Abstract
Traditionally, oxygen therapy has been provided by way of a range of devices such as nasal prongs, face masks, and nose masks, the design of which has changed little since the initial versions were developed more than 80 years ago. Limitations to the provision of oxygen by conventional systems exist, including patient discomfort and intolerance, inaccurate delivery of oxygen, failure to provide flow equivalent to inspiratory demand, drying of the airway, and treatment failure requiring escalation of respiratory support. Nasal high-flow oxygen therapy (NHF) has come to be used widely in the treatment of acute respiratory failure. NHF has been demonstrated to be easy to institute, is comfortable to the patient, and achieves excellent adherence to therapy [1].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Traditionally, oxygen therapy has been provided by way of a range of devices such as nasal prongs, face masks, and nose masks, the design of which has changed little since the initial versions were developed more than 80 years ago. Limitations to the provision of oxygen by conventional systems exist, including patient discomfort and intolerance, inaccurate delivery of oxygen, failure to provide flow equivalent to inspiratory demand, drying of the airway, and treatment failure requiring escalation of respiratory support. Nasal high-flow oxygen therapy (NHF) has come to be used widely in the treatment of acute respiratory failure. NHF has been demonstrated to be easy to institute, is comfortable to the patient, and achieves excellent adherence to therapy [1].
1.1 Nasal High-Flow Oxygen Therapy
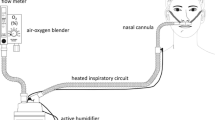
Commercially available NHF systems have rapidly gained popularity among the range of respiratory support devices and oxygen administration systems available to clinicians. Advances in the design of heated delivery tubing and the development of uniquely designed nasal interfaces have allowed the development of systems capable of delivering high-flow rates of heated, humidified, blended air and oxygen directly into the nares, allowing delivery of optimally conditioned gas. Systems comprise an air-oxygen blender (capable of delivering 21–100 % fraction of inspired oxygen (FiO2)), an active heated humidifier chamber, heated single-limb inspiratory delivery tubing (which avoids heat loss and development of condensate in the circuit), and a uniquely designed large-bore nasal interface. This allows delivery of blended air and oxygen at flows up to 60 l/min, heated to 37 °C and optimally humidified to 44 mgH2O/l.
NHF has been suggested as an intermediate form of respiratory support positioned between traditional methods of oxygen delivery such as low-flow nasal cannulas and noninvasive ventilation [2]. It can be used as part of a continuum of respiratory support, either as a tool for escalation of respiratory support in the acute phase of illness or as a means of moving from higher support to lower support when used during the weaning phase. NHF has been shown to reduce respiratory rate, improve oxygenation, reduce carbon dioxide concentrations, and reduce the need for intubation and escalation of respiratory support therapy.
2 Mechanisms of Action
2.1 Delivery of an Accurate FiO2 to Meet or Exceed the Patient’s Peak Inspiratory Flow Demand
The improvement in oxygenation seen with NHF may, in part, be due to less dilution of delivered oxygen. Oxygen dilution occurs in acute respiratory failure as patients breathe with high peak inspiratory flows. As higher gas flows are achieved with NHF, meeting or exceeding patient inspiratory demand, less entrainment of room air and resultant dilution of oxygen concentration occurs. Studies have shown that in healthy volunteers, the oxygen delivered by NHF systems approaches that prescribed when the delivered gas flow rates were greater than the subjects’ peak inspiratory flow rate [3, 4].
2.2 Washout of the Nasopharyngeal Dead Space
NHF removes the air contained in the nasopharyngeal cavity, reducing anatomic dead space and enhancing alveolar ventilation and oxygenation [5, 6]. This dead space washout also may result in higher resting oxygen saturation and potentially enhances CO2 clearance [7]. Experiments suggest that a steady flow assumption within the nasal cavity is invalid during natural breathing; however, it appears valid with NHF. This may support the argument that NHF continuously flushes the nasopharyngeal dead space, which may enhance washout of carbon dioxide [8].
2.3 Provision of Optimal Humidity
Respiratory mechanics may be improved by the delivery of heated humidified gas [6]. Mucosal function is impaired if gas is delivered above or below the optimum level of temperature or humidity [3]. NHF provides conditioned gas that is optimally humidified to 37 °C, 44 mgH2O/l. Improved mucociliary clearance has been found in bronchiectatic patients using NHF for 7 days [9]. Active humidification improves mucociliary function, facilitates secretion clearance, and decreases atelectasis formation, which may improve the ventilation-perfusion ratio and oxygenation [10].
2.4 Provision of a Flow-Dependent Positive Airway Pressure Effect
Studies have demonstrated a degree of positive airway pressure is generated by the provision of NHF. This positive pressure ranges from 2.7 to 7.4 cmH2O at flows of 35–50 l/min and correlates directly with the flow administered; that is, increasing the flow increases the pressure generated. Higher pressure levels were found in female participants and when participants breathed with mouth closed [1, 11]. The degree of positive airway pressure is dependent on the flow delivered, the geometry of the upper airways, lung compliance and resistance, whether the patient is breathing through their mouth or nose, and the absence of significant leak around the nares. Mean (SD) airway pressures of 1.93 (±1.25), 2.58 (±1.54), and 3.31 (±1.05) cmH2O were generated when patients breathed with their mouth closed while receiving 30, 40 and 50 l/min of NHF, respectively [12]. Furthermore, a positive linear relationship was found between the flow delivered and the airway pressure generated. Regression analysis of the mean airway pressure (Fig. 17.1) demonstrated that, for every 10 l/min increase in flow, the mean airway pressure increased by 0.69 cmH2O in the mouth-closed position and by 0.35 cmH2O in the mouth-open position [12].
2.5 Increase in End-Expiratory Lung Volumes
Electrical impedance tomography (EIT) has demonstrated that NHF increases both end-expiratory lung volume (EELV) and tidal volume [13, 14]. Increases in end-expiratory lung impedance (EELI) were significantly influenced by body mass index (BMI), with larger increases associated with higher BMIs. Increases in EELV may result in a reduction in the work of breathing, assist in prevention of small airway closure, and lead to improved oxygenation due to reduced shunting [13]. NHF was found to increase global EELI in both the prone and supine position, which may represent an increase in functional residual capacity [14].
2.6 Enhanced Patient Comfort and Compliance
One of the perceived benefits of NHF is the enhanced patient comfort and tolerability leading to improved compliance with the therapy [10, 15]. Tolerance of NHF has been demonstrated in several studies and is presumed to be due to the provision of optimal heat and humidity during the therapy to patients [10, 16, 17]. The optimal humidity delivered by the system has been shown to reduce mouth and nasal dryness when compared with dry oxygen therapy [10, 18, 19]. Also, because a nasal interface is utilized as opposed to a face mask, patients can eat, drink, sleep, and communicate more easily without removing the device. This has led to improved patient comfort, fewer removals of the interface, and less oxygen desaturation when compared with face mask oxygen therapy [20].
3 Clinical Outcomes and Indications
NHF offers a fast and sustained improvement in respiratory parameters in patients with hypoxemic respiratory failure, ensures patient comfort over extended periods of time, and has been shown to reduce respiratory rate, alleviate dyspnea, and improve oxygen saturation in adult patients presenting to the emergency department and the intensive care unit (ICU) [19, 21]. NHF can effectively be used to manage patients with mild to moderate levels of hypoxemic respiratory failure, may prevent the need for intubation, and can be used to provide respiratory support following extubation [22, 23].
NHF is a useful treatment in patients with acute respiratory failure due to a variety of causes [6, 10, 17, 19]. Evidence suggests NHF may avoid the need for intubation in patients with acute lung injury and acute respiratory distress syndrome as well as hypoxemic respiratory failure. Oxygen therapy after extubation is used to correct residual oxygen impairment. Several studies have demonstrated the use of NHF post extubation to improve gas exchange, reduce respiratory rate, improve comfort, and reduce the need for noninvasive ventilation and reintubation [15, 23, 24].
Patients who are hypoxemic, show signs of increased work of breathing, or require optimal humidity for secretion mobilization may all benefit from a trial of NHF. Patients may be electively extubated to NHF if deemed to be at increased risk for respiratory failure, for example, because of increased BMI, or NHF may be used in alternating cycles with noninvasive ventilation to rest the patient from a face mask and to provide periods for nutrition and oral care. Other indications are described in Table 17.1.
3.1 Instituting Nasal High-Flow Therapy
3.1.1 When Commencing NHF Therapy
-
Explain to the patient that the system will deliver higher flows of warmed air/oxygen by way of the nose. Give them the interface to feel the generated flow and temperature. It may be useful to commence flows at a lower rate to allow the patient to adjust to the sensation of heat, humidity, and flow and then slowly increase flow to desired levels as tolerated. Most commercial systems have a range of interfaces available that must be sized appropriately for the patient to ensure success. All NHF must be adequately warmed to 34–37° to assist with patient comfort and provision of humidity. Ensure tubing is supported so it does not pull on the nasal cannula.
-
Commence at 30–35 l/min and an appropriate FiO2 as determined by need based on oxygen saturations. FiO2 can be set at 21–100 % dependent on measured oxygen saturation and goals for the patient.
-
Encourage patient to breathe in and out through the nose with their mouth closed if possible, thereby slowing inspiratory and expiratory time and maintaining optimum pressure.
-
Increase flow in 5 l/min increments to a maximum of 50–60 l/min, depending on patient need.
-
When weaning, decrease NHF oxygen first to 40 %, then decrease flow in 5 l/min steps to baseline. In practice, patients often “self-wean” when their condition improves.
3.1.2 Monitoring
It is preferable to have continuous monitoring of heart rate, respiratory rate, and oxygen saturations. Blood gas measurements may be undertaken as per local protocol or as clinical need dictates.
3.1.3 Documentation
Staff should ensure that regular documentation of therapy includes the flow and FiO2 delivered, respiratory rate, heart rate, and oxygen saturations. Acceptable parameters should be prescribed describing target oxygen saturations and allowable flow and FiO2.
3.2 Other
Provide regular oral care as per local protocol. Nebulizer spacers or a “T” piece can be used in conjunction with NHF to deliver aerosol therapy. Patients may also be successfully managed on some wards with NHF either in the case of deterioration in respiratory function or following transfer from the ICU where therapy has already been instituted. Care must be taken, however, to set realistic limits on the flow that can be delivered in a ward environment, the FiO2 that is appropriate for ward use, and at what point further advice and management should be sought from specialist ICU teams. For example, call intensive care for further advice if >50 % FiO2 and/or >40 l/min flow.
It is important to recognize that NHF may not be successful in all situations and that an escalation protocol should be made available to staff that encourages higher-level respiratory support in the case of increased respiratory distress, desaturation/apnea, increased PCO2, or further clinical deterioration.
4 Discussion
The utilization of NHF has expanded rapidly since its introduction, and NHF is now seen as a useful treatment option in patients with acute respiratory failure, improving oxygenation and patient comfort and reducing respiratory rate. There is growing evidence that NHF is associated with a number of beneficial mechanisms not typically seen with traditional oxygen therapies.
Further research will help to define appropriate boundaries between nasal high flow and traditional forms of respiratory support such as noninvasive ventilation. Further work is also required to determine optimal patient selection, reliable indicators of success and/or failure, and its place and therapeutic value in novel patient groups such as rapid sequence induction, bronchoscopy, transesophageal echocardiography, and other procedures where sedation is required.
Abbreviations
- BMI:
-
Body mass index
- EELI:
-
End-expiratory lung impedance
- EELV:
-
End-expiratory lung volume
- EIT:
-
Electrical impedance tomography
- FiO2 :
-
Fraction of inspired oxygen
- NHF:
-
Nasal high flow
References
Parke R, McGuinness S. Pressures delivered by nasal high flow therapy during all phases of the respiratory cycle. Respir Care. 2013;58:1621–4.
Chatila W, Nugent T, Vance G, Gaughan J, Criner GJ. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126:1108–15.
Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39:1103–10.
Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63:938–40.
Ricard J. The high flow nasal oxygen in acute respiratory failure. Minerva Anestesiol. 2012;78(7):836–41.
Masclans JR, Roca O. High-flow oxygen therapy in acute respiratory failure. Clin Pulm Med. 2012;19:127–30.
Lee JH, Rehder K, Williford L, Cheifetz I, Turner D. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39:247–57.
Spence C, Buchmann N, Jermy M. Unsteady flow in the nasal cavity with high flow therapy measured by stereoscopic PIV. Exp Fluids. 2012;52:569–79.
Hasani A, Chapman T, McCool D, Smith R, Dilwroth J, Agnew J. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5:81–6.
Roca O, Riera J, Torres F, Masclans J. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–13.
Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20:126–31.
Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56:1151–5.
Corley A, Caruana L, Barnett A, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004.
Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58:589–96.
Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care. 2010;25:463–8.
Chanques G, Constantin J, Sauter M, et al. Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med. 2009;35:996–1003.
Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37:1780–6.
Nicolet J, Poulard F, Baneton D, Rigal JC, Blanloeil Y. High-flow nasal oxygen for severe hypoxemia after cardiac surgery. Ann Fr Anesth Reanim. 2011;30:331–4.
Cuquemelle E, Pham T, Papon J, Louis B, Danin P, Brochard L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxaemic respiratory failure. Respir Care. 2012;57:1571–7.
Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56:265–70.
Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard J. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012;57:1873–8.
Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respir Care. 2013;58:98–122.
Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care. 2014;59:485–90.
Parke R, McGuinness S, Dixon R, Jull A. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth. 2013;111:925–31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Parke, R.L. (2016). High-Flow Nasal Cannula Oxygen in Acute Respiratory Failure After Extubation: Key Practical Topics and Clinical Implications. In: Esquinas, A. (eds) Noninvasive Mechanical Ventilation and Difficult Weaning in Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-319-04259-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-04259-6_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-04258-9
Online ISBN: 978-3-319-04259-6
eBook Packages: MedicineMedicine (R0)