Abstract
This chapter presents an overview of recent contributions that show how fluid mechanics is drastically changing cancer research. The review will mainly focus on the computational modelling of fluid-mediated processes related to cancer dynamics, spanning different representation scales from cells to organs. Fluid mechanics seems to act as a fundamental organizing principle in many aspects of cancer, including its growth, progression, metastasis, and therapy. On the other hand, it is clear that fluid-dynamics modelling can make a huge contribution to many areas of experimental cancer investigation since there is now a wealth of data that requires systematic analysis. The relevance of microfluidics in the isolation, detection, molecular characterization, and migration of tumour cells is also discussed. In the last part of the chapter, future challenges and perspectives are briefly outlined.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microfluidic Device
- Circulate Tumour Cell
- Fluid Mechanic
- Dissipative Particle Dynamic
- Interstitial Pressure
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Cancer cannot be defined as just one disease, but rather as a broad group of more than 200 diseases. From the biological point of view, it is a complex phenomenon that can be characterized by a small set of hallmarks that point to a cascade of events from the molecular to the organismal level (Hanahan and Weinberg 2011). At the molecular level, cancer arises through a series of genetic mutations, which allow cells to grow and divide uncontrollably. An alteration of the DNA molecule can disrupt the genes and produce faulty proteins, causing the cells to become abnormal (or malignant) and lose their restraints on growth.
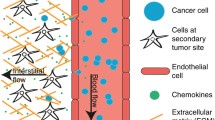
In healthy individuals, the immune system can recognize abnormal cells and destroy them before they get a chance to divide. However, some mutant cells may escape detection and survive to form a tumour (or neoplasm), which looks like a small ball of cells and feeds on oxygen and nutrients that diffuse to its surface. As cells in the core of the tumour become starved of oxygen (hypoxic cells), they release substances (growth factors) that stimulate the growth of new blood vessels; a process called angiogenesis (Dvorak et al. 1988). These angiogenic growth factors activate receptors present on endothelial cells in pre-existing blood vessels. The activated endothelial cells begin to release enzymes (proteases) that allow them to escape from the parent vessel walls. These then proliferate into the surrounding matrix and form solid sprouts connecting neighbouring vessels, which extend towards the tumour, supplying it with blood (Leung et al. 1989; Hanahan and Folkman 1996). For a while, the tumour grows as a cohesive ball of cells with smooth edges. However, eventually some rogue cells break away from the growing tumour and invade the adjacent tissue. This is a key process in the growth of most cancers and an escape route for metastasis—the formation of secondary tumours owing to spreading of cancer cells to more distant parts of the body through the lymphatic system or bloodstream. Metastasis is the main cause of deaths due to cancer (Sporn 1996). For example, as a cause of mortality in the United States, metastatic cancer is second only to heart disease, with one out of four deaths being from cancer.
Cancer invasion occurs through several important steps, involving the interplay between the cells themselves and their microenvironment (Liotta and Kohn 2001): reduction in or loss of cell-cell adhesion, cell adhesion to the extracellular matrix (the surrounding connective tissue), secretion of enzymes that digest the extracellular matrix, and movement (migration) of the cancer cells coupled with their proliferation. Cancer cells experience both self-adhesion (cell-cell adhesion) and cell-matrix adhesion, while cell movement through the surrounding tissue may occur through diffusion with no preferred direction and by directed motion due to the breakdown of the extracellular matrix components (Hanahan and Weinberg 2000; Friedl and Wolf 2003; Weinberg 2007).
Notwithstanding decades of research in cancer biology and medicine, our present ability to predict and treat metastatic cancer is still very limited. The main difficulty to reliably forecast the risk of cancer metastasis for individual patients stems from the fact that cancer itself is the result of a complex interplay between a large number of factors. While biological data continue to pile up at an enhanced rate, a major obstacle to progress lies precisely on how to handle this overwhelming flow of data. As a result of this difficulty, cancer research has commenced to undergo radical changes towards a more quantitative approach, where mathematical models are slowly making their way out as predictive tools using the parameters and information from state-of-the-art experiments. Integrating mathematics, physics, and mechanics with genomic investigations of cancer and its therapy opens a window towards a novel multidisciplinary approach, which encompasses biomathematics and computation, cancer biology, bioengineering, and imaging (Suresh 2007; Michor et al. 2011). Through the use of mathematical modelling and simulation software, this new approach to cancer research has the potential to predict prognosis, optimize surgical and pharmacological treatments for various cancers, and ultimately guide the design of novel therapeutics (Quaranta et al. 2005). For an extensive review on the novel mathematical tools applied to the modelling of cancer onset, evolution, and growth the reader is referred to Bellomo et al. (2008).
A branch of physics and engineering which is transforming the fight against cancer is fluid mechanics. As advocated by Koumoutsakos et al. (2013) in a recent review on the subject: after a century of rapid advances in theory, numerical methods, hardware, and software, the fluid mechanics community has developed a powerful arsenal of multiscale imaging, analysis, and simulation tools that are highly suitable for the investigation of transport processes in cancer. Fluid mechanics has been recognized to play an important role in most aspects of cancer, including tumour inception, growth, metastasis, and therapy. In this chapter, we review the most important contributions that project fluid mechanics as an essential organizing principle for cancer, spanning spatial scales from the gene to the organ and timescales of microseconds, as in gene mutations, to decades, as is pertinent to metastasis. We shall primarily focus on progress achieved in numerical simulation models of aspects of cancer that interface with fluid mechanics and discuss how significant future progress in the area is promising to change dramatically both the way experimental oncology is going on and our understanding of the processes involved from cancer initiation to metastasis and from the molecular to the patient level.
2 The Microscopic Level
The starting point of cancer is the generation of a neoplastic cell through phenotypic alterations, resulting from genetic mutations. However, this concept has not yet been well addressed through mathematical modelling, which so far has mainly focused on angiogenesis and invasion. After the onset of neoplasia, the characterization of the system suggests the identification of three natural scales, which are also connected to different stages of the disease, i.e., processes on the cellular scale (microscopic level) are triggered by signals stemming from the sub-cellular level and these have an impact on the macroscopic scale (organism), when tumours grow and spread.
On the microscopic (cellular) level, fluid-dynamic models have been proposed to simulate the effects of cell-cell interactions. These interactions are fundamental at all stages of tumour formation, whether they are among abnormal cells and host cells, or among abnormal cells themselves. If tumour cells skip recognition and suppression by the action of the immune system, the tumour may evade apoptosis or co-opt host cells, allowing progressive growth. During invasion and metastasis, alterations in cell-cell adhesion between individual tumour cells are key to driving the process. Existing experimental data suggests that tumour cell-adhesion to the endothelium under hydrodynamic shear rate—the change in flow velocity within the micro-capillaries—is a critical step that results in circulation-mediated metastasis (Liang et al. 2008, 2010; Fu et al. 2012).
The mechanism of cell-cell adhesion—a non-local interaction between two cells through transmembrane receptor binding—has naturally suggested the use of discrete cell approaches, which retain the finite cell size and permit incorporation of molecular interactions and/or forces that act between cells. A drawback of these approaches is the significant computational time required to simulate large populations. Therefore, it is desirable to augment such methodologies with continuous models that capture the dynamics of population-level behaviour. The past decade has witnessed the development of a wide variety of discrete models of increasing sophistication that incorporate cell adhesion, which can be classified into two major classes: lattice-based and lattice-free approaches. Examples of the former class include many cellular automata models (Deutsch and Dormann 2005; Moreira and Deutsch 2005) and discrete-continuum techniques (Anderson 2005; Anderson et al. 2006), where the discrete cells are allowed to interact with each other and surrounding continuous fields representing the extracellular matrix densities and growth factor concentrations. In particular, this latter approach has primarily been applied to models of tumour cell invasion, where some models have incorporated the effects of cell-adhesion, cell-migration, and phenotypic mutations (Anderson et al. 2006). These have suggested that invasive fingering is essentially driven by environmental heterogeneity. A spatially extended approach of the lattice-based class is the Cellular Potts Model, which has been adapted and applied to cell populations (Graner and Glazier 1992; Glazier and Graner 1993) and to simulation models of solid tumour growth (Turner and Sherratt 2002) and angiogenesis (Bauer et al. 2007).
In contrast to the above grid-imposed models, lattice-free models allow individual cells to move freely through continuous space. In a number of models of this type, cells are given variable, yet predefined, shapes such as deformable ellipsoids of fixed volume (Dallon and Othmer 2004; Palsson 2008). In more refined models, cells are allowed to shift between spheroidal and polyhedral shapes (Schaller and Meyer-Hermann 2005), or adopt continuously deforming shapes according to their interactions with neighbours and the environment (Newman 2005). Models have also been proposed in which individual cells are described as fluid-elastic structures in which their membrane is represented by a deformable boundary immersed in a fluid (Rejniak 2007; Dillon et al. 2008). In these models, adhesive forces are again represented by force balances that describe the movement and deformation of cells, while channels at their membranes permit the influx of fluid into them required for growth.
Hybrid models aimed at studying the adhesive rolling of leukocytes over a coated surface in parabolic shear flow in microchannels, where the immersed boundary method is used for cell deformation coupled with a Monte Carlo simulation for receptor/ligand interaction, have reproduced the characteristic “stop-and-go” motion of rolling leukocytes and the “tear-drop” shape of adherent leukocytes as observed in experiments (Pappu et al. 2008). A software environment capable of simulating blood flows on cellular scale inside microfluidic devices have been recently proposed, where the blood is modelled as a suspension of liquid blood plasma, immersed blood cells, and magnetic beads (Gusenbauer et al. 2011). The blood flow is represented on a fixed grid by solving the lattice-Boltzmann equations, while the boundary of each suspended object is represented by a set of discrete Lagrangian immersed boundary points that do not need to lie on the fluid grid. A direct application of this model in biomedicine is the use of self-organized magnetic bead chains to isolate circulating tumour cells employing lab-on-a-chip technologies (see Sect. 5 below).
3 Continuous Macroscopic Level
The body of literature devoted to models which link the cellular scale to the macroscopic tissue scale has increased at a high rate during the last few years. We foresee that this trend will continue as cancer research in the immediate near future will focus on refining and improving the existing models, allowing us not only to understand but also diagnose and treat cancer beyond our present technical abilities. While discrete models permit the straightforward incorporation of many intra-, extra-, and inter-cellular processes, they can require a formidable number of cells to describe the transition from the cellular to the tissue level, making the problem computationally intractable. On the other hand, discrete models often resist a thourough analytical investigation that can shed light on generic properties of the system under study. Both of these difficulties can be relaxed by considering continuum-scale models based on fluid-dynamic simulations with genetic and molecular elements, where cells are represented through their density at the tissue level and where relevant aspects of cancer such as tumour inception, growth, metastasis, and therapy that have direct relevance to flow-mediated processes can be thouroughly analyzed. In most of these models, events at the cellular scale are accounted for by the particular choice of terms and parameter functions that enter the governing evolution equations.
3.1 Tumour Onset, Growth, and Invasion
A cell becomes cancerous when a set of mutations is accumulated in its genome. These mutations are linked to oncogenes—genes that have the potential to cause cancer—and to tumour suppressor genes, which in contrast prevent a cell from becoming cancerous. The combination of thousands of mutant genes across different cell lines enables uncontrolled tumour growth. As the cancerous cells accumulate genetic mutations, the rate of mutations increases as the molecular mechanisms of genome maintenance are lost (Negrini et al. 2010). One outcome of this series of mutations is an increase in the proliferation rate and a decrease in the death rate of the cells, giving rise to a tumoral mass consisting of distinct cell types intertwined with the extracellular matrix (Egeblad et al. 2010). However, even a fast growing clump of tumour cells cannot grow beyond a certain size, since there is a balance between cells inside the tumour consuming nutrients and nutrient diffusion into the tumour.
Once cells have formed a tumour mass, its sustained metabolic activity requires oxygen and nutrients, which in the avascular stage (i.e., tumours without blood vessels), are provided by diffusion through the surrounding perfused tissue. At this stage, the tumour has a volume that usually never exceeds 1 mm\(^{3}\) and consists of an inner zone of necrotic cells surrounded by an intermediate zone of quiescent (or dormant) cells, owing to the lack of oxygen and nutrients, and an outer zone of proliferative cells (Koumoutsakos et al. 2013). Tumour substances (angiogenic growth factors), generated by the hypoxic zone near the necrotic one, induce blood vessel growth. During this step, one sees at a macroscopic scale capillary sprouts from existing vasculature moving towards the tumour to feed it and allow its further growth. In particular, sprouting and intussusceptive angiogenesis entail flow-related processes. In the former case, new blood vessels sprout from the existing vasculature and grow to form a new vascular network, characterized by intermittent and low-shear-stress conditions inside the vessel (Song and Munn 2011). The initiation of blood flow leads to active vessel remodelling, maturation, and differentiation into venules and arterioles. In contrast, intussusceptive angiogenesis is the process of transcapillary pillar formation inside existing vessels that result in the formation of new vessels (Styp-Rekowska et al. 2011). It involves three different steps: microvascular growth, arborization, and branching remodelling (Djonov et al. 2003). Its initiation possibly involves the imbalance of forces experienced by endothelial cells due to blood flow, cell-cell adhesion, and the extracellular matrix (Davies 2005).
Tumour vasculature shows increased vascular density and branching patterns, distorted and enlarged vessels, and highly convoluted segments (Goel et al. 2011; Narang and Varia 2011). The presence of large inter-cellular spaces renders the vessels leaky, allowing for enhanced macromolecule transport between the lumen and the extracellular space, offers ways for tumour cells to enter the vasculature, and leads to an increase of the interstitial vasculature (Narang and Varia 2011). The vascular shear rate has been found to influence vascular lumen formation as well as proliferation and migration of endothelial cells (Yamane et al. 2010), while pulsatile flow has been shown to stimulate angiogenesis in an in vitro environment (Cullen et al. 2002). In tumour-associated vasculature, the highly tortuous vessels increase the resistance to blood flow. The leakage of blood plasma leads to an increase in the interstitial pressure, causing vessel occlusion and acute hypoxia, which in turn leads to the persisting release of vascular endothelial growth factor. In response, angiogenesis continues, the network structures changes, and maturation is prevented, promoting vascular leakage. Once the tumour has acquired its own blood supply (vascular stage), peripheral tumour cells can escape via the circulatory system (migration) and set up secondary tumours elsewhere in the body (metastasis). After angiogenesis and metastasis, the patient is left with multiple tumours in different parts of his/her body that are very difficult to detect and even more difficult to treat. From a clinical point of view angiogenesis and vascular tumour growth together with metastasis is what cause the patient to die, and modelling and understanding these different stages is crucial for cancer therapy. However, a recent clinical study has reported a high degree of regression of a nonmelanoma skin tumour, particularly a basal cell carcinoma with a high microvessel density, after photodynamic therapy (Cabrera et al. 2012). While the tumour destruction was induced by the diffusion of cytotoxic agents from the irradiated zone to the neighbourhood of the tumour zone, this may represent a case where the process of angiogenesis may play a beneficial role in the regression of contiguous untreated tumours.
3.2 Fluid-Dynamic Models
In general, computational models at the macroscopic scale are formulated in terms of mass balance equations for the cellular components, coupled to a system of reaction-diffusion equations for the concentration of extracellular chemicals, which can be written in the form (Bellomo et al. 2008):
where \(\rho _{i}\) and \(\phi _{i}\) denote, respectively, the density and concentration of the \(i\)th cellular component (i.e., cells, extra-cellular matrix, or extra-cellular fluid), \(\mathbf{v}_{i}\) is the mass velocity vector of the \(i\)th population, \(c_{k}\) are the concentrations of the chemicals and nutrients, and \(\mathbf{v}_{l}\) is the velocity of the liquid (blood). The term \(\varGamma _{i}\) in Eq. (1) is a source/sink term for each component, including production (cell birth) or destruction (cell death) terms. Tumours constantly produce waste products, mainly water, and a multitude of chemical factors. In particular, when a cell dies, its membrane ruptures releasing its content, which is mostly re-usable organic material. In Eq. (2), \(Q_{k}\) is the diffusion coefficient of the \(k\)th chemical factor and \(\Lambda _{k}\) is a source term for the particular nutrient or chemical. In the language of fluid mechanics \(\phi _{i}\) is just the volume fraction of the \(i\)th constituent so that the tumour is modelled as a multiphase material. The sum of the volume fractions over all constituents must therefore equal one.
In order to close the above system of equations, an equation for the velocity components (\(\mathbf{v}_{i}\)) must be specified. Depending on the choice of this equation, macroscopic models can be defined as phenomenological or mechanical models. Phenomenological models are based on a diffusion equation for cell movement, i.e.,
where \(D_{i}\) is the diffusion coefficient. If this quantity is a positive constant, cell movement will be described by linear diffusion. However, in several models, the motion of cells is described by non-linear diffusion, where \(D_{i}=D_{i}(\phi ,c)\) (Thompson and Byrne 1999; Sherratt and Chaplain 2001). While these models are suitable for describing some interplay between cells such as contact inhibition, they cannot really account for the influence of an elastic membrane. However, they have been successfully used for evaluating the efficacy of therapy or resection in the case of brain tumours (gliomas) (Swanson and Alvord 2002), or the influence of acidity (Gatenby and Gillies 2004). Alternatively, phenomenological models can specify biased movement such as chemotaxis (Chaplain 1996)—the characteristic movement or orientation of a microorganism or cell in response to a chemical concentration gradient either towards or away the chemical stimulus—or haptotaxis (Anderson 2005)—the directional motility or outgrowth of cells towards or along a gradient of chemoattractants or adhesion sites in the extracellular matrix. An extension of the model combining diffusion and haptotactic movement predicted that heterogeneity of the extracellular matrix affects cancer invasion (Perumpanani and Byrne 1999).
As tumour cells proliferate, they push into the surrounding tissue and cause pressure to build. This pressure, along with other mechanical interactions, have very important implications on tumour growth and progression. Incorporation of the physical forces that influence cell motion requires complementing Eqs. (1) and (2) with the momentum balance equations (Bellomo et al. 2008):
for each constituent. Here \(\mathbb {T}_{i}\) is the stress-tensor, \(\mathbf{f}_{i}\) is the body force acting on the \(i\)th constituent, and \(\mathbf{F}_{i}\) is the interaction force with the other constituents. In order to close this system of equations we need to specify constitutive equations that relate the forces to the level of stress and compression. For instance, as a cell undergoes mitosis and divides into two cells, these will generate a pressure on neighbouring cells, causing an increase in tumour size. If cells are assumed to behave as a fluid, the simplest constitutive equation for the stress can be written as
where \(\sigma _{i}\) is the response of the cells to compression and \(\mathbb {I}\) is the unit tensor. Here the implicit assumption is made that cells behave as elastic liquids.
In many instances the filtration of organic liquids through tumours has been simulated by modelling the tumour as a growing and deformable porous medium. If cells move as an elastic fluid within a rigid extracellular matrix, Eqs. (1) and (2) can be closed using Darcy’s law
where \(K\) is the permeability of the matrix. Modifications of this equation for a deformable porous medium and for mass exchange between the constituents are given in De Angelis and Preziosi (2000) and Chaplain et al. (2003). Darcy models have been considered by several authors in simulations of tumour growth (Cristini et al. 2003), of fluid flow in solid tumours (Soltani and Chen 2011), and more recently for describing cancer-therapeutic transport in the lung (Erbertseder et al. 2012). The former models have predicted interstitial velocities in very good agreement with experimental results, while the latter has described the flow, transport, and reaction processes of a therapeutic agent in the pulmonary circulation in healthy and cancerous pulmonary tissue. In this case, the phase moving within the tissue continuum consists of two components, namely the interstitial fluid and the therapeutic agent. While it is assumed that the fluid phase is incompressible, the movement of the dissolved drug molecules in the interstitial tissue of the lung is modelled using a single-phase, two-component approach in a rigid, porous medium. With the additional assumption that the flow within the tissue is creeping, the flow velocity of the interstitial fluid can be very well described by Darcy’s law (Baxter and Jain 1989; Baish et al. 1997; Erbertseder et al. 2012).
Alternatively, the cell-matrix medium can be viewed as a viscous fluid (Stokes flow), where the stress depends on the viscosity (Friedman and Hu 2007), or as a viscoelastic fluid (Holmes and Sleeman 2000). Other models treat the tumour tissue as a mixture of cells living in a porous medium made of extracellular matrix and filled with extracellular liquid (Graziano and Preziosi 2007). Darcy’s law can be used to model both fluid flow and cell motion, where the latter is treated as a granular material flowing in the porous extracellular matrix scaffold. For example, the case of a multicell spheroid can be modelled as a growing poro-elastic medium using Eqs. (1) and (2) coupled to a variant of Eq. (4) for the interstitial pressure \(p\), where the inertial terms are neglected and the stress-tensor of the mixture (\(i=tc\), tumour cells; \(i=l\), extracellular fluid with chemicals and nutrients) is given by \(\mathbb {T}_{m}=-[p+\sigma _{tc}]\mathbb {I}\), and a composite velocity equation (Bellomo et al. 2008). This scheme has been used together with experimental data to show the cell-size reduction by solid stress inside tumour spheroids (Ambrosi and Mollica 2002; Roose et al. 2003). Combining Darcy’s law with Stokes flow gives a further constitutive relation, known as the Brinkman equation. Models based on Darcy-Stokes flow have been used to study tumour morphology and stability (Zheng et al. 2005; Pham et al. 2011). However, models based solely on Stokes flow has been found to be more consistent with experimental data from in vitro three-dimensional multicellular tumour spheroids (Pham et al. 2011).
A number of illustrative mechanical models describing the growth of avascular tumours are reviewed in Roose et al. (2007), and details of some of the existing models can be found in the references therein. Multiscale mechanical models designed for simulating the growth of both avascular and vascular tumours, including environmental conditions, distribution of oxygen, elastic membrane response, membrane degradation, and the dynamics of the motion of the tumour have also started to appear in the literature (Mantzaris et al. 2004; Plank et al. 2004; Macklin et al. 2009; Bresch et al. 2010). While some of these models can be applied to investigate the therapeutic benefits of anti-invasive agents, they provide the basis of a numerical platform for more refined tumour growth simulations. On the other hand, the process of invasion of adjacent tissue by cancer cells has been recently modelled by assuming that cells migrate through a combination of diffusion and haptotaxis as well as undergoing proliferation and by incorporating the effects of cell-cell and cell-matrix adhesion (Chaplain et al. 2011). Multiscale models describing the growth of in vitro multicellular tumour spheroids and in vivo avascular tumour nodules that incorporate heterogeneous population of cells, drug diffusion, drug pharmacokinetics, transitions, and the diffusion of multiple nutrients are being used to formulate effective therapeutic strategies by understanding the interactions between drugs and the heterogeous microenvironments in growing tumours (Venkatasubramanian et al. 2008).
Although macroscopic models based on a fluid-dynamic approach are deeply influencing modern cancer research in that they exhibit the general behaviour of tumour growth, angiogenesis, and invasion, they fail to examine details of the phenomena occurring at the single cell level. In particular, this makes detailed modelling of processes such as angiogenesis difficult because calculating average cell density fails to include the spatial structure of the vascular network. Moreover, it is not completely clear if invasion and metastasis are driven by average population behaviour, or instead by cells which deviate from the mean. For example, it is quite possible that individual rogue cells drive the macroscopic processes of invasion or metastasis. However, their individual behaviour is certainly not captured by a continuum approach. Although the development of multiscale approaches is very recent, future practical models must be based on some modular approach where at a certain scale the processes have to be consistent with the lower and higher scales. In this framework, the overall system can be regarded as a network of several interacting subsystems, each developed at a specific scale, while interactions between contiguous systems need to deal with compatibility (and possibly boundary) conditions at each specific scale. A brief outline of these issues is given in Sect. 6. The interested reader is referred to Bellomo et al. (2008), where perspectives of such a complexity multiscale theory is amply discussed.
4 Models of Vascular Transport and Angiogenesis
The blood flow in microvessels, whose diameters are \(\sim \!\!100\,\upmu \)m or less, is called the microcirculation (Sugihara-Seki and Fu 2005). Microvessels have irregular interconnections that form a network in tissues and are responsible for the exchange of materials between blood and surrounding tissues. Research on the flow through the neoplastic vacuslature of solid tumours has been largely motivated by the desire to understand the role of fluid convection in the treatment of cancer by therapeutic monoclonal antibodies. A key problem in this kind of treatment is the low transport rates into the main body of the tumour across the vasculature, which leads to low and ineffective concentrations of the therapeutic macromolecules. It is a common observation that interstitial fluid pressure is higher in both human and experimental solid tumours than in normal tissue (Heldin et al. 2004). Enhanced interstitial pressure is the result of a richly developed and highly permeable vascular network, combined with facilitated transendothelial fluid transfer (Boucher and Jain 1992; Lee et al. 1994). Clinically, a high interstitial pressure is marked by a reduced delivery and uptake of anticancer drugs (or macromolecules) and, hence, lack of therapeutic effects. Therefore, the analysis of blood flow and transport processes in the growing networks requires accurate modelling of blood flow in microvessels, solute transport, and angiogenesis.
Physically, blood is a suspension of red blood cells, white blood cells (leukocytes), and platelets in plasma. It is an incompressible Newtonian fluid with viscosity of about 1.2 cP at \(37^{\circ }\) C. Red blood cells are the most abundant, with a volume fraction of 40–45%, and therefore they strongly influence the rheology of blood. Because of their flexible viscoelastic membranes, they can easily pass through capillaries with diameters less than their major diameters at rest (\(\sim \!\!8\,\upmu \)m). In fact, the minimum diameter of a cylindrical tube that will allow a normal red cell to pass through intact is as narrow as \(\sim \!\!2.8\,\upmu \)m (Halpern and Secomb 1989). Leukocytes are generally spherical with a mean diameter of \(\sim \!6\)–\(8\,\upmu \)m and are much less deformable than red cells. Despite their relatively small numbers, leukocites can contribute significantly to blood flow resistance (Schmid-Schönbein et al. 1981). The rheological properties of blood flowing in microvessels have been extensively studied by in vitro experiments, using a suspension of red cells flowing through capillary tubes (Sugihara-Seki and Fu 2005).
Accurate numerical simulations of blood flow in microvessels must certainly include detailed models of blood cells as well as the glycocalyx layer attached to the the endothelial surface. The dimensional irregularities of vessel diameters is another important factor. The Reynolds number of the blood flow in microvessels is \(\ll \!1\), so that in general non-linear convective acceleration terms (\(\mathbf{v}\cdot \nabla \mathbf{v}\)) in the momentum-balance equations describing the plasma flow and the cell motion can be neglected (Sugihara-Seki and Fu 2005). Since the plasma is known to be an incompressible Newtonian fluid, its motion is governed by the Navier-Stokes equations
along with the continuity equation
where \(\mathbf{v}\) is the velocity vector, \(p\) is the pressure, \(\rho \) is the density, and \(\mu \) is the dynamic viscosity of the plasma. Early simulations aimed at modelling the flow of red cells in narrow tubes under axisymmetry, the flow fields around cells and shear stress on the cell membrane, and flow resistance due to irregularities of vessel lumen as well as the effects of glycocalyx and leukocytes are reviewed in Sugihara-Seki and Fu (2005), and described in full detail in the references therein. More recent simulations using continuum-based models have shown that coupling of solid components and fluid flow in these models poses a number of challenging problems (Pozrikidis 2005; Noguchi and Gompper 2005; Liu and Liu 2006; Skotheim and Secomb 2007; Wu and Aidun 2010; Fedosov et al. 2012). For example, computational complexity can be reduced by coupling discrete models of red cells with mesoscopic methods for flow discretization such as the lattice Boltzmann method, multiparticle collisional dynamics, and dissipative particle dynamics (Dupin et al. 2008). Numerical simulations have indicated that the effect of leukocyte adhesion to the vessel walls on flow depends strongly on the number of adherent leukocytes and the vessel diameter (Pappu et al. 2008). Owing to many similarities in the process of leukocyte and circulating tumour cell adhesion, models developed for leukocytes can also be applied to circulating tumour cells during the metastasis process.
Microvessel walls consist mainly of endothelial cells. Vascular endothelium is the principle barrier to, and regulator of, material exchange between circulating blood and the body tissues. The ultrastructural pathways and mechanisms whereby endothelial cells and the cleft between the cells modulate microvessel permeability to water and solutes have been a classical question in microvessel transport since the early 1950s. If capillary walls act like semi-permeable membranes, fluid motion across them depends on the net imbalance between the osmotic absorption pressure of the plasma proteins and the capillary hydraulic pressure generated by the heart beating (Levick and Michel 2010). Most existing models of transport through the inter-endothelial clefts are based on continuum approaches. However, it was suggested that more suitable analyses should be based on the molecular nature of the fluid because of the sizes of the mean intermolecular distances (\(\sim \!0.3\) nm) and the cleft width (\(\sim \!\!18\) nm) (Sugihara-Seki et al. 2008). The development of multiscale computational models (Praprotnik and Delle Site 2008), coupling, for example, the molecular structure of the glycocalyx with a continuum description of the flow, is highly suitable in this context. Moreover, solute transport from the vasculature to the cells has been largely modelled as passively transported elements with a flux proportional to the drug concentration. Solute transport inside the tumour was recently analyzed using computational models of diffusion based on high-resolution images (Baish et al. 2011).
On the other hand, tumour-induced angiogenesis has been modelled using both continuum and discrete models (Qutub and Mac Gabhann 2009). In a more recent continuum approach, the extracellular population is modelled by a density function that resolves the vascular branching patterns (Bergdorf et al. 2010). Cell-based and lattice-based discrete models are described in Bauer et al. (2009) and Chaplain (2000), respectively, while a hybrid modelling where a discrete tip-cell representation is coupled to a continuum description of the blood vessels is given in Milde et al. (2008) and Travasso and Corvera Poiré (2011). A model for sprouting angiogenesis based on Poiseuille flow inside a network of connected pipes can be found in McDougall et al. (2002), which was successively extended to account for the variability in blood viscosity and evolving capillary vessels that can dilate and constrict to study the transport of therapeutic agents inside the growing vasculature (Stephanou et al. 2006) and combined with a continuum model of tumour growth (Macklin et al. 2009). An in-depth report on recent simulation models of vascularized tumours is given in Lowengrub et al. (2010), and references therein.
5 Microfluidics in Cancer
Microfluidics typically deals with the manipulation of fluids that are geometrically constrained to a submillimeter scale. Such small scales offer a number of advantages including cost effectiveness, low consumption of reagents, cellular separations and detections with high resolution and sensitivity, and other less obvious features of fluids in microchannels, such as laminar flow (Whitesides 2006). The early development of microfluidics in life science applications has been mainly focused on the analysis of biomolecules from small volumes of fluids (typically nanolitres or less). However, the use of microfluidics in manipulating and analyzing individual cells has notably increased in recent years. Its application to biological systems is compelling because it allows manipulation at the single or even subcellular level. Recently, there has been a push towards applying microfluidic tools to specific biological research areas so that development of these engineering approaches can be better guided.
Microfluidic devices allow for a lab-on-a-chip array to simplify single cell analysis by providing a microenvironment that is of micrometer dimension and containing nanomoles of reagent/media. They also allow for controlled placement of cells and precise delivery factors (Chao and Ros 2008). One conventional system that is commonly used as a model to study cell migration is the transwell Boyden chamber, in which a porous membrane with pore size of \(\sim \!\!5\)–\(10\,\upmu \)m is placed between cells and chemoattractant so that cells are attracted to move across the membrane . Rapid advances in microtechnology have made microfluidic devices easy to design and construct. For instance, polydimethylsiloxane (PDMS) membrane stamps are typically molded off through soft lithography and other rapid prototyping techniques (Xia and Whitesides 1998). Refinements in the fabrication process, such as e-beam lithography, makes it possible to construct channels on the submicron or even nanoscale, which in theory would be able to constrain a fluid volume down to a femtolitre (billionth of microlitre) range (Qin et al. 2010). Recent microfluidic approaches in studying cellular migration are reviewed in Huang et al. (2011).
Another application of microfluidic-based devices is in the isolation, detection, and molecular characterization of circulating tumour cells. Efficient methods for the isolation and characterization of circulating tumour cells can also contribute to a much better understanding of the metastatic process. The development of passive microfluidic cell separation biochips, which can isolate circulating tumour cells from whole blood without the use of antibodies or magnetic beads, is revolutionizing disease detection, diagnosis, and prognosis as cancer cells can be obtained from blood (termed liquid biopsy) rather than via the needle aspiration tumour biopsy, which is invasive, painful, and cannot be performed on a regular basis (Lim 2012). Recent overviews of various methods for circulating tumour cell isolation, detection, and molecular characterization can be found in Hou et al. (2011), Lianidou and Markou (2011), and Yu et al. (2011). Isolation of cells with differential deformabilities remains a great challenge (Wirzt et al. 2011; Gossett et al. 2012). Microfabrication-assisted technology, using microscale arrays of round or rectangular posts, channels, or other simple patterns, has the potential to solve this problem. For instance, a mechanical separation chip, which employs artificial microbarriers in combination with hydrodynamic forces to separate deformable from stiff cells, has been used to demonstrate the separation of: (i) an artificial mixture of two breast cancer cell types (MDA-MB-436 and MCF-7) with distinct deformabilities and matastatic potentials, and (ii) a heterogeneous breast cancer cell line (SUM149), into enriched flexible and stiff sub-populations (Zhang et al. 2012). The flexible phenotype is associated with overexpression of multiple genes involved in cancer cell motility and metastasis.
Microfluidic devices have also been used for studying metastatic cancer cell invasion. Much of the initial work in applying microfluidics to metastasis has focused on studying how cancer cells respond to concentration gradients of chemicals suspected to drive cell motion. For example, with the aid of a PDMS-based device it has been possible to monitor 3D migration of the invasive MDA-MB-231 (mammary carcinoma) cells across extracellular matrix-coated microgaps with real-time light microscopy and map out their migration paths (Chaw et al. 2007). This not only permits to quantify the percentage of migrated cells, but also to obtain information on migration of individual cells. Microdevices for cell isolation and enumeration from blood have also been presented by several other authors (Cheng et al. 2007; Vickerman et al. 2008; Tan et al. 2009). Today, most of these devices has the potential to be used for routine monitoring of cancer development and cancer therapy in a clinical setting. Recently, a microfluidic optical stretcher have been used to study mechanical properties of cells from the inside (Lautenschläger et al. 2009). This helps investigate how the cytoskeleton, cell mechanics, and cell motility may be related, so that we may better understand how to develop therapies that hinder movement of metastatic cells.
6 Future Challenges and Perspectives
In this chapter we have reviewed recent modelling aspects of cancer fluid mechanics at different representation scales. In particular, model simulations of how cellular changes affect macroscopic distributions are especially important when examining sustained angiogenesis, tissue invasion, and metastasis. Although these models have been successful in describing macroscopic evolution properties of cancer, it is well known that they occur through genetic mutations and evolutionary selection; a link that has not yet been fully modelled. On the other hand, while all macroscopic models either assume that cells move through a diffusion-like process or act as an elastic fluid, only discrete models have the ability to track the behaviour of single cells. Therefore, macroscopic continuum models should be derived from the underlying cellular models by suitable asymptotic methods linking inter-cellular distances to those typical of the tissue level. This necessity has recently given rise to multiscale modelling constructs, where the dynamics at the cellular scale is coupled with the continuum mechanics of solid tumours. However, as is the case with very complex systems, all of the components cannot be usually included if we wish to develop practical models.
One possible solution to the above difficulty that has been envisaged is to make use of the so-called theory of modules proposed by Hartwell et al. (1999), where the whole system is decomposed into subsystems (or modules) such that the identification of each module is related to the expression of specific biological functions. However, this modular approach must overcome a number of challenges before becoming a workable and practical multiscale modelling framework. For instance, the analysis of large interacting systems as occurs if the numerous signalling pathways or interacting cytokines are incorporated, which will unavoidably lead to a significant increase in system size and complexity. Other difficult features involve the processes of angiogenesis and metastasis, where a detailed modelling of branching, anastomosis, vascular normalization as well as active cell migration to blood vessels, intravasation, extravasation, and distant site colonization in metastatic spread are completely ignored by present models. In addition, factors as cell geometry, diffusion terms, chemotaxis and haptotaxis, and cell invasion as an active and coordinated process represent future challenges that must be accurately modelled if we want to reproduce cancer in the computer and convert such complexity multiscale models into powerful tools for the diagnosis, prediction, and therapy of cancer.
References
Ambrosi D, Mollica F (2002) On the mechanics of a growing tumour. Int J Eng Sci 40:1297–1316
Anderson ARA (2005) A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol 22:163–186
Anderson ARA, Weaver AM, Cummings PT, Quaranta V (2006) Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127:905–915
De Angelis E, Preziosi L (2000) Advection diffusion models for solid tumours in vivo and related free-boundary problems. Math Models Methods Appl Sci 10:379–408
Baish JW, Netti PA, Jain RK (1997) Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res 53:128–141
Baish JW, Stylianopoulos T, Lanning RM, Kamoun WS, Fukumura D, et al. (2011) Scaling rules for diffusive drug delivery in tumour and normal tissues. Proc Nat Acad Sci USA 108:1799–1803
Bauer AL, Jackson TL, Jiang Y (2007) A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys J 92:3105–3121
Bauer AL, Jackson TL, Jiang Y (2009) Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol 5:e1000445
Baxter LT, Jain RK (1989) Transport of fluid and macromolecules in tumors: I. Role of interstitial pressure and convection. Microvasc Res 37:77–104
Bellomo N, Li NK, Maini PK (2008) On the foundations of cancer modelling: selected topics, speculations, and perspectives. Math Models Methods Appl Sci 18:593–646
Bergdorf M, Milde F, Koumoutsakos P (2010) Continuum models of mesenchymal cell migration and sprouting angiogenesis. In: Deisboeck T, Stamatakos GS (eds) Multiscale cancer modeling. CRC, Boca Raton, pp 213–235
Boucher Y, Jain RK (1992) Microvascular pressure is the principal driving force for interstitial hypertension in solid tumours: Implications for vascular collapse. Cancer Res 52:5110–5114
Bresch D, Colin T, Grenier E, Ribba B, Saut O (2010) Computational modeling of solid tumor growth: the avascular stage. SIAM J Sci Comput 32:2321–2344
Cabrera H, Castro J, Grassi HC, Andrades EDJ, López-Rivera SA (2012) The effect of photodynamic therapy on contiguous untreated tumor. Dermatol Surg 38:1097–1099
Chao T-C, Ros A (2008) Microfluidic single-cell analysis of intracellular compounds. J Royal Soc Interface 5:S139–S150
Chaplain MAJ (1996) Avascular growth, angiogenesis, and vascular growth in solid tumors: the mathematical modelling of the stages of tumor development. Math Comput Model 23:47–87
Chaplain MA (2000) Mathematical modelling of angiogenesis. J Neuro-Oncol 50:37–51
Chaplain MAJ, Graziano L, Preziosi L (2003) Mathematical modelling of the loss of tissue compression responsiveness and its role in solid tumour development. IMA J Math Appl Med Biol 23:197–229
Chaplain MAJ, Lachowicz M, Szymańska Z, Wrzosek D (2011) Mathematical modelling of cancer invasion: the importance of cell-cell adhesion and cell-matrix adhesion. Math Models Methods Appl Sci 21:719–743
Chaw KC, Manimaran M, Tay FE, Swaminathan S (2007) Matrigel coated polydimethylsiloxane based microfluidic devices for studying matastatic and non-metastatic cancer cell invasion and migration. Biomed Microdevices 9:597–602
Cheng SY, Heilman S, Wasserman M, Archer S, Shuler ML, Wu M (2007) A hydrogel-based microfluidic device for the studies of directed cell migration. Lab on a Chip 7:720–725
Cristini V, Lowengrub JS, Nie Q (2003) Nonlinear simulation of tumor growth. J Math Biol 46:191–224
Cullen JP, Sayeed S, Sawai RS, Theodorakis NG, Cahill PA, et al. (2002) Pulsatile flow-induced angiogenesis. Arterioscler, Thromb Vasc Biol 22:1610–1616
Dallon JC, Othmer HG (2004) How cellular movement determines the collective force generated by the Dictyostelium discoideum slug. J Theor Biol 231:203–222
Davies JA (2005) Mechanisms of morphogenesis. Elsevier, Philadelphia
Deutsch A, Dormann S (2005) Cellular automaton modeling of biological pattern formation: characterization, applications, and analysis. Birkhäuser, Boston
Dillon R, Painter KJ, Owen MR (2008) A single-cell based model of cellular growth using the immersed boundary method. In: Koo CB, Li Z, Li P (eds) Moving interface problems and applications in fluid dynamics (Contemporary Mathematics, AMS) pp 1–16
Djonov V, Baum O, Burri P (2003) Vascular remodeling by intussusceptive angiogenesis. Cell and Tissue Res 314:107–117
Dupin MM, Halliday I, Care CM, Munn LL (2008) Lattice Boltzmann modelling of blood cell dynamics. Int J Comput Fluid Dyn 22:481–492
Dvorak HF, Nagy J, Dvorak J, Dvorak A (1988) Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol 133:95–109
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Erbertseder K, Reichold J, Flemisch B, Jenny P, Helmig R (2012) A coupled discrete/continuum model for describing cancer-therapeutic transport in the lung. PLoS One 7:e31966
Fedosov DA, Fornleitner J, Gompper G (2012) Margination of white blood cells in microcapillary flow. Phys Rev Lett 108:028104
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Friedman A, Hu B (2007) Bifurcation for a free boundary problem modeling tumor growth by Stokes equation. SIAM J Math Anal 39:174–194
Fu Y, Kunz R, Wu J, Dong C (2012) Study of local hydrodynamic environment in cell-substrate adhesion using side-view \(\mu \)PIV technology. PLoS One 7:e30721
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Glazier JA, Graner F (1993) Simulation of the differential adhesion driven rearrangement of biological cells. Phys Rev E 47:2128–2154
Goel S, Duda D, Xu L, Munn L, Boucher Y, et al. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, et al. (2012) Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Nat Acad Sci USA 109:7630–7635
Graner F, Glazier JA (1992) Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett 69:2013–2016
Graziano L, Preziosi L (2007) Mechanics in tumour growth. In: Mollica F, Rajagopal KR, Preziosi L (eds) Modeling of biological materials. Birkhäuser, Boston, pp 267–328
Gusenbauer M, Cimrak I, Bance S, Exl L, Reichel F, Oezelt H, Schrefl T (2011) A tunable cancer cell filter using magnetic beads: cellular and fluid dynamic simulations. arXiv:1110.0995v1 [physics.flu-dyn]
Halpern D, Secomb TW (1989) The squeezing of red blood cells through capillaries with near-minimal diameters. J Fluid Mech 203:381–400
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–670
Hartwell HL, Hopfield JJ, Leibner S, Murray AW (1999) From molecular to modular cell biology. Nature 402:C47–C52
Heldin C-H, Rubin K, Pietras K, Östman A (2004) High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer 4:806–813
Holmes MJ, Sleeman BD (2000) A mathematical model of tumour angiogenesis incorporating cellular traction and viscoelastic effects. J Theor Biol 202:95–112
Hou HW, Lee WC, Leong MC, Sonam S, Vedula SRK, Lim CT (2011) Microfluidics for applications in cell mechanics and mechanobiology. Cell Mol Bioeng 4:591–602
Huang Y, Agrawal B, Sun D, Kuo JS, Williams JC (2011) Microfluidics-based devices: new tools for studying cancer and cancer stem cell migration. Biomicrofluidics 5:013412
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Koumoutsakos P, Pivkin I, Milde F (2013) The fluid mechanics of cancer and its therapy. Ann Rev Fluid Mech 45:325–355
Lautenschläger F, Paschke S, Schinkinger S, Bruel A, Bell M, Guck J (2009) The regulatory role of cell mechanics for migration of differentiating myeloid cells. Proc Nat Acad Sci USA 106:15696–15701
Lee I, Boucher Y, Demhartner TJ, Jain RK (1994) Changes in tumour blood flow, oxygenation and interstitial pressure induced by pentoxifylline. Br J Cancer 69:492–496
Leung D, Cachianes G, Kuang W, Goeddel D, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Levick JR, Michel CC (2010) Microvascular fluid exchange and the revised starling principle. Cardiovasc Res 87:198–210
Liang S, Slattery MJ, Wagner D, Simon SI, Dong C (2008) Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Rev Biomed Eng 36:661–671
Liang S, Hoskins M, Dong C (2010) Tumor cell extravasation mediated by leukocyte adhesion in shear rate dependent on IL-8 signaling. Mol Cell Biomech 7:77–91
Lianidou ES, Markou A (2011) Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem 57:1242–1255
Lim CT (2012) Microfluidics for cancer cell detection and diagnosis. IEEE Life Sci Newsl (June 2012)
Liotta LA, Kohn EC (2001) The microenvironment of the tumour-host interface. Nature 411:375–379
Liu Y, Liu WK (2006) Rheology of red blood cell aggregation by computer simulation. J Comput Phys 220:139–154
Lowengrub JS, Frieboes HB, Jin F, Chuang YL, Li X, et al. (2010) Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23:R1–R91
Macklin P, McDougall S, Anderson ARA, Chaplain MAJ, Cristini V, Lowengrub J (2009) Multiscale modelling and nonlinear simulation of vascular tumour growth. J Math Biol 58:765–798
Mantzaris NV, Webb S, Othmer HG (2004) Mathematical modeling of tumor-induced angiogenesis. J Math Biol 49:111–187
McDougall SR, Anderson ARA, Chaplain MAJ, Sherratt JA (2002) Mathematical modelling of flow through vascular networks: implications for tumour-induced angiogenesis and chemotherapy strategies. Bull Math Biol 64:673–702
Michor F, Liphardt J, Ferrari M, Widom J (2011) What does physics have to do with cancer? Nat Rev Cancer 11:657–670
Milde F, Bergdorf M, Koumoutsakos P (2008) A hybrid model for three-dimensional simulations of sprouting angiogenesis. Biophys J 95:3146–3160
Moreira J, Deutsch A (2005) Pigment pattern formation in zebrafish during late larval stages: a model based on local interaction. Dev Dyn 232:33–42
Narang AS, Varia S (2011) Role of tumor vascular architecture in drug delivery. Adv Drug Delivery Rev 63:640–658
Negrini S, Gorgoulis VG, Halazonetis TD (2010) Genomic instability: an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11:220–228
Newman TJ (2005) Modeling multicellular systems using subcellular elements. Math Biosci Eng 2:613–624
Noguchi H, Gompper G (2005) Shape transitions of fluid vesicles and red blood cells in capillary flows. Proc Nat Acad Sci US 102:14159–14164
Palsson E (2008) A 3-D model used to explore how cell adhesion and stiffness affect cell sorting and movement in multicellular systems. J Theor Biol 254:1–13
Pappu V, Doddi SK, Bagchi P (2008) A computational study of leukocyte adhesion and its effect on flow pattern in microvessels. J Theor Biol 254:483–498
Perumpanani AJ, Byrne HM (1999) Extracellular matrix concentration exerts selection pressure on invasive cells. Euro J Cancer 35:1274–1280
Pham K, Frieboes HB, Cristini V, Lowengrub J (2011) Predictions of tumour morphological stability and evaluation against experimental observations. J Royal Soc Interface 8:16–29
Plank MJ, Sleeman BD, Jones PF (2004) A mathematical model of tumor angiogenesis, regulated by vascular endothelial growth factor and the angiopoietins. J Theor Biol 229:435–454
Pozrikidis C (2005) Numerical simulation of cell motion in tube flow. Ann Biomed Eng 33:165–178
Praprotnik M, Delle Site L (2008) Multiscale simulation of soft matter: from scale bridging to adaptive resolution. Ann Rev Phys Chem 59:545–571
Qin D, Xia Y, Whitesides GM (2010) Soft lithography for micro- and nanoscale patterning. Nat Protoc 5:491–502
Quaranta V, Weaver AM, Cummings PT, Anderson ARA (2005) Mathematical modeling of cancer: the future of prognosis and treatment. Clin Chim Acta 357:173–179
Qutub A, Mac Gabhann F (2009) Multiscale models of angiogenesis. IEEE Eng Med Biol Mag 28:14–31
Rejniak KA (2007) An immersed boundary framework for modelling the growth of individual cells: an application to the early tumour development. J Theor Biol 247:186–204
Roose T, Netti PA, Munn LL, Boucher Y, Jain RK (2003) Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc Res 66:204–342
Roose T, Chapman SJ, Maini PK (2007) Mathematical models of avascular tumor growth. SIAM Rev 49:179–208
Schaller G, Meyer-Hermann M (2005) Multicellular tumor spheroid in an off-lattice Voronoi-Delaunay cell model. Phys Rev E 71:051910
Schmid-Schönbein GW, Sung K-P, Tözeren H, Skalak R, Chien S (1981) Passive mechanical properties of human leukocytes. Biophys J 36:243–256
Sherratt JA, Chaplain MAJ (2001) A new mathematical model for avascular tumour growth. J Math Biol 43:291–312
Skotheim JM, Secomb TW (2007) Red blood cells and other nonspherical capsules in shear flow: oscillatory dynamics and the tank-treading-to-tumbling transition. Phys Rev Lett 98:078301
Soltani M, Chen P (2011) Numerical modeling of fluid flow in solid tumours. PLoS One 6:e20344
Song JW, Munn LL (2011) Fluid forces control endothelial sprouting. Proc Nat Acad Sci USA 108:15342–15347
Sporn MB (1996) The war on cancer. Lancet 347:1377–1381
Stephanou A, McDougall SR, Anderson ARA, Chaplain MAJ (2006) Mathematical modelling of the influence of blood rheological properties upon adaptive tumour-induced angiogenesis. Math Comput Model 44:96–123
Styp-Rekowska B, Hlushchuk R, Pries AR, Djonov V (2011) Intussusceptive angiogenesis: pillars against the blood flow. Acta Physiol 202:213–223
Sugihara-Seki M, Fu BM (2005) Blood flow and permeability in microvessels. Fluid Dyn Res 37:82–132
Sugihara-Seki M, Akinaga T, Itano T (2008) Flow across microvessel walls through the endothelial surface glycocalyx and the interendothelial cleft. J Fluid Mech 601:229–252
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3:413–438
Swanson KR, Alvord EC Jr (2002) Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer 86:14–18
Tan SJ, Yobas L, Lee GYH, Ong CN, Lim CT (2009) Microdevice for the isolation and enumeration of cancer cells from blood. Biomed Microdevices 11:883–892
Thompson KE, Byrne WF (1999) Modelling the internalization of labelled cells in tumour spheroids. Bull Math Biol 61:601–623
Travasso RDM, Corvera Poiré E (2011) Tumor angiogenesis and vascular patterning: a mathematical model. PLoS One 6:e19989
Turner S, Sherratt JA (2002) Intercellular adhesion and cancer invasion: a discrete simulation using the extended Potts model. J Theor Biol 216:85–100
Venkatasubramanian R, Henson MA, Forbes NS (2008) Integrating cell cycle progression, drug penetration and energy metabolism to identify improved cancer therapeutic strategies. J Theor Biol 253:98–117
Vickerman V, Blundo J, Chung S, Kamm R (2008) Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab on a Chip 8:1468–1477
Weinberg RA (2007) The biology of cancer. Garland Science, New York
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373
Wirzt D, Konstantopoulos K, Searson PC (2011) The physics of cancer. The role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11:512–522
Wu JS, Aidun CK (2010) Simulating 3D deformable particle suspensions using lattice Boltzmann method with discrete external boundary force. Inter J Numer Methods Fluids 62:765–783
Xia Y, Whitesides GM (1998) Soft lithography. Angew Chem 37:550–575
Yamane T, Mitsumata M, Yamaguchi N, Nakazawa T, Mochisuki K, et al. (2010) Laminar high shear stress up-regulates type IV collagen synthesis and down-regulates MMP-2 secretion in endothelium: A quantitative analysis. Cell and Tissue Res 340:471–479
Yu M, Scott S, Toner M, Maheswaran S, Haber DA (2011) Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 192:373–382
Zhang W, Kai K, Choi DS, Iwamoto T, Nguyen YH, et al. (2012) Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. Proc Nat Acad Sci USA 109:18707–18712
Zheng X, Wise SM, Cristini V (2005) Nonlinear simulation of tumor necrosis, neo-vascularization and tissue invasion via an adaptive finite-element/level-set method. Bull Math Biol 67:211–259
Acknowledgments
D. C. Belisario acknowledges the organizers of the I Workshop of the Venezuelan Society of Fluid Mechanics for inviting me to write part of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Belisario, D.C., Sigalotti, L.D.G. (2014). The Impact of Computational Fluid Mechanics on Cancer Research. In: Sigalotti, L., Klapp, J., Sira, E. (eds) Computational and Experimental Fluid Mechanics with Applications to Physics, Engineering and the Environment. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-00191-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-00191-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00190-6
Online ISBN: 978-3-319-00191-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)