Abstract
Emulsions have a long history of use as potent and effective adjuvants in humans for a range of vaccines, particularly for influenza. Although older mineral oil- and water-in-oil-based emulsion adjuvants did not have an overall safety and tolerability profile to allow them to be acceptable for widespread use, a newer generation of oil-in-water adjuvants has been recently developed, based on the use of the biodegradable oil squalene. These adjuvants have shown particular value in the development of new generation vaccines to offer enhanced protection against both seasonal and pandemic strains of influenza virus. The first oil-in-water emulsion adjuvant included in an approved flu vaccine was MF59, which was originally licensed in Europe in 1997 as an improved influenza vaccine for the elderly. In the very recent past, MF59 and related adjuvants have shown their value by offering the possibility of significant antigen dose reductions and higher potency products in the face of the H1N1 pandemic emergency and other pandemic threats. The recent H1N1 global problem allowed the opportunity for widespread use of emulsion-based adjuvants in a range of population groups in a number of countries, in which strict monitoring of safety was the norm. Importantly, this widespread use allowed the safety profile of squalene-based emulsion adjuvants to be further substantiated in large and diverse populations of humans, including young children and pregnant women. It is our confident prediction that the coming years will see wider use and further licensures for oil-in-water emulsion adjuvants, particularly for improved flu vaccines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Early in the development of nonliving vaccines for widespread human use, modifications were made to enhance their potency. Martin Arrowsmith, the protagonist of the great American novel by the same name, is described as spending time in the laboratory preparing “lipovaccines” and expressing dismay at those promoting the superiority of vaccines suspended in “ordinary salt solutions” [1]. The first generation of lipovaccines, consisting of homogenized dried bacterial cells in lipids, reported in 1916 [2–4], was developed to overcome the relatively poor efficacy of killed bacterial vaccines, which required relatively high doses and multiple injections to induce protective immunity. This was in contrast to an alternative vaccine platform used at the time, live-attenuated organisms (e.g., smallpox) that provided suitable protection from a single dose.
Lipovaccine technology was used in human subjects, including the US military [5, 6], as an approach to increase vaccine potency, which enabled the use of decreasing doses of bacterial cells (dose sparing), as well as decreasing the number of injections required for protection (doseage sparing). These same challenges remain with us today, particularly in relation to influenza vaccines. Currently, a majority of influenza vaccines are produced in eggs, and since this technology is limited in capacity, global supplies are inadequate. Therefore, dose sparing through the use of adjuvants is a safe and practical means to increase vaccine supply. Also, because of the need to respond rapidly to new influenza outbreaks and to reduce the number of doses required to achieve the desired immune response, this represents another role for adjuvants in influenza vaccines. In addition, adjuvants are used to broaden the immune response to emerging influenza variants, as well as to increase responses in the elderly. This early history in lipovaccines has ultimately led to the development of safe emulsions as vaccine adjuvants.
2 Emulsion Technologies
Emulsions are defined as liquid dispersions of two immiscible phases, usually oil and water, either of which may comprise the dispersed phase or the continuous phase to provide water-in-oil (w/o) or oil-in-water (o/w) emulsions, respectively. Emulsions are generally unstable and need to be stabilized by surfactants, which lower interfacial tension and prevent coalescence of the dispersed droplets. Stable emulsions can be prepared through the use of surfactants that orientate at the interface between the two phases and reduce interfacial tensions, since surfactants comprise both hydrophobic and hydrophilic components. Although charged surfactants are excellent stabilizers, nonionic surfactants are widely used in pharmaceutical emulsions due to their lower toxicity and lower sensitivity to the destabilizing effects of formulation additives. Surfactants can be defined by their ratio of hydrophilic to hydrophobic components (hydrophile to lipophile balance, HLB), which gives information on their relative affinity for water and oil phases. At the high end of the scale, surfactants are predominantly hydrophilic and can be used to stabilize o/w emulsions. In contrast, oil-soluble surfactants are at the lower end of the scale and are used mainly to stabilize w/o emulsions. Polysorbates (Tweens) are commonly used surfactants with HLB values in the 9–16 range, while sorbitan esters (Spans) have an HLB in the range of 2–9. Extensive pharmaceutical experience has shown that a mixture of surfactants offers maximum emulsion stability, probably due to the formation of more rigid films at the interface. The physicochemical characteristics of emulsions, including droplet size, viscosity, and so on, are controlled by a variety of factors, including the choice of surfactants, the ratio of continuous to dispersed phases, and the method of preparation. For an emulsion to be used for administration as an injection, stability and viscosity are important parameters, as too is sterility. In general, stability is enhanced by having smaller sized droplets, while viscosity is decreased by having a lower volume of the dispersed phase.
3 The History of Emulsions as Adjuvants
3.1 Water-in-Oil Emulsion Adjuvants
The desire to increase vaccine potency through association with lipid ultimately resulted in the development of emulsions as adjuvants. Following on from the lipovaccine technology, Freund et al. in 1937 [7] and for many years thereafter [8–10] developed and used w/o emulsions, in which antigen was suspended in an aqueous phase and then emulsified into oil. Early versions included paraffin oil, with Arlacel A as the emulsifier, and the inclusion of dried Mycobacterium cells. Such emulsions are still referred to as complete Freund’s adjuvant (CFA or FCA). Typically, these emulsions contain >50% mineral oil. Freund also developed emulsions that did not contain bacteria (incomplete Freund’s adjuvant, FIA or IFA) and applied them experimentally to a number of vaccine preparations [8–11]. However, CFA was found to be unacceptably reactogenic for use in human vaccines, and sensitization to the mycobacterial component compromised its ability to be used in booster immunizations. Painful local reactions with frequent granuloma formation were observed when administered subcutaneously, and extensive granuloma formations and nerve involvement could occur when administered intramuscularly [12].
Improvements in oil-rich emulsions were made through eliminating the mycobacterial cells from the preparations and improving the quality of oils used (IFA) [13]. Unfortunately, preclinical studies suggested that oil-based adjuvants could be tumorigenic [14].
Nevertheless, IFA was developed and tested clinically for influenza vaccines, including landmark papers from Jonas Salk and others [15–17] that were proceeded by nonhuman primate studies for safety evaluation, followed by large-scale human trials [18–20]. Overall these studies demonstrated the acceptable safety and potency of the adjuvanted vaccines, including a dose sparing effect (up to 1,000-fold), the durability of antibody responses, and, in Salk’s studies, a suggestion of increased breadth of response. Importantly, long-term follow-up studies of army recruits (approximately 18,000 having received IFA-adjuvanted influenza vaccine) indicated that there were no serious safety effects attributable to the vaccines [21, 22]. Hence, these studies helped to alleviate the concerns raised in the preclinical models that oil-based adjuvants could be tumorgenic. Nonetheless, human vaccines containing IFA did not manage to gain broad acceptance. During the period of 1964–1965, 900,000 persons in the UK received a licensed seasonal influenza vaccine containing IFA, and 40 individuals developed local nodular reactions, 9 of which required surgical treatment [23]. On the basis of these observations, which had similarities to the reactions observed in experimental animals, the influenza vaccine was withdrawn from the market [23, 24]. This withdrawal essentially killed the future use of mineral oil-based emulsion adjuvants for human vaccines. Nevertheless, subsequent long-term (35 years) analysis of the army recruits who had received the mineral oil emulsions has shown that not only were there no significant adverse events associated with the emulsion, but there was also a statistically significant reduction in certain forms of cancers in the recruits who had received the adjuvant [25].
It had been thought that much of the toxicity associated with the w/o emulsions was related to the presence of free fatty acids, either in the source materials or resulting from hydrolysis over time of the oil or surfactant [26]. Attempts to improve upon these emulsions included the development of adjuvant 65, an emulsion which consisted of approximately 50% peanut oil [27]. This formulation was tested in 182 volunteers with influenza vaccine and gave higher antibody titers of increased duration than those seen in individuals receiving aqueous vaccine, with minimal differences in reactogenicity between the two groups. Follow-up studies led to influenza vaccine formulated in adjuvant 65 being given to more than 16,000 individuals, with increased immune responses, broadening of immune responses, and greater persistence of antibody responses [28]. Local reactions were deemed to be minor with the adjuvant 65 vaccine, comparing favorably to unadjuvanted vaccine. Interestingly, a formulation combining adjuvant 65 with polyI:polyC, now known to activate innate immunity through toll-like receptor 3 (TLR3), significantly added to the potency of the adjuvant for influenza vaccine in monkeys [28]. However, the use of peanut oil emulsions did not advance significantly, partly due to potential safety concerns in individuals with peanut allergies.
More recent w/o emulsions have included the introduction of purified, metabolizable oils and emulsifiers. The most notable of these emulsions is the Montanide adjuvants (Seppic, Paris, France) which are based on purified squalene and squalane, emulsified with a highly purified mannide mono-oleate surfactant. These emulsions have been evaluated in clinical trials, notably for malaria, HIV, and cancer [29]. Several clinical studies with ISA51 and ISA720, two of the Montanide adjuvants, have reported on potent immune responses, although safety results seem to be somewhat questionable, with the incidence of adverse events and severe adverse events increasing with antigen dose and the number of administrations [30, 31].
Despite the ultimate failure of the oil-rich emulsions in terms of their adoption into licensed human vaccines, the use of these preparations demonstrated, over several decades, the value of adjuvanting influenza and other human vaccines. However, the mechanism through which w/o emulsions potentiate the immune response to vaccine antigens is unclear. It had been thought that these high oil content w/o emulsions functioned through a “depot effect,” releasing antigen over time, but such a concept appears inconsistent with the observed kinetics of immune responses following administration of such adjuvanted vaccines. Moreover, studies in which immune response remained strong despite early excision of injections sites in experimental animals would argue against this mechanism of action [32]. The real mechanism of effect may be due to a combination of increased antigen uptake through association with lipid and antigen-presenting cell (APC) activation from the adjuvant components. However, overall safety concerns with high lipid content adjuvants led to emphasis on the development of standardized methods to produce formulations with lower lipid content that would neither form depots nor induce local granuloma and/or ulceration.
3.2 Oil-in-Water Emulsion Adjuvants: The Early Years
Extensive experience in animals and humans, first with lipovaccines, then with w/o emulsions, firmly established the value of formulations containing lipid for adjuvanting nonliving organisms to create more effective vaccines. Attempts to improve the safety of oil-containing adjuvant formulations while maintaining potent immune-stimulatory properties have led to the development of several emulsions with reduced oil content, typically <5% oil. The most advanced of these o/w emulsion adjuvants is MF59, but several others are in various stages of development. The underlying principles behind the development of these new emulsion formulations, in addition to using lower amounts of oil, were to use metabolizable oil (as opposed to mineral oil in the Freund’s formulations), use nontoxic emulsifiers instead of Arlacel A, and retain efficacy while reducing toxicity.
Early work by Ribi et al. [33] described the use of o/w emulsions containing squalene, a metabolizable cholesterol precursor obtained from shark liver and Tween 80 surfactant, to which immunostimulants such as monophosphoryl lipid A (MPL), a glycolipid purified from Gram-negative bacteria and/or mycobacterial-derived components were added to create the Ribi adjuvant systems. Another early program was described by Syntex Corp. (reviewed by Allison [34]), which developed the Syntex adjuvant formulation (SAF), a 5% squalane, prepared by hydrogenation of squalene, o/w emulsion, that included polysorbate (Tween) 80 as an emulsifier. Formulations also included Pluronic 121, a block copolymer, and muramyl dipeptide (MDP), an adjuvant peptide based on a structure derived from mycobacterial cell walls. Thus, SAF and the Ribi adjuvant series (reviewed in [35]) represented a significant advance over the Freund formulations. Both Ribi adjuvants and SAF were tested in a variety of animal models, and the safety and efficacy profile was appropriate to allow clinical evaluation in cancer vaccine trials. Other early emulsions included the Hjorth formulations, which were also squalene based (reviewed in [34]).
The most advanced o/w emulsions currently in development include AS03 (reviewed below) and AS02 from GSK. AS03 is a squalene- and vitamin E-based emulsion, extensively developed for influenza vaccines. AS02 is an emulsion that contains MPL and QS-21, a purified molecule derived from saponin. The addition of MPL and QS-21 results in a formulation that has potent B- and T-cell-stimulating properties and has been extensively tested with malaria vaccine candidates, among others. AS02 and AS01, a liposomal formulation, are in advanced clinical development for malaria, tuberculosis, and other vaccine candidates [29].
3.3 The Development of Oil-in-Water Emulsions as Adjuvants for Flu Vaccines
There are several reasons why adding adjuvants to influenza vaccines is important. These include (1) to enhance protective immune responses in the elderly, the population in which the vast majority of influenza deaths occur, (2) to allow antigen dose sparing to increase the global vaccine supply, (3) to induce a rapid immune response in the case of the emergence of a pandemic, and (4) to induce a broader immune response to protect against serotypes not present in the administered vaccine. Today, the o/w adjuvant formulations represent the best approach to provide the necessary safety profile while fulfilling at least some of these performance criteria.
There are currently two o/w emulsions in licensed influenza vaccines and at least two others in development. By far the greatest experience is with MF59 (Novartis Vaccines and Diagnostics), which is a component of licensed vaccines for both seasonal and pandemic influenza and will be discussed in detail below. The other emulsion adjuvant that is a component of a licensed pandemic influenza vaccine is AS03 (GlaxoSmithKline Biologicals, GSK). Other emulsions in development for influenza vaccines include AF03 (Sanofi Pasteur) and SE (Infectious Disease Research Institute) (Table 1). All of these emulsions are squalene based, generally with a content of 2–4%, with different surfactants to stabilize the emulsions. In addition, AS03 contains α-d-tocopherol (vitamin E), which has been claimed to have adjuvant properties of its own. The mechanisms of action of o/w adjuvants will be discussed later with respect to MF59 but generally include activation of APCs leading to increased antigen uptake, increase of cytokine production, and influencing APC migration to draining lymph nodes through upregulation of chemokine receptors.
AS03 (GSK) is being developed for both seasonal and pandemic influenza vaccines, including prepandemic vaccines to prime individuals against H5 to induce at least partial immunity against related influenza variants. Vaccines containing ASO3 have been evaluated in thousands of individuals. ASO3-H5N1 vaccine has been reported to be safe in both adults and children [36, 37]. The vaccine has been reported to be immunogenic, to be dose sparing, and to induce cross-clade immune responses [38, 39]. Pandemrix™, an AS03-H5N1 pandemic vaccine, and Prepandrix™, a prepandemic AS03-H5N1 vaccine, have been approved in Europe.
In addition to the pandemic influenza studies, AS03 has been developed for enhancing the efficacy of Fluarix™, GSK’s seasonal influenza vaccine, in the elderly. A current trial is in progress to determine the effect of ASO3 in enhancing protection against disease. Early studies compared adjuvanted versus unadjuvanted Fluarix™ and indicated the possibility that including ASO3 could lead to increased T-cell responses and broadened serological responses in elderly subjects (reviewed in [24]). At an earlier stage of development is AF03, the Sanofi Pasteur squalene-based emulsion. This adjuvant has been evaluated as a H5N1 vaccine candidate in 251 healthy adults [40], was found to be adequately safe and immunogenic, and demonstrated both a dose sparing and an immune broadening effect [24]. These results further enforce the utility of o/w emulsions as a safe and effective approach to enhance vaccine potency. The use of such adjuvants comprises an important and necessary solution to develop vaccines to emerging threats, such as pandemic influenza, which cannot be adequately addressed with traditional, unadjuvanted vaccines.
Although emulsions are the most advanced novel adjuvants, many other attempts have been made to develop successful adjuvants based on a range of related technologies. In the 1980s, a number of groups worked on the development of new adjuvant formulations, including emulsions, ISCOMs, liposomes, and microparticles [41]. These approaches had the potential to be more potent and effective adjuvants than insoluble aluminum salts, which were the only adjuvants included in licensed human vaccines at that time. Unfortunately, alum has been shown to be a poor adjuvant for split and subunit influenza vaccines, which comprise the majority of the currently licensed products. Many of the novel adjuvant approaches contained immune potentiators of natural or synthetic origin, which were included to enhance the potency of the adjuvant. However, the inclusion of immune potentiators often raised concerns about the safety of the adjuvant technology. On the basis of the long history of emulsions as adjuvants, including FIA, several groups investigated the development of improved emulsion formulations as adjuvants. As discussed, Syntex developed an o/w emulsion adjuvant (SAF) using the biodegradable oil, squalane, to deliver a synthetic immune potentiator, called N-acetyl-muramyl-l-threonyl-d-isoglutamine (threonyl-MDP) [42]. The closely related immune potentiator, N-acetyl-l-alanyl-d-isoglutamine (MDP), had been originally identified in 1974 as the minimal structure isolated from the peptidoglycan of mycobacterial cell walls, which had adjuvant activity [43]. However, MDP was pyrogenic and induced uveitis in rabbits [44], making it unacceptable as an adjuvant for human vaccines. Therefore, various synthetic derivatives of MDP were produced, in an effort to identify an adjuvant molecule with an acceptable safety profile; threonyl-MDP was one of these synthetic compounds. More recently, it has been shown that MDP activates immune cells through interaction with the nucleotide-binding domain, which acts as an intracellular recognition system for bacterial components [45]. In addition to threonyl-MDP, SAF also contained a pluronic polymer surfactant (L121), which was included to help bind antigens to the surface of the emulsion droplets. Unfortunately, clinical evaluations of SAF as an adjuvant for an HIV vaccine showed it to have an unacceptable profile of reactogenicity [46]. As an alternative to SAF, Chiron vaccines used squalene, a similar biodegradable oil, to develop an o/w emulsion as a delivery system for an alternative synthetic MDP derivative, muramyl-tripeptide phosphatidylethanolamine (MTP-PE). MTP-PE was lipidated to allow it to be more easily incorporated into lipid-like formulations and to reduce toxicity [47]. Unfortunately, clinical testing also showed that emulsions of MTP-PE displayed an unacceptable level of reactogenicity, which made them unsuitable for routine clinical use [48, 49]. Although the emulsion formulation of MTP-PE enhanced antibody responses against influenza vaccine in humans, the level of adverse effects observed made this adjuvant unsuitable for widespread clinical use [48]. Nevertheless, additional clinical studies undertaken at the same time highlighted that the squalene-based emulsion alone (MF59), without any added immune potentiator, was well tolerated and had comparable immunogenicity to the formulation containing the MTP-PE [49, 50]. These observations resulted in the further development of the MF59 o/w emulsion vehicle alone as a vaccine adjuvant.
In preclinical studies with influenza vaccine, it was confirmed that the immune potentiator, MTP-PE, was not required for MF59 to be an effective adjuvant [51]. A key early study highlighted the ability of MF59 adjuvant to enhance protective immunity to flu virus challenge [52]. The use of MF59 adjuvant allowed a dose reduction of flu vaccine (50- to 200-fold lower doses) and improved protection against challenge for more than 6 months after vaccination [52]. MF59-induced enhanced antibody titers in comparison with flu vaccine alone, even at very low antigen dose. Moreover, the addition of MF59 to flu vaccine offered improved survival against challenge with influenza virus in mice and also reduced viral titers in the lungs of challenged mice. The enhanced protection afforded by the inclusion of MF59 in the vaccine was long lived and allowed a significant dose reduction in the amount of antigen needed to induce protection. Moving beyond the mouse model, MF59 was also shown to be an effective adjuvant for flu vaccine in a range of alternative preclinical animal models [51]. Importantly, in follow-up studies, it was shown that MF59 was able to enhance the immune responses to flu vaccines in both young and old animals [53]. Old mice (18 months old in these studies) typically have poor responses to flu vaccines, as do elderly humans, but the inclusion of MF59 in the vaccine restored the response of the old mice back up to the level of response achieved in young mice. Moreover, MF59 was also shown to induce a potent T-cell response to the flu vaccine, in both young and old mice. Pushing the mouse model further, MF59 was also shown to be an effective adjuvant in old mice, which had previously been infected with influenza, a situation more similar to that found in humans, who are often reinfected annually with circulating flu strains [53]. These preclinical studies highlighted the huge potential of MF59 to be used as an adjuvant for an improved flu vaccine, potentially allowing antigen dose reduction, while enhancing protective antibody and T-cell responses, for extended time periods. The ability of MF59 adjuvant to offer a significant reduction in the protective dose for flu vaccines has subsequently become very important in the pandemic flu vaccine setting.
The small droplet size of MF59 adjuvant emulsion, generated through the use of a microfluidizer in the preparation process, is crucial to the potency of the adjuvant, and also enhances emulsion stability and allows the formulation to be sterile filtered for clinical use. Overall, our early clinical experience with o/w emulsions served to highlight the need for careful selection of immune potentiators to be included in adjuvant formulations. The experience with MF59 showed that o/w emulsions can be highly effective adjuvants, with an acceptable safety profile, which may not need the addition of immune potentiators.
4 The Current Status of Emulsion Adjuvants for Flu Vaccines
MF59 is a safe and potent emulsion-based vaccine adjuvant that has been licensed in more than 20 countries, for more than 12 years, for use in an influenza vaccine focused on elderly subjects (Fluad®). The safety profile of MF59 is well established clinically through a large safety database (>26,000 subjects) and through pharmacovigilance evaluations of greater than 55 million doses that have been distributed. The MF59 adjuvant has a significant impact on the immunogenicity of flu vaccines in the elderly, who generally respond poorly to traditional influenza vaccines due to age-related impairment of their immune responses called immunosenescence. Moving beyond the elderly population, the MF59 adjuvant has also been shown to have a significant impact on the immune response to flu vaccines in adults who are chronically ill with a range of diseases and, consequently, also respond poorly to traditional flu vaccines. Moreover, Fluad also shows enhanced immunogenicity in very young subjects, while displaying a similar reactogenicity profile to licensed vaccines in this population. Moving beyond seasonal flu vaccines, MF59 has also been shown to have a significant impact on the immunogenicity of potential pandemic flu vaccines and has enabled vaccines to achieve titers that might be expected to offer protection, with relatively low doses of vaccine. Moreover, the addition of MF59 to the vaccine allows for more broad cross-reactivity against viral strains not actually included in the vaccine. This is a key attribute, since it is difficult to predict exactly which strain might emerge and cause a pandemic. MF59 adjuvant recently received approval for licensure in Europe for all 27 member states for inclusion in a pandemic vaccine against H1N1 (Focetria®) for use in all subjects aged 6 months and older. This same vaccine adjuvant is also under consideration for approval for inclusion in a prepandemic vaccine (Aflunov®). Beyond its use in influenza vaccines, MF59 adjuvant has also been shown to be a potent adjuvant for a wide range of alternative vaccines, including those based on recombinant proteins, particulate antigens, and protein–polysaccharide conjugates. In most studies in which a comparison has been made, MF59 has been shown to be more potent for both antibody and T-cell responses than aluminum-based adjuvants. Moreover, clinical evaluations have established that the MF59 adjuvant is safe in a wide range of subjects from only a few days old to greater than 100 years of age. Hence, MF59 has broad potential to be used as a safe and effective vaccine adjuvant for a broad range of vaccines to be used in populations with a wide age range. The use of o/w adjuvants represents an important and necessary solution to develop vaccines to emerging threats, such as pandemic influenza, which cannot be adequately addressed with traditional, unadjuvanted vaccines.
4.1 The Composition of MF59
MF59 is a low oil content o/w emulsion. The oil used for MF59 is squalene, which is a naturally occurring substance found in plants and in the livers and skin of a range of species, including humans. Squalene is an intermediate in the human steroid hormone biosynthetic pathway and is a direct synthetic precursor to cholesterol. Therefore, squalene is biodegradable and biocompatible, since it is naturally occurring. Shark liver oil comprises 80% squalene and shark liver provides the natural source of the squalene, which is used to prepare MF59. MF59 also contains two nonionic surfactants, Tween 80 and Span 85, which are designed to optimally stabilize the emulsion droplets. Citrate buffer is also used in MF59 to stabilize pH. Although single-vial formulations can be developed with vaccine antigens dispersed directly in MF59, MF59 can also be added to antigens immediately before their administration. Although a less favorable option, combination before administration may be necessary to ensure optimal antigen stability for some antigens but not for flu.
4.2 Manufacturing of MF59
Details of the manufacturing process for MF59 at the 50-l scale have previously been described [54]. The process involves dispersing Span 85 in the squalene phase and Tween 80 in the aqueous phase before high-speed mixing to form a coarse emulsion. The coarse emulsion is then passed repeatedly through a microfluidizer to produce an emulsion of uniform small droplet size (165 nm), which can be sterile filtered and filled into vials. Methods have also been published to allow the preparation of MF59 on a small scale for use in research studies [55]. MF59 is extensively characterized by various physicochemical criteria after preparation.
4.3 The Mechanism of Action of MF59 Adjuvant
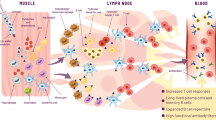
Early studies designed to determine the mechanism of action of MF59 focused on the possibility of the creation of a “depot” effect for coadministered antigen, since there had been suggestions that emulsions may retain antigen at the injection site. However, early work showed that an antigen depot was not established at the injection site and that the emulsion was cleared rapidly [56]. The lack of an antigen depot with MF59 was confirmed in later studies [57], which also established that MF59 and antigen were cleared independently. Subsequently, it was thought that perhaps the emulsion acted as a “delivery system” and was responsible for promoting the uptake of antigen into APCs. This theory was linked to earlier observations with SAF, which contained a pluronic surfactant that was thought to be capable of binding antigen to the emulsion droplets to promote antigen uptake [42]. However, studies with recombinant antigens showed that MF59 was an effective adjuvant, despite no evidence of binding of the antigens to the oil droplets [56]. A direct effect of MF59 on cytokine levels in vivo was also observed in separate studies, suggesting that the delivery method alone was too simplistic an explanation [58]. To gain a better understanding of the mechanism of action of MF59, we have studied the early steps of the immune response on human cells in vitro and in mouse muscle in vivo. We have shown that there are at least two human target cells for MF59, monocytes and granulocytes, and that MF59 has a range of effects, including increased antigen uptake, the release of chemoattractants, and the promotion of cell differentiation. The observation of increased antigen uptake is in line with previous findings in mice [59]. The most readily induced chemoattractant was the chemokine, CCL2, which is involved in cell recruitment. Previous work had shown a reduction of MF59-induced cell recruitment into the muscle in CCR2-deficient mice [60], which is consistent with our observations on human cells. Moreover, experiments on gene expression profiles at the injection site are also consistent with the key role of chemokines [61]. In addition, CCL2 was found in serum after injection of MF59 into mouse muscle, providing further consistency between in vitro and in vivo observations. MF59 also induces phenotypic changes on human monocytes that are consistent with a maturation process toward immature dendritic cells (DCs). There is an impressive consistency between data obtained in vitro from human cells and data obtained in vivo from mouse. These observations suggest that MF59 induces a local proinflammatory environment within the muscle, which promotes the induction of potent immune responses to coadministered vaccines. Figure 1 summarizes the mechanism of action of MF59.
A model for the mechanism of action of MF59 following immunization with the licensed seasonal influenza vaccine containing MF59 (Fluad). Adapted from [62]
Hence, we conclude that during vaccination, adjuvants like MF59 augment the immune response at a range of intervention points. Through induction of chemokines, they increase recruitment of immune cells to the injection site, they augment antigen uptake by monocytes at the injection site, and they enhance differentiation of monocytes into DCs, which represent the gold-standard cell type for priming naive T cells. A particularly important feature of MF59 is that it strongly induces the homing receptor CCR7 on maturing DCs, thus facilitating their migration into draining lymph nodes where they can trigger the adaptive immune response specific to the vaccine antigen. Nevertheless, further studies are necessary to better define the precise mechanism of action of MF59 and these studies are ongoing.
4.4 Preclinical Experience with MF59
Preclinical experience with MF59 is extensive and has been reviewed on several occasions previously [63–65]. MF59 has been shown to be a potent adjuvant in a diverse range of species, in combination with a broad range of vaccine antigens, to include recombinant protein antigens, isolated viral membrane antigens, bacterial toxoids, protein–polysaccharide conjugates, peptides, and virus-like particles. MF59 is particularly effective for inducing high levels of antibodies, including functional titers (neutralizing, bactericidal, and opsonophagocytic titers) and is generally more potent than alum.
In one study, we directly compared MF59 and alum for several different vaccines and confirmed that MF59 was generally more potent, although alum performed well for bacterial toxoids, particularly diphtheria toxoid [66]. MF59 has also shown enhanced potency over alum when directly compared in nonhuman primates with protein–polysaccharide conjugate vaccines [67] and with a recombinant viral antigen [55]. In preclinical studies, MF59 is the most potent adjuvant for flu vaccines in comparison with various readily available alternatives (Fig. 2). In one study, we compared a number of adjuvants for flu vaccine in mice and showed that MF59 significantly outperforms alternatives, including alum, for both antibody and T-cell responses [68]. Moreover, we have recently shown that MF59 offers enhanced protection against challenge with pandemic flu strains in mice [69], which is consistent with our earlier work on interpandemic strains [52]. Moreover, heterologous protection is achieved against challenge strains in ferrets [70]. In addition to immunogenicity studies, extensive preclinical toxicology studies have been undertaken with MF59 in combination with a range of different antigens in a number of species. In these studies, it has been shown that MF59 is neither mutagenic nor teratogenic and did not induce sensitization in an established guinea pig model to assess contact hypersensitivity. The favorable toxicological profile established for MF59 allowed extensive clinical testing for MF59 with a number of different vaccine candidates and the approval of a flu vaccine containing MF59 in Europe in 1997.
Serum hemagglutination inhibition titers in mice against the three strains of influenza virus included in seasonal vaccines (H3N2, H1N1, and B) in combination with adjuvants. The adjuvants evaluated included MF59 o/w emulsion, aluminum (Alum), calcium phosphate (CAP), poly-lactide co-glycolide microparticles (PLG), CpG oligonucleotide (CpG), and the vaccine alone (nil). MF59 was the most potent adjuvant for all three strains
4.5 Clinical Experience with MF59 Adjuvant: Fluad Seasonal Influenza Vaccine
Fluad, an MF59-adjuvanted seasonal influenza vaccine, was licensed in Italy in 1997 and is now registered in 29 countries worldwide. Fluad was approved on the basis of a clinical development program in more the 20,000 subjects that showed the MF59-adjuvanted vaccine was well tolerated and more immunogenic than conventional nonadjuvanted seasonal trivalent inactivated vaccines (TIV). The adjuvanted vaccine was associated with a low incidence of transient local adverse reactions that were mostly mild or moderate in severity and that did not increase in incidence following subsequent immunizations over 3 years [71, 72] (Fig. 3). Compared with unadjuvanted inactivated vaccine comparators, only local pain, erythema, induration, and myalgia occurred significantly more often in the adjuvanted vaccine recipients, while other systemic adverse events including fever and malaise occurred at similar frequencies in both groups [73]. In most countries where it is registered, Fluad is indicated only for adults over 60 or 65 years of age.
MF59-adjuvanted vaccine, Fluad, was well tolerated in the elderly after three consecutive annual vaccinations. A meta-analysis of 20 prospective, randomized, observer-blinded clinical studies in elderly subjects (>65 years); subjects received up to three doses 1 year apart of MF59-adjuvanted subunit influenza vaccine or nonadjuvanted subunit or split vaccines. Adapted from [71]
Fluad was initially developed for vaccination of senior adults to fill the medical need for an improved influenza vaccine for this age group in whom conventional influenza vaccines are less immunogenic and less efficacious compared with younger adults [74, 75]. For this reason, most of the early clinical trials with Fluad were performed in subjects over 65 years old. In this population, the adjuvanted vaccine has induced higher geometric mean titers (GMT), seroconversion rates, and seroprotection rates compared with unadjuvanted vaccine comparators, depending on the vaccine strain composition. GMT HI antibody responses to Fluad typically have been 1.5- to 2.0-fold higher than to unadjuvanted comparators (Fig. 4, right panel). Importantly, higher antibody responses have been seen in the subset of even more frail older adults over 75 years of age [78]. In addition, Fluad has been evaluated in a number of small trials in other patient populations in whom immune response to TIV frequently is lower. Fluad has provided higher HI antibody responses, to varying degrees, in patients with renal transplantation, patients on chronic glucocorticoid therapy, HIV-infected patients on therapy, and senior adults with chronic diseases [79–81].
MF59-adjuvanted vaccine induces strong immune responses in children and the elderly. Left panel shows an observer-blinded, randomized study in healthy children (6 to <36 months; N = 222) involving two doses 4 weeks apart of Fluad (n = 104) or a nonadjuvanted plain split vaccine (n = 118). Right panel shows a randomized, observer-blinded study in elderly (>65 years; N = 192) involving a single dose of Fluad (n = 94) or a nonadjuvanted plain subunit vaccine (n = 98). Adapted from [76] and [77]
Fluad is also more immunogenic in adults 18–60 years old with chronic diseases than nonadjuvanted vaccines. In one published study, geometric mean ratios were higher for the MF59-adjuvanted group (Fluad) for all three vaccine strains [82] (Fig. 5).
Fluad is more immunogenic in adults 18–60 years old with chronic diseases than nonadjuvanted vaccines. Geometric mean ratio of hemagglutination inhibition response in adults (18–60 years of age) with chronic diseases who were immunized with an MF59-adjuvanted subunit influenza vaccine versus a similar group immunized with a vaccine without MF59. Geometric mean ratios were higher for the MF59-adjuvanted group (Fluad) for all three vaccine strains. Adapted from [82]
In addition to augmenting the antibody response in senior adults, MF59 also induces antibody responses that are more broadly cross-reactive [82, 83]. Broader reactivity in a seasonal influenza vaccine is a genuine advantage, as influenza viruses regularly undergo antigenic drift, resulting in periodic mismatches between strains contained in the vaccine and those prevailing in the community. In studies of responses to the H3N2 component of Fluad versus an unadjuvanted vaccine that was otherwise identical (AGRIPPAL), Ansaldi showed that Fluad induced HI titers that were significantly higher, not only to the H3N2 strain contained in the vaccine, but also to the predominant circulating H3N2 strains that circulated in each of the following 3 years, each of which had drifted yet further from the previous year’s strain [84]. Moreover, the Fluad-induced HI responses to the H3N2 strains that circulated 1 and 2 years later would have met CHMP criteria for the annual update for those strains, whereas responses to the unadjuvanted vaccine would not have [85]. The implication of these observations is that the adjuvanted vaccine might mitigate against the poorer antibody responses expected from periodic mismatches of the recommended vaccine composition with strains circulating in the community. To put this in another way, the adjuvanted vaccine induced cross-reactive antibody responses to strains that emerged 1–3 years in the future and that might not yet have circulated in nature at the time the vaccine was manufactured. The fact that these results pertained to H3N2 strains is significant, as that subtype contributes disproportionately more to seasonal influenza morbidity and mortality than the other subtypes.
The higher HI antibody titers induced by Fluad could be expected to lead to increased vaccine efficacy over unadjuvanted TIV. A large-scale observational study of 150,000 senior adults, comparing the effectiveness of Fluad with AGRIPPAL in reducing influenza-related hospitalizations, showed a 23% lower rate of pneumonia and influenza hospitalizations in recipients of Fluad compared to unadjuvanated vaccine [86]. Puig-Barbera et al. have described the effectiveness of Fluad (compared with no influenza vaccination) in preventing emergency admissions for pneumonia, cardiovascular events, and cerebrovascular events in senior adults [87, 88]. The two case–control studies over two seasons showed significantly reduced hospitalization rates for pneumonia, cardiovascular disease, and cerebrovascular disease in adults over 65 years of age with adjusted odds ratios of 0.31, 0.13, and 0.07, respectively. Considerable efforts were made to account for confounding host factors and to focus the analysis on the period when influenza virus circulated in the community, addressing many of the criticisms of observational studies of influenza vaccination in the elderly [74].
At the other end of the age spectrum, young children also have a reduced immune response to TIV, necessitating two doses for primary immunization, and compared with healthy young adults, vaccine efficacy also is lower in this age group [76]. Two trials have evaluated the safety and immunogenicity of Fluad compared with a licensed inactivated split vaccine comparator in young children (6–36 months of age and 6–59 months of age who had never received influenza vaccine). In the first trial, the MF59-adjuvanted vaccine induced significantly higher HI antibody titers at every time point that was studied – 3 weeks after dose one, 3 weeks after dose two, and 6 months after dose two – for all three subtypes (Fig. 4, left panel). In the Fluad group, the proportion of subjects achieving an HI titer of 40 or higher exceeded 70% for all three subtypes, meeting the CHMP criterion for seroprotection in young adults (NB: the CHMP has not established annual update criteria for children). Moreover, for the H3N2 strain, 91% of the Fluad recipients reached an HI titer of ≥40 after just one dose, suggesting the possibility that, for some antigens, the addition of MF59 could sufficiently augment the immune response in immunologically naïve hosts to obviate the need for a two dose primary schedule. The sustained higher HI antibody response, for at least 6 months following the second dose, could be important, as the seasonal influenza vaccine is routinely being delivered in August (in the northern hemisphere), 6 months before the usual peak month of transmission in February of the following calendar year, and 8 months before the usual end of seasonal transmission in April. This is of particular importance for pediatric vaccination as influenza B often is transmitted in the spring, and children under 14 years of age are affected disproportionately by that subtype.
In the same pediatric study, cross-reactive responses to Fluad and the split vaccine comparator also were evaluated in HI tests against strains that were antigenically mismatched to those in the vaccine [76]. As was seen in senior adults, Fluad recipients mounted significantly higher HI antibody titers to mismatched strains of all three subtypes and, for the H1N1 and H3N2 subtypes, CHMP criteria would have been met for geometric mean ratio response (Fig. 6). For the B subtype, however, the antigenic variant that was chosen for testing was not a heterovariant but was a representative of the B/Yamagata lineage while the vaccine contained a B/Victoria lineage strain. The GMT HI titer elicited to the Victoria lineage-virus was just 11, showing that the antigenic distance between the two B lineages is too great for the adjuvant effect of MF59 to bridge.
Fluad induced higher levels of cross-reactive antibodies against heterovariant strains in children. Observer-blinded study in children (6–36 months, n = 222); involving two 0.25-ml doses of Fluad (n = 104) or a nonadjuvanted plain split influenza vaccine (n = 118) administered 4 weeks apart and immune responses were measured against strains not included in the vaccine. Adapted from [76]
A randomized controlled efficacy field trial comparing Fluad versus active comparators in More than 3,000 6-<72 month old children showed that Fluad was 86% efficacious against laboratory confirmed influenza while unadjuvanted influenza vaccine was 43% efficacious, resulting in a relative efficacy of Fluad over unadjuvanted vaccine of 75% (Novartis data on file).
Fluad was shown to be well tolerated among infants and children in these trials (Fig. 7). Although local adverse events occurred more often in the Fluad recipients, only induration at the injection site occurred at a significantly higher frequency [76]. The safety of novel adjuvants in this age group has elicited concern, particularly with respect to the potential for exacerbation of or induction of autoimmune phenomena. All of the pediatric Fluad trials and trials of adjuvanted pandemic influenza vaccines (see below) have been under the oversight of independent data monitoring boards; thus far, none of the trials have been interrupted for safety reasons and no significant safety signals have emerged. Nevertheless, larger scale safety evaluations are needed.
The MF59-adjuvanted influenza vaccine, Fluad, was well tolerated in children. Observer-blinded study in children (6–36 months, n = 269); two doses of Fluad (n = 130) or a nonadjuvanted plain split influenza vaccine (n = 139), 4 weeks apart. Adapted from [76]
4.6 Safety Evaluations of MF59
More than 45 million doses of Fluad have been distributed commercially, and an analysis of pharmacovigilance reports for the product was undertaken for an interval that covered the distribution of approximately 27 million doses [73]. Reports of all adverse events, SAEs, and certain adverse events of specific interest, including allergic events, acute disseminated encephalomyelitis, encephalitis, Guillain–Barré syndrome, other neurologic events, and blood–vascular disorders occurred at a low rate, well below those reported in the literature. Because of the passive nature and low sensitivity of reporting to pharmacovigilance systems, proportional reporting with similar vaccines is a more appropriate indicator of safety signals than comparisons with rates from epidemiological studies. Compared with AGRIPPAL, the unadjuvanted influenza vaccine counterpart to Fluad, reporting rates of the above adverse events were similar, indicating no detectable increase in risk for these adverse advents associated with MF59 (unpublished data, Novartis Vaccines).
A more systematic analysis of the safety of MF59 has been undertaken, by compiling data from 64 clinical trials in which MF59-adjuvanted and unadjuvanted influenza antigens were studied, providing an opportunity to evaluate the safety of MF59 in isolation. The database, comprising approximately 27,998 subjects, included mainly older adults (65%) in the MF59-adjuvanted group [89]. The median duration of follow-up was approximately 6 months. The analysis focused on SAEs, including hospitalizations and deaths, and specific events, such as the new onset of chronic disease, autoimmune disorders, and cardiovascular events. When randomized trials were examined, reports for these outcomes were no higher in the MF59-adjuvanted group compared with the unadjuvanted vaccine recipients.
Additional safety data on events leading to hospitalization will be forthcoming from the observational study in senior adults mentioned above.
Despite the absence of any data indicating the induction of antibodies against squalene contained in vaccines, some members of the public have associated administration of vaccines and of squalene with Gulf War syndrome. Several reviews of the available data and an epidemiological study among Navy Seabees found no association between squalene antibodies and symptoms of Gulf War syndrome [89]. In addition, it is not generally appreciated that naturally occurring antibodies to squalene are present among healthy individuals. A study comparing anti-squalene antibodies in recipients of Fluad and AGRIPPAL found no difference in antibody rises in the two groups, indicating that MF59 adjuvant neither raises the levels of preexisting antibodies nor induces new antibody responses against squalene [90].
4.7 Pandemic Influenza Vaccines Containing MF59: Avian Influenza Viruses
Pandemic viruses, by definition, are of a subtype that is not currently circulating and usually are novel antigens to which the majority of the population is immunologically naïve. Stimulating a protective immune response to such novel antigens has been shown to require two or more primary vaccination doses, and at least for the H5N1 subtype, requires formulations containing a larger quantity of antigen per dose than is present in the 15 μg/strain contained in the seasonal vaccine. The logistical challenges of vaccinating entire populations with two pandemic vaccine doses and the capacity of individual countries and the world to produce sufficient quantities of viral antigen are challenges to public health systems and governments. Moreover, as with seasonal strains, the H5N1 virus continues to evolve into numerous genetically and antigenically distinguishable clades thus far – with further antigenic variation among viruses within the clades.
These difficulties potentially can be ameliorated or even overcome by the use of emulsion-adjuvanted formulations. Ninety micrograms of unadjuvanted H5N1 split HA in two doses provides an HI antibody titer ≥40 in % of healthy young adult subjects, and administering more doses with even higher antigen content is marginally successful in inducing high antibody titers in a suitable proportion of subjects [91]. Substantially less antigen can be used – as little as 1.9 or 3.75 μg of HA is equally or more immunogenic when adjuvanted with an emulsion adjuvant [40, 92–94]. A head-to-head study comparing a subunit H5N1 that was administered unadjuvanted or adjuvanted with alum or with MF59 found that 15 μg of MF59-adjuvanted antigen was significantly more immunogenic than either 45 μg of unadjuvanted or 30 μg of alum-adjuvanted antigen, indicating the potential for MF59 to provide dose sparing, and furthermore, the ineffectiveness of alum as an adjuvant in this circumstance [95].
The antigen dose sparing potential of MF59 was seen even more dramatically in a study of an H9N2 avian influenza virus vaccine in which formulations containing 3.75–30 μg of antigen were studied [96]. All of the adjuvanted formulations provided significantly higher neutralizing antibody titers compared with their unadjuvanted counterparts. Of interest, one dose of the adjuvanted 15-μg formulation was more immunogenic than two doses of the unadjuvanted 15-μg vaccine, suggesting again the potential for MF59 to boost primary antibody responses sufficiently, at least for some antigens, that just one dose could be clinically useful. The ability of 3.75 μg of H5N1 antigen to induce potent immune response in humans in the presence of MF59 adjuvant was recently shown using a flu cell culture-derived influenza vaccine (Fig. 8).
Hemagglutinin inhibition (HI) assay in healthy young adults 3 weeks post-second vaccination with a cell culture-derived H5N1 vaccine. Observer-blinded, randomized study in adults (18–40 years of age; n = 695), involving two doses 21 days apart of MF59-adjuvanted vaccine or nonadjuvanted vaccine with 3.75, 7.5, or 15 μg cell culture-grown influenza A/H5N1 HA. Adapted from [97]
One licensed MF59-adjuvanted H5N1 vaccine has been registered (Focetria®), under the European “mock-up” procedure, as a pandemic vaccine to be used upon a pandemic declaration. Its registration was based on a series of clinical trials using a 7.5-μg HA formulation containing MF59 in the same quantity present in the licensed adjuvanted seasonal vaccine. Two doses in adults 18–64 years old, adolescents 9–17 years old, children 3–8 years old, and infants 6 months to 2 years old provided HI antibody titers meeting CHMP criteria ([58], Novartis data on file). The approved formulation used a reverse genetics-derived clade 1 A/Vietnam/1204/2007 (H5N1) strain; however, formulations using clade 2.2 and clade 2.3.4 also have been manufactured.
Recipients of the clade 1 A/Vietnam/1204 vaccine mentioned above not only made antibodies at putatively protective levels to the vaccine antigen after primary two-dose vaccination, but in young adults, also elicited cross-reactive antibodies to that degree to a clade 2.2 antigen. The responses in senior adults nearly reached those levels. In a pseudotype neutralization assay, primary responses in young adults also were shown to be broadly reactive to clade 2.1 and 2.3.4 antigens [98].
The cross-reactivity of antibody responses of an H5N1 vaccine to viruses in other clades is of considerable importance, as it would be desirable if individuals primed against an antigen in one clade could respond with an anamnestic response to protective antibody levels after a single booster dose of the vaccine produced against the H5N1 virus that actually emerged in a pandemic. Because the emergence of such a pandemic cannot be predicted, induction of persistent immune memory, lasting years, would be desirable.
Data in support of such priming are available from a small cohort of young adults who were immunized with MF59-adjuvanted or unadjuvanted H5N3 vaccine, an antigen that is antigenically related to H5N1 clade 0 viruses [99–101]. The subjects were reconvened 7 years later and boosted with two doses of adjuvanted H5N1 vaccine [102]. Those who previously had received unadjuvanted H5N3 vaccine needed both doses to produce significant neutralizing antibodies to the H5N1 clade 1 antigen. On the other hand, those who previously had been primed with the adjuvanted H5N3 vaccine responded within 7 days of the first adjuvanted H5N1 dose with high levels of neutralizing antibodies not only to the homologous clade 1 antigen but also to the H5N3 (clade 0 equivalent) antigen and to clade 2.1, 2.2, and 2.3.4 antigens, representing the clades responsible for nearly all the reported cases of H5N1 disease. Before booster vaccination, H5N1 viral-specific memory B cells were present more abundantly among the subjects who had been primed years earlier. Memory B cells were higher in number and peaked earlier among the adjuvant-primed subjects at day 21 after vaccination – correlating with the levels of neutralizing antibodies.
These observations are consistent with preclinical data in ferrets, mentioned below, of the priming effect of adjuvanted seasonal vaccine on responses to A/CA/07/2009 (H1N1) antigen. Together, the data suggest that MF59 broadens the primary and memory immune responses to coadministered antigens. This attribute of the adjuvanted response could be of practical importance in the context of prepandemic preparation, as persons at high risk or critical infrastructure workers who were primed potentially could be protected with a single dose of pandemic vaccine as soon as it became available, even if the respective antigens were at some antigenic distance.
Operationally, it is of interest that the priming schedule for the MF59-adjuvanted H5N1 vaccine could be separated between dose one and dose two by as long as 1 year (Novartis data on file). Importantly, when the second dose was derived from a different clade (dose one was a clade 1 antigen, and dose two was a clade 2.2 antigen), responses to the second dose were highly cross-reactive to both antigens, meeting CHMP criteria for viruses in both clades (Novartis data on file). This suggests that annual revaccinations with updated formulations representing newly emerging clades could lead to protection with a single dose. These annual updates could be administered in conjunction with annual seasonal influenza vaccination, as coadministration of the adjuvanted H5N1 vaccine and seasonal inactivated vaccine did not interfere with responses to either seasonal or avian influenza antigens (Novartis data on file).
The still emerging H1N1 pandemic has focused attention on the inadequacy of the global influenza vaccine supply, as the WHO has estimated that only 4.9 billion monovalent doses of vaccine can be produced by all manufacturers within a year. But that assessment is based on the formulations proposed by manufacturers which includes approximately two billion doses that are adjuvanted [103]. The antigen dose sparing potential of MF59 and other emulsion adjuvants on responses to H5N1 virus has been contrasted with alum which has shown variable results. While emulsion adjuvants such as MF59 have provided high immune responses independent of antigen dose above approximately 6 μg, antibody responses to unadjuvanted and alum-adjuvanted vaccines correlate with antigen dose, and to achieve putatively protective levels, amounts of subunit and split antigens greater than 15 μg are needed [104].
As adjuvants themselves must be manufactured with some lead time, minimizing the quantity of adjuvant in a pandemic formulation to its smallest effective dose would be desirable. In fact, when combined with 3.75 or 7.5 μg of subunit H5N1 antigen, half of the usual quantity of MF59 contained in Fluad was as immunogenic as 15 μg of antigen with a full dose of MF59 [97] (Fig. 8).
4.8 Pandemic H1N1 Virus
The distant antigenic and genetic relationship of seasonal H1N1 virus to the novel pandemic H1N1 virus suggested that adjuvanted vaccines would be needed to stimulate protective immunity. The early emerging clinical trial data, however, have indicated that a single 15-μg dose of unadjuvanted hemagglutinin is sufficient to stimulate putatively protective antibody levels in both young and senior adults but that two doses were needed in children [105].
As has been seen with H5N1 virus, the addition of MF59 to subunit H1N1 antigen allowed for considerable antigen sparing [106]. A 3.75-μg antigen dose adjuvanted with a full antigen of MF59 was highly immunogenic in adults after either one or two doses. A combination of 7.5 μg of antigen with half of the usual complement of MF59 was as immunogenic as 15 μg of unadjuvanted antigen and unlike the unadjuvanted vaccine, a single adjuvanted dose was highly immunogenic in children. The former formulation used a cell culture-derived antigen which could prove to be the future of influenza antigen production.
The pandemic H1N1 virus has not yet been observed to drift antigenically from the viruses that were isolated from Mexico and California early in the outbreak. However, as the virus becomes resurgent in the northern hemisphere in the fall of 2009, increased immune pressure from persons who were infected in the spring potentially could lead to emergence of heterovariantsthat might not be neutralized by the current A/CA07/2009 formulated vaccine. When such heterovariants emerge, as they surely will, it will be of considerable interest to determine if the MF59-adjuvanted vaccine provides cross-neutralization.
The public health deployment of these MF59-adjuvanted vaccines in millions of doses will provide additional safety data as well as effectiveness data that should greatly aid further evaluations of the adjuvant in clinical practice. Thus far, no safety signals have emerged with the distribution of more than 100 million doses of MF59-adjuvanted pandemic H1N1 vaccine, which has included tens of thousands of pregnant women and children.
4.9 MF59 with Other Antigens
MF59 has been used in early phase clinical trials with a number of antigens, including herpes simplex, HIV, hepatitis B and C, and cytomegalovirus (CMV) candidate vaccines [107–110]. Of note, MF59 has been used as an adjuvant for pediatric vaccines with CMV and HIV viral antigens [110–113]. Seronegative toddlers immunized with CMV glycoprotein B showed antibody titers that were higher than those found in adults naturally infected with CMV. Moreover, the MF59-adjuvanted vaccine was well tolerated in this age group. A recent phase II study of the MF59-adjuvanted CMV glycoprotein B vaccine in naïve post-postpartum women showed promise, demonstrating 50% efficacy (95% confidence interval, 7–73) in preventing infection, expressed as person-years over a 42-month follow-up interval [114].
4.10 Additional Oil-in-Water Adjuvant Formulations in Development
One of the advantages of o/w emulsion formulations is that they can be used with other immune-stimulating molecules to further improve vaccines to address critical issues for which current vaccines may not be optimal. Examples include combining o/w emulsions with toll-like receptor (TLR) agonists. In development are AS03-MPL (GSK) and MF59 combinations (Novartis), as well as MPL-SE (Infectious Disease Research Institute), which has been clinically evaluated in therapeutic vaccines against leishmaniasis. Such combination adjuvants may help address issues critical to future vaccine development, including rapid response, induction of protective immunological memory, broadening of the immune response, and, of increasing importance, developing more effective vaccines for the elderly. In preclinical studies, combination adjuvant formulations based on MF59 have been shown to induce enhanced immune responses, particularly enhanced T-cell responses with a more Th1 profile [115].
5 The Future
Although MF59 has been used in a licensed seasonal influenza vaccine in Europe for more than a decade, and ASO3 has recently obtained licensure as a pandemic and a prepandemic vaccine, neither of these adjuvants are yet licensed in the USA. The adoption of emulsion-adjuvanted vaccines in the USA will require further demonstrations of safety, particularly if a pediatric indication is sought or if repeated annual exposure is anticipated, as with seasonal influenza vaccine. However, the willingness of regulatory authorities and public health officials to accept the licensure of a vaccine containing the novel MPL adjuvant in a HPV vaccine is encouraging, as it provides a precedent for a licensure path and for clinical acceptance of other novel adjuvants, including emulsions.
References
Lewis S (1924) Arrowsmith. Signet Classics Edition, Chapter 27, p 305
Pinoy LMa (1916) Les vaccins en emulsion dans les corps gras ou ‘lipo-vaccins’. Soc Biol Fil seace du 4 mars, t. LXXIX, 79:201–203
Pinoy LMa (1916) Application a l’homme des vaccines en emulsion dans les corps gras (lipo-vaccins). Soc Biol Fil seance du 6 mars, t. LXXIX, 79:352–354
Achard CaF C (1916) Sur l’emploi des corps gras comme vehicules des vaccines microbiens. Soc Biol Fil seace du 4 mars, t. LXXIX, 79:209–211
Whitmore ER (1919) Lipovaccines, with special reference to public health work. Am J Public Health 9:504–507
Lewis PA, Dodge FW (1920) The sterilization of lipovaccines. J Exp Med 31:169–175
Freund J, Casals J, Hosmer EP (1937) Sensitization and antibody formation after injection of turbecle bacili and paraffin oil. Proc Soc Exp Biol Med 37:509–513
Freund J, McDermott K (1942) Sensitization to horse serum by means of adjuvants. Proc Soc Exp Biol Med 49:548–553
Freund J, Walter A (1944) Saprophytic acidfast bacilli and paraffin oil as adjuvants in immunization. Proc Soc Exp Biol Med 56:47–50
Freund J, Bonanto M (1944) The effect of paraffin oil, lanolin-like substances and killed tubercle bacilli on immunization with diphtheric toxoid and bact. Typhosum. J Immunol 48:325–334
Freund J, Bonanto M (1946) The duration of antibody-formation after injection of killed typhoid bacilli in water-in-oil emulsion. J Immunol 52:231–234
Hilleman MR (1966) Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Prog Med Virol 8:131–182
Jansen T, Hofmans MP, Theelen MJ, Schijns VE (2005) Structure-activity relations of water-in-oil vaccine formulations and induced antigen-specific antibody responses. Vaccine 23:1053–1060
Murray R, Cohen P, Hardegree MC (1972) Mineral oil adjuvants: biological and chemical studies. Ann Allergy 30:146–151
Friedewald WF (1944) Enhancement of the immunizing capacity of influenza virus vaccines with adjuvants. Science 99:453–454
Salk JE, Bailey ML, Laurent AM (1952) The use of adjuvants in studies on influenza immunization. II. Increased antibody formation in human subjects inoculated with influenza virus vaccine in a water in-oil emulsion. Am J Hyg 55:439–456
Salk JE, Laurent AM, Bailey ML (1951) Direction of research on vaccination against influenza; new studies with immunologic adjuvants. Am J Public Health 41:669–677
Henle W, Henle G (1945) Experiments on vaccination of human beings against epidemic influenza. Proc Soc Exp Biol Med 59:181
Stuart-Harris CH, Andrews CH, Andrews BE et al (1955) Antibody responses and clinical reactions with saline and oil adjuvant influenza virus vaccines. Br Med J 2(4950):1229–1232
Salk JE (1953) Use of adjuvants in studies on influenza immunization. 3. Degree of persistence of antibody in human subjects two years aftr vaccination. JAMA 151:169–1175
Beebe GW, Simon AH, Vivona S (1964) Follow-up study on army personnel who received adjuvant influenza virus vaccine 1951–1953. Am J Med Sci 247:385–405
Beebe GW, Simon AH, Vivona S (1972) Long-term mortality follow-up of Army recruits who received adjuvant influenza virus vaccine in 1951–1953. Am J Epidemiol 95:337–346
Stuart-Harris CH (1969) Adjuvant influenza vaccines. Bull World Health Organ 41:617–621
Vogel FR, Caillet C, Kusters IC, Haensler J (2009) Emulsion-based adjuvants for influenza vaccines. Expert Rev Vaccines 8:483–492
Page W (1993) Long-term followup of army recruits immunized with Freund’s incomplete adjuvanted vaccine. Vaccine Res 2:141–149
Page M, Vella C, Corcoran T, Dilger P, Ling C, Heath A, Thorpe R (1992) Restriction of serum antibody reactivity to the V3 neutralizing domain of HIV gp120 with progression to AIDS. AIDS 6:441–446
Smith JW, Fletcher WB, Peters M, Westwood M, Perkins FJ (1975) Response to influenza vaccine in adjuvant 65-4. J Hyg (Lond) 74:251–259
Hilleman MR (1969) The roles of early alert and of adjuvant in the control of Hong Kong influenza by vaccines. Bull World Health Organ 41:623–628
Coler RN, Carter D, Friede M, Reed SG (2009) Adjuvants for malaria vaccines. Parasite Immunol 31:520–528
Lawrence GW, Saul A, Giddy AJ, Kemp R, Pye D, Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA, Moingeon P (1997) Phase I trial in humans of an oil-based adjuvant Seppic Montanide ISA 720. Vaccine 15:176–178
Saul A, Lawrence G, Smillie A, Rzepczyk CM, Reed C, Taylor D, Anderson K, Stowers A, Kemp R, Allworth A et al (1999) Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145–3159
Freund J (1951) The effect of paraffin oil and mycobacteria on antibody formation and sensitization: a review. Am J Clin Pathol 21:645–656
Ribi E, Meyer TJ, Azuma I, Parker R, Brehmer W (1975) Biologically active components from mycobacterial cell walls. IV. Protection of mice against aerosol infection with virulent mycobacterium tuberculosis. Cell Immunol 16:1–10
Allison AC (1999) Squalene and squalane emulsions as adjuvants. Methods 19:87–93
Stills HF Jr (2005) Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J 46(3):280–293
Rumke HC, Bayas JM, de Juanes JR, Caso C, Richardus JH, Campins M, Rombo L, Duval X, Romanenko V, Schwarz TF et al (2008) Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 26:2378–2388
Ballester A, Garces-Sanchez M, Planelles Cantarino MV et al (2008) Pediatric safety evaluation of an AS-adjuvanted H5N1 vaccine in children aged 6–9 years: a phase II study. Presented at 26th annual meeting of the European Society of Infectious Disease, Graz, Austria, 13–17 May, 2008
Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G (2007) Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580–589
Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G (2008) Broad clade immmunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS ONE 3(2):e1665
Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, Leroux-Roels G, Pichon S, Kusters I (2008) An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis 198:642–649
Vogel FR, Powell MF (1995) A compendium of vaccine adjuvants and excipients. In: Powell MF, Newman MJ (eds) Vaccine design: the subunit and adjuvant approach. Plenum, New York, pp 141–228
Allison AC, Byars NE (1986) An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods 95:157–168
Ellouz F, Adam A, Ciorbaru R, Lederer E (1974) Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun 59:1317–1325
Waters RV, Terrell TG, Jones GH (1986) Uveitis induction in the rabbit by muramyl dipeptides. Infect Immun 51:816–825
Fritz JH, Ferrero RL, Philpott DJ, Girardin SE (2006) Nod-like proteins in immunity, inflammation and disease. Nat Immunol 7:1250–1257
Kenney RT, Edelman R (2004) New generation vaccines. Marcel Dekker, New York
Wintsch J, Chaignat CL, Braun DG, Jeannet M, Stalder H, Abrignani S, Montagna D, Clavijo F, Moret P, Dayer JM et al (1991) Safety and immunogenicity of a genetically engineered human immunodeficiency virus vaccine. J Infect Dis 163:219–225
Keitel W, Couch R, Bond N, Adair S, Van Nest G, Dekker C (1993) Pilot evaluation of influenza virus vaccine (IVV) combined with adjuvant. Vaccine 11:909–913
Keefer MC, Graham BS, McElrath MJ, Matthews TJ, Stablein DM, Corey L, Wright PF, Lawrence D, Fast PE, Weinhold K et al (1996) Safety and immunogenicity of Env 2-3, a human immunodeficiency virus type 1 candidate vaccine, in combination with a novel adjuvant, MTP-PE/MF59. NIAID AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses 12:683–693
Kahn JO, Sinangil F, Baenziger J, Murcar N, Wynne D, Coleman RL, Steimer KS, Dekker CL, Chernoff D (1994) Clinical and immunologic responses to human immunodeficiency virus (HIV) type 1SF2 gp120 subunit vaccine combined with MF59 adjuvant with or without muramyl tripeptide dipalmitoyl phosphatidylethanolamine in non-HIV-infected human volunteers. J Infect Dis 170:1288–1291
Ott G, Barchfeld GL, Van Nest G (1995) Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine 13:1557–1562
Cataldo DM, Van Nest G (1997) The adjuvant MF59 increases the immunogenicity and protective efficacy of subunit influenza vaccine in mice. Vaccine 15:1710–1715
Higgins DA, Carlson JR, Van Nest G (1996) MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine 14:478–484
Ott G (2000) The adjuvant MF59: a ten year perspective. In: O’Hagan D (ed) Vaccine adjuvants: preparation methods and research protocols. Humana, Totowa, pp 211–228
Traquina P, Morandi M, Contorni M, Van Nest G (1996) MF59 adjuvant enhances the antibody response to recombinant hepatitis B surface antigen vaccine in primates. J Infect Dis 174:1168–1175
Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G (1995) MF59: design and evaluation of a safe and potent adjuvant for human vaccines. In: Powell MF, Newman MJ (eds) Vaccine design: the subunit and adjuvant approach. Plenum, New York, pp 277–296
Dupuis M, McDonald DM, Ott G (1999) Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine 18:434–439
Valensi JP, Carlson JR, Van Nest GA (1994) Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol 153:4029–4039
Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, Van Nest G, Ott G, McDonald DM (1998) Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol 186:18–27
Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, Ott G (2001) Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol 31:2910–2918
Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E (2008) Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA 23:23
Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A (2008) The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol 180:5402–5412
Ott G (2000) Vaccine adjuvants: preparation methods and research protocols. In: O’Hagan D (ed) Vaccine adjuvants: preparation methods and research protocols. Humana, Totowa
Podda A, Del Giudice G (2003) MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines 2:197–203
Podda A, Del Giudice G, O’Hagan DT (2005) A safe and potent adjuvant for human use. In: Schijns V, O’Hagan DT (eds) Immunopotentiators in modern vaccines. Elsevier, Amsterdam, p 149
Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, Titta F, Contorni M, Volpini G, Del Guidice G et al (2006) A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine 24: 1680–1686
Granoff DM, McHugh YE, Raff HV, Mokatrin AS, Van Nest GA (1997) MF59 adjuvant enhances antibody responses of infant baboons immunized with Haemophilus influenzae type b and Neisseria meningitidis group C oligosaccharide-CRM197 conjugate vaccine. Infect Immun 65:1710–1715
Wack A, Baudner BC, Hilbert AK, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, Del Giudice G, Rappuoli R, O’Hagan DT (2008) Combination adjuvants for the induction of potent, long-lasting antibody and T cell rsponses to influenza vaccine. Vaccine 26:552–561
Subbarao K, McAuliffe J, Vogel L, Fahle G, Fischer S, Tatti K, Packard M, Shieh WJ, Zaki S, Murphy B (2004) Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol 78:3572–3577
Forrest HL, Khalenkov AM, Govorkova EA, Kim JK, Del Giudice G, Webster RG (2009) Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine 27:4187–4195
Podda A (2001) The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine 19:2673–2680
Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P (1999) Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine 17:99–104
Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R (2008) Safety of MF59 adjuvant. Vaccine 26:3209–3222
Goodwin K, Viboud C, Simonsen L (2006) Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169
Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA (2007) Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 7:658–666
Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O’Hagan DT, Podda A (2009) Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J 28:563–571
De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, Senatore F, Verweij P, Fritzell B, Podda A (1999) Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine 17:3094–3101
Squarcione S, Sgricia S, Biasio LR, Perinetti E (2003) Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 21:1268–1274
Banzhoff A, Nacci P, Podda A (2003) A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 49:177–184
Iorio AM, Francisci D, Camilloni B, Stagni G, De Martino M, Toneatto D, Bugarini R, Neri M, Podda A (2003) Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59-adjuvanted influenza vaccine or a conventional non-adjuvanted subunit vaccine during highly active antiretroviral therapy. Vaccine 21:3629–3637
Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R (2007) MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18–60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine 25:3955–3961
Baldo V, Baldovin T, Floreani A, Fragapane E, Trivello R (2007) Response of influenza vaccines against heterovariant influenza virus strains in adults with chronic diseases. J Clin Immunol 27:542–547
Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, Schoendorf I, Borkowski A, Rappuoli R, Podda A (2006) An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine 24:3063–3065
Ansaldi F, Canepa P, Parodi V, Bacilieri S, Orsi A, Compagnino F, Icardi G, Durando P (2009) Adjuvanted seasonal influenza vaccines and perpetual viral metamorphosis: the importance of cross-protection. Vaccine 27:3345–3348
Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P (2008) Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 26:1525–1529
Mannino S, Villa M, Weiss N, Apolone G, Rothman K (2010) Effectiveness of influenza vaccination with FLUAD versus a Sub-unit Influenza Vaccine Society for Epidemiologic Research (SER) Annual Meeting: Seattle, Washington – June 23–26, Ref 00002742
Puig-Barbera J, Diez-Domingo J, Perez Hoyos S, Belenguer Varea A, Gonzalez Vidal D (2004) Effectiveness of the MF59-adjuvanted influenza vaccine in preventing emergency admissions for pneumonia in the elderly over 64 years of age. Vaccine 23:283–289
Puig-Barbera J, Diez-Domingo J, Varea AB, Chavarri GS, Rodrigo JA, Hoyos SP, Vidal DG (2007) Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine 25:7313–7321
Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G (2009) MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 27(49):6959–6965
Phillips CJ, Matyas GR, Hansen CJ, Alving CR, Smith TC, Ryan MA (2009) Antibodies to squalene in US Navy Persian Gulf War veterans with chronic multisymptom illness. Vaccine 27:3921–3926
Del Giudice G, Fragapane E, Bugarini R, Hora M, Henriksson T, Palla E, O’Hagan D, Donnelly J, Rappuoli R, Podda A (2006) Vaccines with the MF59 adjuvant do not stimulate antibody responses against squalene. Clin Vaccine Immunol 13:1010–1013
Beigel JH, Voell J, Huang CY, Burbelo PD, Lane HC (2009) Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J Infect Dis 200:501–509
Leroux-Roels G (2009) Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther 9:1057–1071
Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C et al (2009) MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE 4:e4384
Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H (2008) Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 197:667–675
Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, Pompey J, Cate TR, Couch RB (2006) Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis 43:1135–1142
Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, Tsai TF (2009) Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 28:840–848
Alberini I, Del Tordello E, Fasolo A, Temperton NJ, Galli G, Gentile C, Montomoli E, Hilbert AK, Banzhoff A, Del Giudice G et al (2009) Pseudoparticle neutralization is a reliable assay to measure immunity and cross-reactivity to H5N1 influenza viruses. Vaccine 27:5998–6003
Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC, Windon RG, Chaplin PJ, McWaters P et al (2001) Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937–1943
Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, Katz JM (2005) Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis 191:1210–1215
Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, Zambon M (2003) Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 21:1687–1693
Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T et al (2009) Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA 106:7962–7967
Collin N, de Radigues X, Kieny MP (2009) Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 27:5184–5186
Manzoli L, Salanti G, De Vito C, Boccia A, Ioannidis JP, Villari P (2009) Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis 9:482–492
Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D et al (2009) Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 361:2405–2413
Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I (2009) Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 361:2424–2435
Heineman TC, Clements-Mann ML, Poland GA, Jacobson RM, Izu AE, Sakamoto D, Eiden J, Van Nest GA, Hsu HH (1999) A randomized, controlled study in adults of the immunogenicity of a novel hepatitis B vaccine containing MF59 adjuvant. Vaccine 17:2769–2778
Langenberg AG, Burke RL, Adair SF, Sekulovich R, Tigges M, Dekker CL, Corey L (1995) A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected] [published erratum appears in Ann Intern Med 1995 Sep 1; 123(5):395]. Ann Intern Med 122:889–898
Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr, Handsfield HH, Warren T, Marr L, Tyring S et al (1999) Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group [see comments]. JAMA 282:331–340
Mitchell DK, Holmes SJ, Burke RL, Duliege AM, Adler SP (2002) Immunogenicity of a recombinant human cytomegalovirus gB vaccine in seronegative toddlers. Pediatr Infect Dis J 21:133–138
McFarland EJ, Borkowsky W, Fenton T, Wara D, McNamara J, Samson P, Kang M, Mofenson L, Cunningham C, Duliege AM et al (2001) Human immunodeficiency virus type 1 (HIV-1) gp120-specific antibodies in neonates receiving an HIV-1 recombinant gp120 vaccine. J Infect Dis 184:1331–1335
Borkowsky W, Wara D, Fenton T, McNamara J, Kang M, Mofenson L, McFarland E, Cunningham C, Duliege AM, Francis D et al (2000) Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J Infect Dis 181:890–896
Cunningham CK, Wara DW, Kang M, Fenton T, Hawkins E, McNamara J, Mofenson L, Duliege AM, Francis D, McFarland EJ et al (2001) Safety of 2 recombinant human immunodeficiency virus type 1 (hiv-1) envelope vaccines in neonates born to hiv-1-infected women. Clin Infect Dis 32:801–807
Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C et al (2009) Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360:1191–1199
Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, Hawkins LD, Wack A, O’Hagan DT (2009) MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm Res 26:1477–1485
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Birkhäuser Basel
About this chapter
Cite this chapter
O’Hagan, D.T., Tsai, T., Reed, S. (2011). Emulsion-Based Adjuvants for Improved Influenza Vaccines. In: Rappuoli, R., Del Giudice, G. (eds) Influenza Vaccines for the Future. Birkhäuser Advances in Infectious Diseases. Springer, Basel. https://doi.org/10.1007/978-3-0346-0279-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-0346-0279-2_14
Published:
Publisher Name: Springer, Basel
Print ISBN: 978-3-0346-0278-5
Online ISBN: 978-3-0346-0279-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)