Abstract
To improve the flame retardancy of polymer blends, composites and nanocomposites for extending their application, recent developments in different techniques used for the flame retardancy are reviewed in this chapter. We introduce the fundamentals of experimental methods such as cone calorimetry and UL 94 used to describe fire behavior. Also the pyrolysis process of condensed phrase is presented to prevent further pyrolysis of polymeric materials. Additionally, the combustion process of polymeric materials is described for selecting feasible flame retardants to reduce the amount of flammable volatiles emitted during combustion. At the same time, the smoke formation is discussed during fire for reduce smoke to protect environments and human’s health. Finally, the future trends of different techniques utilized for the flame retardancy are introduced such as nanotechnology, catalysis reaction, vapor phase flame retardant and flame retardant synergy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Polymer blends
- Composites
- Nanocomposites
- Flame retardancy
- Cone calorimetry
- UL 94
- Condensed phase pyrolysis
- Polymer combustion
- Smoke formation
3.1 Introduction
The massive use of polymer blends, composites and nanocomposites is driven by their remarkable combination of properties, low weight and ease of processing. However, the use of organic polymeric materials is limited in many applications because of fire hazard [1]. Such materials are also known for their relatively high flammability; most often accompanied by the production of corrosive or toxic gases and smoke during combustion. Consequently, improving the fire retardant behavior of these materials is a major challenge for extending their use to most applications [2].
The widespread applications of polymer blends, composites and nanocomposites require the use of conventional flame retardants based on halogen and phosphorous compounds to satisfy fire safety regulatory standards. However, these compounds, particularly halogen-based examples, are persistent organic pollutants of global concern and generate corrosive and toxic combustion gaseous products [3].
Considering the eco-friendliness, safety requirements are currently becoming more and more drastic in terms of polymers’ reaction to fire and their fire resistance performances, while various flame retardant additives, such as halogenated additives, are being phased out for their proven or suspected adverse effects on the environment. The combined challenge thus consists in developing effective and environmentally friendly flame retardant systems for above materials. Although the incorporation of non-toxic fillers in materials shows positive potential towards flame retardancy, many obstacles remain [4].
Additionally, combustion of polymeric materials is a complex process involving simultaneous combinations of heat, energy and mass transfer and diffusion, fluid dynamics and degradation chemistry. Some studies on the fire response of such materials are conducted from the perspectives of short-term and long-term fire exposure tests, theoretical modeling or numerical simulation. Also many techniques are used for the flame retardancy of such materials. Hence, there is a need to fundamentally understand the recent development in different techniques used for the flame retardancy [2].
Currently, the most common approach to improve the flame retardancy performance of materials is to add flame retardants, such as halogen-based agents, phosphorous-based compounds, metal hydroxides, intumescent agents, boron and nitrogen-based flame retardants, etc. [5]. Depending on the type and nature of flame retardants, they act chemically and/or physically in the solid, liquid or gas phases and interfere with combustion at different stages (heating, decomposition, ignition, or flame spread). The mechanisms of conventional flame retardants based on halogen, phosphorous and in tumescent compounds have been thoroughly discussed in the past [2].
Simultaneously, smoke suppressants are developed because the majority of human deaths during fire incidents are related to the inhalation of smoke and toxic combustion gases, with carbon monoxide being particularly significant. Some approaches that were adopted to tackle this problem include the use of fillers or additives, surface treatments, and chemical or physical modification. All these are to slow down the thermal decomposition of polymer blends, composites and nanocomposites and reduce the smoke density and gas concentration [3].
Recently, the polymer nanocomposite approach to flame retardancy is the newest technology now in use. Polymer nanocomposites are polymers filled with nanoscale particles finely dispersed in the polymer matrix [6–8]. For flame retardancy, polymer nanocomposites are condensed phase flame retardants that slow the mass loss rate of the polymer during fire conditions through formation of a nanoparticle-rich fire protection barrier [9]. This leads to a lowering of peak heat release rate and inhibition of polymer flow (melting/dripping) during a fire, but it does not lower the total heat release of the fuel; it just spreads it out over a longer time and makes it burn less intensely [10].
In addition, nanocomposites show earlier time to ignition as can be seen in but many other flame retardants show early time to ignition as well, so this effect may or may not be a negative feature [11]. More recently, it has been reported that the presence of nanodispersed particles in polymeric matrices produces a substantial improvement in fire performance [6].
The flammability of polymer materials can be characterized by their ignitability, flame-spread rate and heat release. There are numerous small-, intermediate- or full-scale flammability tests used in industrial or academic laboratories for either screening materials during product development or testing manufactured products, for instance, UL 94 and cone calorimetry.
To protect against fire, it is necessary to (a) understand the flame measurement such as cone calorimetry and UL 94; and (b) know the pyrolysis process of condensed phrase for preventing further pyrolysis of polymeric materials; or (c) describe the combustion process of polymeric materials for selecting feasible flame retardants to reduce the amount of flammable volatiles emitted; or (d) reduce the smoke formation during fire for protecting environments [12].
In this chapter, we present the fundamentals of tests used to describe fire behavior, condensed phrase pyrolysis, polymer combustion, smoke formation and new prospects in different techniques used for the flame retardancy of polymer blends, composites and nanocomposites.
3.2 Cone Calorimetry
The Cone Calorimeter test is at present the most advanced method for assessing materials reaction to fire. This name was derived from the shape of the truncated conical heater that is used to irradiate the test specimen with fluxes in the test. The test apparatus consists of the following components: a conical radiant electric heater; specimen holders; an exhaust gas system with oxygen monitoring and flow measuring instrumentation; an electric ignition spark plug; a data collection and analysis system; and a load cell for measuring specimen mass loss [13]. A photo of the apparatus is shown below. A photo of the apparatus is shown in Fig. 3.1.
The calorimeter is used by having a small sample encased in aluminum foil, wool, and a retainer frame that is ignited below an exhaust hood. A conical heater is placed in between in order for materials to combust. The cone-shaped apparatus outputs high amounts of energy and turns electricity into heat. Without this portion of the device, it would be very difficult to measure the temperature, pressure, and smoke coming off the sample. The conical heater is what makes this device different from the rest, but in reality is only a small part of the entire apparatus [14].
Ventilation is also a very important part of the device, as well as the electrical power to run the conical heater. A small water supply is necessary to cool and regulate the heat in the system of the device. Since temperature and pressure are being evaluated, two different measurement tools are needed in the exhaust tube. Gas samples, smoke measurements, and soot collections are also collected using this device and all need a place to be measured after the exhaust tube [14].
Also, the measurements of smoke and toxic gases can be conducted during this test. The test gives a possibility to evaluate ignitability, combustibility, production of smoke and toxic gases, etc. Recently, the cone calorimeter is a relatively developed to supply an integrated set of physical chemical parameters of the combustion. Currently, the cone calorimeter is the most significant bench scale instrument in the field of fire testing. The test schematic representation of cone calorimeter is shown in Fig. 3.2.
3.2.1 Usage
Cone calorimeter is a modern device used to study the fire behavior of small samples of various materials in condensed phase. It is widely used in the field of fire safety engineering [15]. The cone calorimeter test has been developed for material fire evaluations, computer modeling, design purposes, and development and research to help make real world fire predictions. The test performance uses the bench-scale system to measure fire characteristics associated with heat and smoke output. The measurements can be used directly by researchers or can be used as data input into correlation or mathematical models used to predict fire development. The cone calorimeter became the premier dynamic research tool based on the principle of oxygen consumption calorimetry [15].
The data from the test results can be used for technical modeling bigger fires. The cone calorimeter test is also largely used when testing products that are under development. A whole range of different data can be compared in order to eliminate products that will not have the sufficient fire characteristics. In the test, Various output data are collected including peak rate and average rate of heat release, total heat released, effective heat of combustion, specific extinction area, exhaust flow rate, mass loss rate and final sample mass, time to sustained ignition, O2, CO, CO2, and toxic gas concentrations, and smoke density as a function of time. Heat release is the key measurement required to assess the fire development of materials and products; radiant heat is the major cause of fire spread and the cone measures intensity of the peak rate of heat release and the speed to reach the peak rate; the critical factors in predicting the growth rate of fire [14].
Cone testing can also be utilized by a product manufacturer who is looking to change a component within a product to a new material and wishes to investigate the effect, if any, that change would have on the product’s fire safety. In these cases, testing the new component and the old component in the cone and comparing the thermal properties of the two can be performed in lieu of more expensive full-scale testing. Additionally, the Cone is used as a screening tool for new polymeric materials that are in the development. And the cone can compare the thermal properties of the materials and weed out the worst performers [16].
The different models of the calorimeter can be used to evaluate different aspects of the flammable materials. The research using the cone calorimeters can be used for product safety, environment, and health services. This device is important when dealing with safety issues. It is easier to see how many different materials react with fire using the device. Knowing that information, safety regulations can be made easily to protect the people that come in contact or work with the material often. It is important to know and understand the flammability, heat of combustion, ignitability, heat release, and smoke production of many materials in order to maintain a safe environment, all of which can be found by using a calorimeter [16].
In short, the cone calorimeter is an extremely useful in the department of fire safety and analytical services. The calorimeter is a unique apparatus that is able to study small samples of materials, in order to determine their flammability. The fire characteristics of the material can be determined from several different standard models of the cone calorimeter. A list of various test standards is provided such as ISO 5660, ASTM E1354, ASTM E1474, ASTM E1740, ASTM F1550, ASTM D6113, CAN ULC 135, and BS 476 Part 15 [15, 16].
3.2.2 Test Principle
Cone calorimetry is one of the most effective medium-sized polymer fire behavior tests. The principle of cone calorimeter experiments is based on the measurement of the decreasing oxygen concentration in the combustion gases of a sample subjected to a given heat flux. The surface of the test specimen is exposed to a constant level of heat irradiance, within the range 0–100 kW/m2, from a conical heater (Fig. 3.3). Volatile gases from the heated specimen are ignited by an electrical spark igniter. Combustion gases are collected by an exhaust hood for further analysis. This gas analysis makes it possible to calculate heat release rate and to assess production of toxic gases from the specimen [16].
Also smoke production is assessed by measuring attenuation of a laser beam by smoke in the exhaust duct. The attenuation is related to volume flow, resulting in a measure of smoke density called smoke extinction area [m2/s]. The specimen is mounted on a load cell which records the mass loss rate of the specimen during combustion. A thorough analysis requires testing at several irradiance levels. Typical levels of irradiance are 25, 35, 50 and 75 kW/m2. Three specimens shall be tested at each heat flux level.
3.2.3 Test Specimens
The surface of the specimens shall be essentially flat. The specimens shall be representative of the product, and as far as possible be similar to the final product. Dimensions of the specimens:
Area: 100 mm × 100 mm
Thickness: 50 mm
3.2.4 Test Procedure
A sample with the dimension 100 mm × 100 mm is subjected to a specific irradiance level. The surface of the sample is heated and starts to emit pyrolysis gases that ignite by a spark igniter. The emitted gases are collected in a hood and transported away through a ventilation system. The heat release is measured using the data on measured oxygen concentration in the emitted smoke. The smoke production is measured continually throughout the test with a laser system (See Fig. 3.2).
Device usually allows the test sample to be exposed to different heat fluxes over its surface. It gathers data regarding the ignition time, mass loss, combustion products, heat release rate and other parameters associated with its burning properties. The cone calorimeter introduced a system for measuring smoke optically and soot yield gravimetrically. It is now considered one of the most important devices for fire engineering and safety services, and its usage in research has grown increasingly over the years [16].
3.2.5 Test Report
The cone calorimeter test is very well suited to quantify materials reaction to fire. The test report contains information about dimensions, pretreatment and conditioning of the test specimens, and information about the test conditions. The test results can give information on how to improve the tested product. The following test results are tabulated:
-
Time to ignition [s]
-
Total heat released [MJ/m2]
-
Maximum heat release rate [kW/m2]
-
Average heat release rate after 180 s and after 300 s [kW/m2]
-
Effective heat of combustion [MJ/kg]
-
Average smoke production [m2/s]
-
Production of CO (carbon monoxide) [g]
3.3 UL 94
The UL 94 V test is widely used both in industry and academic research centers, and is intended to meet industrial requirements as well as to hierarchically classify the polymeric materials [17]. UL 94 intends this standard to serve as a preliminary indication of polymer acceptability for use as part of a device or appliance with respect to its flammability. It is not intended to reflect the hazards of a material under actual fire conditions. UL 94 flammability testing is the first step toward obtaining a plastic recognition and subsequent listing in the Plastics Recognized Component Directory [18].
UL 94 flame rating groups materials into categories based on their flammability. UL 94 covers two types of testing: vertical burn and horizontal burn. Vertical burn test includes Vertical Testing (V-0, V-1, V-2), Vertical Testing (5VA, 5VB) and Vertical Testing of Thin Materials (VTM-0, VTM-1, VTM-2). Specimens molded from the plastic material are oriented in either a horizontal or vertical position depending on the specifications of the relevant test method, and they are subjected to a defined flame ignition source for a specified period of time [18].
The set of UL 94 tests has been approved as tests of the flammability of plastic materials for parts in devices and appliances. It includes a range of flammability tests (small and large flame vertical tests, horizontal tests for bulk and foamed materials, radiant panel flame-spread test). In terms of practice and usage, the most commonly used test is UL 94 V for measuring the ignitability and flame-spread of vertical bulk materials exposed to a small flame [17].
3.3.1 Horizontal Testing (HB)
An HB flame rating indicates that the material was tested in a horizontal position and found to burn at a rate less than a specified maximum [19]. A specimen is supported in a horizontal position and is tilted at 45°. A flame is applied to the end of the specimen for 30 s or until the flame reaches the 1 in. mark. If the specimen continues to burn after the removal of the flame, the time for the specimen to burn between the 1 and 4 in. marks are recorded. If the specimen stops burning before the flame spreads to the 4 in. mark, the time of combustion and damaged length between the two marks is recorded. Three specimens are tested for each thickness [19]. The test schematic representation of horizontal testing is shown in Fig. 3.4. Requirements of horizontal rating of horizontal testing are given in Table 3.1.
3.3.2 Vertical Testing (V-0, V-1, V-2)
A specimen is supported in a vertical position and a flame is applied to the bottom of the specimen [17]. The flame is applied for 10 s and then removed until flaming stops at which time the flame is reapplied for another 10 s and then removed. Two sets of five specimens are tested. The two sets are conditioned under different conditions. Test schematic representation of Vertical Testing (V-0, V-1, and V-2) is shown in Fig. 3.5. Requirements of vertical ratings of vertical Testing (V-0, V-1, and V-2) are given in Table 3.2.
The three vertical ratings, V0, V1 and V2, indicate that the material is tested in a vertical position and self-extinguished within a specified time after the ignition source is removed. The vertical ratings also indicate whether the test specimen dripped flaming particles that ignited a cotton indicator located below the sample. These small-scale tests measure the propensity of a material to extinguish or spread flames once it becomes ignited.
3.3.3 Vertical Testing (5VA, 5VB)
UL 94 also describes a method in which the test flame is applied for up to five applications in testing for a 5VA or 5VB classification [18]. Testing is done on both bar and plaque specimens. A bar specimen is supported in a vertical position and a flame is applied to one of the lower corners of the specimen at a 20° angle. The flame is applied for 5 s and is removed for 5 s. The flame application and removal is repeated five times. However the procedure for plaques is the same as for bars except that the plaque specimen is mounted horizontally and a flame is applied to the center of the lower surface of the plaque. Test schematic representation of vertical testing (5VA, 5VB) is shown in Fig. 3.6. Requirements of vertical ratings of vertical testing (5VA, 5VB) are given in Table 3.3.
3.3.4 Vertical Testing (VTM-0, VTM-1, VTM-2)
This test is used for materials that are thin, or are too flexible or may distort, shrink or flex during ordinary vertical testing [20]. An 8 in. × 2 in. specimen is rolled longitudinally around a 1/2 in. diameter mandrel and taped on one end. When the mandrel is removed the specimen forms a cone. The cone is supported in a vertical position and a flame is applied to the bottom of the specimen. The flame is applied for 3 s and then removed until flaming stops at which time the flame is reapplied for another 3 s and then removed. Two sets of five specimens are tested. The two sets are conditioned under different conditions [20]. Test schematic representation of vertical testing (VTM-0, VTM-1, and VTM-2) is shown in Fig. 3.7. Requirements of vertical ratings of vertical testing (VTM-0, VTM-1, and VTM-2) are given in Table 3.4.
Some differences between cone calorimetry and UL 94 will be highlighted below:
-
(1)
Cone calorimeter evaluates the material response to a constant heat flux with time (forced combustion), whereas in UL 94, the response of a material to a removed fire and its self-extinguishing behavior versus time are measured. That is, UL 94 provides information in a local ignition fire scenario, but their safety level is not so clear when exposed to a more aggressive fire scenario [21]. Moreover, the test operator has to follow the polymer with the Bunsen burner flame during the test, and therefore, maintaining a steady ignition source on a moving/curling/dripping thermoplastic is difficult [22]. This obviously induces fluctuations and a degree of uncertainty in the UL 94 test data.
-
(2)
Horizontal versus vertical configuration—another parameter that is highly relevant is the horizontal (cone calorimeter) versus vertical (V-UL 94) configuration; this is particularly important for injection a molded polymers with 2D nanofillers. The differences in the orientation of clay layers will affect the fire performance of the sample. Moreover, in the vertical configuration, dripping should be considered; while in cone calorimeter, the bottom of the material is securely wrapped in an Al-foil.
3.4 Condensed Phase Pyrolysis
Pyrolysis of polymeric materials is a complex process involving simultaneous combinations of heat, energy and mass transfer and diffusion, fluid dynamics and degradation chemistry [23]. The study on polymer combustion and fire retardancy is a complex multidisciplinary topic, encompassing physical and chemical phenomena occurring in the gas and condensed phase. Thus, aspects involved are physical chemistry of flames and thermal degradation of polymers, respectively [24].
The processes occurring in the condensed phase are of primary importance because they originate the volatile species which feed the flame. Techniques and methods of general use in the study of thermal degradation of polymers are applied to the study of condensed phase processes in combustion and fire retardancy [25].
However, thermal degradation of polymers may strongly depend on experimental conditions such as temperature, type of atmosphere, rate of heating, pressure, etc. In order to obtain basic information which is relevant to the understanding of the combustion process, the thermal degradation must be carried out in conditions simulating those to which the polymer is exposed during combustion [25].
Combustion of synthetic polymer materials is characterized by a complex coupling between condensed and gas phase phenomena. Furthermore, the phenomena in each phase consist of a complex coupling of chemical reactions with heat and mass transfer processes. Since the gas phase phenomena, such as chemical reaction, turbulence, soot formation, and so on, have been extensively studied and described elsewhere, this section concentrates on the less-explored condensed phase phenomena [26]. Generally combustion of polymers is a gas-phase process with gaseous fuel supplied by the decomposing solid or liquid polymer. Thus, understanding of polymer pyrolysis is important to understanding the chemistry and physics of polymer combustion and flammability.
3.4.1 Heat Transfer
When polymer is exposed to heat such as a source of ignition, or the combustion flame, the surface temperature of the polymer can rise to a point at which its structure will break down and it will release volatile material [27]. Therefore the polymer behavior in a fire risk situation is the result of a combination of many different physical and chemical processes, which happen in the condensed phase. The kinetics of these processes are particularly important both as a function of temperature and relative to each other. Physical properties are thermal conductivity, heat capacity and the ability to melt back away from an ignition source. As part of the degradation mechanism some polymers will also produce carbonaceous char [27].
The temperature of the solid polymer is raised either due to an external heat source such as radiation or a flame, or by thermal feedback. During the initial exposure to heat thermoplastics, which have a linear chain structure, soften or melt and start to flow. On the other hand, thermosetting plastics have a three-dimensional cross-linked molecular structure which prevents softening or melting. Additional heat causes both types of polymer to pyrolyse and to evolve smaller volatile molecular species. Because of their structure this occurs at higher temperatures for thermosetting as opposed to thermoplastic polymers [28].
Also flame propagation is affected by physical factors, more specifically thermal transfers. Conductive and convective transfers are important in the initial phase of fire development when the height of the flame remains limited to a few tens of centimeters. In a more advanced phase, flame propagation on the surface contributes to a rapid increase in radiative transfer.
3.4.2 Energy Transfer
Pyrolysis is an endothermic process which requires the input of sufficient energy to satisfy the dissociation energies of any bonds to be broken plus any activation energy requirements of the process [29]. In order to burn a polymeric material, thermal energy must be added to the material to raise its temperature sufficiently to initiate degradation. When temperatures near the surface become high, thermal degradation reactions occur and evolve small gaseous degradation products. The majority of the evolved products are combustible. Depending on the nature of the polymer, thermal degradation reactions may proceed by various paths.
The combustion process of polymers is a complex coupling of energy feedback from a flame to the polymer surface with gasification of the polymer to generate combustible degradation products. Combustion is a catalytic exothermic reaction maintained by internally generated free radicals and heat. Provided the supply of radicals and heat exceeds the energy required for combustion, the combustion proceeds at an increasing rate until an explosion occurs [30].
If the energy supply is constant and equals the demand, a stationary equilibrium will be established, i.e. a steady flame occurs. If the available energy is below that required to maintain this equilibrium, the rate of combustion will decrease until the flame extinguishes. The radicals, oxygen and heat necessary to sustain the combustion reach the site by various transport mechanisms.
Combustion reactions liberate the energy stored in the chemical bonds of the molecules of the polymer. A polymer is any substance that will release energy during its reaction with oxygen, usually in air, generally initiated by an external heat source. Thermal decomposition of a polymer is often initiated by dissociation of covalent bonds to form radicals. Bond dissociation energies will depend on the nature of the atoms making up the bond and also the precise structural environment in which the bond occurs [30].
Bond dissociation values can often be used to explain why one bond dissociates in preference to another, and are of particular importance for polymers, which degrade by free radical mechanisms. The mechanisms of polymer degradation and the temperatures at which they occur will depend very much on the polymer’s structure. Polymers at their degradation temperature can form radicals due to bond scission.
3.4.3 Mass Transport
Development of new fire-safe polymeric materials requires a better understanding of the microscopic chemical processes that determine thermal stability and flammability of polymers [31]. As described above, the type of polymer structure, thermal properties, and the amount of heat transferred to the polymer determine the depth over which the polymer is heated sufficiently to degrade. Since the boiling temperatures of some of the degradation products are much less than the polymer degradation temperatures, these products are superheated as they form. They nucleate and form bubbles.
Then, these bubbles grow with the supply of more small degradation products to the bubbles by diffusion from the surrounding molten polymer. Since the polymer temperature is higher near the surface than further below, the polymer sample is more degraded there, and its molecular weight is lower. The net result is a highly complex generation and transport of bubbles containing small molecules from the interior of the polymer melt outward through a strong viscosity gradient that heavily influences bubble behavior [32].
During polymer thermal decomposition, the development of considerable material heterogeneity can be highlighted. A gradient structure tends to form inside the material, arising from the interaction with atmospheric oxygen, coupled with the out-diffusion of reactive species and also concomitant polymer chain breakdown within the material. Several zones inside the material can therefore be identified [33].
The gaseous decomposition products tend firstly to be located in the cavities of this underlayer, and afterwards migrate (through this microporous underlayer) towards the surface, where combustion takes place. The cellular under layer is in direct contact with the thermal decomposition zone of the polymer and lies on the top of another layer in which the polymer remains intact even if it may undergo phase transitions [33]. A schematic representation of thermal decomposition of polymeric materials is present in Fig. 3.8.
At present, it is clear that the subsurface degradation is important for the gasification, but it is not clear what the main transport process for the small degradation products to the sample surface is and also how rapid this transport is. It appears that diffusion of small molecule gases through a polymer is too slow to be responsible for the transport of the products.
3.4.4 Char Formation
Char formation during polymer degradation is generally a complex process and may involve several steps, including chemical fragmentation, conjugated double bond formation, cyclization, aromatization, fusion of aromatic rings, turbostratic char construction and graphitization [34].
Char formation is probably the most important condensed-phase mechanism for modifying the combustion process. It serves as a barrier to heat and mass flow, and as a means of stabilizing carbon, thus preventing its conversion to combustible gases. The efficiency of a char as a barrier in these processes depends greatly on its chemical and physical structure. The ability of char formation to prevent sustained ignition will also depend on its rate of formation in relation to other degradation mechanisms, especially the release of combustible gases. Polymers such as polycarbonate, novolaks and polyphenylene oxide all burn with the formation of a carbon rich residue called char. This char forming property is also reflected during thermogravimetric experiments which show initial degradation producing a more thermally stable material [34].
Char formation rate is important towards flame retardancy or flammability [35]. Phosphorus-based flame retardants can effectively result in char formation in oxygen- or nitrogen-containing polymers. In the absence of reactive groups, polyols like pentaerythritol, mannitol or sorbitol were used as char formers, particularly in intumescent formulations. Boron compounds, another topic mentioned in the preceding, promotes char formation in the burning process.
The mechanism is related to the thermal action of boric acid with alcohol moieties. Addition of dehydrogenation or oxidative dehydrogenation catalysts is another interesting possibility, not yet explored. This is based on the concept that the heat of combustion of a reaction consuming only hydrogen of an aliphatic hydrocarbon polymer molecule is only about one-third that of both hydrogen and carbon [36]. When temperatures near the polymer surface become high, thermal degradation reactions occur and evolve small gaseous degradation products. The majority of the evolved products are combustible. Depending on the nature of the polymer, thermal degradation reactions may proceed by various paths.
Char-forming thermoplastics often swell and intumesce during their degradation/combustion, and one recent flame-retardant approach is to promote the formation of such intumescent char. Degradation of a polymer is often affected by the presence of abnormal structures that are usually less thermally stable than the regular structures. Some such structures are inherent consequences of the method of polymerization. If a vinyl polymer is polymerized with a free radical initiator, termination reactions yield unsaturated end groups and also a head to head linkage within the chain. These abnormal structures were found in PMMA, and it was shown that they lowered the thermal stability of the polymer and reduced ignition delay time and increased burning rate [36].
3.4.5 Char Structure
Char is a highly cross-linked, porous solid and consists of disordered polycyclic aromatic hydrocarbons that become more ordered with increasing temperature (lower amorphous concentration and higher aromaticity). Nevertheless, char formation during combustion is always beneficial whether this points to the materials that are unburned (therefore, not contributing to heat release) or to a higher fire retardancy by serving as a barrier to heat and mass transfer [36].
The char structure is conjugated multiple bonds, transition from a linear to a cross-linked structure, and an increase in the aromaticity of the polymer residue. For polymers containing aromatic carbon and/or heterocyclic links in the main chain of the polymer structure, general features of their pyrolysis and char yield have been derived. Some features are as follows: (1) the thermal stability and the char yield increase with the relative number of aromatic groups in the main chain per repeat unit of the polymer chain; (2) the thermal stability of heterocyclic polymers increases with the aromatic component of the heterocycles; and (3) pyrolysis begins with the scission of the weakest bonds in the bridging groups connecting the aromatic rings or heterocycles [35, 36].
The degree of protection provided by a char during combustion depends on both its chemical and physical structure. Whereas pure graphite is highly stable to heat and oxygen, chars from polymer combustion do not have this property. Although chars are richer in carbon than the original polymer, they are rarely all carbon. The ideal char for fire retardant properties is an intact structure of closed cells containing pockets of gas. For this to happen the bubbles of gas must become frozen into the expanding and thickening polymer melt, which ultimately solidifies to produce the honeycombed structure. This prevents the flow of volatile liquids or vapours into the flame and provides sufficient thermal gradient to keep the remaining polymer or polymer melt below its decomposition temperature [36].
3.5 Polymer Combustion
3.5.1 Combustion Process
Combustion and flammability of polymeric materials are important topics of practical interest directly related to fire safety [37]. Polymer combustion is a complex process involving a multitude of steps and is best described in qualitative terms. In general, four major steps comprise polymer combustion: ignition, pyrolysis, combustion and feedback [38]. Depending on the flammability limit of the material, ignition is normally caused by the presence of an external heat source such as a flame or a spark or, if the temperature is high enough, occurs spontaneously.
Organic polymers can initiate or propagate fires because they undergo thermal degradation to volatile combustible products. If the concentration of the degradation products in the air is within flammability limits, they can ignite either spontaneously, if their temperature is large enough, or by effect of an ignition source such as a spark or a flame. The combustion process continues then to complete consumption of the material, if the heat fed back from the flame to the polymer is sufficient to keep its rate of degradation above the minimum value for feeding the flame itself. Otherwise, the cyclic combustion process stops and the flame extinguish [39].
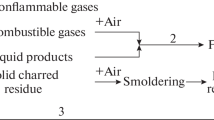
A self-sustaining combustion cycle will be formed after ignition if the heat evolved by the flame is sufficient to keep the decomposition rate of the polymer above that required to maintain the concentration of the combustible volatiles [40]. The duration of combustion cycle depends on the quantity of heat liberated during the combustion. When the amount of heat liberated reaches a certain level, new decomposition reactions are induced in the solid phase, and therefore more combustibles are produced. The combustion cycle is thus maintained as shown in Fig. 3.9.
In fact, combustion of polymeric materials involves simultaneous combinations of heat and mass transfer or diffusion, fluid dynamics and degradation chemistry. The whole combustion process usually starts with an increase in the temperature of the polymeric materials due to a heat source, to such an extent that it induces polymer bond scissions. The heat can be derived by a contribution of thermal energy from an external heat source (radiation, convection or conduction), by a chemical process induced inside the material or by the exothermicity of the combustion reaction initiated. The increase in temperature depends primarily on the heat flow, and the difference in temperature due to the exothermicity of the reactions involved, and the specific heat and thermal conductivity of the polymeric materials [41].
In the presence of a source of sufficient heat (to induce bond scissions), polymers will decompose or pyrolyze evolving flammable volatiles [38]. The exact physics and chemistries that occur in polymer combustion are dictated by the polymer that is burning. The chemical structure of the polymer and how it behaves upon exposure to heat will determine how much heat, smoke, and other gases are released when that polymer burns. In addition, the combustion behavior of polymeric materials is not an intrinsic property depending solely on its chemical structure. In fact, the behavior of the material depends as well on extrinsic factors such as heat irradiance, ventilation, shape, size and density of the specimen, etc.
3.5.2 Products of Combustion
Polymeric materials are made up mainly of carbon and hydrogen which causes such materials to be highly combustible. Polymer combustion is driven by the thermally induced decomposition (pyrolysis) of solid polymer into smaller fragments, which then volatilize, mix with oxygen, and combust. This combustion releases more heat, which reradiates onto the unburned polymer, thus continuing to drive pyrolysis and combustion until a lack of heat/fuel/oxygen causes the fire to extinguish. This is admittedly a simplistic explanation, but it holds basically true for just about all polymeric materials [42].
Thermoplastic polymers have a tendency to drip and flow under fire conditions, which can lead to additional mechanisms of flame spread or propagation whereas thermoset polymers tend to not drip and flow and instead produce pyrolysis gases from the surface of the sample directly into the condensed phase. The volatile fraction of the resulting polymer fragments diffuses into the air and creates a combustible gaseous mixture. This gaseous mixture ignites when the auto-ignition temperature is reached, liberating heat. Alternatively, the combustible volatiles can also ignite at a lower temperature upon reaction with an external source of intense energy [43].
3.5.3 Combustion Mechanism of Polymer
The combustion mechanism of polymer materials highly depends on the weakest bonds, and also on the presence or absence of oxygen in the solid and gas phases. Generally, thermal decomposition is the result of a combination of the effects of heat and oxygen [44]. They combine with air (oxygen) and produce the H2–O2 scheme, which propagates the fuel combustion by the branching reaction below:
The main exothermic reaction that provides most of the energy to maintain the flame is:
In oxidizing thermal conditions, the polymer reacts with oxygen in the air and generates a variety of low molecular weight products: carboxylic acids, alcohols, ketones, aldehydes, etc. Oxidation can lead to crosslinking through recombination reactions of the macromolecular radicals. The propagation rate of the degradation process is controlled by the wrenching reaction of hydrogen atoms from the polymer chains. The oxidation stability of the polymer thus depends on the C–H bond energy [44].
However, non-oxidizing thermal degradation is generally initiated by chain scissions under the simple effect of temperature (pyrolysis). This scission involves varying degrees of material depolymerization. Researchers [45] suggested that at combustion temperatures above 300 °C polymer degradation takes place via non-oxidizing thermal decomposition. Under these conditions, the rate of pyrolysis is much faster than the diffusion of oxygen in the solid phase. Oxidation therefore only occurs in the gas phase due to the presence of low molecular weight compounds produced by thermal decomposition.
The decomposition gases generated by pyrolysis first mix with oxygen by both convection and diffusion into the layer close to the surface, create free radicals, and then ignite. This ignition can be triggered by an external flame (flash ignition) or self-induced (self-ignition) when the temperature is sufficiently high. Ignition depends on several parameters, in particular oxygen concentration. The combustion of the gases increases the polymer temperature and thus supports the pyrolysis and production of new combustible gases. Combustion thus continues even in the absence of an external heat source.
3.5.4 Flame Retardancy Approaches
The fire safety of materials can be enhanced by increased ignition resistance, reduced flame spread rates, lesser heat release rates, and reduced amounts of toxic and smoke products, preferably simultaneously. When considering how to flame retard a polymeric material or protect that same material from a fire, there are three main approaches one can take. These are: engineering approaches, use of inherently low flammable polymers, and flame retardant additives [46].
The first approach, fire safety through an engineering approach, is one of the cheapest to implement. It is a solution, which seeks to find a way to get the polymer out of the fire risk scenario. This can be done with a fire protection shield or changing how the construction of the entire product is used such that it is removed from the fire risk scenario completely. However, the approach is easily implemented and often much cost effective, can be easily defeated [46].
The second approach is to use the polymeric materials with low heat release in a wide range of fire risk scenarios. This tends to be a rather robust method of fire protection, as it does not matter what the fire risk scenario is. Low heat release polymers can be fabricated into a wide range of forms, making them relatively easy to implement in a wide range of applications. However, these same low flammability polymers come with a high cost, and so their use can be limited for economic reasons [47].
The third solution is the addition of flame retardant additives in polymeric materials. The most common approach to enhance fire safety performance is the use of flame retardant additives to inexpensive polymers. The additives must have a minimum impact on physical properties and product cost. Although halogenated flame retardants are highly effective for reducing the heat release rate of commodity polymers, the future use of these retardants faces some questions. The environmental impact of the processing and combustion of certain halogenated flame retardants has become an issue. Flame retardant additives are used to limit the risk of fire and its propagation. They are incorporated in the polymer matrix to increase the time to ignition, improve the self-extinguishing ability of the polymer, decrease the heat release rate during combustion and prevent the formation of flammable drops.
This approach tends to be very cost effective, and is relatively easy to incorporate into a polymer. However, the use of flame retardant additives has its own problems, including potential for leaching into the environment, difficulty with recycling, and often a compromise in reaching a balance in the properties of the polymer [48]. There are six general classes of flame retardant such as halogenated flame retardants, phosphorus-based flame retardants, mineral filler flame retardants, intumescent flame retardants, inorganic flame retardants, and polymer nano-composites. All types of flame retardant chemistries fall into one (or more) of three mechanisms of flame retardant action [48].
-
(1)
Gas phase flame retardants (ex. halogen, phosphorus)
These materials reduce the heat released in the gas phase from combustion by scavenging reactive free radicals.
-
(2)
Endothermic flame retardants (ex. metal hydroxides, carbonates)
These materials function in the gas phase and condensed phase by releasing non-flammable gases (H2O, CO2), which dilute the fuel and cool the polymer through endothermic decomposition of the flame retardant additive.
-
(3)
Char-forming flame retardants (ex. intumescents, nanocomposites)
These materials operate in the condensed phase by preventing fuel release through binding up fuel as non-pyrolyzable carbon (char) and providing thermal insulation for underlying polymer through the formation of char protection layers.
It should be pointed out here that a flame retardant is a chemical applied for a particular application. It is not any different than a chemical applied for curing a disease (a pharmaceutical) or a chemical applied to provide color to a fabric (a pigment). What makes it different than pharmaceutical or pigment chemicals is that its sole purpose is to minimize the flammability.
3.6 Smoke Formation
3.6.1 Smoke Generation
The combustion of polymers involves a variety of processes (both physical and chemical) occurring in several phases. Thus, polymer melting and degradation, heat transfer in both solid and liquid phases and diffusion of the breakdown products through the degrading polymer into the gas phase accompany the various combustion reactions which occur. Polymers with aliphatic backbones, or those that is largely aliphatic and oxygenated, have a tendency toward low smoke generation, while polyenic polymers and those with pendant aromatic groups generally produce more smoke [49].
In the presence of a sufficiently intense heat source a polymer will pyrolyse, breaking down to low molecular weight species. These species diffuse from the solid phase into the gas phase, where they form the smoke observed in the absence of flame. At high heating rates and with ignition, these low molecular weight species fuel the polymer flame. The nature of the cracked species and pyrolyzates generated is thus a major factor in determining smoke formation, given similar conditions of polymer combustion [49].
The relative distribution of pyrolysis products from an individual polymer is dependent on the pyrolysis temperature, the heating rate and the pyrolysis atmosphere. The amount of smoke generated in a nitrogen atmosphere passes through maxima with increasing temperature in several of the polyesters whereas from others the smoke increases steadily with temperature. The structure of a polymer influences both flammability and smoke formation [50].
Visible smoke from burning polymers is generally a result of incomplete combustion. Within a flame, unsaturated hydrocarbon molecules formed by thermal cracking of the fuel will polymerise and dehydrogenate to form carbon, or soot. During these processes, intermediate molecules can form unsaturated species or they can cyclize to form polybenzenoid structures, both of which will lead to soot formation. These polybenzenoid structures take on more importance as intermediates when they are formed directly from aromatic fuels [50].
3.6.2 Smoke Toxicity
The majority of human deaths during fire incidents are related to the inhalation of smoke and toxic combustion gases, with carbon monoxide being particularly significant. It prevents oxygen transport by the formation of carboxyhemoglobin. By comparisons, though carbon dioxide released quantities during combustion of a polymer, is not specifically toxic, its presence in blood stimulates hyper-ventilation, increasing the respiration rate and thus making humans susceptible to the toxic components of the fire gases [51].
Flame retardants working through flame inhibition result in significantly increased smoke yields in the combustion. Both CO production and smoke production result from incomplete combustion. CO production and smoke production are strongly dependent on the material, and also on fire scenario [52].
Irrespective of the functionality of various organic/inorganic additives/fillers, the smoke produced, during burning of polymer materials, is always a mixture of toxic combustion gases along with a suspension of fine particles (mostly inorganic) and soot in the range of nanometers to micrometers, depending on the system. This is a particularly dangerous situation for fire-fighters and incident investigation teams.
3.6.3 Smoke Suppressant
Some of the approaches that were adopted to tackle smoke problem include the use of fillers/additives, surface treatments, and structural modification of the polymers themselves [53]. The idea behind the incorporation of fillers/additives (or conventional flame retardants), as discussed above is to slow down the thermal decomposition of the polymeric material and reduce the smoke density and gas concentration [54].
Polymers and polymer formulations can be modified so that additive smoke suppressing compounds are effective in reducing smoke during burning. Several of the smoke suppressant additives known to be effective in burner fuels are also effective in polymers. Approaches used for reducing smoke during burning have included the use of fillers, additives, surface treatments, and structural modification of the polymers themselves. Certain chemical reactions occurring during combustion processes affect the generation of visible smoke [55].
It is important to realize that polymer decomposition chemistry is very important when trying to address the fire hazard of a polymer through flame retardant approaches. When considering fire protection approaches, one must look at all aspects of the burning polymer: heat release, smoke release, mechanical integrity under fire conditions (such as flow or softening), and how that polymer behaves in a particular fire risk scenario [56].
Based on the functionality of smoke suppressant, fillers are categorized as inert and active. Inert fillers mainly act by diluting the amount of combustible material and to a smaller extent by absorbing heat to reduce the burning rate. While active fillers also promote the dilution process and heat absorption, they absorb more heat per unit weight by endothermic processes. Examples of inert fillers are silica, clays, calcium carbonate; metal hydroxides and oxides are a major class of active fillers. It has been proved that most metal compounds, in particular transition metal compounds such as copper, molybdenum and iron compounds, are the most effective smoke suppressants [55].
3.7 Future Trends
3.7.1 Nanotechnology
The interactions among various entities of the eco-system are highly complex between the environment and humans are multi-faceted. Considering eco-friendliness, the ultimate mechanical or physical properties required for end applications, and processing difficulties, the flame retardant option becomes too narrow. To obtain better flame, smoke and toxicity performance, emphasis is laid on using various nanoparticles as flame retardants, particularly high aspect ratio fillers. Even at low loadings with no additional flame retardants in the system, the flame retardancy of polymeric materials are greatly improved, in addition to huge delay in burning compared to corresponding neat polymers [57].
Despite this, there are many other problems with these materials, such as the total heat released during flame testing, inability to meet existing requirements of the ignition resistance tests, etc. Additionally, only qualitative terms are used to describe the observed phenomena, with little attention focused on quantitative and physical understanding. This obviously has resulted in conflicting or misleading suggestions on the applicability of materials from the perspectives of short-and long-term fire exposure tests [58].
The revolutionary development of chemical/polymer technology and nano-sciences over the past few decades have added to the seriousness of the issue with many direct and indirect effects including waste control and waste management. Recently many flame-retardants, such as bromine, commonly found in polymer blends, composites and nanocomposites are being replaced by other chemicals due to health and environmental risks associated with some brominated flame-retardants.
The analyses of flame retardancy behavior of polymer nanocomposites from the perception of the aforementioned issues will shed light on the use of conventional and potentially harmful flame retardants, and lay the foundation to promote the adoption of “green” and “environmentally benign” materials. It is believed that the polymer nanocomposites, i.e. polymer matrices filled with specific, finely dispersed nanofillers, will undoubtedly pave the way for future materials combining physicochemical and thermo-mechanical performances with enhanced flame retardant behavior.
Additionally, carbon nanotubes are an interesting alternative to the use of conventional flame retardants. The incorporation at low loading rate (<3 wt%) has been reported to improve the flammability of a large range of polymers. Carbon nanotubes display exceptional properties that can potentially be used in many applications ranging from macroscopic material composites down to nanodevices. Thanks to their high aspect ratio, carbon nanotubes percolate to form a network at very low loading in the polymer matrix and lead to substantial enhancement of several functional properties such as mechanical, rheological and flame retardant properties [59].
3.7.2 Catalysis Technique
One possible new area of flame retardant chemistry would be catalysis. Some transition metals at elevated temperatures can form more thermally stable carbon/carbon bonds and so the use of a catalyst to cause a polymer to crosslink and form a more thermally stable char, rather than break apart into smaller monomer pieces may be a promising new direction. There are already some studies out there showing that this technology may have promise, including the use of specific metal complexes [60], nanoscale metal oxides [61], and waste catalysts from olefin crackers [62], which help improve char formation and lower the flammability of the polymer.
3.7.3 Ceramic/Glass Shield
Another area of new flame retardant chemistry would be using ceramic/glass precursors, which melt under fire conditions to form a protective ceramic/glass shield on the top of the burning polymer. Obviously, because glasses and ceramics are already in their highest oxidation state, they cannot be burned further and so if successfully implemented would provide very robust fire safety to a polymeric material. The key to making this technology successful though is to get these inorganic precursors to fuse together at low temperatures (<400 °C) so that they are available to protect the polymer before the polymer undergoes vigorous thermal decomposition. To date, there are only a handful of these systems, which show some promise [63], but there is still much to be done with these materials before they are more successful outside their current niche applications [64].
3.7.4 Vapor Phase Flame Retardant
The last new technology, which may come in the future, would be new vapor phase flame retardants. Currently, this is dominated by halogen, but some other elements have been found to show some vapor phase flame retardant activity, namely phosphorus, and a few metals (tin, iron, manganese) [65] in select forms. However, that appears to be the limit of what has been discovered to date as vapor phase flame retardants. So, if halogen is deselected from use and phosphorus is likewise limited, this just about depletes the choices of vapor phase flame retardants available for use [66].
The aforementioned metal-based vapor phase flame retardants in this paragraph unfortunately are only lab curiosities because they are toxic metal carbonyl compounds. To eliminate an entire flame retardant mechanism (vapor phase) would severely limit the ability to flame retard polymeric materials, and so research in this area is sorely needed. Because this area is so crucial, it seems that it is likely that eventually some new vapor phase flame retardants will be discovered in the future, or someone will discover environmentally friendly and economically viable versions of halogenated flame retardants that can be used instead. Admittedly, this prediction of future flame retardant technology is the hardest to predict and define, but still it seems that something in this area is likely to come out in the coming years [67].
3.7.5 Flame Retardant Synergy
In order to achieve high fire performance levels, it is necessary to develop a flame retardant system based on a combination of different flame retardant agents. The concept of synergism is used to optimize flame retardant formulations and enhance the performance of mixtures of two or more additives. Synergism is achieved when the performance level due to a mixture of additives [68].
As discussed above, polymer flame retardancy can be achieved through one or more chemical and/or physical mechanisms taking place in either the gas or the condensed phase. Synergistic phenomena can be obtained either by a combination of flame retardancy mechanisms, such as char formation by a phosphorated flame retardant combined with a gas phase action by a halogenated flame retardant, or by a combination of flame retardant agents reinforcing the same mechanism, e.g. nanoclays and phosphorated flame retardant agents, both acting in the condensed phase.
3.8 Sources of Further Information and Advice
Most of the information in this chapter is general knowledge to those working in the flame retardancy field of polymer materials and is summarized in the list of books and additional references. Sources of further information are listed as follow.
-
“A review of current flame retardant systems for epoxy resins” Weil, E. D.; Levchik, S. J. Fire. Sci. 2004, 22, 25–40.
-
“Commercial flame retardancy of thermoplastic polyesters: review.” Weil, E. D.; Levchik, S. J. Fire Sci. 2004, 22, 339–350.
-
“Thermal decomposition, combustion and fire-retardancy of polyurethanes—a review of the recent literature” Levchik, S. V.; Weil, E. D. Polym. Int. 2004, 53, 1585–1610.
-
“New developments in flame retardancy of epoxy resins” Levchik, S.; Piotrowski, A.; Weil, E.; Yao, Q. Polym. Degrad. Stab. 2005, 88, 57–62.
-
“Flammability” Tewarson, A. Chapter 42 in Physical Properties of Polymers Handbook, Mark J. E. ed. AIP Press, NY 1996. pp 577–604.
-
“Overview of recent developments in the flame retardancy of polycarbonates” Levchik, S. V.; Weil, E. D. Polym. Int. 2005, 54, 981–998.
-
“Flame retardants in commercial use or in advanced development in polycarbonates and polycarbonate blends” Levchik, S. V.; Weil, E. D. J. Fire Sci. 2006, 24, 137–151.
-
“Flame and smoke retardants in vinyl chloride polymers—commercial usage and current developments” Weil, E. D.; Levchik, S.; Moy, P. J. Fire Sci. 2006, 24, 211–236.
-
“Thermal decomposition, combustion and fire-retardancy of polyurethanes—a review of the recent literature” Levchik, S. V.; Weil, E. D. Polym. Int. 2004, 53, 1585–1610.
-
“Flame retardants for polystyrenes in commercial use or development” Weil, E. D.; Levchik, S. V. J. Fire Sci. 2007, 25, 241–264.
-
“Flame retardants in commercial use or development for polyolefins” Weil, E. D.; Levchik, S. V. J. Fire Sci. 2008, 26, 5–42.
-
“New developments in flame retardancy of styrene thermoplastics and foams” Levchik, S. V.; Weil, E. D. Polym. Int. 2008, 57, 431–448.
-
“Flame retardants in commercial use or development for textiles” Weil, E. D.; Levchik, S. V. J. Fire Sci. 2008, 26, 243–281.
-
“Zinc borates as multifunctional polymer additives” Shen, K. K.; Kochesfahani, S.; Jouffret, F. Polym. Adv. Technol. 2008, 19, 469–474.
-
“Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: a comprehensive review” Singh, H.; Jain, A. K. J. App. Polym. Sci. 2009, 111, 1115–1143.
-
“Fire Properties of Polymer Composite Materials” Eds. Mouritz, A. P.; Gibson, A. G. Springer-Verlag, The Netherlands, 2006. ISBN 978-1-4020-5355-9.
-
“Fire Retardancy of Polymeric Materials”, 2nd Edition. Eds. Wilkie, C. A.; Morgan A. B. 2009, Taylor and Francis. ISBN 978–1420083996.
-
“Flame Retardants for Plastics and Textiles: Practical Applications” Weil, E. D.; Levchik, S. V. 2009, Hanser Gardner Publications, ISBN 978–1569904541.
-
“Fire Retardancy of Polymers: New Strategies and Mechanisms” Hull, T. R.; Kandola B. K. Eds. 2009, Royal Society of Chemistry, ISBN 978–0854041497.
3.9 Conclusion
Stopping the burning of materials is an endeavor as old as recorded history, and as long as we see a need to provide protection against fire, we will have to use flame retardancy in one way or another as a civilization.
Flame retardancy is a much applied field and so understanding all the nuances of the technology is essential to understanding why a particular flame retardant is in use today, as well as what its specific strengths and weaknesses are in its current use. The field of flame retardancy today is dynamic.
Fundamental studies are still needed, but an understanding of the importance of balancing properties and very complex fire risk scenarios and polymer combustion concepts need to be mastered by researchers before they can develop new flame retardant technologies. However, if the reader is just interested in learning the how and why of flame retardant technology, rather than trying to develop a new technology, it is our hope that this review will serve that role nicely.
The flame retardant additive technologies used today may not be utilized in the future. They are suitable and proven for flame retardancy in their respective polymers, but they are not the pinnacles of their respective chemistries; new flame retardant chemistry is likely to be discovered and exploited as more time and money are spent researching this area.
It should be pointed out that, for the most part, flame retardancy is not a topic that most people think about because of successful protection of society provided by the current use of passive flame retardant additives. Fire damages and losses have been reduced by the use of flame retardant additives.
The current trend is to incorporate more rather than less polymeric materials into our modern civilizations, and then new flame retardants will have to be created to address these new tests. Likewise, if improved recycling and lower environmental impact is desired, then new flame retardants will have to be made to meet these requirements. Flame retardant technology is driven by external forces, and these new external forces will greatly change the technology as we know it.
References
Yu, Z., Cai, G., Mai, Y., Dasaria, A.: Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 38, 1357–1387 (2013)
Laoutid, F., Bonnaud, L., Alexandre, M., Lopez-Cuesta, J., Dubois, P.: New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 63, 100–125 (2009)
Kiliaris, P., Papaspyrides, C.D.: Polymer/layered silicate (clay) nanocomposites: an overview of flame retardancy. Prog. Polym. Sci. 35, 902–958 (2010)
Dasari, A., Yu, Z., Cai, G., Mai, Y.: Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 38, 1357–1387 (2013)
Bourbigot, S., Duquesne, S.: Fire retardant polymers: recent developments and opportunities. J. Mater. Chem. 17, 2283–2300 (2007)
Moniruzzaman, M., Winey, K.I.: Polymer nanocomposites containing carbon nanotubes. Macromolecules 39, 5194–5205 (2006)
Utracki, L.A., Sepehr, M., Boccaleri, E.: Synthetic, layered nanoparticles for polymeric nanocomposites (PNCs). Polym. Adv. Technol. 18, 1–37 (2007)
Vaia, R.A., Maguire, J.F.: Polymer nanocomposites with prescribed morphology: going beyond nanoparticle-filled polymers. Chem. Mater. 19, 2736–2751 (2007)
Gilman, J.W., Harris, R.H., Shields, J.R., Kashiwagi, T., Morgan, A.B.: A study of the flammability reduction mechanism of polystyrene-layered silicate nanocomposite: layered silicate reinforced carbonaceous char. Polym. Adv. Technol. 17, 263–271 (2006)
Bartholmai, M., Schartel, B.: Layered silicate polymer nanocomposites: new approach or illusion for fire retardancy? Investigations of the potentials and the tasks using a model system. Polym. Adv. Technol. 15, 355–364 (2004)
Morgan, A.B., Bundy, M.: Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater. 31, 257–283 (2007)
Bourbigot, S., Duquesne, S.: Fire retardant polymers: recent developments and opportunities. J. Mater. Chem. 17, 2283–2300 (2007)
Tata, J., Alongi, J., Carosio, F., et al.: Optimization of the procedure to burn textile fabrics by cone calorimeter: part I. Combustion behavior of polyester. Fire Mater 35(6), 397–409 (2011)
Xu, Q., Majlingova, A., Zachar, M.: Correlation analysis of cone calorimetry test data assessment of the procedure with tests of different polymers. J. Therm. Anal. Calorim. 110(1), 65–70 (2012)
Mariappan, T., Wilkie, C.A.: Cone calorimetric analysis of flame-retarded polyurea for coating applications. J. Fire Sci. 31(4), 330–338 (2013)
Dietenberger, M.: Pyrolysis kinetics and combustion of thin wood by an advanced cone calorimetry test method. J. Therm. Anal. Calorim. 109(3), 1215–1228 (2012)
Wang, Y., Jow, J., Su, K., et al.: Development of the unsteady upward fire model to simulate polymer burning under UL 94 vertical test conditions. Fire Saf. J. 54, 1–13 (2012)
Wang, Y., Zhang, J.: Thermal stabilities of drops of burning thermoplastics under the UL 94 vertical test conditions. J. Hazard. Mater. 246, 103–109 (2013)
Sullalti, S., Colonna, M., Berti, C.: Effect of phosphorus based flame retardants on UL 94 and comparative tracking index properties of poly (butylene terephthalate). Polym. Degrad. Stab. 97(4), 566–572 (2012)
Lin, C.H., Chen, J.C., Huang, C.M., et al.: Side-chain phenol-functionalized poly (ether sulfone) and its contribution to high-performance and flexible epoxy thermosets. Polymer 54(26), 6936–6941 (2013)
Morgan, A.B., Bundy, M.: Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater. 31, 257–283 (2007)
Weil, E.D., Patel, N.G., Said, M.M., Hirschler, M.M., Shakir, S.: Oxygen index: correlations to other fire tests. Fire Mater. 16, 159–167 (1992)
Xu, T., Wang, H., Huang, X., Li, G.: Inhibitory action of flame retardant on the dynamic evolution of asphalt pyrolysis volatiles. Fuel 105, 757–763 (2013)
Popov, K.V., Knyazev, V.D.: Molecular dynamics simulation of C-C bond scission in polyethylene and linear alkanes: effects of the condensed phase. J. Phys. Chem. A 118(12), 2187–2195 (2014)
Dasari, A., Yu, Z.Z., Cai, G.P., et al.: Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 38(9), 1357–1387 (2013)
Schartel, B., Weiß, A.: Temperature inside burning polymer specimens: pyrolysis zone and shielding. Fire Mater. 34(5), 217–235 (2010)
Cevallos, J.G., Bergles, A.E., Bar-Cohen, A., et al.: Polymer heat exchangers—history, opportunities, and challenges. Heat Transf. Eng. 33(13), 1075–1093 (2012)
Liang, J.Z.: Heat Transfer in Polymer Composites Filled with Inorganic Hollow Micro-Spheres: Heat Transfer in Multi-Phase Materials, pp. 163–185. Springer, Berlin (2011)
Strein, E., Colbert, A., Subramaniyan, S., et al.: Charge generation and energy transfer in hybrid polymer/infrared quantum dot solar cells. Energy Environ. Sci. 6(3), 769–775 (2013)
Alsalhi, M.S., Abu Mustafa, Z.S., Masilamani, V.: External energy transfer in amplified spontaneous emissions from MEH-PPV conjugated polymer. Opt. Laser Technol. 43(1), 147–151 (2011)
Kempel, F., Schartel, B., Linteris, G.T., et al.: Prediction of the mass loss rate of polymer materials: Impact of residue formation. Combust. Flame 159(9), 2974–2984 (2012)
Jiang, F.H., Qi, H.Y., De Ris, J.L., et al.: Heat transfer blockage in small scale combustion of polymers. Sci. China Technol. Sci. 54(9), 2457–2467 (2011)
Tibiletti, L., Longuet, C., Ferry, L., et al.: Thermal degradation and fire behaviour of unsaturated polyesters filled with metallic oxides. Polym. Degrad. Stab. 96(1), 67–75 (2011)
Duquesne, S., Bourbigot, S.: Char formation and characterization. In: Wilkie, C., Morgan, A. (eds.) Fire Retardancy of Polymeric Materials, pp. 239–260. CRC Press, Boca Raton (2009)
Gnedin, E.V., Novikov, S.N., Khalturinskij, N.A.: Chemical and physical properties of foamed cokes and their effect on inflammability. Makromol. Chem. Macromol. Symp. 74, 329–333 (1993)
Dasari, A., Yu, Z.Z., Cai, G.P., et al.: Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 38(9), 1357–1387 (2013)
Xu, T., Huang, X.: Combustion properties and multistage kinetics models of asphalt binder filled with flame retardant. Combust. Sci. Technol. 183(10), 1027–1038 (2011)
Green, J.: Mechanisms for flame retardancy and smoke suppression—a review. J. Fire Sci. 14, 426–442 (1996)
Singh, H.: Investigation on ignition, pyrolysis and combustion of commercially important polyurethane foams. Fire Eng. 39(1), 7–11 (2014)
Xu, T., Huang, X.: Combustion properties of asphalt binder containing flame retardant. Fire Mater. 36(2), 97–106 (2012)
Tang, Y., Zhuge, J., Lawrence, J., et al.: Flame retardancy of carbon nanofibre/intumescent hybrid paper based fiber reinforced polymer composites. Polym. Degrad. Stab. 96(5), 760–770 (2011)
Xu, T., Huang, X.: A TG-FTIR investigation into smoke suppression mechanism of magnesium hydroxide in asphalt combustion process. J. Anal. Appl. Pyrol. 87(2), 217–223 (2010)
Azwa, Z.N., Yousif, B.F., Manalo, A.C., et al.: A review on the degradability of polymeric composites based on natural fibres. Mater. Des 47, 424–442 (2013)
Lu, H., Wilkie, C.A.: Fire performance of flame retardant polypropylene and polystyrene composites screened with microscale combustion calorimetry. Polym. Adv. Technol. 22(1), 14–21 (2011)
Xu, T., Huang, X.: Study on combustion mechanism of asphalt binder by using TG–FTIR technique. Fuel 89(9), 2185–2190 (2010)
Morgan, A.B., Gilman, J.W.: An overview of flame retardancy of polymeric materials: application, technology, and future directions. Fire Mater. 37, 259–279 (2013)
Xu, T., Huang, X.: Pyrolysis properties and kinetic model of an asphalt binder containing a flame retardant. J. Appl. Polym. Sci. 119(5), 2661–2665 (2011)
Xu, T., Huang, X.: Investigation into the properties of asphalt and its mixture filled with flame retardant. Fire Saf. J. 46(6), 330–334 (2011)
Lestari, F., Hayes, A.J., Green, A.R., et al.: An alternative method for in vitro fire smoke toxicity assessment of polymers and composites using human lung cells. Fire Mater. 35(6), 411–429 (2011)
Stec, A.A., Rhodes, J.: Smoke and hydrocarbon yields from fire retarded polymer nanocomposites. Polym. Degrad. Stab. 96(3), 295–300 (2011)
Hull, T.R., Carman, J.M., Purser, D.A.: Prediction of CO evolution from small-scale polymer fires. Polym. Int. 49, 1259–1265 (2000)
Babrauskas, V.: The generation of CO in bench-scale fire tests and the prediction for real-scale fires. Fire Mater. 19, 205–213 (1995)
Hornsby, P.R.: Fire retardant fillers for polymers. Int. Mater. Rev. 46, 199–210 (2001)
Cusack, P.A., Hornsby, P.R.: Zinc stannate-coated fillers: novel flame retardants and smoke suppressants for polymeric materials. J. Vinyl Addit. Technol. 5, 21–30 (1999)
Li, B.: Influence of polymer additives on thermal decomposition and smoke emission of poly (vinyl chloride). Polym. Degrad. Stab. 82, 467–476 (2003)
Mouritz, A.P.: Review of smoke toxicity of fiber-polymer composites used in aircraft. J. Aircr. 46, 737–745 (2009)
Kashiwagi, T., Du, F., Douglas, J.F., Winey, K.I., Harris Jr, R.H., Shields, J.R.: Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 4, 928–933 (2005)
Du, B., Ma, H., Fang, Z.: How nano-fillers affect thermal stability and flame retardancy of intumescent flame retarded polypropylene. Polym. Adv. Technol. 22(7), 1139–1146 (2011)
Hapuarachchi, T.D., Peijs, T.: Multiwalled carbon nanotubes and sepiolite nanoclays as flame retardants for polylactide and its natural fibre reinforced composites. Compos. Part A: Appl. Sci. Manuf. 41(8), 954–963 (2010)
Morgan, A.B.: A review of transition metal-based flame retardants: transition-metal oxide/salts, and complexes. In: ACS symposium series 1013—fire and polymers V: materials and concepts for fire retardancy, pp. 312–328. Oxford University Press, New York (2009)
Fontaine G, Turf T, Bourbigot S. Salen copper complexes: a novel flame retardant for thermoplastic polyurethane. In: ACS symposium series 1013—fire and polymers V: materials and concepts for fire retardancy, pp. 329–340. Oxford University Press, New York (2009)
Estevao, L.R.M., Le Bras, M., Delobel, R., Nascimento, R.S.V.: Spent refinery catalyst as a synergistic agent in intumescent formulations: influence of the catalyst’s particle size and constituents. Polym. Degrad. Stab. 88, 444–455 (2005)
Kashiwagi, T., Gilman, J.W.: Silicon based flame retardants. In: Grand, A.F., Wilkie, C.A. (eds.) Flame Retardancy of Polymeric Materials, pp. 353–389. Marcel Dekker, Inc., New York (2000)
Gilman, J.W., Ritchie, S.J., Kashiwagi, T., Lomakin, S.M.: Fire-retardant additives for polymeric materials—I. Char formation from silica gel-potassium carbonate. Fire Mater. 21, 23–32 (1997)
Linteris, G.T., Knyazev, V.D., Babushok, V.I.: Inhibition of premixed methane flames by manganese and tin compounds. Combust. Flame 129, 221–238 (2002)
Linteris, G.T., Katta, V.R., Takahashi, F.: Experimental and numerical evaluation of metallic compounds for suppressing cup-burner flames. Combust. Flame 138, 78–96 (2004)
Morgan, A.B., Gilman, J.W.: An overview of flame retardancy of polymeric materials: application, technology, and future directions. Fire Mater. 37, 259–279 (2013)
Yen, Y.Y., Wang, H.T., Guo, W.J.: Synergistic flame retardant effect of metal hydroxide and nanoclay in EVA composites. Polym. Degrad. Stab. 97(6), 863–869 (2012)
Acknowledgments
The author gratefully thanks National Natural Science Foundation of China (NSFC, Grant No. 51378264) and Ministry of Housing, Urban-Rural Construction of the People’s Republic of China (MOHURD, Grant No. 2013-K5-15), the Start-up-Grant from Nanjing Forestry University (YJ2012-06), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for their financially supporting my research on fire retardancy of polymeric materials.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Xu, T. (2015). Recent Developments in Different Techniques Used for the Flame Retardancy. In: Visakh, P., Arao, Y. (eds) Flame Retardants. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-319-03467-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-03467-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03466-9
Online ISBN: 978-3-319-03467-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)