported adverse effects are malignant neuroleptic syndrome, hypertension, and headache [96]. DOPS (200–400 mg daily) reduces OH with only minor side effects. It is an effective treatment in dopamine β-hydroxylase deficiency. Droxidopa is presently only licenced in the USA, Japan, and surrounding Asian areas.
3.3.2.7 Pyridostigmine
Pyridostigmine is a peripheral cholinesterase inhibitor that potentiates cholinergic transmission when the autonomic ganglia have already been engaged. It can cause a mild increase in standing blood pressure without significantly increasing supine blood pressure. Administering pyridostigmine as needed may improve orthostatic hypotension without contributing to supine hypertension. Adverse effects are cholinergic, including diarrhoea, excessive sweating, and sialorrhoea [38].
The usual initial dose is 30 mg twice daily, which can be increased as tolerated up to 90 mg three times daily.
3.3.2.8 Domperidone
While dopamine agonists are widely used to manage Parkinson’s symptoms, one major side effect is acute orthostatic hypotension after starting the treatment. Domperidone is a peripheral dopamine D2 receptor antagonist that is effective in treating acute orthostatic hypotension induced by dopamine agonists [101]. Adverse effects are worsening of Parkinson’s motor symptoms and hyperprolactinaemia [96]. Domperidone is contraindicated in patients with underlying cardiac conditions because its use increases the risk of prolonged QT syndrome. Prolonged use is not recommended.
3.3.2.9 Yohimbine
Yohimbine is a centrally and peripherally active selective α2-adrenoreceptor antagonist that increases sympathetic nervous system efferent output [36]. Adverse effects are anxiety, tremor, palpitations, diarrhoea, and supine hypertension [96, 102]. Yohimbine increases norepinephrine spill-over from sympathetic nerve endings, leading to a normal increase in plasma norepinephrine levels in control subjects and in patients with MSA, but not in PD patients with OH and autonomic failure.
3.3.2.10 Atomoxetine
Atomoxetine is a norepinephrine transporter blocker thereby increasing synaptic norepinephrine concentration. It increases blood pressure in autonomic failure patients with residual sympathetic activity the first hour after its administration, although its use has not been tested at long term [103]. Adverse effects are nausea, dry mouth, appetite loss, insomnia, fatigue, headache, and cough. It is not licenced for use in OH either in the USA or Europe.
Co-administering Yohimbine with Atomoxetine can then enhance the pressor effect of Atomoxetine by potentiating the activity of the remaining sympathetic efferent fibres.
3.3.2.11 β-Blockers
β1-Selective (cardioselective) adrenoceptor blockers inhibits the activation of mechanoreceptor, leading to a reduction in both resting heart rate and exercise heart rate and decrease in blood pressure. β-Blockers have been used as therapy for neurally mediated syncope [90]. However β-blocker therapy might often worsen PoTS or reflex syncope considerably [90]. Adverse effects are cold hands and feet, tiredness, depression, impotence, vivid dreams, nightmares, and other sleep disturbances.
Take Home Messages
-
OH is defined as fall in BP within 3 min of active standing or on head-up tilt. It is related to a shorter life expectancy.
-
OH is often seen in primary neurodegenerative disorders (PAF, MSA, and PD) and other medical conditions (diabetes mellitus, dehydration) and due to vasoactive and also non-vasoactive drugs.
-
Syncope is the most common autonomic cardiovascular disorder, mainly benign when there is no an underlying cardiac disease.
-
Carotid sinus hypersensitivity should be suspected when the syncope is not preceded by typical warning symptoms, in elderly patients, and when the symptomatic episodes are related to neck movements.
-
PoTS is common in young women, defined as a symptomatic excessive increase in heart rate (more than 30 beats per minute) during the first 10 min of standing or head-up tilt.
-
Nocturnal hypertension can be seen due to the natural progression of the underlying disease or as a side effect of drug treatment.
-
Individually tailored therapy is important in order to improve the patient’s functional capacity and quality of life and preventing injury.
-
Management includes education, advice, and training on various factors that influence blood pressure and special aspects that have to be avoided (foods, habits, positions, and drugs).
-
Countermeasures including leg crossing, squatting, elastic abdominal binders, and stockings are useful.
-
Both OH, syncope, and PoTS benefit from careful exercise.
-
Increased water (1.5–2 l/day) and salt ingestion (6–10 g or 150 mmol/day).
-
Always start with non-pharmacological measurements.
-
First line drug of choice is Fludrocortisone. Sympathomimetics, such as Midodrine is second line.
References
Benarroch EE (2014) The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol 10(7):396–407
Mathias CJ (2003) Autonomic diseases: clinical features and laboratory evaluation. J Neurol Neurosurg Psychiatry 74(Suppl 3):iii31–iii41
Shibao C, Okamoto L, Biaggioni I (2012) Pharmacotherapy of autonomic failure. Pharmacol Ther 134(3):279–286
Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology (1996) Neurology 46(5):1470
Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M (2011) Orthostatic hypotension. In: Gilhus NE, Barnes MP, Brainin M, editors. European handbook of neurological management: volume 1. 2 ed: Blackwell Publishing Ltd.; p. 469–75
Gupta V, Lipsitz LA (2007) Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 120(10):841–847
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I et al (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21(2):69–72
Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME (2007) Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112(3):157–165
Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB et al (2009) Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 30(21):2631–2671
Gibbons CH, Freeman R (2006) Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 67(1):28–32
Claydon VE, Steeves JD, Krassioukov A (2006) Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44(6):341–351
Gibbons CH, Freeman R (2015) Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 85(16):1362–1367
Pavy-Le Traon A, Piedvache A, Perez-Lloret S, Calandra-Buonaura G, Cochen-De Cock V, Colosimo C et al (2015) New insights into orthostatic hypotension in multiple system atrophy: a European multicentre cohort study. J Neurol Neurosurg Psychiatry 87(5):554–561
Mathias CJ, Christensen NJ, Frankel HL, Spalding JM (1979) Cardiovascular control in recently injured tetraplegics in spinal shock. Q J Med 48(190):273–287
Mathias CJ (2006) Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog Brain Res 152:231–243
Frankel HL, Michaelis LS, Golding DR, Beral V (1972) The blood pressure in paraplegia. I. Paraplegia 10(3):193–200
Low PA (2008) Prevalence of orthostatic hypotension. Clin Auton Res 18(Suppl 1):8–13
Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS (1992) Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 19(6 Pt 1):508–519
Senard JM, Brefel-Courbon C, Rascol O, Montastruc JL (2001) Orthostatic hypotension in patients with Parkinson’s disease: pathophysiology and management. Drugs Aging 18(7):495–505
Arnold AC, Shibao C (2013) Current concepts in orthostatic hypotension management. Curr Hypertens Rep 15(4):304–312
Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O (2010) Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 31(1):85–91
Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F (2007) Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets 7(1):63–70
Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M (2006) EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 13(9):930–936
Coupe M, Fortrat JO, Larina I, Gauquelin-Koch G, Gharib C, Custaud MA (2009) Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respir Physiol Neurobi 169(Suppl 1):S10–SS2
Themistocleous AC, Ramirez JD, Serra J, Bennett DLH (2014) The clinical approach to small fibre neuropathy and painful channelopathy. Pract Neurol 14(6):368–379
Treatment Of Orthostatic Hypotension (1998) Continuum (Lifelong Learning in Neurology) 4(2):79–90
Mathias CJ (2002) To stand on one’s own legs. Clin Med 2(3):237–245
Mathias CJ, Fosbraey P, da Costa DF, Thornley A, Bannister R (1986) The effect of desmopressin on nocturnal polyuria, overnight weight loss, and morning postural hypotension in patients with autonomic failure. Br Med J (Clin Res Ed) 293(6543):353–354
Mathias CJ, Young TM (2004) Water drinking in the management of orthostatic intolerance due to orthostatic hypotension, vasovagal syncope and the postural tachycardia syndrome. Eur J Neurol 11(9):613–619
Groothuis JT, van Dijk N, Ter Woerds W, Wieling W, Hopman MT (2007) Leg crossing with muscle tensing, a physical counter-manoeuvre to prevent syncope, enhances leg blood flow. Clin Sci (Lond) 112(3):193–201
Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR (2015) Physical countermeasures to increase orthostatic tolerance. J Intern Med 277(1):69–82
Podoleanu C, Maggi R, Brignole M, Croci F, Incze A, Solano A et al (2006) Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol 48(7):1425–1432
Basso A, Paola LD, Erle G, Boscaro M, Armanini D (1994) Licorice ameliorates postural hypotension caused by diabetic autonomic neuropathy. Diabetes Care 17(11):1356
Schroeder C, Jordan J, Kaufmann H (2013) Management of neurogenic orthostatic hypotension in patients with autonomic failure. Drugs 73(12):1267–1279
Ong AC, Myint PK, Shepstone L, Potter JF (2013) A systematic review of the pharmacological management of orthostatic hypotension. Int J Clin Pract 67(7):633–646
Freeman R (2008) Current pharmacologic treatment for orthostatic hypotension. Clin Auton Res. 18(Suppl 1):14–18
Freeman RR (2003) Treatment of orthostatic hypotension. Semin Neurol 23(4):435–442
Isaacson SH, Skettini J (2014) Neurogenic orthostatic hypotension in Parkinson’s disease: evaluation, management, and emerging role of droxidopa. Vasc Health Risk Manag 10:169–176
Jansen RW, Lipsitz LA (1995) Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med 122(4):286–295
Ong AC, Myint PK, Potter JF (2014) Pharmacological treatment of postprandial reductions in blood pressure: a systematic review. J Am Geriatr Soc 62(4):649–661
Goldstein DS (2006) L-Dihydroxyphenylserine (L-DOPS): a norepinephrine prodrug. Cardiovasc Drug Rev 24(3–4):189–203
Kaufmann HH (2014) Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 83(4):328–335
Mathias CJ (2008) L-dihydroxyphenylserine (Droxidopa) in the treatment of orthostatic hypotension: the European experience. Clin Auton Res. 18(Suppl 1):25–29
Hoeldtke RD, Cavanaugh ST, Hughes JD, Polansky M (1986) Treatment of orthostatic hypotension with dihydroergotamine and caffeine. Ann Intern Med 105(2):168–173
Kroll M, Ring C, Gaus W, Hempel B (2005) A randomized trial of Korodin Herz-Kreislauf-Tropfen as add-on treatment in older patients with orthostatic hypotension. Phytomedicine 12(6–7):395–402
Seyer-Hansen K (1977) Postprandial hypotension. Br Med J 2(6097):1262
Luciano GL, Brennan MJ, Rothberg MB (2010) Postprandial hypotension. Am J Med 123(3):281–286
Mathias CJ, Kimber JR (1998) Treatment of postural hypotension. J Neurol Neurosurg Psychiatry 65(3):285–289
Alagiakrishnan K (2007) Postural and Postprandial Hypotension: Approach to Management. Geriatr Aging 10(5):298–304
Maruta TT (2006) Voglibose inhibits postprandial hypotension in neurologic disorders and elderly people. Neurology 66(9):1432–1434
MARSHALL RJ, SCHIRGER A, Shepherd JT (1961) Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation 24:76–81
Low DA, da Nóbrega ACL, Mathias CJ (2012) Exercise-induced hypotension in autonomic disorders. Auton Neurosci 171(1–2):66–78
Krediet CTP, Wilde AA, Wieling W, Halliwill JR (2004) Exercise related syncope, when it’s not the heart. Clin Auton Res 14(Suppl 1):i25–i36
Kenney MJ, Seals DR (1993) Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 22(5):653–664
MacDonald JR (2002) Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens 16(4):225–236
Lima LCJ, Assis GV, Hiyane W, Almeida WS, Arsa G, Baldissera V et al (2008) Hypotensive effects of exercise performed around anaerobic threshold in type 2 diabetic patients. Diabetes Res Clin Pract 81(2):216–222
Liu S, Thomas SG, Sasson Z, Banks L, Busato M, Goodman JM (2013) Blood pressure reduction following prolonged exercise in young and middle-aged endurance athletes. Eur J Prev Cardiol 20(6):956–962
Rimaud D, Calmels P, Pichot V, Bethoux F, Roche F (2012) Effects of compression stockings on sympathetic activity and heart rate variability in individuals with spinal cord injury. J Spinal Cord Med 35(2):81–88
Aviado DM, Aviado DG (2001) The Bezold-Jarisch Reflex. Ann N Y Acad Sci 940(1):48–58
Figueroa JJ, Basford JR, Low PA (2010) Preventing and treating orthostatic hypotension: as easy as A, B, C. Cleve Clin J Med 77(5):298–306
Endo MY, Kajimoto C, Yamada M, Miura A, Hayashi N, Koga S et al (2012) Acute effect of oral water intake during exercise on post-exercise hypotension. Eur J Clin Nutr 66(11):1208–1213
Butler JE, Ribot-Ciscar E, Zijdewind I, Thomas CK (2004) Increased blood pressure can reduce fatigue of thenar muscles paralyzed after spinal cord injury. Muscle Nerve 29(4):575–584
Nieshoff EC, Birk TJ, Birk CA, Hinderer SR, Yavuzer G (2004) Double-blinded, placebo-controlled trial of midodrine for exercise performance enhancement in tetraplegia: a pilot study. J Spinal Cord Med 27(3):219–225
Jordan J, Biaggioni I (2002) Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens (Greenwich) 4(2):139–145
Arnold AC, Biaggioni I (2012) Management approaches to hypertension in autonomic failure. Curr Opin Nephrol Hypertens 21(5):481–485
Fanciulli A, Göbel G, Ndayisaba JP, Granata R, Duerr S, Strano S et al (2016) Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin Auton Res 26(2):97–105
O’Brien EE (2005) Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 23(4):697–701
Stuebner E, Vichayanrat E, Low DA, Mathias CJ, Isenmann S, Haensch CA (2013) Twenty-four hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson’s disease. Front Neurol 4:49
Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D et al (2013) Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension 61(3):701–706
da Silva RM (2014) Syncope: epidemiology, etiology, and prognosis. Front Physiol 5:471
Calkins HG, Zipes DP (2012) Hypotension and syncope. Braunwald’s heart disease: a textbook of cardiovascular medicine. 10th ed. Saunders: Philadelphia, PA. p. 861–71
Bassetti CL (2014) Transient loss of consciousness and syncope. Handb Clin Neurol 119:169–191
Brignole M (2007) Diagnosis and treatment of syncope. Heart 93(1):130–136
Brignole M, Menozzi C, Moya A, Andresen D, Blanc JJ, Krahn AD et al (2012) Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 125(21):2566–2571
Tan MP, Newton JL, Reeve P, Murray A, Chadwick TJ, Parry SW (2009) Results of carotid sinus massage in a tertiary referral unit--is carotid sinus syndrome still relevant? Age Ageing 38(6):680–686
LOWN B, LEVINE SA (1961) The carotid sinus. Clinical value of its stimulation. Circulation 23:766–789
Sutton R (2014) Carotid sinus syndrome: progress in understanding and management. Glob Cardiol Sci Pract 2014(2):1–8
Healey J, Connolly S, Morillo C (2004) The management of patients with carotid sinus syndrome: is pacing the answer? Clin Auton Res 14(Suppl 1):i80–ii6
Romme JJ, Reitsma JB, Black CN, Colman N, Scholten RJ, Wieling W et al (2011) Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 10:CD004194
van Lieshout JJ, ten Harkel AD, Wieling W (2000) Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res 10(1):35–42
Dicpinigaitis PV, Lim L, Farmakidis C (2014) Cough syncope. Respir Med 108(2):244–251
Smit AA, Wieling W, Fujimura J, Denq JC, Opfer-Gehrking TL, Akarriou M et al (2004) Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 14(3):167–175
Rosen SG, Cryer PE (1982) Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 72(5):847–850
Schondorf R, Low PA (1993) Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43(1):132–137
Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R (2012) Postural tachycardia syndrome–current experience and concepts. Nat Rev Neurol 8(1):22–34
Freeman R, Kaufmann H (2007) Disorders of orthostatic tolerance-orthostatic hypotension, postural tachycardia syndrome, and syncope. Continuum (Lifelong Learning in Neurology) 13(6):50–88
Jordan J (2002) Acute effect of water on blood pressure. What do we know? Clin Auton Res. 12(4):250–255
Wieling W, Ganzeboom KS, Saul JP (2004) Reflex syncope in children and adolescents. Heart 90(9):1094–1100
Abe H, Kondo S, Kohshi K, Nakashima Y (2002) Usefulness of orthostatic self-training for the prevention of neurocardiogenic syncope. Pacing Clin Electrophysiol 25(10):1454–1458
Guzman JC, Armaganijan LV, Morillo CA (2013) Treatment of neurally mediated reflex syncope. Cardiol Clin 31(1):123–129
Smit AA, Halliwill JR, Low PA, Wieling W (1999) Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol 519(Pt 1):1–10
Freeman R (2012) Treatment of orthostatic hypotension. Saunders 2015, Philadelphia
Tanaka K, Tokumiya S, Ishihara Y, Kohira Y, Katafuchi T (2014) Compression stocking length effects on arterial blood pressure and heart rate following head-up tilt in healthy volunteers. Nurs Res 63(6):435–438
Fanciulli A, Goebel G, Metzler B, Sprenger F, Poewe W, Wenning GK et al (2016) Elastic abdominal binders attenuate orthostatic hypotension in Parkinson’s disease. Mov Disord Clin Pract 3(2):156–160
Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN et al (1998) A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology 51(1):120–124
Pathak A, Senard JM (2004) Pharmacology of orthostatic hypotension in Parkinson’s disease: from pathophysiology to management. Expert Rev Cardiovasc Ther 2(3):393–403
Grubb BP, Samoil D, Kosinski D, Kip K, Brewster P (1994) Use of sertraline hydrochloride in the treatment of refractory neurocardiogenic syncope in children and adolescents. J Am Coll Cardiol 24(2):490–494
Lindqvist A, Jalonen J, Laitinen LA, Seppala T, Stromberg C (1996) The effects of midazolam and ephedrine on post-exercise autonomic chronotropic control of the heart in normal subjects. Clin Auton Res 6(6):343–349
Hoeldtke RD, Horvath GG, Bryner KD (1995) Treatment of orthostatic tachycardia with erythropoietin. Am J Med 99(5):525–529
Biaggioni I, Freeman R, Mathias CJ, Low P, Hewitt LA, Kaufmann H (2015) Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension 65(1):101–107
Schoffer KL, Henderson RD, O’Maley K, O’Sullivan JD (2007) Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson’s disease. Mov Disord 22(11):1543–1549
Senard JM, Rascol O, Durrieu G, Tran MA, Berlan M, Rascol A, et al. Effects of yohimbine on plasma catecholamine levels in orthostatic hypotension related to Parkinson disease or multiple system atrophy. Clinical neuropharmacology. 1993;16(1):70–6.
Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L et al (2014) Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 64(6):1235–1240
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hagen, E.M., Navarro-Otano, J. (2017). The Diagnosis and Management of Cardiovascular Autonomic Dysfunction and Disease. In: Struhal, W., Lahrmann, H., Fanciulli, A., Wenning, G. (eds) Bedside Approach to Autonomic Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-05143-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-05143-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05142-0
Online ISBN: 978-3-319-05143-7
eBook Packages: MedicineMedicine (R0)