Abstract
The Bayer process is the primary technology utilized for alumina production but it is limited by its stringent requirements on the quality of the bauxite raw material. Due to the absence of a reasonable and effective large-scale utilization method, a substantial amount of high-alkali red mud generated from the process is kept in storage yards, occupying significant land space and posing grave threats to both the environment and human health. Based on prior research, this paper comprehensively summarizes and scrutinizes the current state of alumina production as well as the management of red mud. Commencing with the phase structure of alumina production, this paper delves into the development of the Bayer process and the Calcification-carbonization method. The aforementioned approaches offer pioneering concepts for promoting sustainable development within the alumina industry.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
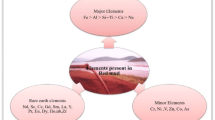
Bayer red mud is an insoluble alkaline residue that results from the manufacture of alumina during the Bayer alkaline leaching process. Red mud is a waste material consisting largely of iron oxide and other insoluble substances. Although commonly red due to its high Fe2O3 content, the color can vary from brown to grayish-white depending on specific iron oxide concentrations [1]. The chemical composition of red mud is not uniform and is influenced by the varying chemical makeup of bauxite and different production processes. Typically, red mud generated through the Bayer process contains Al2O3 (8˗30%), Fe2O3 (6–72%), SiO2 (5–30%), Na2O (2–10%), CaO (0–23%), and TiO2 (4–25%). Additionally, bauxite ores from diverse regions introduce elements such as potassium, magnesium, arsenic, chromium, and a range of rare earth elements. A comprehensive list of the main components of Bayer process red mud, categorized by country of origin, is presented in Table 1.
Statistically, for every ton of alumina produced, between 1.5 and 2.5 tons of red mud is generated. Figure 1 illustrates the discharge and comprehensive utilization quantity of red mud in China from 2011 to 2022. By 2022, the global stockpile of red mud will surpass 5 billion tons. Notably, China's stockpile alone accounted for 1.6 billion tons, approximately one-third of the global total. The annual discharge of red mud is increasing by approximately 100 million tons annually [8]. Historically, coastal countries opted for sea dumping to dispose of red mud. However, with the tightening of global environmental regulations in recent years, this method has been explicitly banned. Presently, dam construction serves as the primary storage solution for untreated red mud.
Red mud is a problematic waste material that poses a two-fold challenge: it consumes large swathes of land while requiring extensive and costly storage sites. In addition, the highly alkaline red mud waste liquid can pollute groundwater and increase environmental pressure, leading to water and soil alkalization [9]. A red mud storage dam breach occurred on October 4, 2010 at the Ajka alumina plant in Hungary, resulting in the leakage of an estimated 1 million cubic meters of red mud [10]. This event caused extensive damage to surface water and land, leading to the most severe safety accident in the history of the aluminum industry and prompting widespread panic across several European countries. This catastrophe also served as a clarion call for the Chinese aluminum oxide industry, compelling relevant national departments to prioritize the issue of solid waste storage. The “Twelfth Five-Year Plan for the Comprehensive Utilization of Bulk Industrial Solid Waste,” published in 2012, identified the comprehensive utilization of red mud as one of the top ten key projects during the “Twelfth Five-Year Plan” period.
This paper provides a systematic study of the current status of alumina production and the reasons for red mud generation from the perspective of phase transformation. Furthermore, it discusses the current state of clean production processes for alumina and the harmless utilization of red mud. Finally, focusing on the calcification-carbonization process, this paper summarizes the process parameters and experimental results obtained by numerous researchers in our laboratory. It evaluates the treatment costs and economic benefits of the calcification-carbonization method, providing new insights for promoting the sustainable development of the alumina industry.
Alumina Production and Red Mud Generation
Bayer Process
In 1889, Bayer discovered that sodium aluminate solution, from which most of the aluminum hydroxide had precipitated, could selectively dissolve the alumina contained in bauxite when bauxite was pressurized and heated in an autoclave. This process is known as the leaching of bauxite, and by alternating leaching and precipitation processes, bauxite can be continuously processed to produce aluminum hydroxide products, forming the Bayer cycle [11]. This process is characterized by its simplicity, low energy consumption, and high product quality. It is economically more efficient for processing high-grade bauxite compared to other methods [12]. The reaction principle is illustrated in Eq. 1 (forward reaction for dissolution and reverse reaction for precipitation).
The Bayer process, as illustrated in Fig. 2 can be divided into several main steps: ore slurry preparation, high-pressure leaching, dilution of the leached slurry, separation and washing of red mud, decomposition of seed crystals, classification and washing of aluminum hydroxide, calcification of aluminum hydroxide, evaporation of the mother liquor, and causticization [13]. The Bayer process is characterized by its simplicity, a relatively small number of production steps, and its suitability for large-scale equipment and process automation. It offers energy efficiency advantages when processing bauxite with good leaching performance. However, the Bayer process requires high-grade bauxite, and it is particularly beneficial when processing bauxite with a high aluminum-to-silicon ratio (A/S > 8).
In the Bayer process, a portion of the alumina in bauxite reacts with silica and caustic soda to form sodium aluminosilicate hydrate (Na2O·Al2O3·1.7SiO2·n H2O, n ≤ 2), which enters the red mud. The reaction is represented by Eq. 2, where the mass ratio of alumina, silica, and sodium oxide is 1:1:0.608. This means that for every 1 kg of silica in bauxite, there is a loss of 1 kg of alumina and 0.608 kg of sodium oxide. Therefore, when the grade of bauxite decreases, that is, when the aluminum-to-silicon ratio decreases, the economic efficiency of the Bayer process is significantly affected. The influence of the aluminum-to-silicon ratio in bauxite and red mud on the alumina dissolution rate can be illustrated by plotting the data obtained from the alumina dissolution calculation formula (Eq. 3).
The impact of the aluminum-to-silicon ratios (A/S)O and (A/S)R on the alumina dissolution rate is shown in Fig. 3. Here, (A/S)O represents the original aluminum-to-silicon ratio in bauxite, and (A/S)R represents the aluminum-to-silicon ratio in red mud. From the graph, it can be observed that the alumina dissolution rate reaches 80% only when the aluminum-to-silicon ratio in bauxite is greater than 5, considering that the minimum aluminum-to-silicon ratio in red mud is theoretically 1. However, due to practical conditions in alumina production, the actual aluminum-to-silicon ratio in red mud is usually greater than 1. If the aluminum-to-silicon ratio in red mud is 1.4, the aluminum-to-silicon ratio in the original bauxite needs to be greater than 7 to achieve an 80% alumina dissolution rate. On the other hand, if the aluminum-to-silicon ratio in red mud is reduced to 0.4, an aluminum-to-silicon ratio of 2 in bauxite is sufficient to achieve an 80% dissolution rate. Therefore, there are two ways to improve the alumina dissolution rate: increasing the aluminum-to-silicon ratio in bauxite and reducing the aluminum-to-silicon ratio in red mud.
Based on the aforementioned ideas, the current research progress in improving the alumina dissolution rate in the Bayer process mainly focuses on the following two directions:
-
1.
Enhancing the aluminum-to-silicon ratio in bauxite through physicochemical methods to achieve predesilication before dissolution.
-
2.
Exploring ways to alter the structure of red mud to decrease the aluminum-to-silicon ratio and sodium-to-silicon ratio.
Enhancing the Aluminum-to-Silicon Ratio of Bauxite Ore
Improving the aluminum-to-silicon ratio is a key objective in bauxite ore processing to enhance the quality and efficiency of alumina extraction. At present, there are some methods to improve the aluminum-silicon ratio of bauxite, such as ore beneficiation methods, chemical desiliconization methods and biological desiliconization methods for the predesiliconization of raw ore to improve the grade.
Ore Beneficiation Methods
Ore beneficiation methods primarily exploit the differences in physical properties (particle size, density) and chemical properties (selectivity of beneficiation reagents) between aluminum- and silicon-bearing minerals in bauxite ore. Several methods, including screening, gravity separation, and flotation, are used to achieve the separation of aluminum and silicon minerals.
The application of screening methods depends largely on the microscopic mineralogy of the ore. Rio Tinto discovered that the bauxite ore in the Weipa region of Australia exists in the form of bean-shaped particles, with decreasing silica content as the particle size increases. Therefore, the company's beneficiation plant crushes the mined bauxite ore to below 19 mm and performs wet screening using a 10-mesh sieve, with the tailings returned to the mining area for backfilling to avoid adverse effects on the subsequent processing caused by SiO2. If there is no significant difference in particle size between the target minerals, separation cannot be achieved through screening [14].
Gravity separation exploits the density differences between the target ore minerals for separation. Currently, it is mainly applied to certain local mono-hydrated alumina ores due to their simple relationship between aluminum and silicon minerals, allowing separation through gravity methods. Conversely, trihydrated gibbsite ores have a tighter association between aluminum and silicon minerals, making effective separation unachievable.
Flotation relies on the selectivity of beneficiation reagents to achieve desilication in bauxite ore. Since most added beneficiation reagents are high-molecular-weight organic compounds, some may enter the subsequent production process with the concentrate. After a certain enrichment level, they can affect the settling characteristics of red mud, evaporation of the mother liquor, seed decomposition rate, and product granularity [15].
Chemical Desiliconization
In the 1940s, the Lauta plant in Germany proposed a chemical desilication method for processing high-silica bauxite ores in Yugoslavia, Austria, and other regions [16]. This desilication method is particularly effective for bauxite ores with fine-grained intergrowth or close association between kaolinite and bauxite, which are difficult to separate. The main process of this method includes preroasting, alkali leaching desilication, and solid‒liquid separation. The addition of thermal activation in the chemical desilication process led to an improved recovery rate of aluminum oxide. This process also effectively removes impurities such as organic matter, sulfur, and carbonates from the ore. However, it should be noted that the calcification process significantly increases the energy consumption of the overall process.
Biological Desiliconization
The bioleaching method primarily utilizes heterotrophic bacteria to decompose silicates or aluminosilicates in bauxite ores. Through bacterial decomposition, SiO2 is transformed into soluble substances, while Al2O3 remains insoluble [17]. For instance, silicate bacteria can breakdown kaolinite into silica dioxide and aluminum oxide. Compared to other methods, this approach can achieve favorable economic and technical indicators while simultaneously avoiding or minimizing environmental pollution [18]. Bioleaching methods are more suitable for treating gelatinous and ultrafine bauxite ores. However, this method faces challenges such as a slow bacterial leaching rate, long process cycles, stringent environmental requirements, low productivity, and technical issues related to the removal and degradation of heterotrophic bacteria. Therefore, there is still a considerable gap between this method and its widespread industrial application.
Modifying the Balanced Structure of Red Mud
Lime Bayer Process
The Lime Bayer process is a new aluminum oxide production technology developed by the Zhengzhou Research Institute of Aluminum Corporation of China Limited and the Shenyang Aluminum and Magnesium Design and Research Institute, specifically tailored to the characteristics of China's bauxite resources. The main principle of the lime Bayer process is to add a significant amount of lime during the high-temperature Bayer leaching process, transforming the balanced structure of red mud from sodium aluminosilicate hydrate in the form of sodalite to calcium aluminosilicate hydrate in the form of hydrous garnet. This transformation eliminates alkali from the leaching desilication product, resulting in a substantial reduction in alkali consumption. The Lime Bayer process can utilize the existing Bayer process system with minimal changes to the process equipment. It only requires an increase in lime dosage to treat medium-grade bauxite, producing alumina with lower alkali consumption and energy consumption compared to the conventional Bayer process. Therefore, this process has low investment requirements and is relatively simple to implement. Currently, the Shanxi Branch of Aluminum Corporation of China Limited has completed the construction of a three-phase Lime Bayer process production line with a capacity of 800,000 tons. The experimental results from July 2002, which lasted for 119 h, showed that when using bauxite with an aluminum-to-silicon ratio of 6.5 to 7, the aluminum-to-silicon ratio of the red mud after leaching was 1.7, and the sodium-to-silicon ratio was 0.26, the actual recovery rate of alumina is over 70%. These technical indicators were close to the design specifications.
High-Pressure Hydrothermal Method
The high-pressure hydrothermal method involves leaching under high temperature (above 280 ℃), high alkaline concentration (mother liquor alkali concentration above 400 g/L of Na2O), and high molecular ratio (mother liquor molecular ratio of Na2O/Al2O3 was 30–35). The leaching solution is evaporated to a sodium oxide concentration of approximately 500 g/L, and hydrated sodium aluminate is crystallized out. This is then dissolved to obtain a sodium aluminate solution with a lower molecular ratio, which can be used to produce aluminum hydroxide through seeding and precipitation. Silicon in the red mud exists in the form of NaCa(HSiO4), which can be hydrolyzed to recover Na2O. The equilibrium solid phase of the red mud is CaO·SiO2·H2O, with an A/S ratio of less than 0.5 and a Na2O mass fraction of less than 1%. This method does not cause the loss of Al2O3 and Na2O, but the evaporation process of the leaching solution consumes a considerable amount of energy, with an evaporated water quantity of 18 t/t Al2O3. Meanwhile, the hydrolysis and Na2O recovery process of NaCa(HSiO4) is complex and involves high temperature and alkaline conditions, making the overall process intricate.
The Sub-molten Salt Method
The sub-molten salt method is employed for processing low-grade bauxite, utilizing a high-concentration alkali solution (Na2O > 600 g/L), temperatures ranging from 150 to 180 ℃, and near atmospheric pressure. This technique yields a leaching solution with a high molecular ratio, along with red mud primarily composed of hydrated sodium silicoaluminate. The leaching solution is diluted, subjected to liquid‒solid separation, and subsequently evaporated for the crystallization and precipitation of solid hydrated sodium aluminate. This solid is then dissolved to acquire a sodium aluminate solution with a lower molecular ratio, serving as a precursor for the production of Al(OH)3 through seeding and precipitation. The separated red mud is treated with hot water washing and lime to remove alkali, resulting in the formation of hydrated garnet as the equilibrium solid phase in the final red mud. Experimental findings indicate that the sub-molten salt method achieves an alumina leaching rate of 96.4% when processing bauxite with an A/S ratio of 8.62.
However, the production process of the sub-molten salt method requires a high-alkali concentration of Na2O exceeding 600 g/L, placing high demands on equipment resistance to alkali. Additionally, the leaching solution exhibits high viscosity and surface tension, posing challenges for subsequent liquid‒solid separation.
The primary objective of increasing the alumina-to-silica ratio in bauxite is to reduce the amount of silica entering the Bayer process, thereby lowering the consumption of caustic soda and alumina associated with silica. Modifying the structure of the leached residue (red mud) is primarily aimed at altering the bonding of aluminum, silicon, and sodium to achieve a balanced red mud structure with lower alkalinity and aluminum content, consequently reducing caustic soda and alumina losses.
Harmless Utilization of Red Mud
The problem of red mud storage can be addressed by finding a cost-effective method for large-scale resource utilization. The basic principles of comprehensive red mud utilization are to extract valuable metals effectively and utilize red mud as a whole without generating secondary waste [19]. Currently, research on the comprehensive utilization of red mud primarily focuses on three aspects: recovery of valuable elements, preparation of construction materials, and preparation of adsorbent materials. Different processes generate red mud suitable for different comprehensive utilization methods. For example, red mud from the sintering process contains a high content of active components such as 2CaO·SiO2 due to the addition of limestone, making it suitable for cement production. On the other hand, red mud from the Bayer process contains a large amount of adhered and structural alkalis but lacks active components such as 2CaO·SiO2, making it unsuitable for the construction materials industry. Therefore, alternative approaches are needed to achieve comprehensive utilization.
Extraction of Valuable Elements from Red Mud
Red mud is a waste residue from the metallurgical industry, and its application in the metallurgical field is an important aspect of comprehensive red mud treatment. The primary application of red mud in the metallurgical industry is the extraction and recovery of valuable components, including iron, aluminum, sodium, and other major valuable elements, as well as trace elements such as titanium, scandium, tantalum, niobium, gallium, vanadium, thorium, and uranium. It is inferred that the recovery of metal elements such as iron, titanium, scandium, gallium, and vanadium from red mud can meet the growing demand for these metals. Given the current situation of gradually depleting mineral resources, the recovery of valuable metals from red mud holds significant value [20].
In recent years, the recovery and extraction of valuable elements from red mud have shown technical feasibility. However, most studies have focused on individual metal elements or elements within the same group, resulting in high costs. Additionally, the remaining residues generated after processing create a new form of solid waste, hindering the achievement of large-scale red mud disposal objectives.
Overall Utilization of Red Mud for Building Materials
Both domestic and international practices have demonstrated the significant demand for raw materials in the construction industry. Red mud, with its potential to be used in the production of various construction materials, offers a viable solution for large-scale utilization [21]. However, there are two limiting factors in utilizing red mud for construction material production: high-alkali content and production costs. The high-alkali content in cement can lead to an alkali-aggregate reaction, where alkalis from cement, admixtures, and the environment react with active SiO2 in aggregates (sand and stone), forming alkali silicate gel. This gel can cause concrete to expand and exhibit spider-web-like cracking, leading to structural damage. One possible solution is to use calcium oxide to remove alkalis from red mud, thereby reducing their impact on cement quality. The production costs of using red mud as a cement raw material primarily include red mud treatment and transportation costs. The high moisture content of red mud (50% to 85%) necessitates further dehydration before direct calcification, thereby increasing costs. To mitigate transportation costs, it is advisable to establish cement plants near alumina plants, thereby reducing transportation expenses.
Application of Red Mud in the Environmental Protection Field
The application of red mud in the field of environmental protection primarily utilizes its potential to sequester metals and metalloid elements such as arsenic. Both raw and modified red mud can be applied in environmental protection and agriculture. The main characteristics of red mud can be categorized into two types: (1) high alkalinity, which facilitates the hydrolysis or precipitation of metals into hydroxides and carbonates, and (2) a high content of iron, aluminum, and titanium oxides, which provides active surface sites for the adsorption of metals and metalloids. Therefore, red mud holds potential application value for substances and areas contaminated by metals and metalloids [22].
Calcification-Carbonization Process
To achieve clean production of aluminum oxide and the harmless utilization of red mud, it is necessary to explore a new method for the low-alkalinity discharge of red mud in the Bayer process for aluminum oxide production. This requires changing the equilibrium phase structure of red mud in the Bayer process, specifically disrupting the balance between sodium, aluminum, and silicon in red mud and seeking a new type of red mud that does not contain sodium and aluminum. Based on this, the Special Metallurgy Team at Northeast University invented a low-cost and large-scale method for the disposal of red mud called the “calcification-carbonization” process. The key steps of the calcification-carbonization process for treating bauxite and red mud include the following: first, calcification is used to transfer all the silicon in bauxite or red mud into hydrated garnet (calcification process), which can remove most of the Na2O from bauxite or red mud; second, CO2 is used to carbonize the calcification-transformed slag (containing hydrated garnet), resulting in carbonized slag mainly composed of calcium silicate, calcium carbonate, and aluminum hydroxide (carbonization transformation); finally, the carbonized slag is subjected to low-temperature aluminum dissolution to obtain a new structure of red mud mainly composed of calcium silicate and calcium carbonate (aluminum dissolution process). The reactions involved in each major process are as follows:
-
(1)
Calcification process:
$$ {\text{Na}}_{{2}} {\text{O}}\cdot{\text{Al}}_{{2}} {\text{O}}_{{3}} \cdot{\text{xSiO}}_{{2}} \cdot\left( {{6} - {\text{2x}}} \right){\text{H}}_{{2}} {\text{O}} + {\text{3CaO}} + {\text{H}}_{{2}} {\text{O }} \to {\text{3CaO}}\cdot{\text{Al}}_{{2}} {\text{O}}_{{3}} \cdot{\text{xSiO}}_{{2}} \cdot\left( {{6} - {\text{2x}}} \right){\text{H}}_{{2}} {\text{O}} + {\text{2NaOH}} $$ -
(2)
Carbonization process:
$$ {\text{3CaO}}\cdot{\text{Al}}_{{2}} {\text{O}}_{{3}} \cdot{\text{xSiO}}_{{2}} \cdot\left( {{6} - {\text{2x}}} \right){\text{H}}_{{2}} {\text{O}} + \left( {{3} - {\text{2x}}} \right){\text{CO}}_{{{2} }} \to {\text{xCa}}_{{2}} {\text{SiO}}_{{4}} + \left( {{3} - {\text{2x}}} \right){\text{CaCO}}_{{3}} + {\text{2Al}}\left( {{\text{OH}}} \right)_{{3}} + \left( {{3} - {\text{2x}}} \right){\text{H}}_{{2}} {\text{O}} $$ -
(3)
Aluminum dissolution process:
$$ {\text{Al}}\left( {{\text{OH}}} \right)_{{3}} + {\text{ NaOH }} \to {\text{ NaAl}}\left( {{\text{OH}}} \right)_{{4}} $$
The processed red mud from the Bayer process primarily consists of calcium silicate (Ca2SiO4) and calcium carbonate (CaCO3) in a new structure. Theoretically, this new structure of red mud does not contain alkaline or aluminum components, making it suitable as a raw material in the cement industry. This solution addresses the fundamental issues of land occupation and environmental pollution caused by red mud, thereby significantly improving the current situation of high solid waste emissions in the aluminum oxide industry. The specific process flow is illustrated in Fig. 4.
The flow chart of the calcification-carbonization method [23]
Drawing upon the fundamental principles underpinning calcification-carbonization treatment technology and processes, subsequent comprehensive and methodical explorations have been undertaken across multiple avenues. These encompass the domains of thermodynamics, kinetics, intrinsic material systems, tangible mineral systems, reactor design, geographically diverse red mud samples, process simulation, scaling strategies, and the sustainable harnessing of calcification-carbonization byproducts.
Exploration and Optimization of the Calcification-Carbonization Method
Pan Lu (2011) employed the calcification-carbonization method to treat low-grade monohydrate bauxite and Bayer red mud, comparing the effects of alkali-carbonation and CO2-carbonation on the extraction efficiency of alumina from low-grade monohydrate bauxite [24]. Guo Fangfang (2013) investigated the influence of various parameters in the calcification and carbonization processes on the treatment effectiveness of Bayer monohydrate bauxite red mud and trihydrate bauxite red mud [25]. Li Qiji (2019) studied the process of alumina production through post-calcium addition and calcification-carbonization, determining the impact of the post-calcium addition process on the rate of alumina dissolution and the sodium content in the red mud [26]. Chen Yang [27] utilized calcium carbide slag as a calcium source in the calcification process, conducting a comparative analysis of the effects of diverse calcium sources, such as calcium oxide and calcium carbide slag, on the calcification-carbonization transformation of Bayer red mud. Zhu Xiaofeng [28] conducted comprehensive studies on the calcification transformation and carbonization decomposition processes of trihydrate bauxite ore and red mud using the calcification-carbonization method. They established the process parameters and successfully conducted scaled-up experiments in a 200 L reactor.
The Reaction Mechanism of the Calcification-Carbonization Method
The core of the calciumization-carbonization method lies in the conversion of sodium silicate slag into hydrated garnet. To investigate the transformation mechanism, Zheng Chaozhen [29] conducted a study on the synthesis of hydrated garnet using pure substances. This research aimed to understand the generation process and regularities of hydrated garnet and put forth three methods for calculating the silicon saturation coefficient of hydrated garnet. Additionally, an exploration was undertaken to investigate the carbonization stability and decomposition mechanism of hydrated garnet. Liu Guanting [30] conducted a systematic investigation into the reaction mechanisms of carbonization decomposition and low-temperature aluminum dissolution in monohydrate bauxite red mud. The study meticulously analyzed the behavioral patterns of sodium, aluminum, silicon, calcium, and other elements during the carbonization process. Building upon these findings, novel schemes for process optimization were proposed, resulting in an enhanced utilization rate of aluminum and silicon resources in red mud. Chen Yongchao [31] and Guo Yong [32], focusing on the primary silicon-bearing minerals in bauxite, namely, kaolinite and illite, conducted research on the calciumization transformation mechanisms of these minerals. Li Xiaoqi [33] carried out a comprehensive analysis and study of the thermodynamics, process conditions, and kinetics involved in the calciumization and carbonization processes of Bayer red mud. The study determined the thermodynamic conditions and kinetic control steps for each stage of the investigated processes.
Research and Development of Calcified Carbonization Reactor
According to the liquid–solid reaction law of calcification carbonization reaction, the laboratory independently developed a new type of stack tube stirring dissolution reactor and Venturi jet flow carbonization reactor [34] and used the reactor to carry out a 200 L scale expansion experiment. After treatment, the aluminum-silicon ratio of trihydrate ore was 4.24, and the aluminum-silicon ratio of secondary carbonization slag was 0.89. The content of Na2O in the slag was 1.01%. The ratio of Al to Si and the content of Na2O decreased from 1.47 and 10.27 wt% to 0.76 and 0.35 wt%, respectively. The recovery rates of alumina and sodium oxide were 48.3% and 96.6%, respectively.
Comprehensive Utilization of New Red Mud After the Calcified Carbonization Method
To achieve clean alumina production and harmless utilization of red mud, Wang Kun [35, 36] conducted vortex melting reduction to recover iron from iron-containing tailings after calcification-carbonation. However, complete utilization of the remaining slag was still not achieved. Therefore, Yanxiu Wang [37, 38] used calcification-carbonation red mud as a raw material to prepare silicate cement and iron-aluminum cement. After appropriate component allocation, they were calcined at 1450 °C and 1300 °C. The strength of the silicate cement clinker exceeded that of 425-grade cement and reached the standard of 525-grade cement. The addition of calcification-carbonation red mud was 42.37%, which was 20% higher than that in existing research. The strength of the iron-aluminum cement met the requirements of 325-grade cement, with a calcification-carbonation red mud addition ratio of 65%.
The calcification-carbonation method effectively reduces the alkalinity and salinity of Bayer process red mud, rendering it comparable to natural soil in terms of salinity levels. Consequently, this method serves as a viable solution to mitigate the environmental repercussions stemming from red mud soilization. Wang Yanxiu [39] conducted a comparative analysis of the soil properties of Bayer process red mud, calcification-carbonation red mud, natural soil, and nutrient soil. The study revealed that both the original red mud and calcification-carbonation red mud fulfill the criteria for agricultural soil in terms of bulk density, total porosity, water retention, and fertility. According to the international soil texture classification, the former was categorized as sandy clay, while the latter exhibited a superior soil texture classified as sandy loam in comparison to Bayer process red mud. Nevertheless, red mud exhibits deficiencies in total nitrogen content, organic matter concentration, soil macroaggregates, and an imbalance in the proportion of readily available nutrients when compared to total nutrients.
To address these shortcomings, Chaoxi [40, 41] employed a one-step KOH hydrothermal transformation of red mud to generate potassium silicate minerals. The resulting transformed slag contained a mass fraction of 10.63% effective K2O, 18.32% mass fraction of SiO2, and various mineral elements such as Ca, Mg, and Fe. It satisfied the requirements outlined in the “Organic‒Inorganic Compound Fertilizer Standard” (GB/T 18,877–2020), specifically meeting the Type I standard. When applied as a red mud slag-potassium silicate mineral fertilizer, it exhibited pronounced promotion of Chinese cabbage growth, establishing itself as a high-quality silicate mineral fertilizer that enhances nutrient absorption by plants.
Optimization of Calcified Carbonization Method
During the industrial promotion of the calcification-carbonation method, several issues have emerged. The calcification transformation requires the addition of nearly 40% lime, which significantly increases the proportion of solid raw materials and reduces material flowability. To address these challenges, Li Qiji made improvements to the existing calcification-carbonation process. By adding lime after leaching, Li Qiji effectively resolved issues such as poor slurry circulation, pipe scaling, and blockages. Additionally, the heat released during flash evaporation cooling and pressure reduction was fully utilized. Under conditions that did not affect the original reaction and production status, lime addition was adjusted to occur after slurry leaching, replacing flash evaporation. The heat from the residual steam after leaching was utilized to facilitate the calcification transformation. Xie Liqun employed continuous calcification-carbonation treatment on monohydrate bauxite red mud to reduce the calcification-carbonation process. The solution obtained after calcification was directly subjected to carbonation without solid‒liquid separation. Both stepwise and continuous processing achieved the desired effect of aluminum extraction and alkali removal. The residual Na2O resulting from continuous processing without solid‒liquid separation after calcification did not affect the final outcome. Furthermore, the low solubility of alumina in the solution obtained after aluminum leaching in the calcification-carbonation method leads to the formation of a large amount of sodium aluminate solution with a high ratio of sodium aluminate to alumina molecules. If this solution were to be reintroduced into the Bayer process system like the calcification solution, it would increase the molecular ratio of sodium aluminate in the Bayer process and increase the energy consumption for evaporation. To address this issue, Lv [23] conducted research on the treatment process of monohydrate bauxite red mud using calcium aluminate as a calcium source in the calcification-carbonization method. They determined the process conditions and parameters while analyzing the reaction mechanism of aluminum precipitation and the cyclic utilization of calcium aluminate.
Conclusions
After nearly a decade of relentless effort, the calcification-carbonization method has undergone systematic laboratory research and scaled-up experiments on over 20 representative raw materials both domestically and internationally. These materials include monohydrate bauxite and red mud from Henan and Shanxi Provinces, as well as high-iron monohydrate bauxite and red mud from Yunnan Province. Additionally, trihydrate bauxite and red mud from regions such as Indonesia, Malaysia, Australia, and Guinea were also investigated. A series of Chinese and international patents have been obtained, affirming the efficacy of this method. It has the potential to increase China's bauxite resource reserves by 4–5 times, effectively mitigating the ecological threats posed by the traditional Bayer process. Furthermore, this method enables clean production of alumina and the low-cost large-scale utilization of Bayer process red mud, injecting new vitality into the alumina industry.
References
Xue S, Liu Z, Fan J, et al. Insights into variations on dissolved organic matter of bauxite residue during soil-formation processes following 2-year column simulation. Environmental Pollution, 2022, 292:118326.
Liu X, Han Y, He F, et al. Characteristic, hazard and iron recovery technology of red mud—A critical review. Journal of Hazardous Materials, 2021, 420:126542.
Snars K, Gilkes R J. Evaluation of bauxite residues (red muds) of different origins for environmental applications. Applied Clay Science, 2009, 46(1):13–20.
Collazo A, Fernández D, Izquierdo M, et al. Evaluation of red mud as surface treatment for carbon steel prior painting. Progress in Organic Coatings, 2005, 52(4):351–358.
Komnitsas K, Bartzas G, Paspaliaris I. Efficiency of limestone and red mud barriers: laboratory column studies. Minerals Engineering, 2004, 17(2):183–194.
Jústiz-Smith N, Buchanan V E, Oliver G. The potential application of red mud in the production of castings. Materials Science and Engineering: A, 2006, 420(1):250–253.
Altundogan H, Altundoğan S, Tumen F, et al. Arsenic Adsorption From Aqueous Solutions by Activated Red Mud. Waste Management, 2002, 22:357–363.
Deng B, Li G, Luo J, et al. Selectively leaching the iron-removed bauxite residues with phosphoric acid for enrichment of rare earth elements. Separation and Purification Technology, 2019, 227:115714.
Huang L, Chunlei L I, Wang H, et al. Research Progress on Comprehensive Utilization of Red Mud. Journal of Physics: Conference Series, 2021, 2009(1):012021–012026.
Novais R M, Carvalheiras J, Seabra M P, et al. Innovative application for bauxite residue: Red mud-based inorganic polymer spheres as pH regulators. Journal of Hazardous Materials, 2018, 358:69–81.
Narayan A, Mac-Quhae C, Rosales J, et al. Does Alumina-Refining Waste Increase the Nutrient Level in Tropical Mesotrophic Floodplain Lakes? Bulletin of Environmental Contamination Toxicology, 2021, 107:506–513.
Mishra B, Gostu S. Materials sustainability for environment: Red-mud treatment. Frontiers of Chemical Science Engineering, 2017, 11(003):483–496.
Bartlett R W. Alumina production process. Comprehensive Utilization of Minerals, 1989, (5):5.
Peter Smith. The processing of high silica bauxites-Review of existing and potential processes. Hydrometallugy, 2009, 98(1–2):162–176.
Andrew P.I., Anishchenko N.M., Mishchenkova N.P. Mechanism of the anionic flotation of chamosite and gibbsite. Tsvetnye Metally, 1973, 116(6):16–20.
Barnes M C, Jonas A M, Gerson A R. The kinetics of desilication of synthetic spent Bayer liquor seeded with cancrinite and cancrinite/sodalite mixed-phase crystals. Journal of Crystal Growth. 1999, 200:251–264.
Roth L G, Berns D S, Chang-Hwei C, et al. Biobeneficiation of bauxite using bacillus polymyxa: calcium and iron removal. International Journal of Mineral Processing, 1996, 48(1):34–41.
Banashettappa V. Biobeneficiation of bauxite ore using bacillus polymyxa. 2012, 49(1):51–60.
Shaohan Wang, Huixin Jin, Yong Deng, Yuandan Xiao. Comprehensive utilization status of red mud in China: A critical review. Journal of Cleaner Production. 2021, 289:125136.
Xiaolin Pan, Hongfei Wu, Zhongyang Lv, Haiyan Yu, Ganfeng Tu.Recovery of valuable metals from red mud: A comprehensive review.Science of The Total Environment, 2023, 904:166686.
Jizhe Zhang, Zhanyong Yao, Kai Wang, Fei Wang, Hongguang Jiang, Ming Liang, Jincheng Wei, Gordon Airey. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Construction and Building Materials. 2021, 270:121419.
Mengfan Wang, Xiaoming Liu. Applications of red mud as an environmental remediation material: A review. Journal of Hazardous Materials. 2021, 408:124420.
Guo-zhi Lu, Ting-an Zhang, Li-nan Ma, Yan-xiu Wang, Wei-guang Zhang, Zi-mu Zhang, Long Wang. Utilization of Bayer red mud by a calcification–carbonation method using calcium aluminate hydrate as a calcium source. Hydrometallurgy. 2019, 188:248–255.
Pan Lu. Basic Research on Treating Low-grade Bauxite and Red Mud by Lime-carbonation Process [D]. Shengyang: Northeastern University, 2011.
Guo Fangfang. Research on Calcification-Carbonation Process for Red Mud [D]. Shengyang: Northeastern University, 2015.
Li JIqi. New Process for Alumina Production with Back Feeding CaO Technology in Calcification Carbonation Method [D]. Shengyang: Northeastern University, 2019.
Chen Y, Lv G, Zhang TA, et al. Carbide Slag as a Calcium Source for Bauxite Residue Utilization via Calcification–Carbonization Processing. Russ J Non-Ferr Met, 2022, 63(2):132–145.
Zhu XF, Zhang TA, et al. Kinetics of carbonated decomposition of hydrogarnet with different silica saturation coefficients. International Journal of Minerals,Metallurgy and Materials, 2020, 27(4):11.
Zheng Chaozhen. Research on the Generation Process and Carbonation Properties of Hydrogarnet [D]. Shengyang: Northeastern University, 2015.
Liu GT, Liu Y, Lv GZ, et al. Wet grinding of calcified slag to improve alumina extraction from red mud by the calcification-carbonization method. JOM, 2020, 72(2):970–977
Zhang ZM, Lv GZ, Chen YC, et al. Alumina extraction from kaolinite via calcification carbonation process. Russ J Non-Ferr Met, 2020, 61(3):248–256.
Guo Yong. Research on Illite Transformation under Calcification-Carbonation Process [D]. Shengyang: Northeastern University, 2020.
Li Xiaoqi. Research on Calcification-Carbonation Process and Kinetics of Red Mud in Bayer Process [D]. Shengyang: Northeastern University, 2016.
Li RB, Zhang TA, Liu Y, et al. Characteristics of red mud slurry flow in carbonation reactor. Powder Technology, 2017, 311:66–76.
Kun Wang, Yan Liu, Ting-an Zhang, Xiao-fei Li, and Xin Chen. Investigationof thesmelting reduction mechanism and of iron extraction from high-iron red mud. MaterialsResearch Express, 2020, 7:126514.
Kun Wang, Yan Liu, Guozhi Lyu, Xiaofei Li, Xin Chen, and Ting’an Zhang. Recoveryofiron from high-iron Bayer red mud by smelting reduction. TMS Annual Meeting, TMS Light Metals 2020, 92–97.
Y.X. Wang, T.A. Zhang, Y.H. Zhang, et al. Mineral transformation in treating low-grade bauxite by the calcification-carbonization process and preparing cement clinker with obtained residue. Minerals Engineering, 2019, 138:139–147.
Wang Y, Zhang TA, Zhang Y, et al. Transformation and Characterization of Cement Clinker Prepared from New Structured Red Mud by Sintering. JOM: the journal of the Minerals, Metals & Materials Society, 2019, 71(8).
Wang Y, Zhang TA, Lv G, et al. Assessment of Bauxite Residue for Reclamation Purposes After Calcification–Carbonization Treatment. JOM: the journal of the Minerals, Metals & Materials Society, 2019, 71(9).
Xi Chao, Ting‒an Zhang, Guozhi Lyu, Zhipeng Liang, Yang Chen. Sustainable application of sodium removal from red mud: Cleaner production of silicon-potassium compound fertilizer. Journal of Cleaner Production, 2022, 352:131601.
Xi Chao, Ting‒an Zhang, Guozhi Lyu, Yang Chen, Qiuyue Zhao, Xuewei Yang, Fangqin Cheng. Research on the mechanism of sodium separation in bauxite residue synergy preparation of potassium-containing compound fertilizer raw materials by the hydrothermal method. Journal of Environmental Management, 2022, 317:115359.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Xin, H., Guo-zhi, L., Ting-an, Z., Song, W., Long, W. (2024). Research of Cleaner Production of Alumina and Harmless Utilization of Red Mud. In: Wagstaff, S. (eds) Light Metals 2024. TMS 2024. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50308-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-50308-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50307-8
Online ISBN: 978-3-031-50308-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)