Abstract
Two patients are here presented with symptomatic stenosis of anastomosis after esophageal resection for cancer. Previous to the operation both patients were treated by neoadjuvant therapy. Therapeutic options are extensively treated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
First Patient (Intrathoracic Anastomosis)
Diagnosis and Indication for Surgery
A 60-year-old female was seen in the outpatient clinic because of dysphagia and 10 kg weight loss. Endoscopic and pathologic evaluation revealed squamous cell carcinoma of the distal esophagus. Further investigation by CT scan, endoscopic ultrasound together with PET-CT revealed a T2N0M0 squamous cell carcinoma of the esophagus. She was treated with neoadjuvant chemotherapy. No concurrent radiotherapy was given because of previous radiation for treating breast cancer. Chemotherapy was followed by curative esophagectomy.
Operation
An Ivor Lewis procedure included laparoscopy and thoracoscopy to create the gastric conduit, a dissection of the intrathoracic esophagus, engagement of a two-field lymphadenectomy, and intrathoracic anastomosis. Conversion to thoracotomy was needed because of adhesions in the right thorax probably due to pneumonia a few weeks before surgery. The intrathoracic anastomosis was performed end-to-side by means of a 25 mm circular stapler.
Pathology
Radically resected T2N0 adenocarcinoma of the esophagus.
Postoperative Course: Identification and Treatment of the Complication
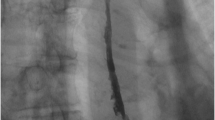
During the postoperative course, the patient suffered from dysphagia because of pyloric spasm, which was treated with a 20 mm balloon dilatation. After this dilatation, she could increase her oral intake and was subsequently discharged with combined feeding (oral and by jejunostomy). The jejenunostomy could be retired 4 weeks later because of optimal oral intake. Yet, 6 months later, she was suffering from progressive dysphagia and lost 3 kg of her weight. Swallow X-ray showed a relative stenosis at the site of anastomosis of the esophagus, treated by endoscopic dilatation (Fig. 5.1). Five dilatations with Savary dilatators up to 20 mm were needed to successfully treat the stenosis. After the last dilatation, the patient could swallow normally and regained weight. No more dilatations were needed.
Discussion
The major reason for midterm morbidity after esophagectomy with esophagogastric anastomosis is the development of benign anastomotic strictures [1]. An incidence of 48 % for hand sewn anastomoses and 35 % for semi-mechanical anastomoses in the neck is described [2]. It is important to distinguish two types of anastomotic strictures: the early strictures responding to few dilatations without restenosis and the so-called refractory stenosis, where over ten dilatations are needed on a frequent basis to treat and to avoid restenosis. Risk factors for refractory stenosis are anastomotic leakage, neoadjuvant chemoradiotherapy, and diagnosis of the stricture within 90 days after surgery [1]. In the above-mentioned case there was no leakage of the anastomosis in the postoperative period. The patient had received neoadjuvant chemotherapy only because of previous radiotherapy for breast cancer. Chemotherapy alone has not been mentioned as a risk factor for stenosis. Strictures developed over 1 year postoperatively are usually malignant strictures [3].
Cervical anastomoses are described to be risk factors for benign strictures [3]. However, a recent review showed no differences in anastomotic strictures between cervical and intrathoracic anastomosis [4].
We conclude that despite the improvement of survival after esophagectomy over the last years, the long-term complications still importantly influence the quality of life of these patients. More focus is needed on the improvement of the esophagogastric anastomosis techniques in order to decrease postoperative anastomotic complications.
Second Patient: Benign Stenosis of the Cervical Esophagogastric Anastomosis After Esophagectomy
Diagnosis and Indication for Surgery
A 62-year-old female presented to the gastroenterologist with severe dysphagia for liquids. Nine years before she had undergone a laparoscopic cardiomyotomy for achalasia, which was converted to a laparotomy due to perforation of the stomach. No fundoplication was added to the procedure. The years following, she underwent several dilatations for recurrent dysphagia and peptic stenosis of the distal esophagus. Manometry showed a hypotensive lower esophageal sphincter and 100 % simultaneous contractions of the esophagus. A barium swallow showed a dilated esophagus (but no mega- or sigmoid esophagus) and slow passage of contrast across the esophagogastric junction after 10 min. Endoscopy showed no peristalsis of the esophagus, stasis of gastric juice, and the esophagogastric junction could be easily passed. No Z-line could be determined, and there were no signs of hiatal hernia or esophagitis. After discussing all the surgical treatment options with the patient, she was determined to undergo an esophagectomy with a gastric tube reconstruction in order to relieve dysphagia in a definitive way, and the endosopic surveillance of the esophagus could be abandoned.
Operation Description
An open transhiatal esophagectomy was performed with an end-to-end esophagogastrostomy with PDS 3/0 running suture.
Postoperative Course: Identification and Treatment of the Complication
The postoperative course was uncomplicated and the patient was discharged on day 7.
Seven weeks after discharge, the patient reported dysphagia for solids and liquids; subsequently, an endoscopy showed at 20 cm from the incisors a stenosis of the anastomosis with edema and inflammation. At week 10, she underwent a dilatation over a guidewire with Savary dilators up to 9 mm (Fig. 5.2). The weeks following, she underwent one to two weekly dilatations up to 16 mm, but the stenosis recurred and dysphagia persisted. At week 17, three longitudinal short incisions (precut) at the site of the persistent stenosis were made, followed 1 week later by a dilatation up to 16 mm (Fig. 5.3). This was again followed by four more endoscopic sessions in which precut and dilatation ware performed. Despite these interventions, symptomatic stenosis recurred within a few days following treatment. At week 30, another precut was done at three sites of the stenosis followed by dilatation up to 17 mm and injection of 1 cc steroids in the submucosa (Kenacort® 10 mg/mL) One more dilatation was performed 4 weeks later and since then the anastomosis remained wide open and easy to be passed with an endoscope (Fig. 5.4).
Discussion
This case shows that benign stenosis of the esophagogastrostomy can be refractory to repeated dilatation and incisional therapy. During the treatment the patient was intermittently unable to swallow solid foods and had remained on a liquid diet. Weight loss and decreased quality of life were reported by the patient and generate important issues to address. By adding a corticosteroid injection to the precut and dilatation session, we observed that the restenosis was resolved and the patient is well 2 years after surgery.
Incidence and Risk Factors for Benign Stenosis
Benign strictures of a cervical anastomosis occur frequently and incidence rates of 26–42 % are reported [1]. Strictures seem to be more common and more severe after gastric pull up when compared to colon interposition. Most strictures are becoming symptomatic and referred for treatment around 2–3 months after the surgery. Factors involved in the development of benign strictures are diverse: postoperative anastomotic leakage, neoadjuvant therapy, and a history of cardiac disease are reported to increase the risk. The location of the anastomosis (neck versus chest) does not seem to play a major role. But since anastomoses in the neck tend to leak more often this might induce anastomotic stricture with time. There are also some reports that relate stricture formation with the use of stapler devices and in particular with the size of the staple device employed [5]. A meta-analysis comparing hand-sewn and stapled anastomosis for the development of strictures however found no difference between the two techniques [6]. When performing a hand-sewn anastomosis, the end-to-side anastomosis is associated with a lower anastomotic stricture rate, compared to the end-to-end anastomosis. However, prevention of stricture formation was at high costs with increased anastomotic leakage and longer in-hospital stay [7].
Treatment
The preferred treatment of benign strictures is endoscopic mechanical dilatation, which is an established and safe treatment option (Illustration 5.1a,b). Most strictures respond well to dilatation and need three to eight dilatations for successful treatment. A proportion of patients with benign cervical strictures, however, suffer from a refractory stricture requiring more than ten and sometimes up to 30 dilatations. Possibly, patients at increased risk (neoadjuvant treatment, leakage, early stricturing [<90 days]) for refractory strictures might benefit from routine performance of endoscopy postoperatively and dilatation in an earlier stage. In case of complex strictures or simple strictures not responding to wire-guided bougie or through-the-scope balloon dilatation, one can consider other endoscopic treatment techniques such as intralesion steroid injection, incision therapy, argon plasma, or placement of a temporary self-expandable stent. Steroid injection in the stenosis combined with dilatation may be effective for refractory strictures with longer dilatation-free time intervals and decreased overall number of dilatations [8]. Incision therapy appeared to be effective—especially in short-segment anastomotic stenosis. Patients remained symptom-free during 1-year of follow-up after a single treatment session [9]. Patients with a longer-segment stenosis usually require more than one incision treatment. Whether this technique is better in combination with steroid injection in the stenosis or alone has not yet been clarified. The third option is placement of a temporary self-expandable stent, where the stent remains in place for 6–8 weeks. Long-term resolution of refractory stenosis up to 80 % has been described [10]. By placement of a plastic or fully covered metal stent, the stent can easily and non-traumatically be removed. However, stent migration remains a concern and is seen in up to 20 % of the patients.
Prevention
The use of a semi-mechanical side-to-side cervical anastomosis has been suggested for significantly reducing the development of strictures, possibly due to the larger cross-sectional surface area of the anastomosis [2, 11]. However, the drawback of this technique is that by using a linear stapler a longer esophageal remnant is needed in order to perform the side-to-side anastomosis. Therefore, patients with tumors located at the higher upper third of the esophagus might not be appropriate for this technique. Second, when the esophageal lumen is already obviously dilated at operation, it is not necessary to apply this technique for enlarging the anastomotic orifice further. Besides less stenosis, applying a semi-mechanical technique with use of the linear stapler also seems to decrease the leakage rates. Orringer has reported a decrease of clinical significant leaks from 10 to 15 % (manually sewn anastomosis) to 2.7 % after introduction of the linear stapling technique in over 100 patients [2].
A recent randomized study in 291 patients comparing the wrapping of the omental pedicle flap around the esophagogastric anastomosis with a stapled technique, reported only a decreased incidence of anastomotic leakage and strictures in the omental wrapping group [12]. The theory behind this phenomenon is that adhesions form between the omentum and the gastric tube, thereby helping to seal microscopic leaks and aid in tissue remodeling. The pool of histiocytes, monocytes, and granulocytes in the omentum may also contain the local infective process, thereby protecting the anastomosis. Gastric ischemic conditioning prior to esophagogastrostomy might improve gastric perfusion and hence decrease the rate of leakage and strictures [13]. But this has still to be proven in randomized controlled trials. There are more techniques and modifications of existing techniques described, such as the mucosal tube technique and supercharge technique. However, there is a paucity of trials that compare one with the other technique. The best surgical technique and location of the anastomosis (neck versus chest) therefore remains to be determined. Recently, it was hypothesized that benign anastomotic stricture formation is related to reflux of erosive gastric contents from the gastric tube. In the 40 patients randomized to receiving proton pump inhibitors (PPIs) after a circular stapled anastomosis in the chest, benign anastomotic strictures developed in 13 % as compared to 45 % of patients not on PPIs [14].
References
van Heijl M, Gooszen JA, Fockens P, et al. Risk factors for development of benign cervical strictures after esophagectomy. Ann Surg. 2010;251:1064–9.
Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg. 2000;119:277–88.
Sutcliffe RP, Forshaw MJ, Tandon R, et al. Anastomotic strictures and delayed gastric emptying after esophagectomy: incidence, risk factors and management. Dis Esophagus. 2008;21:712–7.
Biere SSAY, Maas KW, Cuesta MA, van der Peet DL. Cervical or intrathoracic anastomosis after esophagectomy for cancer: a systematic review. Dig Surg. 2011;28:29–35.
Dresner SM, Lamb PJ, Wayman J, et al. Benign anastomotic stricture following transthoracic subtotal oesophagectomy and stapled oesophago-gastrostomy: risk factors and management. Br J Surg. 2000;87:362–73.
Urschel JD, Blewett CJ, Bennett WF, et al. Handsewn or stapled esophagogastric anastomoses after esophagectomy for cancer: meta-analysis of randomized controlled trials. Dis Esophagus. 2001;14:212–7.
Nederhof N, Tilanus HW, Tran TC, et al. End-to-end versus end-to-side esophagogastrostomy after esophageal cancer resection: a prospective randomized study. Ann Surg. 2011;254:226–33.
Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointest Endosc. 2002;56:829–34.
Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc. 2006;63:157–63.
Repici A, Conio M, De Angelis C, et al. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc. 2004;60:513–9.
Xu QR, Wang KN, Wang WP, et al. Linear stapled esophagogastrostomy is more effective than hand-sewn or circular stapler in prevention of anastomotic stricture: a comparative clinical study. J Gastrointest Surg. 2011;15:915–21.
Dai JG, Zhang ZY, Min JX, Huang XB, Wang JS. Wrapping of the omental pedicle flap around esophagogastric anastomosis after esophagectomy for esophageal cancer. Surgery. 2011;149:404–10.
Varela E, Reavis KM, Hinojosa MW, Nguyen N. Laparoscopic gastric ischemic conditioning prior to esophagogastrectomy: technique and review. Surg Innov. 2008;15:132–5.
Johansson J, Oberg S, Wenner J, et al. Impact of proton pump inhibitors on benign anastomotic stricture formations after esophagectomy and gastric tube reconstruction: results from a randomized clinical trial. Ann Surg. 2009;250:667–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Maas, K., Wijnhoven, B.P.L., Spaander, M.C.W. (2014). Case on Benign Stenosis of the Intrathoracic and Cervical Esophagogastric Anastomosis After Esophagectomy. In: Cuesta, M., Bonjer, H. (eds) Case Studies of Postoperative Complications after Digestive Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-01613-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-01613-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-01612-2
Online ISBN: 978-3-319-01613-9
eBook Packages: MedicineMedicine (R0)