Abstract
Hydro-cracking slurry bubble column design, scale-up, and operation are strongly influenced by a fluid-dynamic parameter known as volumetric phase distribution. This parameter depends on the operating conditions (gas flow, liquid flow, pressure, and temperature) as well as on the gas, liquid, and solid physical properties. Experiments were carried out at ambient temperature and atmosphere pressure (cold conditions) in a 120 mm inner diameter plexiglas column (without any gas sparger) with air and \(\mathrm{{CO}}_{2}\), mineral oil, and coke with average particle sizes of 630 microns. The column was operated to up-flow continuous recirculation with superficial gas velocities ranging from 3 to 10 cm/s and a constant liquid-solid flow about 29 l/h. Experimental measurements were done by two methods: direct phase trapping and pressure drop. Measurement results indicate that the volumetric gas phase is highly affected by the superficial gas velocity. However, the superficial gas velocity effect on solid concentration is negligible. The experimental results were also compared with experimental data from other authors, obtaining a good agreement. A gas volumetric phase correlation was proposed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Slurry bubble column reactors (SBCR) are used nowadays as part of hidro-cracking units, having a notably importance for heavy crude oil processing. Several advantages of their use include: high heat and mass transfer rates, high conversion levels by strong gas-liquid-solid interactions, and improvement of catalyst recovery processes (Chengtian et al. 2008). Also, these reactors reduce maintenance costs due to the absence of moving parts or without any internal and improved control of the phase residence time inside the equipment (Shah et al. 1982).
The design and scale-up of these equipments is strongly influenced by the fluid-dynamic parameters. Some of the parameters with a major impact are the gas-liquid-solid phase volumetric distribution, the pressure drop, the flow pattern, and the mixing regime. Several of these variables, validated in an operation range and under certain physical conditions, have been cleared through empirical and semi-empirical correlations, which predominate as the bases of slurry bubble column reactor design. However, there is not enough to get from a universal methodology to describe the whole behaviour of a slurry bubble column (Hikita et al. 1981; Akita and Yoshida 1973; Shah et al. 1982). Additionally, process variables, like gas and liquid superficial velocities and material physical properties, influence the fluid-dynamic performance, which is a particular characteristic in each kind of process.
This work deals with the study of the gas-liquid-solid phase distribution based on two physical properties (specific particle range size and gas density) and one operation condition. For hydro-conversion processes, it is relevant to know the gas-liquid-solid volumetric phase distribution since it affects the conversion rate, which can be considered as the most important variable in these units.
2 Methodology and Experimental Setup
2.1 Experimental System and Materials
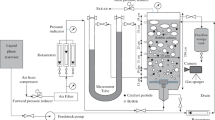
A schematic diagram of the slurry bubble column (SBC) used in this work is presented in Fig. 1. The core of the system is a plexiglas column of 120 mm internal diameter (ID) with an entrance at the bottom and an exit at the top, without any internal parts. The experimental equipment has a slurry piston pump for the feedstock, a slurry centrifugal pump for recycle, a mechanical agitated tank for the feedstock, and two discharge tanks. The measurement variable system has a mass flow-meter transmitter (FT) for gas Endress \(+\) Hauser Promass 83A 4–20 mA output range 0–450 kg/h error \(\pm \) 0.50 %, a pressure transmitter (PT) ABB 2600T model 261T dual wire 4–20 mA output range 13.7–397 mbar accuracy \(\pm \) 0.15 %, a pressure differential transmitter (DPT) Rosemount model 115T Smart 4–20 mA output range 0–20 bar error \(\pm \) 0.25 %, and videographer recorder ABB model Screen Master 200 with four input channel.
Commercial mineral oil VASSA LP-90 was used as the liquid phase because it has low toxicity and due to the fact that its physical properties (density and viscosity) at ambient temperature are similar to those of heavy oil, residual oil, or any other heavy hydrocarbon in operation conditions of hidro-cracking processes. Air and carbon dioxide were used at atmospheric pressure in the injection into the column. The air was supplied from the compression system from pilot plant services, which has about 6.8 bar, and carbon dioxide was supplied from gas cylinders, which have about 55.1 bar, connected to a pressure regulator to make pressure drop to 6.8 bar. The solid phase was ground petroleum coke. The particles sizes were measured by laser scattering of HORIBA device. The properties of fluids and solids are shown in Tables 1 and 2.
2.2 Experimental Methodology
The unit was operating in recycling mode. In order to start the unit, a concentration of 1.5 wt % of solid with respect to the feedstock was mixed in the stirred tank. Then valves 2, 3, and 4 were opened (see Fig. 1), and after that pumps were started to begin the loading of the column.
The volumetric phase fraction was determined by phase trapping. This method consists in the rapid closing of the inlet and outlet valves of the column. This allows for an instantaneous and direct measure of the gas phase fraction inside the column. In operation, once the column reaches a steady value of pressure drop, the volume of gas phase is determined between the inlet and outlet valves of the column. This method has been used in vertical and horizontal pipes for oil production and transportation (Hagedorn and Brown 1965; Beggs and Brill 1973; Griffith et al. 1975) and also in the study of cold models in bubble column and slurry bubble column reactors (Tai-Ming et al. 1987; Wenge et al. 1995; Jena et al. 2008).
The experimental measurements were done when the stabilization time was reached, defined as the time when the differential pressure, between the fluidized zone, achieved an almost constant value. The estimated stabilization time was 12–16 h, approximately. Once the system has stabilized, the sampling procedure consists in closing the inlet valve just before the lower cone, and simultaneously opening the by-pass valve number 2. After that, it is necessary to keep the column for at least 12 h, until the phases have separated inside it. Finally, the volumes are measured directly through observation.
In addition, during the tests with air/oil/coke particles the gas density effect on the gas volumetric fraction was tested. After each test, the gas phase was switched to \(\mathrm{{CO}}_{2}\) during 30 min. This time was considered enough to get gas stabilization in this case.
3 Results and Analysis
The gas-liquid-solid phase distribution was determined in a slurry bubble column by means of cold modelling, following the experimental procedure described above. The temperature was kept at 25 \(\pm \) 2 \(^\circ \) C at atmospheric pressure. Figure 2 shows that the gas volume fraction increases with the gas flow. This agrees with the results reported by Akita and Yoshida (1973); Shah et al. (1982); Pino et al. (1990a, b, 1992); Hoefsloot and Krishna (1993); Wenge et al. (1995) and Jena et al. (2008). The accumulated gas volume holds an average value between 0.17 and 0.30 v/v for all superficial gas velocities and solid phases tested.
Figure 2 also shows comparisons between two and three-phase studies reported by Pino et al. (1990a, 1992) and these experiments. The former authors used a bubble column of 100 mm internal diameter without gas sparger. The gas superficial velocity was varied in the range between 2 and 10 cm/s. In continuous mode operation, the average slurry superficial velocity was 0.5 cm/s. The solid concentration was 126 kg/m\(^{3}\) with a particle range size of 3–180 microns and density of 4,530 kg/m\(^{3}\). In the air-oil system mode, the gas volumetric phase is higher than in the air-kerosene system. This behaviour is due to the effect of the superficial liquid velocity, which is higher in the air-kerosene test. However, this effect is rather small in the two systems. On the other hand, the gas volumetric phase in the air-kerosene-solid system is lower than in the air-oil-coke testing. This performance is due to the fact that the solid concentration in the air-kerosene-solid system, is greater than in the air-oil-coke test, which increases the occurrence of bubble coalescence, thus inducing the formation of large bubbles with a high rise velocity and, consequently, a fast disengagement of the column, which then allows the gas volumetric phase to decrease (Kantarci et al. 2005; Behkish et al. 2007). Moreover, a lower average particle size facilitates particle movement throughout the bubble column, thus increasing the area of the solid-liquid contact. This also causes a decrease of the gas volumetric phase.
In Fig. 3, the effect of the gas density on the gas volumetric fraction is shown as a function of the gas velocity for the air/oil/coke and the CO\(_{2}\)/oil/coke systems. The trend shows that the gas volume increases with the gas velocity a little more (between 1 and 2 %) for the CO\(_{2}\) system than for the air system, suggesting a small effect of the gas density between these two gases at the operation conditions tested. However, in systems with a pressure higher than atmospheric, it was found that the gas volumetric fraction increased and the number of fine bubbles formed with residence time in the system was significantly higher. For columns with a pressure higher than atmospheric, the gas density can be affected in a higher proportion (Krishna et al. 1991). A large gas-phase density increases the kinetic energy and momentum of the bubbles, and this leads to an increase of the collision energy which then promotes bubble rupture (Inga and Morsi 1999). These parameters could increase appreciably the gas volumetric fraction.
According to the data plotted in Fig. 4, the average volume fraction of liquid decreases from 0.79 to 0.68 v/v as the gas velocity increases from 3 to 10 cm/s. There is a difference in the average of the liquid volume between 1 and 2 %, for the CO\(_{2}\) system when compared with the air system, for gas superficial velocities of 6, 8, and 10 cm/s. This effect is, however, smaller at lower gas velocities of 3 and 4 cm/s.
Looking at Fig. 5, we may see that the solid volume also decreases as the superficial gas velocity increases in a range between 0.03 and 0.02 v/v for carbon particles at the gas superficial velocity tested. The low accumulation of coke inside the column, for all gas superficial velocities tested here, indicates a high solid drag exerted by the liquid. Moreover, due to the low solid concentration inside the column, the influence on the average volume fractions of liquid is small. However, the solids might cause an antifoaming effect, decreasing the gas volume and increasing the liquid volume in the column (Guitian and Joseph 1997).
Figure 6 shows the error bars with three experimental points for the air/oil/coke and the CO\(_{2}\)/oil/coke tests. There can be seen a standard deviation of 0.5 % for the air/oil/coke test and of 0.7 % for the CO\(_{2}\)/oil/coke test. From the analysis of the experimental data obtained the following correlation was developed for the three-phase systems:
This correlation takes into account particle properties, which appear to improve the correlation between the experimental and predicted gas volume fractions. It has an average error of 2 %, a standard deviation of 1 %, and a maximum error of 4 %, which is lower than the values obtained from other correlations in the literature as, for example, those reported by Hikita et al. (1980); Smith et al. (1984); Fan et al. (1999) and Urseanu et al. (2003) for different systems. Hikita et al. (1980) correlation had the lower average error (5 %), assuming for the gas volume fraction calculation that the slurry properties were given by the liquid properties, because their correlation applies only to two-phase systems (i.e., gas-liquid). As expected, from Fig. 7 we may see that the parity for the correlation developed here is higher than for the other correlations.
Finally, Fig. 8 shows a parity graph between the correlation obtained in this work and the experimental data of Pino et al. (1992). This latter correlation has an average error of 34.5 % in this case. This occurs because there are appreciable differences in the solid particle characteristics, the concentration of particles, and the superficial liquid velocity between both sets of data.
Parity between experimental average gas volume fractions of Pino et al. (1992) and the predicted ones
4 Conclusions
The gas, liquid, and solid phase distribution was estimated in a bubble column under cold conditions. It was found that the gas density does not affect significantly the volumetric fractions of gas and liquid. The low solid concentration obtained during the experiments denotes a high drag exerted by the liquid and a small effect of the superficial gas velocity on solid concentration. On the other hand, the comparison between two-phase and three-phase systems along with experimental data from the literature indicates that in systems with a tendency to foaming, the solids, even in small amounts, reduce the gas volume fraction. A new correlation for predicting the gas volume fraction in three-phase systems was developed, which has shown good agreement with experimental data that takes into account particle properties.
References
Akita K, Yoshida F (1973) Gas holdup and volumetric mass transfer coefficient in bubble columns. Ind Eng Chem Process Des Dev 12:76–80
Beggs HD, Brill J (1973) A study of two-phase flow in inclined pipes. J Petrol Technol 25:607–617
Behkish A, Lemoine R, Sehabiague L, Oukaci R, Morsi BI (2007) Gas holdup and bubble size behavior in a large-slurry bubble column reactor operating with an organic liquid under elevated pressure and temperatures. Chem Eng J 128:69–84
Chengtian W, Kelsey S, Muthanna H (2008) Bubble dynamics investigation in a slurry bubble column. American Ins Chem Eng 54:1203–1212
Chiu T-M, Hoh Y-C, Wang W-K (1987) Dispersed-phase hydrodynamic characteristics and mass transfer in three-phase (liquid-liquid) fluidized beds. Ind Eng Chem Res 26:712–718
Dargar P, Macchi A (2006) Effect of surface-active agents on the phase holdups of three-phase fluidized beds. Chem Eng Process 45:764–772
Fan L-S, Yang GQM, Lee DJ, Tsuchiya K, Luo X (1999) Some aspects of high-pressure phenomena of bubble in liquids and liquid-solid suspensions. Chem Eng Sci 54:4681–4709
Griffith P, Woou LC, Cheong HP, Pearson JF (1975) Two phase pressure drop in inclined and vertical oil wells. M.I.T. Cambrige, USA (SPE 5667-MS), p 1–22
Guitian J, Joseph D (1997) How bubbly mixture foam and foam control using a fluidized bed. Int J Multiph Flow 21:1–16
Hagedorn AR, Brown KE (1965) Experimental study of pressure gradients occurring during continuous two-phase flow in small-diameter vertical conduits. J Petroleum Tech 17:475–484
Hikita H, Asai K, Tanigawa K, Segawa K, Kitao M (1980) Gas hold-up in bubble columns. Chem Eng J 20:59–67
Hikita H, Asai K, Tanigawa K, Segawa K, Kitao M (1981) The volumetric liquid-phase mass transfer coeficient in bubble colums. Chem Eng J 22:61–69
Hoefsloot H, Krishna R (1993) Influence of gas density on the stability of homogeneous flow in bubble columns. Ind Eng Chem Res 32:747–750
Inga J, Morsi B (1999) Effect of operating variables on the gas holdup in a large-scale slurry bubble column reactor operating with an organic liquid mixture. Ind Eng Chem Res 38:928–937
Jena H, Sahho B, Roy G, Meikap B (2008) Prediction of gas holdup in a three-phase fluidized bed from bed pressure drop measurement. Chem Eng Res Des 86:1301–1308
Kantarci N, Borak F, Ulgen KO (2005) Bubble column reactors. Process Biochem 40:2263–2283
Krishna R, Wilkinson PM, van Dierendock LL (1991) A model for gas holdup in bubble columns incorporating the influence of gas density on flow regine transitions. Chem Eng Sci 46:2491–2496
Mitra-Majumadar D, Farouk D, Shah T, Macken N, Oh Y (1998) Two- and three-phase flows in bubble columns: tumerical predictions and measurements. Ind Eng Chem Res 37:2284–2292
Murray P, Fan L (1989) Axial solid distribution in slurry bubble columns. Ind Eng Chem Res 28:1697–1703
Pino LZ, Yépez MM, Sáez AE, De Drago G (1990) An experimental study of gas holdup in two-phase bubble columns with foaming liquids. Chem Eng Comm 89:155–175
Pino LZ, Yépez MM, Sáez AE (1990) Hydrodynamics of a semi-batch slurry bubble column a foaming liquid. AIChE J 36:1758–1962
Pino LZ, Solari RB, Siquier S, Estévez LA, Yépez MM, Saez AE (1992) Effect of operating conditions on gas holdup in slurry bubble columns with a foaming liquid. Chem Eng Comm 177:367–382
Shah YT, Kelkar BG, Godbole SP (1982) Design parameters estimations for bubble column reactors. AlChE J 28(3):353–379
Smith DN, Fuchs W, Lynn RJ, Smith DH, Hess M (1984) Bubble behavior in a slurry column reactor model. Chemical and catalytic reactor modeling. ACS Symp Ser 237:125–147
Tai-Ming C, Ying C, Wai Ko W (1987) Dispersed-phase hydrodynamic characteritics and mass transfer in three-phase (liquid-liquid) fluidized beds. Ind Eng Chem Res 26:712–718
Urseanu M, Guit RPM, Stankiewicz A, van Kranenburg G, Lommen JHGM (2003) Influence of operating pressure on the gas hold-up in bubble columns for high viscous media. Chem Eng Sci 58:697–704
Wenge F, Yusuf C, Murray M (1995) A new method for the measurement of solids holdup in gas-liquid-solid three-phase systems. Ind Eng Chem Res 34:928–935
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Paiva-Rojas, M.V., Contreras-Andrade, V., Araujo, S.C. (2014). Gas-Liquid-Solid Volumetric Phase Distribution Estimation in a Cold Slurry Bubble Column System for Hydro-Conversion Processes. In: Sigalotti, L., Klapp, J., Sira, E. (eds) Computational and Experimental Fluid Mechanics with Applications to Physics, Engineering and the Environment. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-00191-3_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-00191-3_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00190-6
Online ISBN: 978-3-319-00191-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)