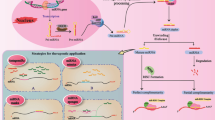
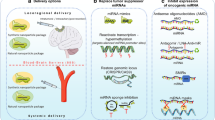
Abstract
Glioblastoma (GBMs) are the most common and aggressive primary brain tumors. Current treatments include surgery, radiation therapy, and chemotherapy. Unfortunately, prognosis remains extremely poor and the median survival of 12–14 months in GBM has not changed significantly. GBMs contain a small subpopulation of glioma stem cells (GSCs) that are implicated in therapeutic resistance and tumor recurrence. Thus, the development of new therapeutic strategies to target these cells is of utmost significance. MicroRNAs (miRNAs) have been reported to play a major role in cancer development and progression and are now emerge as potential therapeutic targets in various tumors. This chapter reviews the expression of miRNAs in GBM and GSCs and their use as diagnostic and prognostic biomarkers and discusses the role of specific miRNAs in GSC functions and their potential use as therapeutic targets. Since the clinical use of miRNAs is hindered by lack of effective delivery mechanisms to tumor sites, a particular focus is placed on the possible modes of delivery of miRNA mimics or antagonists focusing on viral vectors, nanoparticles, exosomes and stem cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Gliomas are the most common form of primary brain tumors, accounting for 80 % of malignant CNS tumors [1]. These tumors exhibit a marked malignant progression characterized by high level of infiltration throughout the brain, resistance to traditional chemotherapy and radiotherapy and high level of angiogenesis [1, 2]. Malignant gliomas are classified on the basis of histopathological features, clinical presentation and genetic alterations as astrocytomas, oligodendrogliomas, or tumors with morphological features of both astrocytes and oligodendrocytes, termed oligoastrocytomas [3]. Astrocytic tumors are graded based on the WHO consensus-derived scale of I to IV according to their degree of malignancy as judged by various histological features accompanied by genetic alterations [3, 4]. Grade I tumors are biologically benign and can be cured if they can be surgically resected; grade II tumors are low-grade malignancies that may follow long clinical courses, but their infiltration into the surrounding brain parenchyma renders them incurable by surgery; grade III tumors exhibit increased anaplasia and proliferation and grade IV tumors (glioblastoma, GBM), exhibit more advanced features of malignancy, including vascular proliferation, necrosis and resistance to radio and chemotherapy [5]. On the basis of clinical presentation, GBMs have been further subdivided into primary or secondary subtypes [6]. Primary GBMs account for the great majority of tumors in older patients, while secondary GBMs are less common and tend to occur in patients under the age of 45. Remarkably, primary and secondary GBMs are morphologically and clinically indistinguishable as reflected by a similar poor prognosis when adjusted for patient age. However, although these GBM subtypes share a common phenotypic endpoint, recent genomic profiles have revealed strikingly different transcriptional patterns and DNA copy number aberrations between primary and secondary GBMs [6]. Based on these studies, a recently expanded group of novel markers has been identified, which allow a better classification of gliomas and can serve as novel prognostic markers [7–9]. In addition, specific miRNAs have been also identified as diagnostic and prognostic markers in GBM and as novel therapeutic targets [10, 11].
1.1 The New Classification of GBM
A recent study by Phillips et al. [12] reported the identification of three high-grade astrocytoma subsets, identified by differential expression of markers associated with outcome, and named them proneural, proliferative and mesenchymal in recognition of the key features of the molecular signatures associated with each group. This study showed that the proneural subtype is distinguished by markedly better prognosis and expresses genes associated with normal brain and the process of neurogenesis. The two other subtypes, the proliferative and mesenchymal, are characterized by a resemblance to either highly proliferative cell lines or tissues of mesenchymal origin and show activation of gene expression programs indicative of cell proliferation or angiogenesis, respectively.
More recently, using an unsupervised approach to classify data from The Cancer Genome Atlas (TCGA) project, Verhaak [13] reported the existence of four GBM subtypes, termed proneural, neural, classical and mesenchymal. The four GBM subtypes have distinct molecular markers and three of the subtypes identified in this study were demonstrated to have a strong association with specific genomic alterations; the proneural subtype exhibiting IDH1 mutations and/or PDGFRA amplification, the classical subtype exhibiting amplification and/or mutation of EGFR, and the mesenchymal subtype showing loss and/or mutation of NF1.
While additional subtypes likely exist, only the proneural and the mesenchymal GBMs have been consistently identified in both supervised and unsupervised classification of GBM and have been reported to have both prognostic and predictive values [12, 13]. Recent studies also identified specific miRNAs that are characteristic of the mesenchymal GBM [14, 15].
1.2 The Mesenchymal Transformation of GBM
The epithelial to mesenchymal transformation (EMT) is a process that allows polarized epithelial cells to undergo multiple biochemical changes that enable them to acquire a mesenchymal cell phenotype which includes enhanced migratory capacity, invasiveness and increased resistance to apoptosis [16]. Activation of EMT is important for cancer cell dissemination and for the promotion of tumor metastasis in epithelial tumors [17]: however, the importance of this process in the neuro-epithelial context is much less characterized. The results from recent studies suggest that primary GBM, GSCs and glioma cells can undergo mesenchymal transformation [18–21]. During this process, which strongly resembles EMT of epithelial tumors, glioma cells acquire a mesenchymal stem cell-like properties which include increased ability to migrate, upregulation of mesenchymal markers and the ability to differentiate to the mesenchymal lineage.
The clinical relevance of the mesenchymal transformation of GBM is of utmost importance, as several studies showed that GBM patients whose tumors have a proneural phenotype have better survival compared with those that have mesenchymal phenotype [12, 13] and upon recurrence, tumors frequently transform toward the mesenchymal phenotype which is characterized as the most aggressive and therapy resistant subtype [12, 20, 22, 23].
1.3 The Factors Underlying the Mesenchymal Transformation of GBM
Using gene regulatory network analyses, the transcription factors signal transducer and activator of transcription 3 (STAT3) and CCAAT enhancer-binding protein β (C/EBPβ) have been recently identified as synergistic initiators and master regulators of the mesenchymal transformation in glioma [24]. Analysis of regulatory networks of available expression microarray data sets of GBM, the transcriptional co-activator TAZ has been identified as another major regulator of mesenchymal differentiation in malignant glioma [25]. In addition, a recent study reported that the TNF-1/NF-KB pathway activated by macrophages/microglia, also plays a role in the mesenchymal transformation of proneural glioma stem cells [23]. Similarly, specific miRNAs have been also identified as important regulators of the EMT process [26] and of the mesenchymal transformation of GBM [27, 28].
2 Cancer Stem Cells (CSCs)
2.1 General Aspects of Cancer Stem Cells
CSCs represent a subset of tumor cells that has the ability to self-renew, generate the diverse cells that comprise the tumor and to continually sustain tumorigenesis. CSCs share important characteristics with normal tissue stem cells, including self-renewal (by symmetric and asymmetric divisions) and differentiation capacity, albeit in an aberrant mode [29]. The first evidence for the existence of CSCs came from acute myeloid leukemia in which a rare subset comprising 0.01–1 % of the total population could induce leukemia when transplanted into immunodeficient mice [30]. CSCs are distinct from the cell of origin, which specifically refers to the cell type that receives the first oncogenic hit(s). Moreover, CSCs do not necessarily originate from the transformation of normal stem cells but may arise from restricted progenitors or more differentiated cells that have acquired self-renewing capacity [31]. One implication of this model is that there are mechanistic parallels between the self-renewal programs of normal stem cells and CSCs. It has been presumed in many cases that the cells in which cancer originate share committed cells that have undergone some degree of differentiation [32].
2.2 Glioma Stem Cells (GSCs)
Glioma stem cells (GSCs) were one of the first CSCs isolated from solid tumors. GBM contain a small subpopulation of self-renewing and tumorigenic CSCs which are implicated in tumor infiltration, resistance to conventional therapies and tumor recurrence [33–38]. Interestingly, GSCs isolated from human tumors and cultured in vitro showed remarkable similarities to normal neural stem cells (NSCs), expressing neural stem/progenitor markers such as Nestin, Sox2, and Olig2 and upon induction, could be differentiated to cells expressing neuronal or glial markers [36, 39]. Transplantation of GSCs into immunodeficient mice yielded tumors that shared similar histology and global gene expression patterns with their parental tumors [40]. Understanding the mechanisms associated with the stemness and oncogenic features of these cells is essential for the development of therapeutic approaches that can eradicate GSCs and may provide the basis for the development of novel therapeutic approaches for GBM patients [41–43].
2.3 miRNAs in Cancer
miRNAs are 19–25 nucleotide non-coding small RNAs that can play important regulatory roles in animals and plants by targeting mRNAs for cleavage or translational repression [44–46]. miRNAs induce gene silencing by partial sequence homology and thus a single miRNA can have hundreds of targets and therefore regulate diverse cellular functions such as pathways involved in stem cell function, cell proliferation, migration and oncogenic transformation [47, 48]. Indeed, aberrantly expressed miRNAs have been described in various types of tumors including GBM [49–51]. The expression and function of specific miRNAs in astrocytic tumors have been studied with regards to mechanisms of gliomagenesis, patient prognosis and their use as novel therapeutic targets. [52, 53].
Mechanisms of miRNA deregulation have been described at various levels including genetic, epigenetic, transcriptional, and processing levels [54]. Amplification and deletion of many miRNAs are associated with their location at regions that are either amplified or deleted in human cancers [55]. Repression of some miRNAs is mediated by CpG hypermethylation in different tumors [56], whereas other miRNAs are regulated by transcription factors such as p53, E2F, STAT3 or via deregulation of Dicer or Drosha as has been observed in many cancers [57]. Various studies documented that miRNAs can act as tumor suppressor or oncogenes based on the function of their major target genes [58]. In addition, they have been shown to regulate a variety of cellular functions such as cell proliferation, cell death and apoptosis, cell migration and invasion, metastasis, angiogenesis, tumor microenvironment, tumor immunology and chemoresistance as well as many aspects of cancer stem cell biology [48–52, 59].
2.4 miRNAs as Biomarkers in GBM
Various studies have been performed to identify biological markers for the detection and risk stratification of gliomas. miRNA expression and signature have been also associated with the diagnosis and prognosis of patients with different types of tumors [60–62]. Genome-wide profiling studies indicated that the miRNA signature can contribute to the distinguishing of different types of cancers, the identification of tissue of origin of purely characterized tumors or of metastasis of undefined origin and the classification of tumor histological subtypes [63, 64]. In addition, specific miRNAs have been demonstrated to have great potential as early minimally invasive biomarkers in different tumors due to their stability, abundance and accessibility in various fixed and fresh tissues, different biologic fluids and circulating exosomes [65, 66].
Various gene profiling studies suggested the existence of multiple GBM classes but their characteristics are not fully defined. Recent studies employed also the use of miRNAs as biomarkers in GBM [66]. A study by Kim et al., analyzed 261 miRNA expression profiles from TCGA and identified five clinically and genetically distinct subclasses of glioblastoma based on their miRNA signature. These subgroups were related to different neural precursor cell types resembling those of radial glia, oligoneuronal precursors, neuronal precursors, neuroepithelial/neural crest precursors, or astrocyte precursors [11]. In addition, this study also identified specific miRNAs as potent regulators of subclass-specific gene expression networks in GBM. One of the miRNAs, miR-9, was found to suppress the mesenchymal differentiation of glioblastoma by inhibiting JAK kinases and of STAT3 [11].
To identify significant miRNA-mRNA correlations in gliomas, a study by Ma et al. [67] analyzed the miRNA and mRNA signatures and the relationship between them in 82 glioma specimens. Statistical analysis showed that expression of miR-128a, -504, -124a, and -184 was negatively correlated with the expression of mesenchymal markers in GBM. Functional analysis of miR-128a and -504 demonstrated that mesenchymal signaling in GBM may be negatively regulated by miR-128a and -504 [67].
In another study, Li et al., described an integrated approach to identify miRNA functional targets during glioma malignant progression by combining the paired expression profiles of miRNAs and mRNAs across 160 glioma patients. They identified miR-524-5p and miR-628-5p as protective factors and their expression decreased during glioma progression [68]. miRNA profiling of glioma was also analyzed in cerebrospinal fluid (CSF) in a study by Baraniskin et al. [69] which identified miR-21 and miR-15b as potential markers in CSF for glioma that can distinguish these patients from normal individuals and from patients with brain lymphoma and metastatic brain tumors.
A recent review that summarized the expression of specific miRNAs in GBM based on the recent literature identified 253 upregulated and 95 downregulated miRNAs. Both of the oncogenic and tumor suppressor miRNAs were found to target genes involved in cell migration, invasion, angiogenesis and proliferation [28].
In addition to studies aiming at analyzing the miRNA signatures of GBM, there have been multiple studies reporting the expression of specific miRNAs in different tumor grades. miR-21 has been shown to exhibit both diagnostic and prognostic values in different tumors including gliomas [70]. The expression of miR-26a has been associated with poor prognosis [71]. The decreased expression of miR-328 is associated with unfavorable prognosis in glioma [72] and miR-181d expression acted as a predictive biomarker for temozolomide response [73]. We recently reported that both miR-145 [74] and miR-137 [75] were downregulated in glioblastoma as compared to normal brain specimens and that the decrease of miR-137 was attributed to hypermethylation of pre-miR-137 promoter [75].
2.5 miRNA Functions in GBM
In addition to their diagnostic application, specific miRNAs have been also implicated as potential therapeutic targets and tools [76, 77]. The most appealing advantage of using miRNAs for the treatment of cancer therapy is their ability to affect multiple target genes in the context of a network, making them suitable especially for the treatment of glioma that is a complex heterogeneous tumor. The miRNAs that are overexpressed in a deregulated manner in tumors are considered as oncogenes and are called oncomiRs [71, 78]. They are associated with the inhibition of tumor suppressor genes or those which control cell proliferation, differentiation, migration and apoptosis.
Various studies have highlighted the importance of miRNA deregulation in several aspects of the pathogenesis of GBM including cell cycle control, invasion, migration, resistance to chemotherapy and radiotherapy and cell apoptosis [79–81]. Specific miRNAs control some of the core signaling pathways in GBM such as EGFR signaling and those related to p53, PTEN/PI3K/AKT and the Notch pathways. Moreover, several in vitro and pre-clinical studies demonstrated the therapeutic benefits of either expressing tumor suppressor miRNAs or inhibiting OncomiRs [82–85].
Various modification of anti-miRNA oligonucleotides have been reported to successfully decrease miRNA expression in pre-clinical models, including conjugation to peptides, addition of 2-O-methoxyethyl and locked nucleic acid (LNA)-oligonucleotides anti-miRNA oligonucleotides [86, 87]. In addition, various reports described the simultaneous silencing of multiple miRNAs that share the same seed by using seed-targeting tiny LNAs [88]. Similarly, miRNA sponges, which are transcripts with repeated miRNA antisense sequences that contain tandem siRNA binding sites can also sequester miRNA from their endogenous targets and can block an entire miRNA seed family [89].
The delivery of miRNA mimics can be used for increasing the expression of downregulated or tumor suppressor miRNAs [90]. Studies using adeno-associated virus, lipid-based nanoparticles, and the combination of miRNA expression vectors and lipid-based nanoparticles, demonstrated effective biological impact in various xenograft systems [91]. However, difficulties in the delivery of miRNA mimics may be due to difficulties in targeting a specific tissue and the fate and function of the passenger strand that sometimes acts as an anti-miRNA.
2.5.1 Oncogenic miRNAs in GBM
Different oncogenic miRNAs have been described in GBM. This paragraph highlights some of these miRNAs and their cellular functions.
-
The miR-21 – miR-21 is located on chromosome 17 within TMEM49, a transmembrane protein that is overexpressed in various tumors [70] and is also associated with the malignancy of glioma [92]. miR-21 is considered an oncogenic miRNA and it regulates cell migration by inhibiting RECK and TIMP3 [93] and cell apoptosis [94]. Silencing of miR-21 decreases glioma cell migration and growth, induces cell apoptosis and inhibits the growth of glioma xenografts [95].
-
miR-26a – miR-26a was reported to be amplified in glioma and to promote glioma cell growth and transformation. MAP3K2 and MEKK2 have been implicated as potential targets of miR-26a that eventually lead to cell proliferation and inhibition of cell apoptosis via targeting of the RBI, Pi3K/AKT and the JNK pathways [95].
-
miR-221/222 – These two miRNAs are highly expressed in GBM and regulate glioma cell proliferation by targeting p27 and cell apoptosis by targeting the survivin 1 homologs BIRC1 and NIAP. These miRNAs also promote resistance of glioma cells to cytotoxic T cells by targeting ICAM-1 [96].
Some of the additional oncomiRs that were described in gliomas include miR-10b, miR-125b, miR-182, miR-296, miR-196a [52].
2.5.2 Tumor Suppressor miRNAs in GBM
Some miRNAs exhibit tumor suppressive activities by targeting oncogenes or signal pathways associated with proliferation, migration or resistance to cell apoptosis. Some of the known glioma tumor suppressor miRNA include the following miRNAs.
-
miR-181 – miR-181a, 181b and 181c were described as tumor suppressor miRNAs in glioma and overexpression of miR-181a sensitizes glioma cells to radiation by targeting Bcl2 [97].
-
miR-124 – miR-124 inhibits glioma cell growth by downregulating SOS1. miR-124 also inhibits the STAT4 signaling pathway and enhances T cell mediated immune clearance of glioma [98].
-
miR-145 – This miRNA is downregulated in glioma specimens and have been shown to inhibit glioma cell migration by targeting CTGF [74] and NEDD9 [99]. Additional studies demonstrated that miR-145 also targeted Sox9 and adducin 3 in glioma cells [100].
-
miR-146a – miR-146a is another miRNA that inhibits the migration and invasion of glioma cells probably by targeting MMP16.
Additional miRNAs in this group include, miR-128, miR-137, miR-17 and miR-184, miR-218 and miR-219-5p [52].
2.6 miRNAs in Glioma Stem Cells
Despite the large number of publications related to the expression and function of specific miRNAs in glioma cells, much less is known about the role of miRNAs in GSCs. Three main approaches were undertaken to identify miRNAs important for the function of GSCs, comparative analysis of miRNA expression in GSCs vs. normal neural stem cells (NSCs), comparing subpopulations of GSCs such as CD133+ and CD133− and comparing undifferentiated (neurospheres) vs. differentiated GSCs.
2.6.1 GSCs Versus NSCs
As described in Sect. 2.2, a potential origin of GSCs is attributed to transformed NSCs [39]. Therefore, one of the approaches to identify miRNAs that play a role in the initiation, maintenance and function of GSCs is to study their expression and function in comparison with that of NSCs. The similarity in some of the characteristics of NSCs and GSCs raises the possibility that these two cell types share some common miRNA-based regulatory pathways but differ in others. Indeed, NSCs and GSCs share partially overlapping miRNA profiles in particular those related to self-renewal and proliferation, whereas miRNAs related to differentiation, migration and response to apoptotic stimuli are expected to be quite different. A study that compared the miRNA profiles of glial tumors, embryonic stem cells (ESCs), neural precursor cells (NPCs), and normal adult brain tissues of both human and mouse origin, reported that gliomas display a microRNA expression profile reminiscent of neural precursor cells [101]. Half of these miRNAs were clustered as miR-17-92, miR-106b-25, miR-106a-363, miR-183-96-182, miR-367-302, miR-371-373 and the large miRNA cluster in the Dlk1-Dio3 region [101]. Fifteen miRNAs exhibited disparate expression between stem cells and glioma specimens; ten were associated with stem cell specific clusters and five (miR135b, miR-141, miR205, miR-200c, miR-301c) were associated with malignancy [101].
In a recent study a genomic-wide miRNA expression profiling in GSCs and NSCs using combined miRNA microarray and deep sequencing analysis identified eight miRNAs (miR-10a, miR-10b, miR-140-5p, miR-204, miR-424, miR-34a, miR-193-aP and miR-455-5p) that were up-regulated and two miRNAs (miR-124 and miR-874) that were downregulated in GSCs relative to NSCs. These modified miRNAs inhibited the expression of respective target genes that were involved in either tumor suppression or progression [102].
2.6.2 miRNAs Associated with CD133+ and CD133− Cells
Another method for identifying miRNAs that play a role in the stemness of GSCs is to compare the expression of miRNA signature between CD133+ and CD133− cells. Using this approach a number of miRNAs were identified including miR-425, miR-451 and miR-486. miR-451 was reported to target the LKB1/AMPK pathway and to sensitize cells to glucose deprivation [52, 103].
2.6.3 miRNAs Associated with the Differentiation of GSCs
One of the approaches to target GSCs is by their differentiation which abolishes their stemness and tumorigenic potential. Therefore differentiation of GSCs in the presence of serum and absence of growth factors can reveal miRNAs that are associated with the stemness inhibition of these cells. Using this approach, Fareh et al. [104] identified that the miR-302-367 cluster was induced during the differentiation of GSCs and that stable expression of this cluster inhibited self renewal and stemness of GSCs by targeting the CXCR4 pathway upstream of sonic hedgehog (SHH)-GLI-NANOG. Similarly, a recent study analyzing the miRNAs of differentiated GSCs identified miR-1275 as a major miRNA that was downregulated during the differentiation process along with up-regulation of its target gene claudin 11 [105]. miR-137 was also reported to be upregulated during NSC and GSC differentiation along with downregulation of its target gene RTVP-1/GLIPR1 [75].
Additional miRNAs have been identified as important in regulating various functions of GSCs.
-
miR-7 – miR-7 was reported to affect the proliferation and invasion of a GSC line and to inhibit the expression of EGFR and AKT [84].
-
miR-9/9* and miR-17 – These miRNAs, in addition to miR-106b, are highly abundant in CD133+ GSCs. Their inhibition decreases neurosphere formation and induces GSC differentiation. Both miR-9/9* and miR-106b target calmodulin-binding transcription activator 1 (CAMTA1) [106]. Interestingly, the inhibitor of differentiation 4 (ID4), induces dedifferentiation of human glioma cells to glioma stem-like cells and enhances SOX2 expression by suppressing miR-9* [107].
-
miR-124 – miR-124 is one of the major neuronal miRNA and has reported to promote neuronal differentiation by targeting the PTBP1, Sox9 and the REST pathways (27, 114). In addition, miR-124 decreases neurosphere formation, CD133+ population and stem cell marker expression in GSCs by targeting SNAI2 [108].
-
miR-137 – This miRNA has been also reported to promote the neuronal differentiation of NSCs and GSCs by targeting CDK6 [109]. In addition, we recently reported that miR-137 targets RTVP-1 that regulated the stemness of GSCs upstream of the CXCR4-Gli- pathway [75].
-
miR-34a – miR-34 which is downregulated in glioma, exerts tumor suppressive effects in GSCs by targeting c-Met, Notch-1 and Notch-2. The Notch pathways plays a major role in the stemness, proliferation and radioresistance of GSCs. miR-34 induced GSC differentiation, and inhibited GSC proliferation, migration and survival [110, 111].
-
miR-326 – miR-326 is another miRNA that targets the Notch pathway but very interestingly its expression is regulated by its target gene [112]. miR-326 exerted cytotoxic effects on GSCs in vitro and in vivo [112].
-
miR-17-92 cluster – This miRNA cluster has been implicated in the regulation of GSC apoptosis, proliferation and differentiation. Target genes of the miR-17-92 cluster including CDKN1A, E2F1, PTEN and CTGF have been identified [113].
-
miR-128 – miR128 has been identified as a tumor suppressor miRNA in glioma [27, 52] and was reported to inhibit the self-renewal and stemness of GSCs by targeting the polycomb transcriptional repressor Bmi [114].
-
miR-145 and miR-143 – miR145 expression is significantly decreased in GSCs compared to NSCs and it decreases the migration of these cells by targeting CTGF and its downstream signaling protein SPARC [74]. In addition, miR-145 has been reported to target the stemness-related protein SOX2 in GSCs [115]. Similarly, the expression of miR-143 was decreased in GBM and this miRNA inhibited glycolysis and the stemness of GSCs [116].
2.7 miRNA Delivery into Brain Tumors
The potential therapeutic use of miRNAs is hindered by the lack of effective delivery approaches into target tissues. The main challenges are associated with the ability of therapeutic miRNAs to enter the cell cytoplasm without encountering the endosomal vesicles, evading kidney filtration and excretion and removal from the bloodstream by phagocytic cells. This difficulty is further amplified in the case of brain tumors due to the presence of the blood brain barrier (BBB), which prevents the entry of RNA molecules and potential RNA-based therapy. Although the BBB is compromised in areas of tumors, it is still intact in areas of tumor infiltration. Various methods including the use of stereotactic or direct intra-tumoral injection, convection-enhanced delivery, intrathecal and intra-ventricular injection and intravascular infusion with or without modification of the blood-tumor-barrier were described for the delivery of drugs into the brain [117]. In addition, recent studies demonstrated intranasal delivery of stem cells, exosomes, nanoparticles and viruses to the brain in animal models of brain inflammation and glioma xenografts [117].
2.8 Vectors for miRNA Delivery
Vectors for gene therapy and the delivery of miRNAs can be divided into two categories: viral and non-viral vectors. Viral vectors include adenovirus, adeno-associated virus, lentivirus and retrovirus vectors. Indeed, various reports demonstrated the ability of viral vectors to successfully deliver miRNAs to tumor sites [118, 119]. However, despite multiple reports demonstrating that modified viral vectors are effective in gene delivery, the immune response to adenovirus vectors remains a problem and lentivirus vectors have the potential for insertional mutagenesis, which may be a concern when used for therapy [118, 119]. Therefore, non-viral vectors, which retain biocompatibility, targeting efficacy and enhanced transfection efficiency, are a more suitable alternative to viruses for achieving successful miRNA delivery without side effects [120, 121].
In addition, RNA nanoparticles, in which the scaffold, the ligand and the therapeutic tool are all composed of RNA, have been recently described to specifically target tumors with low toxicity and low immunogenicity [122].
2.9 Exosomes for miRNA Delivery
In addition to the biological and chemical vehicles described so far, a recent approach for RNA delivery has been recently explored using extracellular vesicles and in particular exosomes, due to their natural adaptation for the transport of various substances, including nucleic acids [123].
Exosomes are membrane vesicles of endocytic origin, 50–100 nm in diameter, and are secreted by most cells into the extracellular environment [124]. They can impact the function of neighboring cells through intercellular transfer of mRNAs, microRNAs, receptors and enzymes, and are involved in the communication of immune responses [125]. Exosomes have multiple advantages over existing microRNA delivery vehicles such as low immunogenicity since they can be derived from a patient’s own cells [126]. More importantly, exosomes are natural carriers for miRNAs, which make them excellent delivery systems for these molecules [127]. We recently showed that synthetic miRNA mimics are delivered by mesenchymal stem cells (MSCs) to glioma cells via exosomes [115]. Similarly, exosomes isolated from MSCs have been reported to deliver miR-146 and miR-9 to glioma cells [128, 129].
These and other studies suggest that the future advances in the manipulation and targeting of miRNA delivery by exosomes may lead to the development of efficient cancer-specific therapy.
2.10 Stem Cells for the Delivery of miRNAs
An alternative approach to current therapies of GBM with a potential to target infiltrative tumor cells is the use of stem cells, which exhibit homing to tumor and injury sites in the brain. Indeed, numerous studies demonstrated tropism of NSCs to infiltrating glioma cells in the brain and their therapeutic benefits [130]. Another source of stem cells that exhibit tropism to tumor cells is adult human mesenchymal stromal stem cells (MSCs) that can be obtained from autologous bone marrow (BM) and adipose tissue or from cord or placenta. MSCs exhibit homing abilities, which enable them to migrate to sites of injury, inflammation and tumors [131, 132]. Specifically, MSCs have been shown to cross the blood brain barrier and migrate to sites of experimental GBM when administered intra-arterially and intravenously and can deliver cytotoxic compounds and exert anti-tumor effects [133, 134].
The ability of MSCs to cross the blood brain barrier, to home to tumor cells and deliver therapeutic molecules render these cells excellent delivery vehicles for the targeted therapy of brain tumors. We recently demonstrated the ability of MSCs to deliver miRNA mimic to glioma cells and GSCs in vitro and in vivo. The delivery of the miRNA mimic was mediated by exosomes and had an impact on the expression of target genes, cell migration and invasion [115]. In addition, recent studies also demonstrated the use of MSC-derived exosomes to deliver miRNA mimics to glioma cells [128, 129].
2.11 Pre-clinical Studies in Glioma and GSC-Derived Xenografts
Different viral vectors have been utilized for the treatment of glioma in pre-clinical studies. A recent study combined adenoviral vectors expressing hTERT-targeting ribozyme controlled HSV-tk expression together with the overexpression of miR-145. Intratumoral injection of the adenovirus vector expressing the HSV-tk expression cassette plus miR-145, combined with intraperitoneal injection of ganciclovir increased animal survival [135]. In another study, Wang et al., employed adenovirus vector expressing siRNAs that silenced the expression of miR-221 and miR-222 in glioma cells and increased the expression of p27kip1 that led to cell cycle arrest in G1 and cell apoptosis [136]. Similarly, we recently demonstrated the effectiveness of lentivirus vectors expressing pre-miR-124, pre-miR-137 and pre-miR-145 in the transduction and function of glioma stem cells [74, 75, 115].
In addition to the use of viral vectors there have been studies describing the use of nanoparticles for the delivery of miRNA mimics or siRNAs. Stable nucleic acid lipid particles (SNALPs), which target GBM cells were generated by covalent coupling of the peptide chlorotoxin (CTX) to the liposomal surface [137]. These CTX-coupled SNALPs efficiently and specifically delivered encapsulated anti-miR-21 oligonucleotides to cultured U87 GBM cells and into established intracranial tumors.
In another study, Yang et al., employed cationic polyurethane (PU)-shortbranch PEI (PU-PEI) to deliver miR-145 into CD133+ GSCs which decreased their oncogenic potential and induced their differentiation into CD133-cells. Intravenous administration of the nanoparticle-formulated miR-145 increased the sensitivity of CD133+ GSC-derived xenografts to temozolomide and radiation and prolonged animal survival [138].
In another study, systemic administration of miR-7 encapsulated in cationic liposomes resulted in the decreased growth of glioma xenografts and metastatic nodules by targeting the EGFR [139].
3 Conclusions
miRNAs have been reported to play major roles in a variety of cellular processes and biological system and their deregulation has been implicated in the pathogenesis of many diseases including cancer. Multiple studies have demonstrated the use of miRNAs as potential diagnostic and prognostic markers in GBM and as important regulators of GSC functions. Moreover, preclinical studies demonstrated their impact on tumor growth and invasiveness and novel approaches have been developed for the delivery of miRNA mimics or antagonists to tumor sites. However, despite these significant advances, there are still major issues that need to be addressed prior to the clinical application of miRNA-based therapy in GBM. These include specific target validations and prevention of undesired off-target effects, and the development of delivery approaches for the targeting infiltrating glioma cells and GSCs. Nonetheless, the increasing numbers of discoveries and reports contribute to our understanding of the mechanisms involved in the biology of GBM and GSCs and are likely to make a significant therapeutic impact in the near future.
References
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
James CD, Carlbom E, Dumanski JP, Hansen M, Nordenskjold M, Collins VP, Cavenee WK (1988) Clonal genomic alterations in glioma malignancy stages. Cancer Res 48:5546–5551
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Cloughesy TF, Cavenee WK, Mischel PS (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9:1–25
Maher EA, Brennan C, Wen PY, Durso L, Ligon KL, Richardson A, Khatry D, Feng B, Sinha R, Louis DN, Quackenbush J, Black PM, Chin L, DePinho RA (2006) Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res 66:11502–11513
Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, Samuel C, Shetty M, Tandon A, Pandey P, Hegde S, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Kondaiah P, Somasundaram K, Rao MR (2008) Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res 14:2978–2987
Olar A, Aldape KD (2012) Biomarkers classification and therapeutic decision-making for malignant gliomas. Curr Treat Options Oncol 13:417–436
Farias-Eisner G, Bank AM, Hwang BY, Appelboom G, Piazza MA, Bruce SS, Sander Connolly E (2012) Glioblastoma biomarkers from bench to bedside: advances and challenges. Br J Neurosurg 26:189–194
Lawler S, Chiocca EA (2009) Emerging functions of microRNAs in glioblastoma. J Neurooncol 92:297–306
Kim TM, Huang W, Park R, Park PJ, Johnson MD (2011) A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs. Cancer Res 71:3387–3399
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, CGAR Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Masui K, Cloughesy TF, Mischel PS (2012) Review: molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol 38:271–291
Lin J, Teo S, Lam DH, Jeyaseelan K, Wang S (2012) MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis 3:e398
Franco-Chuaire ML, Magda Carolina SC, Chuaire-Noack L (2013) Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin 54:186–205
Tiwari N, Gheldof A, Tatari M, Christofori G (2012) EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol 22:194–207
Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, Nelson SF (2006) Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res 4:607–619
Ricci-Vitiani L, Pallini R, Larocca LM, Lombardi DG, Signore M, Pierconti F, Petrucci G, Montano N, Maira G, De Maria R (2008) Mesenchymal differentiation of glioblastoma stem cells. Cell Death Differ 15:1491–1498
Rieske P, Golanska E, Zakrzewska M, Piaskowski S, Hulas-Bigoszewska K, Wolańczyk M, Szybka M, Witusik-Perkowska M, Jaskolski DJ, Zakrzewski K, Biernat W, Krynska B, Liberski PP (2009) Arrested neural and advanced mesenchymal differentiation of glioblastoma cells-comparative study with neural progenitors. BMC Cancer 9:54
deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, Mikkelsen T (2010) Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells 28:181–190
Beier CP, Kumar P, Meyer K, Leukel P, Bruttel V, Aschenbrenner I, Riemenschneider MJ, Fragoulis A, Rümmele P, Lamszus K, Schulz JB, Weis J, Bogdahn U, Wischhusen J, Hau P, Spang R, Beier D (2012) The cancer stem cell subtype determines immune infiltration of glioblastoma. Stem Cells Dev 2:2753–2761
Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WF, Boddeke HW, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K (2013) Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346
Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A (2010) The transcriptional network for mesenchymal transformation of brain tumours. Nature 463:318–325
Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, Turski A, Azodi Y, Yang Y, Doucette T, Colman H, Sulman EP, Lang FF, Rao G, Copray S, Vaillant BD, Aldape KD (2011) The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev 25:2594–2609
Yan J, Gumireddy K, Li A, Huang Q (2013) Regulation of mesenchymal phenotype by MicroRNAs in cancer. Curr Cancer Drug Targets 13:930–934
Karsy M, Arslan E, Moy F (2012) Current progress on understanding MicroRNAs in glioblastoma multiforme. Genes Cancer 3:3–15
Møller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M (2013) A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol 47:131–144
Magee JA, Piskounova E, Morrison SJ (2012) Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21:283–296
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Venere M, Fine HA, Dirks PB, Rich JN (2011) Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer’s hierarchy. Glia 59:1148–1154
Vescovi AL, Galli R, Reynolds BA (2006) Brain tumour stem cells. Nat Rev Cancer 6:425–436
Stiles CD, Rowitch DH (2008) Glioma stem cells: a midterm exam. Neuron 58:832–846
Park DM, Rich JN (2009) Biology of glioma cancer stem cells. Mol Cells 28:7–12
Swartling FJ, Bolin S, Phillips JJ, Persson AI (2013) Signals that regulate the oncogenic fate of neural stem cells and progenitors. Exp Neurol (in press)
Chen J, McKay RM, Parada LF (2012) Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149:36–47
Sampetrean O, Saya H (2013) Characteristics of glioma stem cells. Brain Tumor Pathol 30(4):209–214
Stopschinski BE, Beier CP, Beier D (2013) Glioblastoma cancer stem cells – from concept to clinical application. Cancer Lett 338:32–40
Ahmed AU, Auffinger B, Lesniak MS (2013) Understanding glioma stem cells: rationale, clinical relevance and therapeutic strategies. Expert Rev Neurother 13:545–555
Zeng Y (2006) Principles of micro-RNA production and maturation. Oncogene 25:6156–6162
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Rana TM (2007) Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 8:23–36
Mitra CK, Korla K (2014) Functional, structural, and sequence studies of MicroRNA. Methods Mol Biol 1107:189–206
Alvarez-Garcia I, Miska EA (2005) MicroRNA functions in animal development and human disease. Development 132:4653–4662
Iorio MV, Croce CM (2012) MicroRNA involvement in human cancer. Carcinogenesis 33:1126–1133
Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E, Ren H, Pestell RG (2014) MicroRNAs and cancer stem cells: the sword and the shield. Oncogene (in press)
Godlewski J, Newton HB, Chiocca EA, Lawler SE (2010) MicroRNAs and glioblastoma; the stem cell connection. Cell Death Differ 17:221–228
Zhang Y, Dutta A, Abounader R (2012) The role of microRNAs in glioma initiation and progression. Front Biosci (Landmark Ed) 17:700–712
Suh SS, Yoo JY, Nuovo GJ, Jeon YJ, Kim S, Lee TJ, Kim T, Bakàcs A, Alder H, Kaur B, Aqeilan RI, Pichiorri F, Croce CM (2012) MicroRNAs/TP53 feedback circuitry in glioblastoma multiforme. Proc Natl Acad Sci U S A 109:5316–5321
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 101:2999–3004
Baer C, Claus R, Plass C (2013) Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res 73:473–477
Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, Johnstone CN, Megraw MS, Adams S, Lassus H, Huang J, Kaur S, Liang S, Sethupathy P, Leminen A, Simossis VA, Sandaltzopoulos R, Naomoto Y, Katsaros D, Gimotty PA, DeMichele A, Huang Q, Bützow R, Rustgi AK, Weber BL, Birrer MJ, Hatzigeorgiou AG, Croce CM, Coukos G (2008) Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A 105:7004–7009
Kent OA, Mendell JT (2006) A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene 25:6188–6196
Pencheva N, Tavazoie SF (2013) Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol 15:546–554
Di Leva G, Croce CM (2013) miRNA profiling of cancer. Curr Opin Genet Dev 23:3–11
Srivastava SK, Bhardwaj A, Leavesley SJ, Grizzle WE, Singh S, Singh AP (2013) MicroRNAs as potential clinical biomarkers: emerging approaches for their detection. Biotech Histochem 88:373–387
Riddick G, Fine HA (2011) Integration and analysis of genome-scale data from gliomas. Nat Rev Neurol 7:439–450
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X (2010) Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 127:118–126
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838
Iorio MV, Croce CM (2012) MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 4:143–159
Hermansen SK, Kristensen BW (2013) MicroRNA biomarkers in glioblastoma. J Neurooncol 114:13–23
Ma X, Yoshimoto K, Guan Y, Hata N, Mizoguchi M, Sagata N, Murata H, Kuga D, Amano T, Nakamizo A, Sasaki T (2012) Associations between microRNA expression and mesenchymal marker gene expression in glioblastoma. Neuro Oncol 14:1153–1162
Li Y, Xu J, Chen H, Bai J, Li S, Zhao Z, Shao T, Jiang T, Ren H, Kang C, Li X (2013) Comprehensive analysis of the functional microRNA-mRNA regulatory network identifies miRNA signatures associated with glioma malignant progression. Nucleic Acids Res 41:e203
Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zöllner H, Schmiegel W, Hahn S, Schroers R (2012) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14:29–33
Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao H, Sun Q, Yan F, Yan C, Li H, Ren X (2014) Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis. Gene 533:389–397
Kim H, Huang W, Jiang X, Pennicooke B, Park PJ, Johnson MD (2010) Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A 107:2183–2188
Wu Z, Sun L, Wang H, Yao J, Jiang C, Xu W, Yang Z (2012) MiR-328 expression is decreased in high-grade gliomas and is associated with worse survival in primary glioblastoma. PLoS One 7:e47270
Zhang W, Zhang J, Hoadley K, Kushwaha D, Ramakrishnan V, Li S, Kang C, You Y, Jiang C, Song SW, Jiang T, Chen CC (2012) miR-181d: a predictive glioblastoma biomarker that downregulates MGMT expression. Neuro Oncol 14:712–719
Lee HK, Bier A, Cazacu S, Finniss S, Xiang C, Twito H, Poisson LM, Mikkelsen T, Slavin S, Jacoby E, Yalon M, Toren A, Rempel SA, Brodie C (2013) MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLoS One 8:e54652
Bier A, Giladi N, Kronfeld N, Lee HK, Cazacu S, Finniss S, Xiang C, Poisson L, de Carvalho AC, Slavin S, Jacoby E, Yalon M, Toren A, Mikkelsen T, Brodie C (2013) MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1. Oncotarget 4:665–676
Auffinger B, Thaci B, Ahmed A, Ulasov I, Lesniak MS (2013) MicroRNA targeting as a therapeutic strategy against glioma. Curr Mol Med 13:535–542
Hummel R, Maurer J, Haier J (2011) MicroRNAs in brain tumors: a new diagnostic and therapeutic perspective? Mol Neurobiol 44:223–234
Cheng CJ, Slack FJ (2012) The duality of oncomiR addiction in the maintenance and treatment of cancer. Cancer J 18:232–237
Chistiakov DA, Chekhonin VP (2012) Contribution of microRNAs to radio- and chemoresistance of brain tumors and their therapeutic potential. Eur J Pharmacol 684:8–18
Besse A, Sana J, Fadrus P, Slaby O (2013) MicroRNAs involved in chemo- and radioresistance of high-grade gliomas. Tumour Biol 34:1969–1978
Palumbo S, Miracco C, Pirtoli L, Comincini S (2014) Emerging roles of microRNA in modulating cell-death processes in malignant glioma. J Cell Physiol 229:277–286
Chen L, Zhang W, Yan W, Han L, Zhang K, Shi Z, Zhang J, Wang Y, Li Y, Yu S, Pu P, Jiang C, Jiang T, Kang C (2012) The putative tumor suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in glioma. Carcinogenesis 33:2276–2282
Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T, Holland EC (2009) The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23:1327–1337
Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B (2008) MicroRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res 68:3566–3572
Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438:685–689
Fabbri E, Brognara E, Borgatti M, Lampronti I, Finotti A, Bianchi N, Sforza S, Tedeschi T, Manicardi A, Marchelli R, Corradini R, Gambari R (2011) miRNA therapeutics: delivery and biological activity of peptide nucleic acids targeting miRNAs. Epigenomics 3:733–745
Ørom UA, Kauppinen S, Lund AH (2006) LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene 372:137–141
Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S (2011) Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet 43:371–378
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721–726
Yang G, Yin B (2014) The advance of application for microRNAs in cancer gene therapy. Biomed Pharmacother 68:137–142
Fabbri M (2013) MicroRNAs and cancer: towards a personalized medicine. Curr Mol Med 13:751–756
Papagiannakopoulos T, Shapiro A, Kosik KS (2008) MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res 68:8164–8172
Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28:5369–5380
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033
Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K (2007) MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res 67:8994–9000
Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, McDonald HA, Potter DM, Hamilton RL, Lotze MT, Khan SA, Sobol RW, Okada H (2009) Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci U S A 106:10746–10751
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, Hu W (2010) MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep 23:997–1003
Wei J, Wang F, Kong LY, Xu S, Doucette T, Ferguson SD, Yang Y, McEnery K, Jethwa K, Gjyshi O, Qiao W, Levine NB, Lang FF, Rao G, Fuller GN, Calin GA, Heimberger AB (2013) miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res 73:3913–3926
Speranza MC, Frattini V, Pisati F, Kapetis D, Porrati P, Eoli M, Pellegatta S, Finocchiaro G (2012) NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget 3:723–734
Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D, Shiras AS (2013) MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro Oncol 15:1302–1316
Lavon I, Zrihan D, Granit A, Einstein O, Fainstein N, Cohen MA, Zelikovitch B, Shoshan Y, Spektor S, Reubinoff BE, Felig Y, Gerlitz O, Ben-Hur T, Smith Y, Siegal T (2010) Gliomas display a microRNA expression profile reminiscent of neural precursor cells. Neuro Oncol 12:422–433
Lang MF, Yang S, Zhao C, Sun G, Murai K, Wu X, Wang J, Gao H, Brown CE, Liu X, Zhou J, Peng L, Rossi JJ, Shi Y (2012) Genome-wide profiling identified a set of miRNAs that are differentially expressed in glioblastoma stem cells and normal neural stem cells. PLoS One 7:e36248
Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE (2010) MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 37:620–632
Fareh M, Turchi L, Virolle V, Debruyne D, Almairac F, de-la-Forest Divonne S, Paquis P, Preynat-Seauve O, Krause KH, Chneiweiss H, Virolle T (2012) The miR 302–367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ 19:232–244
Katsushima K, Shinjo K, Natsume A, Ohka F, Fujii M, Osada H, Sekido Y, Kondo Y (2012) Contribution of microRNA-1275 to Claudin11 protein suppression via a polycomb-mediated silencing mechanism in human glioma stem-like cells. J Biol Chem 287:27396–27406
Schraivogel D, Weinmann L, Beier D, Tabatabai G, Eichner A, Zhu JY, Anton M, Sixt M, Weller M, Beier CP, Meister G (2011) CAMTA1 is a novel tumour suppressor regulated by miR-9/9* in glioblastoma stem cells. EMBO J 30:4309–4322
Jeon HM, Sohn YW, Oh SY, Kim SH, Beck S, Kim S, Kim H (2011) ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res 71:3410–3421
Xia H, Cheung WK, Ng SS, Jiang X, Jiang S, Sze J, Leung GK, Lu G, Chan DT, Bian XW, Kung HF, Poon WS, Lin MC (2012) Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem 287:9962–9971
Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6:14
Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, Lopes B, Schiff D, Purow B, Abounader R (2009) MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res 69:7569–7576
Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D, Purow B, Abounader R (2010) MicroRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle 9:1031–1036
Kefas B, Comeau L, Floyd DH, Seleverstov O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca EA, Lee J, Fine H, Abounader R, Lawler S, Purow B (2009) The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci 29:15161–15168
Ernst A, Campos B, Meier J, Devens F, Liesenberg F, Wolter M, Reifenberger G, Herold-Mende C, Lichter P, Radlwimmer B (2010) De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene 29:3411–3422
Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S (2008) Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res 68:9125–9130
Lee HK, Finniss S, Cazacu S, Bucris E, Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, Mikkelsen T, Slavin S, Brodie C (2013) Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget 4:346–361
Zhao S, Liu H, Liu Y, Wu J, Wang C, Hou X, Chen X, Yang G, Zhao L, Che H, Bi Y, Wang H, Peng F, Ai J (2013) miR-143 inhibits glycolysis and depletes stemness of glioblastoma stem-like cells. Cancer Lett 333:253–260
Serwer LP, James CD (2012) Challenges in drug delivery to tumors of the central nervous system: an overview of pharmacological and surgical considerations. Adv Drug Deliv Rev 64:590–597
Liu YP, Berkhout B (2011) miRNA cassettes in viral vectors: problems and solutions. Biochim Biophys Acta 1809:732–745
Couto LB, High KA (2010) Viral vector-mediated RNA interference. Curr Opin Pharmacol 10:534–542
Zhang Y, Wang Z, Gemeinhart RA (2013) Progress in microRNA delivery. J Control Release 172:962–974
Muthiah M, Park IK, Cho CS (2013) Nanoparticle-mediated delivery of therapeutic genes: focus on miRNA therapeutics. Expert Opin Drug Deliv 10:1259–1273
Shu Y, Pi F, Sharma A, Rajabi M, Haque F, Shu D, Leggas M, Evers BM, Guo P (2014) Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv Drug Deliv Rev 66C:74–89
Lee Y, El Andaloussi S, Wood MJ (2012) Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 21:R125–R134
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841:108–120
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Azmi AS, Bao B, Sarkar FH (2013) Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 32:623–642
Rayner KJ, Hennessy EJ (2013) Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res 54:1174–1181
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M (2013) Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335:201–204
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P (2013) Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids 2:e126
Binello E, Germano IM (2012) Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro Oncol 14:256–265
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF (2005) Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65:3307–3318
Choi SA, Lee JY, Wang KC, Phi JH, Song SH, Song J, Kim SK (2012) Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer 48:129–137
Gondi CS, Veeravalli KK, Gorantla B, Dinh DH, Fassett D, Klopfenstein JD, Gujrati M, Rao JS (2010) Human umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropism in vitro and in vivo. Neuro Oncol 12:453–465
Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F (2008) Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 15:730–738
Lee SJ, Kim SJ, Seo HH, Shin SP, Kim D, Park CS, Kim KT, Kim YH, Jeong JS, Kim IH (2012) Over-expression of miR-145 enhances the effectiveness of HSVtk gene therapy for malignant glioma. Cancer Lett 320:72–80
Wang X, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Shen C, Kang C (2011) Adenovirus-mediated shRNAs for co-repression of miR-221 and miR-222 expression and function in glioblastoma cells. Oncol Rep 25:97–105
Costa PM, Cardoso AL, Mendonça LS, Serani A, Custódia C, Conceição M, Simões S, Moreira JN, Pereira de Almeida L, Pedroso de Lima MC (2013) Tumor-targeted Chlorotoxin-coupled nanoparticles for nucleic acid delivery to glioblastoma cells: a promising system for glioblastoma treatment. Mol Ther Nucleic Acids 2:e100
Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, Chen MT, Chiou SH (2012) Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials 33:1462–1476
Wang W, Dai LX, Zhang S, Yang Y, Yan N, Fan P, Dai L, Tian HW, Cheng L, Zhang XM, Li C, Zhang JF, Xu F, Shi G, Chen XL, Du T, Li YM, Wei YQ, Deng HX (2013) Regulation of epidermal growth factor receptor signaling by plasmid-based microRNA-7 inhibits human malignant gliomas growth and metastasis in vivo. Neoplasma 60:274–283
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Brodie, C., Buchris, E., Lee, H.K. (2014). miRNA Expression and Functions in Glioma and Glioma Stem Cells. In: Sarkar, F. (eds) MicroRNA Targeted Cancer Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-05134-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-05134-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05133-8
Online ISBN: 978-3-319-05134-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)