Abstract
Skeletal muscle has the ability to regenerate following injury, and this response implicates a specific type of resident muscle stem cell, the satellite cell. Three main phases have been identified in the process of muscle regeneration, including (I) a destruction phase with the initial inflammatory response, (II) a repair phase with the activation of satellite cells, and (III) a remodeling phase with the maturation of the regenerated myofibers. Nevertheless, in severe muscle injuries, we also observed the formation of fibrosis that impairs muscle function. Various strategies, including the use of growth factors, transplantation of muscle stem cells, or antifibrotic therapies, may become therapeutic alternatives to improve functional recovery after severe muscle injuries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
Human skeletal muscle is about 40 % of the body mass and is formed by bundle of contractile muscle fibers. Muscle fibers are multinucleated cells resulting from the fusion of myoblast, the muscle progenitor cells. Myofibers are surrounded by the sarcolemma, the plasma membrane of muscle fibers. Located between the plasma membrane and the basal lamina, we find satellite cells, i.e., the reserve adult muscle stem cells, which play a key role in the muscle regeneration process [19, 26]. After muscle injury, satellite cells are activated and form myoblasts, then fuse into myotubes, and mature into new myofibers that participate in the muscle regeneration process.
2.2 Muscle Injury
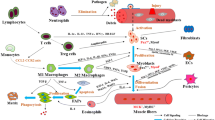
Muscle injuries can stem from a variety of events, including direct trauma (i.e., muscle lacerations, contusions, or strains) and indirect causes (i.e., ischemia or neurological dysfunction) [10, 15, 18, 23, 31]. Muscle injury is one of the most common injuries in professional and recreational sports. In fact, muscle injuries constitute between 10 and 55 % of all injuries sustained by athletes, depending on the type of sport [33]. Whereas relatively minor muscle injuries, such as strains, can heal completely without intervention, severe muscle injuries typically result in the formation of dense scar tissue that impairs muscle function and can lead to muscle contracture and chronic pain. Injured muscle undergoes a sequential cycle of healing phases. Three phases have been identified in this process (Fig. 2.1):
-
I.
Destruction phase, including muscle degeneration/inflammation: Characterized by the rupture and then necrosis of the myofibers, formation of a hematoma, and an important inflammatory reaction.
-
II.
Repair phase: In this phase, we observed phagocytosis of the damaged tissue, followed by regeneration of the myofibers, leading to activation of the satellite cells.
-
III.
Remodeling phase: A period during which we observed maturation of the regenerated myofibers with recovery of the functional capacity of the muscle (III b) but also a period where we can observed fibrosis deposition (III a).
2.2.1 Muscle Degeneration and Inflammation
Active muscle degeneration and inflammation occur within the first few days after injury. In injured muscle, mechanical trauma destroys the integrity of the myofibers. The injured ends of the myofibers undergo rapid necrosis. Similar to cell necrosis, the inflammation starts with invasion of mononuclear cells, activated macrophages, and lymphocytes at the injury site [45]. Necrotic debris of the damaged myofibers are phagocytized by macrophages, which simultaneously secrete growth factors that enhance muscle regeneration by favoring satellite cells activation and proliferation [7].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to relieve pain after muscle injury. However, the effect of this group of drugs on the muscle healing process remains largely controversial. Human studies are lacking, but some studies have been performed in animal models. It appears that short-term use of different NSAIDs had no major adverse effect on muscle healing [28].
2.2.2 Muscle Regeneration
Muscle regeneration usually starts during the first week after injury, peaks at 2 weeks, and then gradually diminishes 3–4 weeks after injury. Regeneration is linked to the activation of the satellite cells. Satellite cells proliferate, form myoblasts, and fuse with each other to form new multinucleated myotubes that will participate in the muscle regeneration process.
2.2.3 Muscle Fibrosis
Despite the fact that the majority of skeletal muscle lesions heal without formation of an extensive scar tissue, we often observe formation of a dense scar tissue that can prevent the skeletal muscle regeneration process in severe muscle injuries or muscle re-ruptures. Fibrosis usually starts between the second and third week after muscle injury. The amount of scar tissue increases in size over time due to excessive fibroblast proliferation and an increase in production of type I collagen [22].
2.3 Improving Muscle Healing
2.3.1 Growth Factors
Many reports have shown that growth factors play a variety of roles during muscle regeneration [16, 30]. Although hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) are of interest because of their capacity to stimulate satellite cells [1, 39, 47], insulin-like growth factor-1 (IGF-1) appears to be of particular importance for the muscle regeneration process notably because IGF-1 stimulates myoblasts proliferation and differentiation [13]. IGF-1 is implicated in the regulation of muscle growth [38]. In a mouse model, direct injections of human recombinant IGF-1 at 2, 5, and 7 days after injury have enhanced muscle healing in lacerated, contused, and strain-injured muscle [20, 30]. However, the efficacy of direct injection of recombinant proteins (growth factors) is limited by the high concentration of the factor typically required to elicit a measurable effect. This is mainly due to the bloodstream’s rapid clearance of these molecules and their relatively short biological half-lives. Gene therapy may prove to be an effective method by which to deliver high, maintainable concentrations of growth factor to injured muscle [2, 3, 32]. Although we observed improved muscle healing, histology of the injected muscle revealed muscle fibrosis within the lacerated site, despite the production of a high level of IGF-1 [21]. Some studies suggest that the stimulatory action of IGF-1 on myofibroblast proliferation and the deposition of extracellular matrix (ECM-scar tissue) might interfere with the ability of this growth factor to improve muscle healing after injury, even at high concentrations [11].
2.3.2 Stem Cell Therapy
Transplantation of myogenic precursor cells represents a promising therapeutic strategy for treatment of extensive skeletal muscle destruction. Myogenic precursor cells can participate directly in the muscle regeneration process but also create a reservoir of secreting molecules that may impact the different stages of muscle healing. Despite encouraging results obtained in animal models [36], the subsequent clinical trials of myoblast transfer in human patients have been disappointing due to rapid death, limited spread of the injected cells, and rejection of transplanted myoblasts [17, 29, 40]. Although the use of myoblasts for cell therapy applications is prevalent, concerns associated with myoblast proliferation, cell migration, and the limited life span of these cells have brought the usage of stem cells to the forefront of such applications. Stem cells are defined as cells that can both self-renew and give rise to clonal progeny with the ability to differentiate [46]. Isolation of muscle stem cells that can overcome these limitations would enhance the success of muscle cell transplantation significantly.
A population of murine muscle-derived stem cells (MDSC) displayed a high transplantation capacity in both skeletal and cardiac muscles [34, 37]. The MDSCs’ ability to proliferate in vivo for an extended period of time combined with their capacity for long-term proliferation, strong capacity for self-renewal, resistance to stress, ability to undergo multilineage differentiation, and ability to induce neovascularization at least partially explains the high regenerative capacity of these cells in various musculoskeletal tissues including skeletal muscle [12, 34, 37]. Recently, it has been demonstrated that blood vessel progenitors (including myo-endothelial cells and pericytes) share a number of features with MDSC [9, 42]. In particular, they share cell-type marker profiles and have high myogenic potentials in vitro and in vivo. The use of such myogenic progenitors cells for improving muscle healing may become an interesting therapeutic alternative [8, 43, 44].
2.3.3 Antifibrotic Therapy
Some reports indicate that scar tissue formation precludes complete regeneration of muscle tissue. Although various studies have implicated TGF-β1 in the onset of fibrosis [24, 41], very few reports have examined the role of this cytokine in skeletal muscle fibrosis. It has been demonstrated that TGF-β1 is expressed at high levels and is associated with fibrosis in the injured skeletal muscle [6, 24]. These results support the hypothesis that TGF-β1 expression in skeletal muscle plays an important role in the fibrotic cascade that occurs after the onset of muscle injury. Therefore, neutralization of TGF-β1 expression in injured muscle could inhibit the formation of scar tissue. Indeed, the use of antifibrotic agents (i.e., decorin, relaxin, antibody against TGF-β1) that inactivate TGF-β1 appears to reduce muscle fibrosis and, consequently, improves muscle healing, leading to a near-complete recovery of the lacerated muscle [14, 25] (Fig. 2.2). Losartan, an angiotensin II receptor antagonist, has recently been demonstrated to neutralize the effect of TGF-β1 and reduce fibrosis, making it the treatment of choice, since it already has FDA approval to be used clinically [4, 35].
Four weeks after injury, decorin-treated muscle (a, b) exhibits a greater number of regenerating myofibers (significantly higher numbers of centronucleated myofibers) and contained significantly less fibrosis (less collagen deposition, area in blue) than the control muscle (c, d) (Adapted from Li et al. [25])
2.4 Clinical Implementation After Muscle Injury: From the Bench to the Sport Field
Muscle injuries constitute one of the most frequent sports lesions. Prevention of muscle strain includes proper conditioning and warm-up and good management of fatigue. However, most muscle strains occur in sports competition requiring velocity and force. Muscle injuries are currently identified as mild, moderate, and severe injuries based on muscle impairment (from few muscle fibers contusion to the entire muscle with complete loss of muscle function). In clinical practice, treatment regimens have been designed based upon empiricism and experience.
The objective in the treatment of a muscle strain is to create the best mechanical and biological environment to allow rapid and complete healing and thereby prevent a re-tear.
Treatment must start within minutes after the injury, following the algorithm known as PRICE (Protection, Rest, Ice, Compression, Elevation) to prevent further damage and limit hematoma formation. Protection is a crucial step for the first 2–3 days (crutches or even immobilization) to prevent excessive scar formation and re-rupture at the injury site. In the coming years, the use of IGF-1 injection may improve and accelerate the healing process. Recently, a new treatment approach came from basic science research. From days 3 to 5, the athlete is advised to perform a light exercise for 20′ per day (Fig. 2.3). Berg and Bang [5] have demonstrated a 27 % increase of IGF-1 after 10′ moderate exercise (10–28 μg/l), favoring thus the environment of the initial healing. Moreover, such exercise may increase satellite cell numbers and, thus, appear as an efficient strategy to improve muscle function and repair after injury [27].
After this protective phase, which can extend up to 5 days in severe injuries, controlled isometric, isotonic, and isokinetic contractions of the injured muscle group are performed with increasing intensity. At the same time, one should begin general reconditioning of the athlete, either by activation of the upper extremity in the presence of a lesion of the lower extremity or by activation of the contralateral limb. Reconditioning of the injured muscle group is mandatory. Gentle, progressive, and pain-free sports-specific reprogramming is rapidly begun. The criteria for time to return to sports include: (a) the ability to stretch the injured muscle as much as the contralateral healthy muscle, (b) pain-free use of the injured muscle in sports-specific movements, (c) comparable strength between injured and healthy muscles, and (d) the recovery of the proprioceptive and coordination capacity in the injured segment as well as the reprogramming of the sports movement. There is an obvious lack of evidence in determining these criteria, and these guidelines are mostly empirical.
In patients with a true muscle rupture, surgical reinsertion and repair should be considered, particularly with lesions in the proximal hamstrings or distal pectoralis major. The surgical management of these injuries permits a reduction in the length and degree of functional disability. The means to reduce the length of disability in athletes with muscle strains are the following: (a) Take them off the sports field; do not even permit them to play; (b) apply the proper treatment immediately and protect the injured muscle; (c) start controlled motion and general reconditioning; (d) recondition the injured muscle and rapidly begin sports-specific reprogramming; (e) surgically reinsert and repair a muscle rupture (especially hamstrings proximally); and (f) consider the use of hyperthermia which appears to be a promising technique to reduce the length of disability.
References
Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311–5.
Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–48.
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95(26):15603–7.
Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36(8):1548–54.
Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Horm Res. 2004;62 Suppl 1:50–8.
Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96(2):1137–44.
Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163(5):1133–43.
Chen CW, Corselli M, Peault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439.
Crisan M, Huard J, Zheng B, Sun B, Yap S, Logar A, Giacobino JP, Casteilla L, Peault B. Purification and culture of human blood vessel-associated progenitor cells. Curr Protoc Stem Cell Biol. 2008;Chapter 2:Unit 2B.2.1–2B.2.13.
Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM. A muscle contusion injury model. Biomechanics, physiology, and histology. Am J Sports Med. 1994;22(5):702–10.
Damon SE, Haugk KL, Birnbaum RS, Quinn LS. Retrovirally mediated overexpression of insulin-like growth factor binding protein 4: evidence that insulin-like growth factor is required for skeletal muscle differentiation. J Cell Physiol. 1998;175(1):109–20.
Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323–33.
Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135(2):431–40.
Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29(4):394–402.
Garrett Jr WE. Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc. 1990;22(4):436–43.
Grounds MD. Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol. 1999;12(5):535–43.
Huard J, Bouchard JP, Roy R, Malouin F, Dansereau G, Labrecque C, Albert N, Richards CL, Lemieux B, Tremblay JP. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve. 1992;15(5):550–60.
Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–32.
Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports exerc. 1992;24(2):197–205.
Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland MS, Fu FH, Buranapanitkit B, Watkins SS, Huard J. Use of growth factors to improve muscle healing after strain injury. Clin Orthop Relat Res. 2000;370:272–85.
Lee C, Fukushima K, Usas A, Xin L, Pelinkovic D, Martinek V, Huard J. Biological intervention based on cell and gene therapy to improve muscle healing after laceration. J Musculoskelet Res. 2000;4(4):256–77.
Lehto M, Duance VC, Restall D. Collagen and fibronectin in a healing skeletal muscle injury. An immunohistological study of the effects of physical activity on the repair of injured gastrocnemius muscle in the rat. J Bone Joint Surg Br. 1985;67(5):820–8.
Lehto MU, Jarvinen MJ. Muscle injuries, their healing process and treatment. Ann Chir Gynaecol. 1991;80(2):102–8.
Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–19.
Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15(9):1616–22.
Lipton BH, Schultz E. Developmental fate of skeletal muscle satellite cells. Science. 1979;205(4412):1292–4.
Macaluso F, Myburgh KH. Current evidence that exercise can increase the number of adult stem cells. J Muscle Res Cell Motil. 2012;33(3–4):187–98.
Mackey AL, Mikkelsen UR, Magnusson SP, Kjaer M. Rehabilitation of muscle after injury – the role of anti-inflammatory drugs. Scand J Med Sci Sports. 2012;22(4):e8–14.
Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333(13):832–8.
Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82(1):131–7.
Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med. 1999;27(2):222–9.
Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27(2):195–200.
Nikolaou PK, Macdonald BL, Glisson RR, Seaber AV, Garrett Jr WE. Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987;15(1):9–14.
Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12(6):1130–41.
Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, et al. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-beta and fibrosis in skeletal muscle injury. Cell Transplant. 2012;21(11):2407–24.
Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337(6203):176–9.
Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–64.
Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1(1):4.
Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23(2):239–45.
Skuk D, Caron NJ, Goulet M, Roy B, Tremblay JP. Resetting the problem of cell death following muscle-derived cell transplantation: detection, dynamics and mechanisms. J Neuropathol Exp Neurol. 2003;62(9):951–67.
Sporn MB, Roberts AB. A major advance in the use of growth factors to enhance wound healing. J Clin Invest. 1993;92(6):2565–6.
Tavian M, Zheng B, Oberlin E, Crisan M, Sun B, Huard J, Peault B. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50.
Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25(5):597–603.
Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11–9.
Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37(4):284–6.
Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–68.
Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111(4):1623–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Laumonier, T., Menetrey, J., Huard, J. (2014). Basic Principles of Muscle Healing. In: Kerkhoffs, G., Servien, E. (eds) Acute Muscle Injuries. Springer, Cham. https://doi.org/10.1007/978-3-319-03722-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-03722-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03721-9
Online ISBN: 978-3-319-03722-6
eBook Packages: MedicineMedicine (R0)