Abstract
Shales form the principal source rock for most of the conventional and unconventional petroleum systems. In India, both the prolific and prospective sedimentary basins have excellent to fair development of shales that range in age from the Proterozoic to Cenozoic. Deposition, preservation and maturation of organic content in shales define the source rock characteristics and its hydrocarbon generation potential, leading to a prolific/non prolific oil and gas play. Useful insight on development and hydrocarbon generation potential of shales and other organic rich source rocks is obtained through the geochemical study of sedimentary organic matter. In the present work, organic richness, kerogen type and thermal maturity of potential shales from few sedimentary basin of India, namely—Vindhyan, Krishna-Godavari, Kutch, Cambay and Jammu and Kashmir have been studied, to understand their effectiveness as source rocks in the particular basins. The studied shales show high Total organic carbon (TOC) content and contain dominantly Type III kerogen with partial contributions from Type II, in varying stages of hydrocarbon generation from immature to post mature, depending upon the thermal history of respective basins. Quantitative approaches involving the use of kinetic parameters for thermal cracking of organic matter (kerogen) into hydrocarbons, in conjunction with the source rock quality can account for improved understanding of the hydrocarbon resources in these basins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Shales form the principal source rock for most of the conventional and unconventional petroleum systems around the globe (Hunt 1996). In India, both the producing as well as the frontier basins exhibit excellent to fair development of shales that range in age from the Proterozoic to Cenozoic. The source potential of shale rocks depends primarily upon the organic facies, which has been defined as ‘a mappable subdivision of a designated stratigraphic unit, distinguished from the adjacent subdivisions on the basis of the character of its organic constituents, without regard to the inorganic aspects of the sediment’ (Jones and Demaison 1982; Jones 1983). Palynological studies of the organic facies in source rocks has been used since earlier days to investigate the organic matter content, source input and depositional environment. However; the geochemistry of source rocks was developed in relatively recent times, and it added a precise quantitative hand to the palynological and sedimentological studies of the source rocks. Advances in disciplines like molecular and stable isotope geochemistry have led to infer reliably on abundance, origin, type and maturity of organic matter, which are key controls in development of petroleum source rocks.
In India, hydrocarbons occur across a large temporal range of geological setup from Cambrian (Bikaner-Nagaur), Permian (Krishna-Godavari), Mesozoic (Cauvery; Krishna-Godavari; Jaisalmer; Barmer-Sanchor), to Cenozoic (Assam-Arakan, Cambay, Mumbai offshore; Krishna-Godavari), with majority of production coming from the Cenozoic sediments (Bhowmick and Misra 2012; DGH 2014). The Mesozoics, despite of sufficient sedimentary thicknesses in basins like Kutch and Saurashtra, have added meagrely to the Indian hydrocarbon reserves due to inherent challenges in understanding of their sub-basaltic petroleum generative aspects. Gondwana sequences are now being targeted in basins like Krishna-Godavari, Assam, and Damodar Valley. About 80 % of the Indian sedimentary cover is under different stages of initial exploration (DGH 2014). Petroleum geochemistry essentially adds to the much needed information towards determining of the presence and properties of hydrocarbons trapped in the source rocks or the potential to generate such hydrocarbon (Chandra et al. 2001; Eden and Mungo 2013; Mani et al. 2011a, b; Peters et al. 2005). Useful insights on basic source rock characterization, genetic correlations of oil and gas and kinetic parameters of thermal cracking of hydrocarbons can be obtained though the properties of organic content and/or hydrocarbon components.
Giving an overview on the transformation of sedimentary organic matter into hydrocarbons, we present the organic geochemical characteristics of shales, an important source rock in majority of oil and gas plays from few sedimentary basins of India, namely—Vindhyan, Kutch, Cambay, Jammu and Kashmir and Krishna-Godavari. The results presented include the qualitative and quantitative aspects of shale’s total organic carbon (TOC) content and thermal maturity of the different kerogen types. Also, the kinetic parameters derived from the cracking of kerogen into hydrocarbons are discussed for the shales from the Kutch region.
2 Organic Matter, Source Rock Development and Hydrocarbon Generation
Of less than 0.1 % of the carbon that escapes the carbon cycle only to get incorporated in the sediments and in later stages, under given conditions of sufficient thermal exposure and burial, matures to generate the hydrocarbons (Mani et al. 2013). Photosynthesis has been the basis for mass production of organic carbon and its preservation in sedimentary basins is governed by the paleo depositional environments and oxygenation conditions, sedimentation rates and grain size (Tissot and Welte 1984). Successive burial of organic matter to different depths causes the physical and chemical transformations and evolution of kerogen into hydrocarbons (Fig. 1).
Generalized evolution of sedimentary organic matter upon increasing burial and temperature (not to scale) (after, Mani et al. 2013)
Initially, diagenetic processes operate in recently deposited sediments at shallow depths and low temperatures (typically <50 °C). The biopolymers such as proteins and carbohydrates progressively evolve into geopolymer—the kerogen. Successive deposition of sediments with time results in burial of previous beds and exposure of sediments to subsurface conditions of increasing temperature (~50–150 °C) and pressure (~300–1000 bars) depending on the overburden sedimentary column and tectonic activity (Hunt 1996). Thermal degradation of kerogen leads to generation of liquid petroleum, wet gas and condensate. With still higher temperature ranges (150–200 °C), organic molecules are cracked to gas in the process of metagenesis (Fig. 1). The last stage of evolution of sediments is metamorphism resulting in greenschist and amphibolite facies (Tissot and Welte 1984).
Around 10–20 % of the petroleum is formed directly from the hydrocarbons synthesized by the once living organisms. These include C15+ compounds with recognizable biological structures (biomarkers or the chemical fossils), which get trapped in sediments with no or minor changes during the diagenesis processes associated with sedimentation. These are easily soluble in the organic solvents and constitute the bitumen fraction of organic matter. It represents the first source of hydrocarbons. The second and the major source (80–90 %) of hydrocarbons involve the conversion of the lipids, proteins and carbohydrates of living organisms into organic matter of sedimentary rocks or the kerogen. When buried deeper at high temperatures kerogen cracks to bitumen, which degrades to give oil and gas (Hunt 1996; Tissot and Welte 1984).
The organic richness and maturity of sedimentary organic matter are directly related to the hydrocarbon generation potential of a source rock. Those having TOC greater than 2 % are considered very good source rocks; and that of <0.5 % are considered to be poor with very low to negligible hydrocarbon generation potential. Depending upon the source organic input and depositional environment, the carbon (C), hydrogen (H) and oxygen (O) contents of the kerogen differs, resulting its classification into Type I, II, III and IV (van Krevelen 1961; Hunt 1996; Tissot and Welte 1984). Where the depositional and early diagenetic environment is highly oxygenated, the total-organic-carbon (TOC) content is low. The remaining kerogen contains large quantity of oxygen with a negligible generative capacity for hydrocarbons, despite a relatively high hydrocarbon/TOC ratio in the immature state. An anoxic depositional, early diagenetic environment can result in the preservation of organic-rich, fine-grained sediments that can develop into excellent source rock. Thermal maturity, which is the extent of temperature time driven reactions that convert the sedimentary organic matter or kerogen to oil and gas, is equally important for generation of hydrocarbons. In general, thermally immature source rocks do not have pronounce effect of temperature (~60 °C; 0.2–0.6 % Ro). The bacterial and plant organic matter is converted to kerogen and bitumen and biogenic methane is generated. Thermally mature kerogen generates oil (~60–160 °C; 0.6–1.35 % Ro) where as the post mature kerogen (>160 °C; >1.35 % Ro) is in wet condensate and dry gas zones (Tissot and Welte 1984; Peters and Casa 1994).
The petroleum evaluation in a sedimentary basin involves a quantitative approach, taking into account the amount of oil and gas generated by primary cracking of kerogen when temperature increases through time (Fig. 2; Tissot and Espitalie 1975). The degradation of kerogen into hydrocarbons is described by a series of n parallel chemical reactions, each of which obey a first order kinetics, characterized by Arrhenius Law.
- \({\text{dXi}}/{\text{dt}}\) :
-
hydrocarbon generation rate
- A:
-
arrhenius constant
- Ei:
-
activation energy
- R:
-
molar gas constant
- T:
-
absolute temperature
- Xi:
-
residual petroleum potential
General scheme of kerogen degradation (after, Tissot and Espitalie 1984)
The total initial petroleum potential of kerogen (Hydrogen Index) HI in mg HC/gTOC is expressed by \({\text{HI}} = \sum\nolimits_{{{\text{i}} = 1}}^{\text{n}} {{\text{Xi}}_{\text{o}} }\) . Thus, the amount Q of generated hydrocarbons is (mg HC/gTOC) is
The kinetic parameters so obtained are used in basin modelling to predict the amount and timing of generated hydrocarbons as function of time and temperature (Tissot and Espitalie 1975).
3 Analytical Procedures
An array of geochemical screening and follow up analyses of organic rich source rocks using advanced techniques such as programmed pyrolysis, biomarker separation and analysis, and compound specific stable isotopic composition measurements are used to understand the source rock’s quality and quantitative hydrocarbon generation.
3.1 Rock Eval Pyrolysis
Rock Eval pyrolysis is one of the most basic organic geochemical screening methods and is used to estimate the petroleum potential of rocks by open system cracking of kerogen according to a programmed temperature pattern (Behar et al. 2001). The complete process takes place in the two ovens, pyrolysis and oxidation (combustion), respectively of Rock Eval pyrolyzer. The pyrolyzed hydrocarbons are monitored by a flame ionization detector (FID), forming the so-called peaks S1 (thermovaporized free hydrocarbons) and S2 (pyrolysis products from cracking of kerogen). The method is completed by combustion of the residual rock recovered after pyrolysis up to 850 °C, under artificial air (N2/O2). During pyrolysis and combustion, released CO and CO2 are monitored on line by means of an infra-red cell. This complementary data acquisition enables determination of the organic and mineral carbon content of samples, labelled TOC and MinC, respectively. The Tmax value is a maturity parameter. It corresponds to the temperature at which maximum amount of hydrocarbons are released from the thermal degradation of kerogen, i.e. the temperature at which S2 peak reaches its maximum. Among various calculated parameters of Rock Eval, the hydrocarbon potential or hydrogen index, (HI) is defined by 100 × S2/TOC. The oxygen index (OI) is defined as 100 × S3/TOC, where S3 is the CO2 released during the pyrolysis. These indices help in defining kerogen types and maturation. Details on Rock Eval functioning, parameters acquired, and interpretive guidelines have been discusses by several workers (Behar et al. 2001; Espitalie et al. 1987; Lafargue et al. 1988; Peters 1986; Peters and Cassa 1994).
3.1.1 Operational Parameters for Rock Eval
Pyrolysis of shales was carried out using the Rock Eval 6 pyrolyser, Turbo version (Vinci Technologies). After obtaining a stable signal for the detectors, the instrument was calibrated in standard mode using the IFP standard, 160000 (Tmax = 416 °C; S2 = 12.43). The samples were weighed in pre-oxidized crucibles depending upon the organic matter content (~50–70 mg of shale; and 8–15 mg of coaly shale). The shale samples were run under analysis mode using the bulk rock method and basic cycle of Rock Eval 6 and the data was reported on dry weight basis (Mani et al. 2014).
3.2 Gas Chromatography (GC)
Gas Chromatography is an instrumental technique for the qualitative and quantitative identification of chemical compounds (Grob 2004). The separation and identification of the chemical mixture takes place in a gas chromatograph, which consists of three main components (i) an injector: it is a port meant for injecting the samples into the system, (ii) a column: where the analyte gets separated into its components depending upon its affinity with the stationary phase and the mobile carrier gas phase and (iii) the detector: where the qualitative and quantitative determination of the analyte takes place. The technique is based on the partitioning of the molecules between the stationary phase of the column and the mobile phase of the carrier gas. A carrier gas is usually an inert or un-reactive gas such as helium or nitrogen that carries the sample through the column to the detector. The column consists of the stationary phase made up of polymeric material and is contained in a heated oven to maintain the column temperature. Depending upon the nature of analyte, columns of different types such packed or capillary columns with specific polymer are used. The mobile phase elutes the components to reach the detector at different time, which is very specific for each component under a particular condition of pressure and temperature and is called the retention time (Rt) of respective component. The flame ionization detector (FID) is used for the detecting the hydrocarbon concentration. In an FID, the organic compounds are burnt in a flame of hydrogen and air and the electrons produced are collected over a collecting electrode. The resulting current is the response of the detector in the form of series of signal peaks and is recorded using peak area or peak height as basis (Grob 2004).
3.2.1 Operational Parameters for GC
Varian CP-3380 gas chromatograph was used for the determination of concentration of gaseous hydrocarbons. Nitrogen, with a flow rate of 30 ml/min, was used as a carrier gas. The temperature of injector port was maintained at 120 °C. The GC was equipped with packed column: Porapak ‘Q’ of length 1.8 m and diameter: 1/8″ × 2.0 mm. The column oven program is given in Table 1. The temperature of the detector (FID) was maintained at 200 °C. The fuel gases used to burn the flame at FID were hydrogen and zero air with flow rate of 300 ml/min. Star Workstation was used for the data acquisition. The calibration of the gas chromatograph was done using external standard (BOC standard gas mixture), comprising of methane, ethane, propane, i-butane, n-butane, i-pentane and n-pentane, of four different concentration levels (Mani et al. 2012a).
3.3 Organic and Isotope Ratio Mass Spectrometry
3.3.1 Organic/Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS is used to detect and quantify organic compounds using relative gas chromatographic retention times, elution patterns, and the mass spectral fragmentation patterns characteristic of their structures (Sneddon et al. 2007). A typical GC-MS system performs (1) compound separation by gas chromatography; (2) transfer of separated compounds to the ionizing chamber of the mass spectrometer; (3) ionization; (4) mass analysis; (5) detection of the ions by the electron multiplier; (6) acquisition, processing, and display of the data by computer (Sneddon et al. 2007). As the individual organic compounds elute from the GC, they enter the MS, typically and most common is the electron ionization detector. Here, they are bombarded by a stream of electrons causing them to fragment. The mass of the fragment divided by the charge is the mass charge ratio (m/z). Almost always the charge is +1, and m/z ratio represents the molecular weight of the fragment. In case of a quadrupole GC-MS, a group of four electromagnets (a quadrupole), focus each fragment through a slit into the detector. These quadrupoles are programmed by a computer to direct only certain fragments, one at a time (scan) until the range of m/z is recovered. This produces the mass spectrum, which is a graph of signal intensity (relative abundance) versus m/z ratios (essentially molecular weight). Each compound has a unique fingerprint and software is readily available to provide a library of spectra for unknown compounds (Sneddon et al. 2007).
3.3.1.1 Solvent Extraction
Prior to the analysis of organic compounds on GC-MS, the total organic matter is extracted from the sediments through the use of organic solvents at raised temperature and pressure. Extraction of organic matter from selected shale samples was carried out using Buchi Speed Extractor E-914. 3 g of sample was mixed with 3 g of sand and placed between the top and bottom layers of 1.5 g sand each, in 40 ml extraction cell. The extraction was done in atmosphere of nitrogen with maximum temperature and pressure of 100 °C and 100 psi, respectively using dichloromethane (DCM) and methanol in ratio of 9:1. The Speed Extractor was programmed for three extraction cycles. The collected organic extracts were reduced to about 1 ml on the Buchi Rotavap V-855. n-pentane was added to the extract for the separation of asphaltene, following which the HCl treated Cu turning were added for the removal of sulphides. The extracts were cleaned up using silica column chromatography and separate fractions of the extracts, saturate and aromatic, were analyzed for the n-alkane and PAH components (Mani et al. 2015).
3.3.1.2 Operational Parameters for GC-MS
A Varian 320 Quadrupole Mass Spectrometer coupled to Varian CP-3800 Gas Chromatograph, equipped with Br-5 MS column (30 m × 0.25 mm × 0.25μm) was used to analyze the organic extracts for the n-alkanes and PAH in full scan and selective ion monitoring (SIM) modes, respectively. In the SIM mode, the GC oven was programmed at 70 °C with a hold time of 4 min and increased to 300 °C @20 °C/min with a hold time of 15 min. The MS was operated under 70 eV with source temperature at 240 °C and transfer line at 280 °C. The manifold was maintained at 40 °C. In the full scan mode, the column oven was programmed at 40 °C with a hold time of 1 min and increased to 310 °C @5 °C/min with a hold time of 15 min. The calibration for n-Alkanes was done using C14–C32 (even carbon numbered homologues + pristine/phytane) standard from Chiron AS and that of PAH standard from Accustandard (PAH mix). The sample spectra and retention times were compared with characteristic published spectra (Philp 1985a, b; Peters and Moldowan 1993) and/or procured standard spectra (Mani et al. 2012b, 2015).
3.3.2 Isotope Ratio Monitoring Spectrometry (IRMS)
IRMS are widely used to determine the ratio of stable isotopes of Carbon (C) and Oxygen (O), i.e. 13C/12C and 18O/16O, respectively in geological samples. With the advancement of hyphenated techniques, the separation power of gas chromatograph has been coupled to the mass spectrometer along with introduction of sample combustion interface into the gas chromatograph-isotope ratio mass spectrometer (Platzner 1997). The separated products of the sample mixture in the stream of helium at the output of the gas chromatograph are passed through an oxidation/reduction reactor and then introduced into the ion source of mass spectrometer for precise concentration determination. The basic mass spectrometer comprises of the (i) ion source for fragmentation of sample molecule into ions and (ii) mass analyzer for separating the ion beam according to the mass of the respective ions. The open split-coupling device ensures that only a part of the sample/reference gas containing carrier gas is fed into the ion source of the MS. In this way, pulse injection of sample gas can be analyzed, reducing the volume constraints and sample size (Platzner 1997).
Continuous Flow-IRMS is a generic term for IRMS instruments that are coupled on-line to preparation lines or instruments. This includes the (i) Gas Chromatography-Combustion-IRMS (GC-C-IRMS), used for the compound specific analysis of hydrocarbon components. (ii) Gas Bench-IRMS (GB-IRMS), used for the C and O isotope ratio determinations on carbonates. (iii) Elemental Analyzer-IRMS (EA-IRMS), used for the determination of coexisting organic matter in shale samples, with IRMS, being common to all the above mentioned peripherals.
3.3.2.1 GC-C-IRMS
The δ13C analysis of gaseous hydrocarbons was carried out using GC-C-IRMS. An Agilent 6890 GC coupled to a Finnigan-Delta PlusXP IRMS via a GC combustion III interface was used for the determination of carbon isotope ratios. One ml of the ortho-phosphoric acid desorbed gas (Horvitz 1985) was injected into the GC, equipped with “Pora Plot Q” capillary column 25 m in length and a diameter of 0.32 mm, in splitless mode with helium as carrier gas at a fixed oven temperature of 28 °C. The chromatographically separated hydrocarbon gases after eluting from GC column enter a pre-oxidized Cu-Ni-Pt combustion reactor maintained at 960 °C, where they get converted into carbon dioxide and water. The water was removed using Nafion membrane tube prior to their entry into the mass spectrometer. The purified CO2 after combustion enters into the mass spectrometer for 13C/12C ratio measurement of the respective hydrocarbon component. The GC-C-IRMS was calibrated to the international standard Vienna Pee Dee Belemnite (VPDB) using Natural Gas Standard (NGS-1) mixture (Mani et al. 2011a).
4 Case Studies
Of the twenty six sedimentary basins in India, shales of varied age and lithology are prolific source rocks in six basins, whereas there are several frontier basins in which the potential source rocks are yet being characterized at different stages of exploration. Geochemical attributes such as organic richness, kerogen type and thermal maturity of shales are important parameters, which define a potential source rock and provide useful insights onto the geological processes under which the carbonaceous sediments got deposited and were well preserved, leading to generation of hydrocarbons. Organic geochemical parameters studied for the shales from some of the sedimentary basins of India are described below.
4.1 Vindhyan Basin
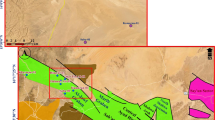
The Proterozoic Vindhyan basin consists of more than 5000 m thick sedimentary sequence, which is characterized by the presence of rich fossil assemblage, organic rich black shales containing microbial mats and stromatolitic limestones. The existence of Infra-Cambrian reservoir sequences, source–seal relationships and the widespread presence of carbonaceous shale and stromatolitic carbonate render promising opportunities for hydrocarbons in the basin (DGH 2014; Ojha 2012). The Vindhyan succession is divided into the Lower Vindhyan (Semri Group) which is overlain unconformably by the Upper Vindhyan (Kaimur, Rewa, and Bhander Groups) (Banerjee et al. 2002, 2006; Dutta et al. 2006; Sharma 2006; Sastri 1984). Evaluation of organic matter in the outcrop shale samples from the Semri and Kaimur Groups of Vindhyan basin was carried out using Rock Eval pyrolysis, wherein thirty-two rock samples from the Khachhuhar, Murlipahar, Banjari (Rohtas Formation, the Semri Group) and Amjhore (Bijaigarh Formation, the Kaimur Group) were studied for their organic richness and kerogen type (Fig. 3) (Dayal et al. 2014).
Geological map of the Vindhyan basin showing the outcrops of the Semri and Kaimur rocks along with the sample locations (after, Dayal et al. 2014)
The Total Organic Carbon (TOC) content in shales ranges between 0.04 and 1.43 %. The S1 (thermally liberated free hydrocarbons) values range between 0.01 and 0.09 mg HC/g rock (milligram hydrocarbon per gram of rock sample), whereas the S2 (hydrocarbons from cracking of kerogen) show the values between 0.01 and 0.14 mg HC/g rock. The HI and Tmax correlation, which is a modified form of van Krevelen diagram (Hunt 1996; Tissot and Welte 1984; van Krevelen 1961) is used here to determine the quality and maturity of kerogen from Semri and Kaimur Groups of rocks (Fig. 4). In general, Tmax values lower than 435 °C indicate immature stage of kerogen. Tmax values between 435 and 455 °C indicate “oil window” conditions (mature kerogen). Values between 455 and 470 °C are considered transitional. A Tmax higher than 470 °C represents the wet-gas zone and over mature kerogen (Peters 1986). The thermal maturity of the shales from Semri Group of rocks is between immature to mature range, whereas the shales from the Muralipahar and Banjari hills are thermally more mature compared to the Amjhore. The kerogen is characterized by Type III gas prone kerogen (Dayal et al. 2014).
Hydrogen Index (HI) versus Tmax (temperature at highest yield of S2) plot of shales from the Semri and the Kaimur Groups, Vindhyan basin (after, Dayal et al. 2014)
The Semri Group of rocks was deposited in marine environment and show profuse development of organo-sedimentary structure, the microbialites (Sharma 2006). These shales, as indicated by their Rock Eval data, suggest good to excellent gas generation potential (Dayal et al. 2014). The presence of elevated concentrations of adsorbed light gaseous hydrocarbons (methane, ethane, propane) in the near surface sediments of Vindhyan basin (Dayal et al. 2014) indicates that hydrocarbons have been generated in the subsurface. The adsorbed soil gas, CH4 (C1), C2H6 (C2), C3H8 (C3) and nC4H10 (nC4), concentrations measured in the soil samples from the eastern part of Vindhyan basin (Son Valley) vary from 0 to 186 ppb, 0 to 4 ppb, 0 to 5 ppb, and 0 to 1 ppb, respectively (Dayal et al. 2014; Ojha 2012). The light hydrocarbon concentrations (C1–C4) in near surface soils of the western Vindhyan basin around Chambal Valley have been reported to vary between 1–2547 ppb, 1–558 ppb, 1–181 ppb, 1–37 ppb and 1–32 ppb, respectively with high concentrations around Baran-Jhalawar-Bhanpur-Garot regions (Kumar et al. 2006). The microseepage of light hydrocarbons of thermal origin is indicated by the compound specific isotopic analysis of C1 and C2 compounds (−45.7‰ to −25.2‰ and −35.3‰ to −20.19‰; VPDB) (Fuex 1977; Dayal et al. 2014). The organically rich shales with sufficient thermal maturity might be the source for these hydrocarbons.
4.2 Krishna-Godavari Basin
The Krishna-Godavari (KG) on the eastern coast of India occupies an important place amongst the Gondwana basins. The basin evolved from the rifting of the Gondwanaland along the eastern continental margin of India during the Early Mesozoic (Sastri et al. 1981; Veevers 2004). Gondwana sediments along with the Tertiary sequences form rich source rocks, making KG one of the most promising petroliferous provinces of India. The Raghavapuram (R), Gollaplli (G), and Tirupati (T), form a dominant Cretaceous petroleum system R-G-T-R (!) with Razole (R) acting as basaltic caprock in the west of KG basin. Ten carbonaceous shales were collected from the exposed Raghavapuram Formation spanning the top, middle and bottom sections of the hillock, near Ramnujampuram village (Fig. 5) (Mani et al. 2012a, 2015). Organic geochemical studies comprising of TOC, Rock-Eval pyrolysis, and biomarker separation and analysis were carried on the Raghavapuram shales to understand their paleo and depositional environment and its implications toward hydrocarbon generation in the basin (Mani et al. 2012a, 2015).
Geological map of Krishna-Godavari basin showing the exposures of Raghavapuram shales (after, Gupta 2006)
The TOC content varies between 0.01 and 0.5 % in the shales. Rock Eval pyrolysis studies of the selected shales show the S1 values to range between 0.01 and 0.06 mg HC/g rock, whereas, the S2 values vary between 0.01 and 0.05 mg HC/g rock. The thermal maturity parameter, Tmax ranges between 302 and 497 °C. The hydrogen index (HI) is between 50 and 500 mg HC/g TOC. The HI versus Tmax plot for the majority of shales studied here indicate a Type III kerogen with immature to post mature stage of hydrocarbon generation (Fig. 6). Type-III kerogen with low H/C range (H/C = 0.7 to 1.0) generates primarily gas, condensates and some waxes and contains mostly condensed poly-aromatics and oxygenated functional groups, with minor aliphatic chains. The organic matter is mostly derived from terrestrial higher plants. This is in agreement with the paleoecological conditions which existed during the deposition of the Raghavapuram sediments (Bhalla 1968; Lakshminarayana 2002; Mani et al. 2015).
HI versus Tmax plot indicating the kerogen type in the Raghavapuram shale, KG basin (after, Mani et al. 2015)
The extractable organic matter from the Raghavapuram shales was used to study the polyaromatic and n-alkanes hydrocarbons. The PAH analysis on GC—showed the presence of naphthalene, acenapthene acenaphthylenes, phenanthrene, anthracene, fluoranthene, pyrene, benzoanthracene and chrysene; however the alkylated homologues are not present in significant measurable concentration (Table 2) (Mani et al. 2012a, 2015). Distribution of PAHs is often used as an indicator for depositional environment and lithology (Simoneit 1992; Simoneit and Fetzer 1996). Two types of PAHs, those derived from combustion and those sourced from land plant have been proposed (Killops and Massoud 1992). Phenanthrene and chrysene may arise in recent sediments through both combustion and diagenetic processes, probably from terpenoids (Jiang et al. 1998). The high phenanthrene and chrysene concentrations observed in the Raghavapuram shales indicate high terrestrial source organic matter input. Benzoanthracenes, acenapthenes and acenapthylenes are present in three samples in significant concentrations. These compounds are present naturally in bituminous fossil fuels, such as coal and crude oil deposits, as a result of diagenesis, which involves low temperature (100–150 °C) transformation of organic material over a significant span of time.
The Raghavapuram shales are characterized by the presence of n–C11–C18, including n-tricosane and phytanes (Table 3) (Mani et al. 2012a, 2015). Alkane biomarkers have been widely applied to study the source, paleoenvironmental conditions and maturity of sedimentary organic matter (Allan and Douglas 1977; Philip 1985a, b). The n-alkanes from the organic matter in sedimentary rocks originate from lipids in bodies of plants and animals. The source of organic input in sediments can be inferred through the odd and even predominance of n-alkanes (Peters and Moldowan 1993; Peters et al. 2005). Input of terrigenous source material is indicated by a strong predominance of high molecular weight odd-numbered alkanes (n–C25 to n–C31), which are associated with leaf waxes (Brooks and Smith 1967). The n-alkanes, C11 to C23, obtained in the organic matter extracts from the Raghavapuram shales indicate marine inputs to the sedimentary organic matter. Pristane, phytane (Pr/Ph) ratio has been proposed as an indicator of redox potential of source sediments by Didyk et al. (1978). It has been widely utilized in many studies to infer oxicity or anoxicity of depositional environments and source of organic matter. The mass spectra of the extracted organic matter from Raghavpuram shales show the occurrence of phytanes, indicating the oxygen low conditions during the deposition and preservation of these sediments (Mani et al. 2015).
The organic geochemical proxies indicate the source organic matter to be derived from near-shore terrestrial environment and its deposition in strongly reducing conditions. The organic matter richness and maturity derived from a favourable depositional setting has its bearing upon the Gondwana sediments globally, and also provides promising petroleum exploration opportunities, particularly in the Raghavapuram sequence of the KG basin (Mani et al. 2012a, 2015).
4.3 Kutch Basin
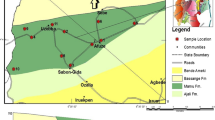
The Kutch basin in the western margin of Indian subcontinent encompasses one of the best developed and well preserved Cenozoic sequences comprising of organic rich Tertiary shales. The basin has identified hydrocarbon prospectivity (DGH 2014; Patil et al. 2013). Cenozoic sequences comprising of lignite and interbedded carbonaceous shales occur as discrete mappable units in the north western part of Kutch, chiefly at Panandhro, Akrimota, Umarsar Mata-no-Madh, Lakhpat-Dhedhadi in Lakhpat Taluka (Biswas 1992; Dutta et al. 2011). The study area forms part of a condensed stratigraphic section of ~900 m thickness, ranging from Paleocene to Pliocene and is exposed in open cast mines of Mata-no-Madh, Panandhro and Umarsar (Fig. 7). Twenty five shale samples were collected from the interbedded shale horizons within the lignite sequences to determine the TOC content and kerogen type in the shales using Rock-Eval pyrolysis (Misra et al. 2013a; Dayal et al. 2013).
Map of Kutch basin along with the location of lignite mines (after, Misra et al. 2013a)
The Total Organic Carbon (TOC) content from Mata-no-Madh shales ranges between 0.35 and 30.99 %. The S1 values range between 0.01 and 3.71 mg HC/g. The S2 values range between 0.02 and 74.01 mg HC/g. The Tmax values lie between 383 and 428 °C respectively. The hydrogen index (HI) is ranging in values between 3 and 312 mg HC/g TOC, where as the oxygen index (OI) shows variegated values ranging between 18 and 151 mg CO2/g TOC (Misra et al. 2013a). For the Umarsar shales, the TOC varies between 0.79 and 25.43 %. The S1 values are between 0 and 3.05 mg HC/g Rock and the S2 values range between 0.24 to 91.13 mg HC/g Rock. The Tmax varies between 421 and 426 °C, whereas the HI values vary between 30 and 258 mg HC/g TOC. The OI values range between 29 and 58 mg CO2/g TOC (Misra et al. 2013a).
The Panandhro shales shows TOC content ranging between 1.08 and 1.35 %. The S1 values range between 0.05 and 0.34 mg HC/g Rock, S2 values between 0.65 and 1.56 mg HC/g Rock. Tmax varies between 423 and 452 °C. The HI and OI values range between 53–116 mg HC/g TOC and 40–214 mg CO2/g TOC respectively. The mineral carbon content lies between 0.09 and 3.96 % (Misra et al. 2013a).
The gas generation potential of the shales depends upon the organic richness, basically TOC, and its thermal maturity. A TOC content (wt%) <0.50 is considered poor; 0.50–1.0 as fair; 1.0–2.0 as good and that >2.0 as excellent for the source rocks (Hunt 1996; Peter and Cassa 1994). In general, the shales from Mata-no-Madh and Umarsar area have TOC >2 %. These values indicate good source rock potential.
The Tertiary shales from the Kutch basin show a higher hydrogen index from the samples belonging to the Mata-no-Madh and Umarsar areas, where as the Panandhro shales show comparatively lower HI values (Misra et al. 2013a; Dayal et al. 2013). The HI values vary linearly in positive direction with the TOC content, whereas the OI values show a negative correlation. This is an accepted trend for the variation of hydrogen and oxygen indices with the TOC for hydrocarbon source rocks which are deposited in low oxygen conditions and having well preserved organic matter (Tissot and Welte 1984). The HI versus OI correlations for the shales from Mata-no-Madh and Umarsar shows that the kerogen is characterized by Type-II/Type-III and Type-III kerogen, whereas that of Panandhro shales show Type-III kerogen.
The HI versus Tmax (pyrolysis temperature) plot for the shales from the Kutch Basin indicates the kerogen to be characterized by Type-II/III to Type-III kerogen (Fig. 8) (Misra et al. 2013a; Dayal et al. 2013). The Tertiary shales from the Kutch Basin show an immature stage for the kerogen towards hydrocarbon generation and could possibly be in early diagenetic stage. These shales can be thermally mature with suitable hydrocarbon generation potential if buried deeply elsewhere in the basin. Naredi Formation is reported to have a subsurface extent of about 40 m (Biswas 1992). The subsurface extent of the Formation containing the shale horizons and the petrological and petrophysical details along with the organic geochemical attributes of the shales on subsurface core samples shall help in precise delineation of horizons for the shale gas plays.
HI versus Tmax plot for the shales from Matanomadh, Panandhro and Umarsar, showing the maturity (after, Misra et al. 2013a)
4.4 Cambay
The petroliferous Cambay basin in western India with interbedded carbonaceous shales in its thick Tertiary sequence forms a potential prospect for the shale gas. Organic-rich, fine grained clastic Cambay, Tarapur and other Tertiary shales have sourced the oil and gas for the basin. The interbedded shale formations within the Middle Eocene lignite sequences, referred to as Cambay Formation, (Nagori et al. 2013), in the Tadkeshwar and Rajpardi mines of Surat and Bharuch districts (Fig. 9), respectively, have been sampled to study the organic matter properties (Misra et al. 2013b; Dayal et al. 2013). In general, the shales from Rajpardi area show a high TOC content along with other Rock Eval parameters compared to that of Tadkeshwar. The TOC content from Rajapardi shales ranges between 9.35 and 26.03 %. The S1 values range between 3.29 and 7.12 mg HC/g rock and are characteristic of the entire sample suit. The S2 values show an elevated range between 60.6 and 190.11 mg HC/g Rock. The Tmax ranges between 429 and 435 °C. The HI is high ranging in values between 648 and 754 mg HC/g TOC, whereas OI for all studied samples is near to 15 mg CO2/g TOC. The mineral carbon content is <0.5 %. The organic matter in shales is characterized by Type II kerogen, suitable for the generation of oil and gas (Fig. 10a) (Misra et al. 2013b).
Geological sample location map of lignite mines in Gujarat (after, Misra et al. 2013b)
For the Tadkeshwar shales, the TOC varies between 0.19 and 47.39 %. The S1 values are between 0.05 and 3.58 mg HC/g Rock and the S2 values range between 0.14 and 78.84 mg HC/g rock. The Tmax varies between 342 and 450 °C, whereas the HI values vary between 33 and 241 mg HC/g TOC. The variation of HI versus Tmax suggests an immature to mature stage for the hydrocarbons. The organic matter in shales is characterized by Type III kerogen, suitable for the generation of gas (Fig. 10b) (Misra et al. 2013b).
4.5 Jammu and Kashmir
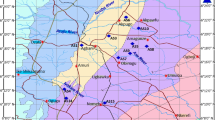
The Himalayan Foreland basin is considered prospective for hydrocarbon generation and entrapment, owing to its favorable tectonics and geological setting (DGH, 2014). The Karewa and Spiti-Zanskar basins are categorized potentially prospective due to their analogy with similar hydrocarbon producing basins of the world (Jokhan Ram 2005; DGH 2014). Organic richness and kerogen properties of the Eocene Subathu shales obtained from the outcrops and underground mines of Jammu region have been evaluated using Rock Eval pyrolysis (Mani et al. 2014). Twenty-nine shales/coaly shales and few coals were collected from the interbedded shale horizons of underground mines in coal fields of Kalakot, Kotla and Mahogla and outcrops at Salal, Kanthan and Kalimitti areas of Jammu for the organic matter characterization (Fig. 11).
Geological map of Jammu region showing the sample collection points for the Subathu shales (after, Mani et al. 2014)
The Eocene shales/coaly shales show quite high Total Organic Carbon (TOC) content ranging from 3.2 to 77.8 %. The S1 values range from 0.01 to 2.6 mg HC/g rock. S2 shows an elevated value ranging from 0.51 to 71.62 mg HC/g rock. The HI ranges between 2 and 113 mg HC/g TOC, where as the oxygen index (OI) for all studied samples is low (<30 mg HC/g CO2). A modified van Krevelen diagram (van Krevelen 1961) indicates that organic matter is characterized by Type III kerogen. The Tmax of the shale samples ranges from 490 to 515 °C suggesting a post mature phase for the hydrocarbons (Fig. 12). The coals and coaly shales have TOC content >30 %, and Tmax above 550 °C suggesting high levels of maturity as compared to the carbonaceous shales. The organic matter in majority of Subathu samples consists of Type III kerogen and has generation potential for the gaseous hydrocarbons (Fig. 13).
HI versus Tmax plot indicating the thermal maturity of kerogen in the Subathu shales, Jammu (after, Mani et al. 2014)
Source rock characteristics as interpreted by the relationship between remaining hydrocarbon potential (S2) and TOC (wt%) for the Subathu shales from Jammu (after, Mani et al. 2014)
Stratigraphic equivalents of Subathu, the Lower Dharamsala Formation of Himachal Pradesh, indicated gas shows during exploratory drilling (Karunakaran and Rangarao 1979; DGH 2014). The gases of Jwalamukhi and Nurpur wells are methane rich with low nitrogen concentration and are dry and thermogenic in nature (C2+ < 2 %) (Mittal et al. 2006). Carbon isotopic composition of methane from these wells suggest a deep over mature source for the gases (δ13C1 ~ −32.0‰) (Mittal et al. 2006). The knowledge of the distribution, facies, and thickness of the Subathu Group shale put together with petro-physical and lithologic properties and stratigraphic heterogeneity due to faults and fractures can provide criteria for precise defining of the gas shale horizons.
5 Quantitative Approach to Petroleum Potential of a Basin
A mathematical model of petroleum generation based on kinetics of kerogen degradation and utilizing the general scheme of kerogen evolution explicitly for geological time was proposed by Tissot (1969), Tissot and Espitalie (1975). The primary cracking of kerogen is based on several parallel first order reactions obeying Arrhenius law (Fig. 2; Eq. 1). The kinetic parameters (Ei, A, q0i) in these reactions can be determined by optimising the pyrolysis parameters obtained by the open system pyrolysis, such as that of Rock Eval.
An example of quantitative estimates on hydrocarbon generation from the pyrolysis study of Tertiary shales from the Kutch basin is presented through the use of optimization program OPTKIN and Rock Eval parameters—S2, HI, Time, Temperature and heating rate of pyrolysis (5, 15, 30 °C). The representation, for a kerogen, of its activation energies (E1, E2, …, Ei) of cracking is described through a histogram in Fig. 14. With increasing depth and temperature, the various bonds corresponding to successive activation energies are broken, roughly in order of increasing energies. A Type II/III kerogen has a wide distribution of activation energies which are generally unsymmetrical, due to the complex nature of kerogen with lesser aliphatic components and large quantity of aromatic structures.
For each pyrolysis curve used for optimization, a comparison is obtained between:
(i) measured and computed petroleum potential HI (in mg/g TOC); (ii) measured and computed S2 peak temperature Tmax (in deg. C); (iii) measured and computed amounts Q of generated hydrocarbon (in mg/g TOC, from 0 to HI); (iv) measured and computed Hydrocarbon generation rates dQ/dt (in mg/g TOC/deg. C); (v) measured and computed transformation ratios TR from Kerogen to Hydrocarbon (in %, from 0 to 100 %); TR = Q/HI. The amount of generated hydrocarbons Q, (Eq. 2) obtained from the computation and optimization of open system pyrolysis parameters of the Tertiary shales is shown in Fig. 15. A good match between the measured and computed values of the generated hydrocarbons, at a maximum value of 180 mg HC/g TOC, is obtained within temperature range between 380 and 490 °C.
The principle use of such calibrated kinetic models of thermal cracking of kerogen into hydrocarbons is that along with thermal and burial history data of a basin, 1D geological basin models can be created, which provide quantitative estimates on oil and gas generated as a function of time. These models help in determination of the timing of petroleum formation for comparison with the age of structural or stratigraphic traps and in general, offer a holistic approach towards evaluation of hydrocarbon potential of a basin.
6 Summary
Organic geochemical characterization of varied age shales from the Vindhyan, Krishna-Godavari, Kutch, Cambay and Jammu and Kashmir basins indicate high TOC content, with dominantly type III kerogen in immature to post mature stage, depending upon the depositional and thermal history of the respective basin. The Raghavapuram shales along with its stratigraphic equivalents, is inferred to be main source rock for much of the prolific KG basin. The extractable organic matter comprising of n-alkanes and PAH, along with the kerogen pyrolysis studies suggest promising petroleum opportunities for the Raghavapuram sequence. Amongst the frontier basins, the Semri and Kaimur Group shales from the Vindhyan basin show a gas prone Type III kerogen, where as the Tertiary shales from the Kutch and Cambay basin show organic rich, mixed Type II and III kerogen. The Subathu shales from Jammu show a post mature Type III kerogen suitable for a late metagenetic gas generation. The frontier basins are characterized by varied geology, age, tectonics, and depositional environments and the potential source rock data controls, in particular, are poor. The studied organic geochemical parameters can be used to map the pod of potential/active source rocks, regional variation in organic facies, and volume of generated hydrocarbons, which are critical in defining a basin’s hydrocarbon reserve.
References
Allan J, Douglas AG (1977) Variations in the content and distribution of n-alkanes in a series of carboniferous vitrinites and sporinites of bituminous rank Geochim. Cosmochim Acta 41:1223–1230
Banerjee A, Pahari S, Jha M, Sinha AK, Jain AK, Kumar N, Thomas NJ, Misra KN, Chandra K (2002) The effective source rocks in the Cambay basin. India. AAPG Bull 86(3):433–456
Banerjee S, Dutta S, Paikaray S, Mann U (2006) Stratigraphy, sedimentology and bulk organic geochemistry of black shales from the Proterozoic Vindhyan Supergroup, Central India. J Earth Syst Sci 115(1):37–47
Behar F, Penteado V, Beaumont HL, De B (2001) Rock-Eval 6 technology: performances and developments. Oil Gas Sci Technol—Rev IFP 56(2):111–134
Bhalla SN (1968) Paleoecology of the Raghavapuram Shales (Early Cretaceous), East Coast Gondwanas, India. Palaeogeogr Palaeoclimatol Palaeoecol 5(4):345–357
Bhowmick PK, Misra R (2012) Indian oil and gas potential. In: Banerjee DM, Singhvi AR (eds) Proceedings of the INSA glimpses of geoscience research in India, vol 78, pp 218–228
Biswas SK (1992) Tertiary stratigraphy of Kutch. J Palaeontol Soc India 37:1–29
Brooks JD, Smith JW (1967) The diagenesis of plant lipids during the formation of coal, petroleum and natural gas-I. Changes in the n- paramhydrocarbons. Geochim Cosmochim Acta 31:2389–2397
Chandra K, Raju DSN, Bhandari A, Mishra CS (2001) Petroleum systems in the Indian sedimentary basins: stratigraphic and geochemical perspectives. Bull Oil Nat Gas Corp 38(1):1–45
Dayal AM, Mani Devleena, Mishra Snigdharani, Patil DJ (2013) Shale Gas prospects of the Cambay Basin, Western India. Geohorizon 18(1):26–31
Dayal AM, Mani D, Madhavi T, Kavitha S, Kalpana MS, Patil DJ, Sharma M (2014) Organic geochemistry of Vindhyan sediments: implications for hydrocarbons. J Asian Earth Sci 91:329–338
Didyk BM, Simoneit BRT, Brassell SC, Eglinton G (1978) Organic geochemical indicators of palaeoenvironmental conditions of sedimentation. Nature 272:216–222
Directorate General of Hydrocarbons (DGH) (2014) Retrieved from www.dghindia.org
Dutta S, Steiner M, Banerjee S, Erdtmann B, JeevanKumar S, Mann U (2006) Chuaria circularis from the early Mesoproterozoic Suket Shale, Vindhyan Supergroup, India: insights from light and electron microscopy and pyrolysis–gas chromatography. J Earth Syst Sci 115(1):99–112
Dutta S, Mathews RP, Singh BH, Tripathi SM, Singh A, Saraswati PK, Banerjee S, Mann U (2011) Petrology, palynology and organic geochemistry of Eocene lignite of Matanomadh, Kutch Basin, Western India: Implications to depositional environment and hydrocarbon source potential. Int J Coal Geol 85:91–102
Eden H, Mungo D (2013) Geochemistry-the dark horse upstream. GeoExpro 10(1):22–25
Espitalie J, Marquis F, Sage L (1987) Organic geochemistry of the Paris Basin. In: Brooks J, Glennie K (eds) Petroleum geology of north west Europe. Graham and Totman, London, pp 71–86
Fuex AN (1977) The use of stable carbon isotope in hydrocarbon exploration. J Geochem Explor 7:155–188
Grob RL (2004) Theory of chromatography. In: Grob RL, Barry EF (eds) Modern practice of gas chroamtography, 4th edn. Willy, New York, 743p
Gupta SK (2006) Basin architecture and petroleum system of Krishna-Godavari basin, east coast of India. Lead Edge 25(7):830–837
Horvitz L (1985) Geochemical exploration for petroleum. Science 229(4716):821–827
Hunt JM (1996) Petroleum geochemistry and geology, 2nd edn. W.H. Freeman and company, USA, 715p
Jiang C, Alexander R, Kagi RI, Murray AP (1998) Polycyclic aromatic hydrocarbons in ancient sediments and their relationships to palaeoclimate. Org Geochem 29(5–7):1721–1735
Jokhan Ram J (2005) Hydrocarbon exploration in onland frontier basins of India: perspective and challenges. J Palaeontol Soc India 50(1):1–16
Jones RW, Demaison GJ (1982) Organic facies-stratigraphic concept and exploration tool. In: Salvidar-Sali A (ed) Proceedings of the second ASCOPE conference and exhibition, Manila, Oct 1981, pp 51–68
Jones RW (1983) Organic matter characteristics near the shelf-slope boundary. Soc Econ Paleontol Mineral (SEPM) 33:391–405 (special publication)
Karunakaran C, Ranga Rao A (1979) Status of exploration for hydrocarbon in the Himalayan region—contributions to stratigraphy and structure. In: Proceeding of Himalayan geology seminar, 13–17 Sept 1979. Miscellaneous publications no 41, Part V, vol 1–6(6). Geological Survey of India, New Delhi
Killops SD, Massoud MS (1992) Polycyclic aromatic hydrocarbons of pyrolytic origin in ancient sediments: evidence for Jurassic vegetation fires. Org Geochem 18(1):1–7
Kumar B, Raju SV, Patil DJ, Kalpana G, Vishnu Vardhan C (2006) Integrating surface geochemical expressions with geological, geophysical and remote sensing data: case histories from selected Proterozoic Basins of India. http://www.searchanddiscovery.com/documents/2006/06088houston_abs/abstracts/kumar.htm, downloaded on 16 Dec 2012
Lafargue E, Marquis F, Pillot D (1988) Rock-Eval 6 applications in hydrocarbon exploration, production and soil contamination studies, vol 53(4). In: Juillet-Août (1998). IFP, Pondicherry
Lakshminarayana G (2002) Evolution in Basin Fill Style during the Mesozoic Gondwana Continental break-up in the Godavari Triple Junction, SE India. Gondwana Res 5(1):227–244
Mani D, Patil DJ, Dayal AM (2011a) Stable carbon isotope geochemistry of near surface adsorbed alkane gases in Saurashtra basin. Chemical Geology 280(1–2):144–153
Mani D, Dayal AM, Rasheed MA, Satish Kumar T, Rao TG, Balaram V (2011b) Soil Iodine determination in Deccan Syneclise Basin of India: implications for near surface geochemical hydrocarbon prospecting. Nat Resour Res 20(1):75–88
Mani D, Ratnam B, Patil DJ, Kalpana MS, Dayal AM (2012a) Geochemical proxies for the interpretation of sedimentary paleoenvironment: preliminary results from Krishna Godavari basin, India. In: IGCP-3rd annual symposium response of asian rivers to climate change—past, present and future scenario, 14–16 Nov 2012. CSIR-National Geophysical Research Institute, Hyderabad
Mani D, Patil DJ, Kalpana MS, Dayal AM (2012b) Evaluation of hydrocarbon prospects using surface geochemical data with constraints from geological and geophysical observations in Jamnagar area, Saurashtra Basin, India. J Pet Geol 35(1):67–84
Mani D, Patil DJ, Dayal AM (2013) Evolution of sedimentary organic matter and formation of oil and gas: a geochemical approach. In: International seminar on earth science for society, 7–8 Dec 2012. CSIR-National Geophysical Research Institute, Hyderabad
Mani D, Dayal AM, Patil DJ, Hafiz M, Hakoo N, Bhat GM (2014) Gas potential of Proterozoic and Phanerozoic shales from NW Himalaya, India: inferences from new pyrolysis data. Int J Coal Geol 128–129:81–95
Mani D, Ratnam B, Patil DJ, Kalpana MS, Dayal AM (2015) Elemental and organic matter characteristics of the Gondwana sediments from the Krishna–Godavari Basin, India. Chemie der Erde-Geochemistry (accepted)
Misra SR, Mani D, Kavita S, Kalpana MS, Patil DJ, Vyas DU, Dayal AM (2013a) Organic matter characteristics and gas generation potential of the Tertiary shales from the NW Kutch basin, India. J Pet Sci Eng (under review)
Misra SR, Mani D, Kavita S, Patil DJ, Kalpana MS, Dayal AM (2013b) Pyrolysis results of shales from the South Cambay basin, India: implications for gas generation potential. J Geol Soc India (accepted article)
Mittal AK, Pandey HC, Singh RR (2006) Geochemistry of gas seeps from surface shows and wells of the Himalayan foreland Basin. In: Proceedings of 6th international conference and exposition on petroleum geophysics, Kolkata, pp 235–291
Nagori ML, Khosla SC, Jakhar SR (2013) Middle Eocene Ostracoda from the Tadkeshwar Lignite Mine, Cambay Basin, Gujarat. JGeol Soc India 81:514–520
Ojha PS (2012) Precambrian sedimentary basins of India: an appraisal of their petroleum potential. In: Bhat G, Craig J, Thurow JW, Thusu B, Cozzi A (eds) Geology and hydrocarbon potential of Neoproterozoic–Cambrian Basins in Asia, vol 366. Geological Society, London. doi: 10.1144/sp366.11 (special publications)
Patil DJ, Mani D, Madhavi T, Sudarshan V, Srikarni C, Kalpana MS, Sreenivas B, Dayal AM (2013) Near surface hydrocarbon prospecting in Mesozoic Kutch sedimentary basin, Gujarat, Western India–A reconnaissance study using geochemical and isotopic approach. J Pet Sci Eng 108:393–403
Peters K (1986) Guidelines for evaluating petroleum source rocks using programmed pyrolysis. Am Assoc Pet Geol 70:318–329
Peters KE, Cassa MR (1994) Applied source rock geochemistry. In: Magoon LB, Dow WG (eds) The petroleum system—from source to trap: Tulsa, Okla. Am Assoc Pet Geol Memoir 60:93–117
Peters KE, Moldowan JM (1993) The biomarker guide—interpreting molecular fossils in petroleum and ancient sediments. Prentice Hall, New Jersey
Peters KE, Walters CC, Moldowan JM (2005) The biomarker guide: biomarkers and isotopes in the environment and human history, vol 1. Cambridge Press, Cambridge
Philp RP (1985a) Fossil fuel biomarkers applications and spectra. Elsevier, The Netherlands
Philp RP (1985b) Biological markers in fossil fuel production. Mass Spectrom Rev 4:1–54
Platzner IT (1997) Modern isotope ratio mass spectrometry. Wiley, Chichester
Sastri MVA, Moitra AK (1984) Vindhyan stratigraphy—a review, memoir. Geol Surv India 116(II):109–148
Sastri VV, Venkatachala BS, Narayanan V (1981) The evolution of the east coast of India. Palaeogeogr Palaeoclimatol Palaeoecol 36:23–54
Sharma M (2006) Late Proterozoic (Statherian) carbonaceous film from the olive (Koldaha shale), Semri Group, Vindhyan Supergroup, India. J Paleontol Soc India 51(2):27–35
Simoneit BRT, Fetzer JC (1996) High molecular weight polycyclic aromatic hydrocarbons in hydrothermal petroleums from the Gulf of California and Northeast Pacific Ocean. Org Geochem 24:1065–1077
Simoneit BRT (1992) Natural hydrous pyrolysis-petroleum generation in submarine hydrothermal systems. In: Whelan JK, Farrington JW (eds) Productivity, accumulation and preservation of organic matter in recent and ancient sediments. Columbia University Press, New York, pp 368–402
Sneddon J, Masuram S, Richert JC (2007) Gas chromatography-mass spectrometry-basic principles, instrumentation and selected applications for detection of organic compounds. Anal Lett 40(6):1003–1012. doi:10.1080/00032710701300648
Tissot B (1969) Premières données sur les mécanismes et la cinetique de la formation du pétroledans les sédiments. Simulation d’un schéma réactionnel sur ordinateur (First data on the mechanisms and kinetics of the formation of petroleum in sediments. Simulation of a reaction scheme on a computer) Revue de Institut Français du Pétrole, vol 24, pp 470–501
Tissot BP, Welte DH (1984) Petroleum formation and occurrence. A new approach to oil and gas exploration. Springer, Berlin, p 538pp
Tissot B, Espitalie J (1975) L’evolution thermique de la matiere organique des sediments; applica-tions d’une simulation mathematique; Potentiel petrolier des basins sedimentaires et reconstitu-tion de l’histoire thermique des sediments: Revue de l’Institut Francais du Petrole et Annales des Combustibles Liquides, vol 30, pp 743–777
Van Krevelen DW (1961) Coal: typology-chemistry-physics-constitution. Elsevier Science, Amsterdam
Veevers JJ (2004) Gondwanaland from 650–500 Ma assembly through 320 Ma merger in Pangea to 185–100 Ma breakup: supercontinental tectonics via stratigraphy and radiometric dating. Earth Sci Rev 68:1–132
Acknowledgments
The authors acknowledge Oil Industry Development Board, New Delhi for providing financial aid in setting up of the laboratory. The Director, NGRI is acknowledged for permitting the publication of this work. DM acknowledges CSIR for the Senior Research Associate ship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mani, D., Patil, D.J., Dayal, A.M. (2015). Organic Properties and Hydrocarbon Generation Potential of Shales from Few Sedimentary Basins of India. In: Mukherjee, S. (eds) Petroleum Geosciences: Indian Contexts. Springer Geology. Springer, Cham. https://doi.org/10.1007/978-3-319-03119-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-03119-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03118-7
Online ISBN: 978-3-319-03119-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)