Abstract
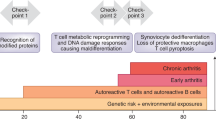
The sequence of events required for the development of rheumatoid arthritis (RA) remains poorly defined. Evidence strongly supports a genetic predisposition for disease development, and an association between certain class II major histocompatibility complex (MHC) alleles and the development and severity of RA has been established. Clearly, environmental factors are also likely to contribute to the disease process, although these etiological agents in RA have not been identified. Early histopathological alterations in the RA joint include morphological and phenotypic changes in the vascular endothelium, and hypertrophy of the synovial lining cells. However, also present at this stage is a modest perivascular accumulation of lymphocytes, including T lymphocytes. As disease progresses, this T cell infiltration becomes more pronounced and frequently more organized, and is accompanied by an influx of B lymphocytes, macrophages, dendritic cells and fibroblasts. Cytokines, primarily of macrophage and fibroblast origin, are secreted in abundance within the synovia and contribute in multiple ways to the progression of chronic inflammation, synovial cell proliferation and expansion, and cartilage destruction.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Firestein GS, Zvaifler NJ (1990) How important are T cells in chronic rheumatoid syn-ovitis? [published erratum appears in Arthritis Rheum 1990 Sep;33(9):14371. Arthritis Rheum 33: 768–773
Panayi GS, Lanchbury JS, Kingsley GH (1992) The importance of the T cell in initiat-ing and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum 35: 729–735
Cush JJ, Lipsky PE (1988) Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum 31: 1230–1238
Iannone F, Corrigall VM, Kingsley GH, Panayi GS (1994) Evidence for the continuousrecruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol 24: 2706–2713
Yokota A, Murata N, Saiki O, Shimizu M, Springer TA, Kishimoto T (1995) High avid-ity state of leukocyte function-associated antigen-1 on rheumatoid synovial fluid T lymphocytes. J Immunol 155: 4118–4124
Hernández-Garcia C, Fernández-Gutiérrez B, Morado IC, Bañares AA, Jover JA (1996)The CD69 activation pathway in rheumatoid arthritis synovial fluid T cells. Arthritis Rheum 39: 1277–1286
MacDonald KPA, Nishioka Y, Lipsky PE, Thomas R (1997) Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest 100: 2404–2414
Keystone EC, Poplonski L, Miller RG, Gorczynski R, Gladman D, Snow K (1988) Reactivity of T-cells from patients with rheumatoid arthritis to anti-CD3 antibody. Clin Immunol Immunopathol 48: 325–337
Verwilghen J, Vertessen S, Stevens EAM, Dequeker J, Ceuppens JL (1990) Depressed T-cell reactivity to recall antigens in rheumatoid arthritis. J Clin Immunol 10: 90–98
Kotzin BL, Leung DYM, Kappler J, Marrack P (1993) Superantigens and their potential role in human disease. Adv Immunol 54: 99–166
Trentham DE, Townes AS, Kang AH (1977) Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med 146: 857–868
Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B (1980) Immunisation against heterologous type II collagen induces arthritis in mice. Nature 283: 666–668
Verheijden GFM, Rijnders AWM, Bos E, Coenen-de Roo CJ, van Staveren CJJ, Miltenburg AMM, Meijerink JH, Elewaut D, de Keyser F, Veys E et al (1997) Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum 40: 1115–1125
Glant TT, Mikecz K, Arzoumanian A, Poole AR (1987) Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum 30: 201–212
Theofilopoulos AN, Dixon FJ (1985) Murine models of systemic lupus erythematosus. Adv Immunol 37: 269–390
Cohen PL, Eisenberg RA (1991) Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 9: 243–269
Hang L, Theofilopoulos AN, Dixon FJ (1982) A spontaneous rheumatoid arthritis-like disease in MRL/I mice. J Exp Med 155: 1690–1701
O’Sullivan FX, Fassbender H-G, Gay S, Koopman WJ (1985) Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/1 mice. I. The histomorphologic basis of joint destruction. Arthritis Rheum 28: 529–536
O’Sullivan FX, Vogelweid CM, Besch-Williford CL, Walker SE (1995) Differential effects of CD4+ T cell depletion on inflammatory central nervous system disease, arthritis and sialadenitis in MRL/lpr mice. J Autoimmun 8: 163–175
Nepom GT, Nepom BS (1992) Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen genotyping. Rheum Dis Clin North Am 18: 785–792
Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30: 1205–1213
Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364: 33–39
Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC (1994) Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 368: 215–221
Dessen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC (1997) X-ray crystal structure of HLA-DR4 (DRA’’0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity 7: 473–481
Brown JH, Jardetzky T, Saper MA, Samraoui B, Bjorkman PJ, Wiley DC (1988) A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules [published erratum appears in Nature 1988 Jun 23;333(6175):786]. Nature 332: 845–850
O’Sullivan D, Arrhenius T, Sidney J, del Guercio M-F, Albertson M, Wall M, Oseroff C, Southwood S, Colón SM, Gaeta FCA et al (1991) On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol 147: 2663–2669
Chicz RM, Urban RG, Lane WS, Gorga JC, Stern LJ, Vignali DA, Strominger JL (1992) Predominant naturally processed peptides bound to HLA-DR1 are derived from MHCrelated molecules and are heterogeneous in size. Nature 358: 764–768
Hammer J, Takacs B, Sinigaglia F (1992) Identification of a motif for HLA-DR1 binding peptides using M13 display libraries. J Exp Med 176: 1007–1013
Chicz RM, Urban RG, Gorga JC, Vignali DAA, Lane WS, Strominger JL (1993) Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med 178: 27–47
Hammer J, Valsasnini P, Tolba K, Bolin D, Higelin J, Takacs B, Sinigaglia F (1993) Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell 74: 197–203
Sette A, Sidney J, Oseroff C, del Guercio M-F, Southwood S, Arrhenius T, Powell MF, Colon SM, Gaeta FCA, Grey HM (1993) HLA DR4w4-binding motifs illustrate the biochemical basis of degeneracy and specificity in peptide-DR interactions. J Immunol 151: 3163–3170
Marshall KW, Liu AF, Canales J, Perahia B, Jorgensen B, Gantzos RD, Aguilar B, Devaux B, Rothbard JB (1994) Role of the polymorphic residues in HLA-DR molecules in allele-specific binding of peptide ligands. J Immunol 152: 4946–4957
Wucherpfennig KW, Strominger JL (1995) Selective binding of self peptides to disease-associated major histocompatibility complex (MHC) molecules: a mechanism for MHClinked susceptibility to human autoimmune diseases. J Exp Med 181: 1597–1601
Hammer J, Gallazzi F, Bono E, Karr RW, Guenot J, Valsasnini P, Nagy ZA, Sinigaglia F (1995) Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med 181: 1847–1855
Woulfe SL, Bono CP, Zacheis ML, Kirschmann DA, Baudino TA, Swearingen C, Karr RW, Schwartz BD (1995) Negatively charged residues interacting with the p4 pocket confer binding specificity to DRB1*0401. Arthritis Rheum 38: 1744–1753
Rosloniec EF, Brand DD, Myers LK, Whittington KB, Gumanovskaya M, Zaller DM, Woods A, Altmann DM, Stuart JM, Kang AH (1997) An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med 185: 1113–1122
Rosloniec EF, Brand DD, Myers LK, Esaki Y, Whittington KB, Zaller DM, Woods A, Stuart JM, Kang AH (1998) Induction of autoimmune arthritis in HLA-DR4 (DRB1*0401) transgenic mice by immunization with human and bovine type II collagen. J Immunol 160: 2573–2578
Fugger L, Rothbard JB, Sonderstrup-McDevitt G (1996) Specificity of an HLADRB1*0401-restricted T cell response to type II collagen. Eur J Immunol 26: 928–933
Goronzy JJ, Weyand CM (1993) Interplay of T lymphocytes and HLA-DR molecules in rheumatoid arthritis. Curr Opin Rheumatol 5: 169–177
Malhotra U, Spielman R, Concannon P (1992) Variability in T cell receptor V13 gene usage in human peripheral blood lymphocytes. Studies of identical twins, siblings, and insulin-dependent diabetes mellitus patients. J Immunol 149: 1802–1808
Hawes GE, Struyk L, van den Elsen PJ (1993) Differential usage of T cell receptor V gene segments in CD4+ and CD8+ subsets of T lymphocytes in monozygotic twins. J Immunol 150: 2033–2045
Davey MP, Meyer MM, Bakke AC (1994) T cell receptor Vß gene expression in monozygotic twins. Discordance in CD8 subset and in disease states. J Immunol 152: 315–321
Walser-Kuntz DR, Weyand CM, Weaver AJ, O’Fallon WM, Goronzy JJ (1995) Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity 2: 597–605
Kohsaka H, Nanki T, Oilier WER, Miyasaka N, Carson DA (1996) Influence of the rheumatoid arthritis-associated shared epitope on T-cell receptor repertoire formation. Proc Assoc Am Physicians 108: 323–328
Penzotti JE, Nepom GT, Lybrand TP (1997) Use of T cell receptor/HLA-DRB1’04 molecular modeling to predict site-specific interactions for the DR shared epitope associated with rheumatoid arthritis. Arthritis Rheum 40: 1316–1326
Hiraiwa A, Yamanaka K, Kwok WW, Mickelson EM, Masewicz S, Hansen JA, Radka SF, Nepom GT (1990) Structural requirements for recognition of the HLA-Dw14 class II epitope: a key HLA determinant associated with rheumatoid arthritis. Proc Natl Acad Sci USA 87: 8051–8055
Penzotti JE, Doherty D, Lybrand TP, Nepom GT (1996) A structural model for TCR recognition of the HLA class II shared epitope sequence implicated in susceptibility to rheumatoid arthritis. J Autoimmun 9: 287–293
Nepom GT, Ou D, Lybrand TP, DeWeese C, Domeier ME, Buckner JH, Mitchell LA, Tingle AJ (1996) Recognition of altered self major histocompatibility complex molecules modulated by specific peptide interactions. Eur J Immunol 26: 949–952
Roudier C, Auger I, Roudier J (1996) Molecular mimicry reflected through database screening: serendipity or survival strategy? Immunol Today 17: 357–358
Roudier J, Petersen J, Rhodes GH, Luka J, Carson DA (1989) Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR (3–1 chain and the Epstein-Barr virus glycoprotein gp110. Proc Natl Acad Sci USA 86: 5104–5108
La Cava A, Nelson JL, 011ier WER, MacGregor A, Keystone EC, Thorne JC, Scavulli JF, Berry CC, Carson DA, Albani S (1997) Genetic bias in immune responses to a cassette shared by different microorganisms in patients with rheumatoid arthritis. J Clin Invest 100: 658–663
Albani S, Carson DA (1996) A multistep molecular mimicry hypothesis for the pathogenesis of rheumatoid arthritis. Immunol Today 17: 466–470
Salvat S, Auger I, Rochelle L, Begovich A, Geburher L, Sette A, Roudier J (1994) Tolerance to a self-peptide from the third hypervariable region of HLA DRB1*0401 in rheumatoid arthritis patients and normal subjects. J Immunol 153: 5321–5329
Albani S, Keystone EC, Nelson JL, 011ier WER, La Cava A, Montemayor AC, Weber DA, Montecucco C, Martini A, Carson DA (1995) Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med 1: 448–452
Auger I, Escola JM, Gorvel JP, Roudier J (1996) HLA-DR4 and HLA-DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70-kD heat shock proteins. Nat Med 2: 306–310
Auger I, Roudier J (1997) A function for the QKRAA amino acid motif: mediating binding of DnaJ to DnaK. Implications for the association of rheumatoid arthritis with HLADR4. J Clin Invest 99: 1818–1822
Zanelli E, Gonzalez-Gay MA, David CS (1995) Could HLA-DRB1 be the protective locus in rheumatoid arthritis? Immunol Today 16: 274–278
Nepom GT, Erlich H (1991) MHC class-II molecules and autoimmunity. Annu Rev Immunol 9: 493–525
Gonzalez-Gay MA, Nabozny GH, Bull MJ, Zanelli E, Douhan J, Griffiths MM, Glimcher LH, Luthra HS, David CS (1994) Protective role of major histocompatibility complex class II Ebd transgene on collagen-induced arthritis. J Exp Med 180: 1559–1564
Nabozny GH, Baisch JM, Cheng S, Cosgrove D, Griffiths MM, Luthra HS, Davis CS (1996) HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J Exp Med 183: 27–37
Bradley DS, Nabozny GH, Cheng S, Zhou P, Griffiths MM, Luthra HS, David CS (1997) HLA-DQB1 polymorphism determines incidence, onset, and severity of collagen-induced arthritis in transgenic mice. Implications in human rheumatoid arthritis. J Clin Invest 100: 2227–2234
Zanelli E, Krco CJ, Baisch JM, Cheng S, David CS (1996) Immune response of HLADQ8 transgenic mice to peptides from the third hypervariable region of HLA-DRB1 correlates with predisposition to rheumatoid arthritis. Proc Natl Acad Sci USA 93: 1814–1819
Zanelli E, Krco CJ, David CS (1997) Critical residues on HLA-DRB1“0402 HV3 peptide for HLA-DQ8-restricted immunogenicity: implications for rheumatoid arthritis predisposition. J Immunol 158: 3545–3551
Jawaheer D, Thomson W, MacGregor AJ, Carthy D, Davidson J, Dyer PA, Silman AJ, Ollier WER (1994) “Homozygosity” for the HLA-DR shared epitope contributes the highest risk for rheumatoid arthritis concordance in identical twins. Arthritis Rheum 37: 681–686
Weyand CM, Xie C, Goronzy JJ (1992) Homozygosity for the HLA-DRB1 allele selects for extraarticular manifestations in rheumatoid arthritis. J Clin Invest 89: 2033–2039
Weyand CM, Hicok KC, Conn DL, Goronzy JJ (1992) The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med 117: 801–806
Evans TI, Han J, Singh R, Moxley G (1995) The genotypic distribution of shared-epitope DRB1 alleles suggests a recessive mode of inheritance of the rheumatoid arthritis disease-susceptibility gene. Arthritis Rheum 38: 1754–1761
Moreno I, Valenzuela A, García A, Yélamos J, Sánchez B, Hernánz W (1996) Association of the shared epitope with radiological severity of rheumatoid arthritis. J Rheumatol 23: 6–9
Weyand CM, McCarthy TG, Goronzy JJ (1995) Correlation between disease phenotype and genetic heterogeneity in rheumatoid arthritis. J Clin Invest 95: 2120–2126
Berg LJ, Frank GD, Davis MM (1990) The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell 60: 1043–1053
Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher H-P, Zinkernagal RM, Tonegawa S (1994) Evidence for a differential avidity model of T cell selection in the thymus. Cell 76: 651–663
Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR (1994) T cell receptor antagonist peptides induce positive selection. Cell 76: 17–27
Ricalton NS, Paliard X, Kappler J, Marrack P, Kotzin BL (1996) Analysis of TcRBV expression in human populations for evidence of superantigen exposure. The Immunologist 4: 106–108
McDermott M, Kastner DL, Holloman JD, Schmidt-Wolf G, Lundberg AS, Sinha AA, Hsu C, Cashin P, Molloy MG, Mulcahy B et al (1995) The role of T cell receptor 13 chain genes in susceptibility to rheumatoid arthritis. Arthritis Rheum 38: 91–95
Mu H, Charmley P, King M-C, Criswell LA (1996) Synergy between T cell receptor [3 gene polymorphism and HLA-DR4 in susceptibility to rheumatoid arthritis. Arthritis Rheum 39: 931–937
Cornélis F, Hardwick L, Flipo RM, Martinez M, Lasbleiz S, Prud’Homme JF, Tran TH, Walsh S, Delaye A, Nicod A et al (1997) Association of rheumatoid arthritis with an amino acid allelic variation of the T cell receptor. Arthritis Rheum 40: 1387–1390
Hall FC, Brown MA, Weeks DE, Walsh S, Nicod A, Butcher S, Andrews LJ, Wordsworth BP (1997) A linkage study across the T cell receptor A and T cell receptor B loci in families with rheumatoid arthritis. Arthritis Rheum 40: 1798–1802
Charmley P, Nepom BS, Concannon P (1994) HLA and T cell receptor (3-chain DNA polymorphisms identify a distinct subset of patients with pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum 37: 695–701
Maksymowych WP, Gabriel CA, Luyrink L, Melin-Aldana H, Elma M, Giannini EH, Lovell DJ, Van Kerckhove C, Leiden J, Choi E et al (1992) Polymorphism in a T-cell receptor variable gene is associated with susceptibility to a juvenile rheumatoid arthritis subset. Immunogenetics 35: 257–262
Luyrink L, Gabriel CA, Thompson SD, Grom AA, Maksymowych WP, Choi E, Glass DN (1993) Reduced expression of a human Vß6.1 T-cell receptor allele. Proc Natl Acad Sci USA 90: 4369–4373
Acha-Orbea H, Mitchell DJ, Timmermann L, Wraith DC, Tausch GS, Waldor MK, Zamvil SS, McDevitt HO, Steinman L (1988) Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell 54: 263–273
Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, Ando DG, Sercarz EE, Hood L (1988) Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell 54: 577–592
Offner H, Buenafe AC, Vainiene M, Celnik B, Weinberg AD, Gold DP, Hashim G, Vandenbark AA (1993) Where, when, and how to detect biased expression of disease-relevant V(3 genes in rats with experimental autoimmune encephalomyelitis. J Immunol 151: 506–517
Keystone EC, Minden M, Klock R, Poplonski L, Zalcberg J, Takadera T, Mak TW (1988) Structure of T cell antigen receptor)3 chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum 31: 1555–1557
Stamenkovic I, Stegagno M, Wright KA, Krane SM, Amento EP, Colvin RB, Kurnick JT (1988) Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci USA 85: 1179–1183
Duby AD, Sinclair AK, Osborne-Lawrence SL, Zeldes W, Kan L, Fox DA (1989) Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci USA 86: 6206–6210
Miltenburg AMM, van Laar JM, Daha MR, de Vries RRP, van den Elsen PJ, Breedveld FC (1990) Dominant T-cell receptor (3-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol 31: 121–126
Cooper SM, Dier DL, Roessner KD, Budd RC, Nicklas JA (1991) Diversity of rheumatoid synovial tissue T cells by T cell receptor analysis. Oligoclonal expansion in interleukin-2-responsive cells. Arthritis Rheum 34: 537–546
van Laar JM, Miltenburg AMM, Verdonk MJA, Daha MR, de Vries RRP, van den Elsen PJ, Breedveld FC (1991) Lack of T cell oligoclonality in enzyme-digested synovial tissue and in synovial fluid in most patients with rheumatoid arthritis. Clin Exp Immunol 83: 352–358
van Laar JM, Miltenburg AMM, Verdonk MJA, Daha MR, de Vries RRP, van den Elsen PJ, Breedveld FC (1992) T-cell receptor (3-chain gene rearrangements of T-cell populations expanded from multiple sites of synovial tissue obtained from a patient with rheumatoid arthritis. Scand J Immunol 35: 187–194
Bucht A, Oksenberg JR, Lindblad S, Grönberg A, Steinman L, Klareskog L (1992) Characterization of T-cell receptor a(3 repertoire in synovial tissue from different temporal phases of rheumatoid arthritis. Scand J Immunol 35: 159–165
Fischer D-C, Opalka B, Hoffmann A, Mayr W, Haubeck H-D (1996) Limited heterogeneity of rearranged T cell receptor Va and Vß transcripts in synovial fluid T cells in early stages of rheumatoid arthritis. Arthritis Rheum 39: 454–462
Williams WV, Fang Q, Demarco D, VonFeldt J, Zurier RB, Weiner DB (1992) Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest 90: 326–333
Bröker BM, Korthäuer U, Heppt P, Weseloh G, de la Camp R, Kroczek RAE, Emmrich F (1993) Biased T cell receptor V gene usage in rheumatoid arthritis. Oligoclonal expansion of T cells expressing V03B12 genes in synovial fluid but not in peripheral blood. Arthritis Rheum 36: 1234–1243
Lunardi C, Marguerie C, So AK (1992) An altered repertoire of T cell receptor V gene expression by rheumatoid synovial fluid T lymphocytes. Clin Exp Immunol 90: 440–446
Maruyama T, Saito I, Miyake S, Hashimoto H, Sato K, Yagita H, Okumura K, Miyasaka N (1993) A possible role of two hydrophobic amino acids in antigen recognition by synovial T cells in rheumatoid arthritis. Eur J Immunol 23: 2059–2065
Pluschke G, Ricken G, Taube H, Kroninger S, Melchers I, Peter HH, Eichmann K, Krawinkel U (1991) Biased T cell receptor Va region repertoire in the synovial fluid of rheumatoid arthritis patients. Eur J Immunol 21: 2749–2754
Uematsu Y, Wege H, Straus A, Ott M, Bannwarth W, Lanchbury J, Panayi G, Steinmetz M (1991) The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci USA 88: 8534–8538
Sottini A, Imberti L, Gorla R, Cattaneo R, Primi D (1991) Restricted expression of T cell receptor V(3 but not Va genes in rheumatoid arthritis. Eur J Immunol 21: 461–466
Struyk L, Kurnick JT, Hawes GE, van Laar JM, Schipper R, Oksenberg JR, Steinman L, de Vries RRP, Breedveld FC, van den Elsen P (1993) T-cell receptor V-gene usage in synovial fluid lymphocytes of patients with chronic arthritis. Hum Immunol 37: 237–251
Davey MP, Munkirs DD (1993) Patterns of T-cell receptor variable 13 gene expression by synovial fluid and peripheral blood T-cells in rheumatoid arthritis. Clin Immunol Immunopathol 68: 79–87
Jenkins RN, Nikaein A, Zimmermann A, Meek K, Lipsky PE (1993) T cell receptor V(3 gene bias in rheumatoid arthritis. J Clin Invest 92: 2688–2701
Pluschke G, Ginter A, Taube H, Melchers I, Peter HH, Krawinkel U (1993) Analysis of T cell receptor V(3 regions expressed by rheumatoid synovial T lymphocytes. Immunobiol 188: 330–339
Cooper SM, Roessner KD, Naito-Hoopes M, Howard DB, Gaur LK, Budd RC (1994) Increased usage of Vß2 and Vß6 in rheumatoid synovial fluid T cells. Arthritis Rheum 37: 1627–1636
Howell MD, Diveley JP, Lundeen KA, Esty A, Winters ST, Carlo DJ, Brostoff SW (1991) Limited T-cell receptor (3-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci USA 88: 10921–10925
Paliard X, West SG, Lafferty JA, Clements JR, Kappler JW, Marrack P, Kotzin BL (1991) Evidence for the effects of a superantigen in rheumatoid arthritis. Science 253: 325–329
Sioud M, Kjeldsen-Kragh J, Quayle AJ, Wiker HG, Sorskaar D, Natvig JB, Forre O (1991) Immune responses to 18.6 and 30-kDa mycobacterial antigens in rheumatoid patients, and Vß usage by specific synovial T-cell lines and fresh T cells. Scand J Immunol 34: 803–812
Zagon G, Tumang JR, Li Y, Friedman SM, Crow MK (1994) Increased frequency of Vß17-positive T cells in patients with rheumatoid arthritis. Arthritis Rheum 37: 1431–1440
Li Y, Sun G-R, Tumang JR, Crow MK, Friedman SM (1994) CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest 94: 2525–2531
Pannetier C, Even J, Kourilsky P (1995) T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today 16: 176–181
Hingorani R, Monteiro J, Furie R, Chartash E, Navarrete C, Pergolizzi R, Gregersen PK (1996) Oligoclonality of Vß3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J Immunol 156: 852–858
Lim A, Toubert A, Pannetier C, Dougados M, Charron D, Kourilsky P, Even J (1996) Spread of clonal T-cell expansions in rheumatoid arthritis patients. Hum Immunol 48: 77–83
Ikeda Y, Masuko K, Nakai Y, Kato T, Hasanuma T, Yoshino S-I, Mizushima Y, Nishioka K, Yamamoto K (1996) High frequencies of identical T cell clonotypes in synovial tissues of rheumatoid arthritis patients suggest the occurrence of common antigen-driven immune responses. Arthritis Rheum 39: 446–453
Alam A, Lambert N, Lulé J, Coppin H, Mazières B, de Préval C, Cantagrel A (1996) Persistence of dominant T cell clones in synovial tissues during rheumatoid arthritis. J Immunol 156: 3480–3485
Kato T, Kurokawa M, Masuko-Hongo K, Sasakawa H, Sekine T, Ueda S, Yamamoto K, Nishioka K (1997) T cell clonality in synovial fluid of a patient with rheumatoid arthritis: persistent but fluctuant oligoclonal T cell expansions. J Immunol 159: 5143–5149
Masuko-Hongo K, Sekine T, Ueda S, Kobata T, Yamamoto K, Nishioka K, Kato T (1997) Long term persistent accumulation of CD8+ T cells in synovial fluid of rheumatoid arthritis. Ann Rheum Dis 56: 613–621
Goronzy JJ, Bartz-Bazzanella P, Hu W, Jendro MC, Walser-Kuntz DR, Weyand CM (1994) Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest 94: 2068–2076
Schmidt D, Goronzy JJ, Weyand CM (1996) CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 97: 2027–2037
Waase I, Kayser C, Carlson PJ, Goronzy JJ, Weyand CM (1996) Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum 39: 904–913
Struyk L, Hawes GE, Dolhain RJEM, van Scherpenzeel A, Godthelp B, Breedveld FC, van den Eisen PJ (1994) Evidence for selective in vivo expansion of synovial tissue-infiltrating CD4+ CD45RO+ T lymphocytes on the basis of CDR3 diversity. Int Immunol 6: 897–907
Struyk L, Hawes GE, Chatila MK, Breedveld FC, Kurnick JT, van den Eisen PJ (1995) T cell receptors in rheumatoid arthritis. Arthritis Rheum 38: 577–589
Rock EP, Sibbald PR, Davis MM, Chien Y-H (1994) CDR3 length in antigen-specific immune receptors. J Exp Med 179: 323–328
Chien Y-H, Davis MM (1993) How aß T-cell receptors `see’ peptide/MHC complexes. Immunol Today 14: 597–602
Fox DA (1997) The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum 40: 598–609
Hingorani R, Choi I-H, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK (1993) Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol 151: 5762–5769
Posnett DN, Sinha R, Kabak S, Russo C (1994) Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy” [published erratum appears in J Exp Med 1994 Mar 1; 179(3): 1077]. J Exp Med 179: 609–618
Fitzgerald JE, Ricalton NS, Meyer A-C, West SG, Kaplan H, Behrendt C, Kotzin BL (1995) Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol 154: 3538–3547
DerSimonian H, Sugita M, Glass DN, Maier AL, Weinblatt ME, Rème T, Brenner MB (1993) Clonal Va12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med 177: 1623–1631
Wang ECY, Lawson TM, Vedhara K, Moss PAH, Lehner PJ, Borysiewicz LK (1997) CDBhigh+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum 40: 237–248
Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K (1993) Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol 11: 729–766
Elson CJ, Barker RN, Thompson SJ, Williams NA (1995) Immunologically ignorant autoreactive T cells, epitope spreading and repertoire limitation. Immunol Today 16: 71–76
Lanzavecchia A (1995) How can cryptic epitopes trigger autoimmunity? J Exp Med 181: 1945–1948
Martens PB, Goronzy JJ, Schaid D, Weyand CM (1997) Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum 40: 1106–1114
Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H (1991) Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65: 305–317
Röcken M, Urban JF, Shevach EM (1992) Infection breaks T-cell tolerance. Nature 359: 79–82
Oldstone MBA (1997) Viruses and autoimmune diseases. Scand J Immunol 46: 320–325
Barnaba V, Sinigaglia F (1997) Molecular mimicry and T cell-mediated autoimmune disease. J Exp Med 185: 1529–1531
Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC (1996) Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384: 134–141
Wucherpfennig KW, Strominger JL (1995) Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80: 695–705
Hemmer B, Fleckenstein BT, Vergelli M, Jung G, McFarland H, Martin R, Wiesmüller KH (1997) Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med 185: 1651–1659
Londei M, Savill CM, Verhoef A, Brennan F, Leech ZA, Duance V, Maini RN, Feldmann M (1989) Persistence of collagen type II-specific T-cell clones in the synovial membrane of a patient with rheumatoid arthritis. Proc Natl Acad Sci USA 86: 636–640
Melchers I, Jooss-Rüdiger J, Peter HH (1997) Reactivity patterns of synovial T-cell lines derived from a patient with rheumatoid arthritis. I. Reactions with defined antigens and auto-antigens suggest the existence of multireactive T-cell clones. Scand J Immunol 46: 187–194
Solinger AM, Bhatnagar R, Stobo JD (1981) Cellular, molecular, and genetic characteristics of T cell reactivity to collagen in man. Proc Natl Acad Sci USA 78: 3877–3881
Klareskog L, Forsum U, Scheynius A, Kabelitz D, Wigzell H (1982) Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci USA 79: 3632–3636
Cuesta IA, Sud S, Song Z, Affholter JA, Karvonen RL, Fernández-Madrid F, Wooley PH (1997) T cell receptor (V(3) bias in the response of rheumatoid arthritis synovial fluid T cells to connective tissue antigens. Scand J Rheumatol 26: 166–173
Snowden N, Reynolds I, Morgan K, Holt L (1997) T cell responses to human type II collagen in patients with rheumatoid arthritis and healthy controls. Arthritis Rheum 40: 1210–1218
van Schooten WCA, Devereux D, Ho CH, Quan J, Aguilar BA, Rust CJJ (1994) Joint-derived T cells in rheumatoid arthritis react with self-immunoglobulin heavy chains or immunoglobulin-binding proteins that copurify with immunoglobulin. Eur J Immunol 24: 93–98
Leroux J-Y, Guerassimov A, Cartman A, Delaunay N, Webber C, Rosenberg LC, Banerjee S, Poole AR (1996) Immunity to the G1 globular domain of the cartilage proteoglycan aggrecan can induce inflammatory erosive polyarthritis and spondylitis in BALB/c mice but immunity to G1 is inhibited by covalently bound keratan sulfate in vitro and in vivo. J Clin Invest 97: 621–632
Singer II, Kawka DW, Bayne EK, Donatelli SA, Weidner JR, Williams HR, Ayala JM, Mumford RA, Lark MW, Glant TT et al (1995) VDIPEN, a metalloproteinase-generated neoepitope, is induced and immunolocalized in articular cartilage during inflammatory arthritis. J Clin Invest 95: 2178–2186
Mikecz K, Glant TT, Buzás E, Poole AR (1990) Proteoglycan-induced polyarthritis and spondylitis adoptively transferred to naive (nonimmunized) BALB/c mice. Arthritis Rheum 33: 866–876
Guerassimov A, Duffy C, Zhang Y, Banerjee S, Leroux J-Y, Reimann A, Webber C, Delaunay N, Vipparti V, Ronbeck L et al (1997) Immunity to cartilage link protein in patients with juvenile rheumatoid arthritis. J Rheumatol 24: 959–964
Blass S, Haferkamp C, Specker Ch, Schwochau M, Schneider M, Schneider EM (1997) Rheumatoid arthritis: autoreactive T cells recognising a novel 68k autoantigen. Ann Rheum Dis 56: 317–322
Nyirkos P, Golds EE (1990) Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem J 269: 265–268
Hakala BE, White C, Recklies AD (1993) Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 268: 25803–25810
Johansen JS, Jensen HS, Price PA (1993) A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol 32: 949–955
Toyosaki T, Tsuruta Y, Yoshioka T, Takemota H, Suzuki R, Tomita T, Ochi T (1998) Recognition of rheumatoid arthritis synovial antigen by CD4+, CD8- T cell clones established from rheumatoid arthritis joints. Arthritis Rheum 41: 92–100
Vaughn JH (1995) The Epstein-Barr virus in autoimmunity. Springer Sem Immunopathol 17: 203–230
Birkenfeld P, Haratz N, Klein G, Sulitzeanu D (1990) Cross-reactivity between the EBNA-1 p107 peptide, collagen, and keratin: implications for the pathogenesis of rheumatoid arthritis. Clin Immunol Immunopathol 54: 14–25
David-Ameline J, Lim A, Davodeau F, Peyrat MA, Berthelot JM, Semana G, Pannetier C, Gaschet J, Vie H, Even J et al (1996) Selection of T cells reactive against autologous B lymphoblastoid cells during chronic rheumatoid arthritis. J Immunol 157: 4697–4706
Scotet E, David-Ameline J, Peyrat MA, Moreau-Aubry A, Pinczon D, Lim A, Even J, Semana G, Berthelot JM, Breathnach R et al (1996) T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med 184: 1791–1800
van Eden W, Thole JER, van der Zee R, Noordzij A, van Embden JDA, Hensen EJ, Cohen IR (1988) Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331: 171–173
Jones DB, Coulson AFW, Duff GW (1993) Sequence homologies between hsp60 and autoantigens. Immunol Today 14: 115–118
Holoshitz J, Klajman A, Drucker I, Lapidot Z, Yaretzky A, Frenkel A, van Eden W, Cohen IR (1986) T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet 2: 305–309
Res PCM, Schaar CG, Breedveld FC, van Eden W, van Embden JDA, Cohen IR, de Vries RRP (1988) Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet 2: 478–480
Quayle AJ, Wilson KB, Li SG, Kjeldsen-Kragh J, Oftung F, Shinnick T, Forre O, Capra JD, Natvig JB (1992) Peptide recognition, T cell receptor usage and HLA restriction elements of human heat-shock protein (hsp) 60 and mycobacterial 65-kDa hsp-reactive T cell clones from rheumatoid synovial fluid. Eur J Immunol 22: 1315–1322
Celis L, Vandevyver C, Geusens P, Dequeker J, Raus J, Zhang J (1997) Clonal expansion of mycobacterial heat-shock protein-reactive T lymphocytes in the synovial fluid and blood of rheumatoid arthritis patients. Arthritis Rheum 40: 510–519
Pope RM, Lovis RM, Gupta RS (1992) Activation of synovial fluid T lymphocytes by 60-kd heat-shock proteins in patients with inflammatory synovitis. Arthritis Rheum 35: 43–48
De Graeff-Meeder ER, van der Zee R, Rijkers GT, Schuurman H-J, Kuis W, Bijlsma JWJ, Zegers BJ, van Eden W (1991) Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet 337: 1368–1372
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1998 Springer Basel AG
About this chapter
Cite this chapter
Falta, M.T., Kotzin, B.L. (1998). T Cells as primary players in rheumatoid arthritis. In: Miossec, P., Firestein, G.S., van den Berg, W.B. (eds) T Cells in Arthritis. Progress in Inflammation Research. Birkhäuser, Basel. https://doi.org/10.1007/978-3-0348-8823-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-0348-8823-3_11
Publisher Name: Birkhäuser, Basel
Print ISBN: 978-3-0348-9787-7
Online ISBN: 978-3-0348-8823-3
eBook Packages: Springer Book Archive