Abstract
With increasing amount of high silica bauxites used as Bayer refinery feed, the re-precipitation of dissolved silicates results in greater volumes of desilication product (commonly known as DSP) which corresponds to elevated caustic consumption and issues with bauxite residue neutralisation and storage. Furthermore, incomplete desilication of pregnant Bayer liquor also results in silicate reactor and piping scaling as well as the possibility of contamination of the alumina product. Optimization of silicate management in the Bayer process is therefore a high priority. Understanding the chemistry of silicate leaching and precipitation of silicate in Bayer process underpins potential process improvements. This literature review summarises the chemistry of DSPs, with a focus on chemical-thermodynamics and reaction kinetics.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Since the Bayer process was patented in 1888 by Australian chemist Karl Bayer, this technology has been the major industrial process for the producing of alumina from bauxite ores [1, 2]. Even after 135 years, this process remains unchanged for four major key sections: digestion of alumina-rich minerals (gibbsite, boehmite, and diaspore) into hot caustic solution, clarification of the insoluble phases (bauxite residue/red mud), and precipitation of gibbsite and calcination of the gibbsite to alumina.
Bauxite is also comprised of iron phases such as goethite and hematite, titanium oxides, clay minerals comprised of quartz (SiO2), and kaolinite (Al2O3⋅2SiO2⋅2H2O) as well as other impurities such as organics [1, 3, 4]. During bauxite digestion, in addition to extraction of aluminium from bauxite ores (Eqs. 1 and 2), there is also the desilication products (DSP) from clay minerals in solution as shown by Eqs. 3 and 4.
Alumina-Bearing Minerals Digestion
The reactive silica in the bauxite, mainly kaolinite, reacts with the sodium hydroxide to form the sodium metasilicate solution. Once sufficiently supersaturated, the silicates then re-precipitate as insoluble sodium aluminosilicate DSP. Based on the different types of X anions and solution temperature, DSP could be hydroxysodalite, Cl-sodalite and cancrinite [1, 5, 6]. While DSP is costly, it plays a beneficial role in the Bayer process in terms of purifying the liquor of impurities such as sulphate, carbonate, and chloride, and therefore a certain amount of DSP is helpful to ensure a high degree of recyclability of the Bayer liquor.
Reactive Silica Dissolution
Dissolved Silica Precipitation
X = OH−, Cl−, SO42− and CO32−.
The principles of Bayer desilication have been described in many studies [7, 8]. Impurities in bauxite dissolve and accumulate in the Bayer circuit, contributing to an array of process issues thus making it difficult to operate and control [9] with silicate phases being a primary contributor. Silicates such as kaolinite, halloysite, chamosite, and quartz exhibit different solubilities and reaction kinetics [1, 10]. Quartz typically does not significantly dissolve during the extraction of alumina from bauxite, unless the grain structure is fine and the digestion temperature and residence time are high. However, if quartz does dissolve it is especially costly as it leads to additional loss of dissolved aluminium. Halloysite is reactive but tends to be a minor component if present at all. Kaolinite is the most commonly found reactive silica and dissolves readily in caustic soda [5, 11, 12]. In the absence of crystallization seed, most of the reactive silica forms soluble sodium silicate which then precipitates as DSP during the predesilication and digestion stages of the Bayer process [13].
This review summarises the factors that influence thermodynamics solubility and kinetic DSP crystallisation, and mineral phase transformation during Bayer processes. The objective is to bring together the relevant fundamental information about reaction equilibrium and kinetics to enable the optimisation or improvement of predesilication and digestion in terms of economic and environmental outcomes.
Thermodynamic Research of Desilication Products
Despite several empirical solubility correlations proposed for silicates [14, 15], there is a lack of reliable chemical-thermodynamic data relevant to the Bayer process. This data is essential for predictive solubility modelling of DSPs. Only a single report has been found on the measurement of thermodynamic data for anhydrous sodalite (Na6(AlSiO4)6*NaCl2) by Komada et al. [16]. Using the group contribution method for calculating properties such as enthalpies, free energies of formation, and heat capacities proposed by Mostafa et al. [17, 18], Park and Englezos [19] estimated the equilibrium constant for sodalite solubility as it was not available in the literature. The results showed that the equilibrium constant ln(K) was 88.7 with large uncertainty ±10.4 with 95% confidence level. The major contribution in the uncertainties was due to the uncertainty in the Gibbs energy of formation of the DSP phase. Later, Moloy et al. [20] reported the formation and hydration enthalpies of the hydroxysodalite family by hydrothermally synthesising the material from a zeolite phase. More recently, Zeng and Li [21] determined the solubility of sodalite in NaOH-NaAl(OH)4 solutions at temperatures between 30 and 75 °C by dissolving synthetic sodalite into solution. By data regression, they reported sodalite enthalpies and Gibbs energies of formation at standard conditions. For cancrinite, Kurdakova et al. [22] estimated the thermodynamic properties of synthetic sodium carbonate cancrinite at 27 °C using the reported thermodynamic data for calcium cancrinite by Liu et al. [23] and Ogorodova et al. [24]. In this section, the solid (synthesised under hydrothermal conditions) is different from the phases that would be formed during the Bayer process. No reports have been identified that include the enthalpies and Gibbs energies of formation for sodalite and cancrinite at Bayer process conditions. Furthermore, there is no reliable report of the heat capacity–temperature relationship for the high-alumina, semi-crystalline DSPs forming in the Bayer process.
Empirical Solubility Models
The equilibrium concentration of sodium aluminosilicate in sodium aluminate solutions has been the subject of a number of studies. There is a considerable amount of evidence in literature to suggest that the desilication kinetics are directly proportional to the liquor SiO2 supersaturation ratio. Therefore, in order to optimise desilication it is important to accurately predict equilibrium SiO2 values. The supersaturation ratio is defined in Eq. 5 [25]:
where [SiO2]t is the silicate concentration (expressed as SiO2 equivalent) at time t and [SiO2]eq is the equilibrium silicate concentration. Previous research has led to development of models predicting equilibrium silicate concentration. However, most of these models are empirical in nature and not based on any experimentally determined thermodynamic parameters, which strictly limits their application to a set range of factors such as temperature, solution ionic strength, caustic concentration, and liquor impurities [26]. Models used in industry (which are confidential and therefore not published) are also limited to their specific refinery due to the empirically determined nature [27]. When conditions fall outside their parameterisation, the model predictions become almost meaningless [28]. Fundamental understanding of silicate precipitation behaviour and accurate prediction of the products could enable novel, efficient methods of desilication to be realised. Table 1 outlines the correlations for SiO2 solubility in open literature and their range of applicability. As these are empirical models, they have expressed the equilibrium concentration of silicate as [SiO2]eq.
Thermodynamics Solubility Models
Outside of empirical correlations, only a few studies have attempted to construct a silica solubility model from a thermodynamics perspective. The earliest thermodynamic-based model found in literature was created by Jamialahmadi and Müller-Steinhagen [10]. They used the equilibrium constant for the precipitation reaction in Eq. 6 to provide a fit to experimental data.
A later study by Park and Englezos modelled silicate solubility from a chemical-thermodynamics perspective at conditions applicable to Kraft pulp mills. In their model, a slightly different precipitation reaction was used to give the corresponding equilibrium constant in Eq. 7 [19]:
Although both models were reported to have good predictions, they consistently predicted higher values than the measured data, especially at higher temperatures. When considering solubility from thermodynamic first principles, the equilibrium product Kp is dependent exactly on the nature of the chemical species. Given newly understood speciation, the equilibrium constant Kc can be written as Eq. 8 which considers silicate species in terms of H2SiO42− molality—the predominant silicon ion in Bayer process conditions [36]:
where α is the activity, m is the molality, and γ is the activity coefficient of the species.
Considerable attention must be paid to speciation in Bayer liquors, especially at high ionic strength and temperature. When considering Eq. 8 as the equilibrium constant equation for DSP, good agreement has been observed with reported solubility values at extremely dilute conditions and low temperature, however fails when increasing either of these conditions. Equally and perhaps more important to DSP modelling than the aluminium speciation is the speciation of silicon. Problems associated with Si speciation modelling can be traced back to its origins in geochemical modelling [37]. It is generally accepted that the predominant silicate species at high pH such as in the Bayer process is the monomeric H2SiO42− although other polymeric and aluminosilicate species may exist. According to the speciation diagram in Fig. 1, the H2SiO42− ion accounts for at least 90% of the total silicate species in solution and is a generalised form of silicate ion shown in the equations as it is the dominant species [38, 39].
A systematic study undertaken by Smirnov [30] into aluminate solutions that revealed three distinguishable regions in a characteristic ‘U’-shaped solubility curve as shown in Fig. 2: (I) at low Al(OH)3 concentrations where a decrease in the alumina concentration leads to a considerable increase in solubility of DSP (higher SiO2); (II) an intermediate zone where the solubility is practically independent of Al(OH)3 concentration; and (III) at high Al(OH)3 concentrations the equilibrium silica concentration increases with increasing alumina concentration. Note that at gibbsite precipitation conditions (≈3.3–3.8 M Al(OH)3), a sharp decrease in silicate solubility is inevitable as aluminate concentration decreases [40]. As plant liquors typically do not fall below 100 g/L Al2O3, the empirical correlations do not parameterise a U-shaped curve and instead show only a positive relationship as shown in Fig. 2 Region III.
The solubility of silicate (as g/L SiO2) in sodium aluminate solution as a function of aluminate concentration (as g/L Al(OH)3). Graph was taken and modified from [38]
Problems arise when considering both Al and Si species in highly alkaline solution as neither Fig. 1 nor 2 adequately describe their co-existence. Pokrovski et al. were among the first group to investigate Al-Si speciation in basic solutions [41]. It found that the species AlSiO(OH)6− and its formation through Eq. 9 accounted for at least 80% of the total dissolved aluminium in the presence of silicate through 27Al nuclear magnetic resonance measurements.
Gout et al. later conducted comprehensive Raman spectroscopic investigations on Al-Si complexation in ultrabasic solutions at 20 °C with higher OH− molality than previous studies [38]. Through measuring band intensities, they derived the amounts of complexed Al and Si in solution shown in Fig. 3. The dimer species AlSiO3(OH)43− was suggested to be the dominant complex formed through Eq. 10:
Complexed Al and Si concentration as a function of the product of concentrations of the free silicate and aluminate [38]
Zeng and Li modelled silicate speciation using OLI’s mixed solvent electrolyte (MSE) [42] and the AlSiO3(OH)43− species proposed by Gout et al. [43]. Their model appeared to fit their low-temperature data well and suggested it to be the predominant anion at Bayer conditions. However, even sophisticated electrolyte solubility calculations such as that often do not always predict the unusually high Al and SiO2 concentrations of Bayer liquors and nuclear waste tank concentrates [44]. With the abundance of conflicting species, further studies into the system at elevated temperature are required for accurate modelling.
The chemical-thermodynamic approach for estimating solubility can be a powerful predictive tool, provided the model is based on a complete and accurate data set. There is limited data available for certain aqueous species such as aluminate-silicate polyanions and the heat capacity–temperature relationships have not been established for some important components, including sodalite. Reliably relating the thermodynamic predictions to practical solution phase concentrations requires an estimate of the activity coefficient for the aqueous species which is also currently lacking for aqueous silicates. The systems are further complicated when considering the wide range of other solution components and ion substitutions in the DSP.
Crystallization Mechanisms and Kinetics of DSPs
The mechanisms of crystallization during Bayer process, including nucleation, growth, DSP metastable phase transformations, and agglomeration, are complicated. Understanding these mechanisms is crucial for increasing the particle size of DSP and enable adequate recycling of otherwise lost sodium and aluminium.
As shown in Eq. 11, mass deposition is split into a diffusion step and a first-order reaction step. Here m = mass deposited in time t; A = crystal surface area; c = solute concentrations in the bulk solution; ci = at the interface and c* = equilibrium saturation; kd and kr = deposition/reaction mass transfer coefficients; and g = exponent g, the order of the overall crystal growth process [45, 46]. These reactions can be combined by approximating the overall driving force or concentration difference and introducing an overall crystal growth coefficient KG.
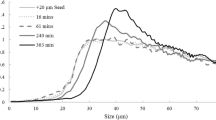
The nucleation and crystal growth of DSP can be determined from the desilication rate and the supersaturation order. Smith et al. proposed that the order of the reaction (n) depends on the supersaturation ratio, with n equal to 1 at lower ratios and 2 or 3 at higher ratios [34]. However, even when the initial silicate concentration is the same, the seed mass or surface area can also affect the order of the reaction. Ruan et al. suggest that desilication with a third-order or greater dependence on Si supersaturation is due to nucleation, either primary heterogeneous or secondary, while less than third-order dependence is predominantly due to aluminosilicate crystal growth [47, 48]. The nucleation and crystal growth rates of DSP during Bayer process vary with kaolinite dissolution in caustic solutions [11, 12]. As shown in Fig. 4, kaolinite from bauxite completely dissolves into caustic solutions within hours, which is coupled with an early increase of SiO2 concentration in the solutions. The SiO2 concentration starts to decrease after complete dissolution of kaolinite due to precipitation of DSP which consumes the SiO2 component in the solution [49]. Nucleation and growth rate for DSP have historically been studied in terms of changes in silicate supersaturation, but not in terms of fundamental parameters. Furthermore, evidence of growth rate dispersion and growth as a function of particle size has not been investigated for DSP.
The reactivity of kaolinite on the precipitation step to form DSP has also been studied. Kotte states that experiments have shown that kaolinite from most bauxites will dissolve rapidly (less than 15 min) provided the temperature is kept close to the atmospheric boiling point [49]. However, this is dependent on the particle size and kaolinite form present. Smith et al. similarly found that DSP precipitation rate is limited by the dissolution of kaolin [50]. Kaolinite can also be heat treated to form meta-kaolin, which reacts rapidly forming DSP phases seen under similar conditions using a soluble silicate source [11, 12, 51, 52].
Desilication Products Phase Transformation
The DSPs have much lower solubility than the kaolinite in Bayer liquor. Many researchers have investigated the structure of DSP [6, 13, 50, 53,54,55,56]. Depending on the solution chemistry and plant operating conditions, various types of DSP can be formed. The silicon-oxygen tetrahedron is a basic structural element of most DSPs. Based on the different types of anion incorporation and solution temperature, the structure of DSP could be either that of a sodalite (Na6(AlSiO4)6*2NaOH*2H2O) or cancrinite (Na6(AlSiO4)6*Na2CO3*2H2O) [1, 5, 6] with molar soda to a silicate ratio (Na2O/SiO2) of 2/3. These two types of DSPs, on the other hand, are known to form as a result of a series of solution-mediated silica-rich phase transformations (e.g., kaolinite) [45, 57,58,59]. This sequence was explained by Peng et al. in the context of thermodynamic driving forces and the observed crystallization pathway occurs via the Ostwald successive transformation step rule, which was proposed as amorphous sodium aluminosilicate → zeolite Linde Type A (LTA) → sodalite → cancrinite [39, 60, 61]. It suggests that Bayer process heat exchanger scale is in fact possibly made up of all phases including amorphous material, zeolite A, sodalite, and cancrinite, but their proportions will be dependent on temperature and conversion rates. This phase transformation sequence has received support from several research groups [1, 62, 63] and is regarded as the ‘correct’ phase transformation sequence in synthetic Bayer liquor. Radomirovic et al. [64] and Peng et al. [60] also observed DSP formation beginning with an amorphous phase under Bayer conditions. In most of the studies investigating phase transformation, a soluble silicate source such as sodium metasilicate pentahydrate or waterglass was used. Some studies have concluded that the starting silicate source influences the precipitating phase, implying that the true phase transformation pathway originating from kaolin is still unknown. It is suggested that DSP is initially heterogeneously nucleated on the kaolin and as kaolin further dissolves, these nuclei are released, allowing for secondary nucleation. Vogrin et al. recently concluded that the DSP formed at alumina digestion concentrations directly precipitates as a high-alumina cancrinite-type structure that can remain stable at equilibrium [65]. This was the proposed phase of DSP in bauxite residues compared to heat exchangers. Generally, in the Bayer liquor, truncated octahedral cages (toc units) are formed by crosslinking AlO4 and SiO4 tetrahedral blocks [66] and these are the fundamental building blocks of the different DSP phases after kaolinite dissolution at temperature less than 100 °C [11, 67, 68].
Anion Effects on the Formation of DSPs
Anion impurities present in Bayer liquors are known to have a detrimental effect on the efficiency of the Bayer process. These anions originate from partial dissolution of sulphur- or carbonate-containing minerals, organic material, process additives, as well as the diffusion of atmospheric carbon dioxide [69]. The presence of different anions can impact the phase transformation rates [50, 52, 61, 70, 71] and trace amounts of impurities (~10–3 M) can cause significant effects on the crystal formation process. As Bayer liquor is recirculated in the process there is a tendency for impurity levels to build up, reducing liquor productivity by affecting alumina solubility, yield, liquor density, and viscosity [72]. As a result, they require purging to maintain reasonable steady-state concentration in the process. Although generally considered undesirable, the precipitation of sodium aluminosilicate shows that various anions can become enclathrated within the framework, including Cl−, CO32−, SO42−, etc. DSP is both paradoxically problematic and useful as the inclusion can actually remove significant amounts of impurities from Bayer liquors [73]. It has been reported that inclusions in DSP may account for up to 75% of Na2SO4 exiting the Bayer circuit, while chloride and carbonate are seemingly lower at about 25% [72]. As anions are able to replace the hydroxide ion, the potential to reduce caustic losses by charging sodium salts after digestion has been suggested and patented in the literature [74, 75].
Only a few studies have been conducted into anion incorporation in DSP that are relevant to Bayer process temperatures, caustic and sodium aluminate concentrations [7, 76, 77]. Fundamental studies by Seimiya et al. into desilication in the presence of impurities revealed that the percentage of anions enclathrated in DSP increases with higher synthesis temperatures and longer times [78,79,80]. Sodalite synthesised with added Na2CO3 contained up to 300% more CO32− than control tests without impurity addition. The results also suggested that there is some preference to incorporate not only CO32−, but also Cl− and SO42− into the crystal lattice over OH− and Al(OH)4− [80].
Comprehensive studies on anion impurities and their effects on DSP were conducted over two decades [13, 52, 54, 61, 71, 81]. The composition of sodalite formed after predesilication and digestion in liquors containing added sodium salts such as NaCl, Na2CO3, or Na2SO4 salts was investigated, which shows that the magnitude of anion incorporation into sodalite under pseudo-Bayer conditions follows the trend: OH− < Al(OH)4− < Cl− < CO32− < SO42−. The incorporation of chloride (Cl−) and sulphate (SO42−) was also observed to follow a Langmuir-type isotherm with increasing anion concentration, which was later supported by Whittington et al., Smith et al., and Vogrin et al. [13, 81, 82]. These studies were beneficial as they modelled quantitative anion incorporation into DSP, although they did not relate to other fundamental factors such as reaction kinetics.
In addition to being incorporated into the DSP structure, anions can promote or suppress the phase transformation sequence. For example, Seimiya et al. discovered DSP in bauxite residue transformed from zeolite A to sodalite after desilication with the addition of NaCl, Na2CO3, and Na2SO4 [80]. Breuer et al. demonstrated that the addition of sodium salt such as sodium oxalate, carbonate, and sulphate promoted formation of cancrinite over sodalite at 200 °C [7]. However, other studies have found that specific salts might inhibit the transformation of zeolite A to other phases [62]. In summary, it is critical to understand the behaviour of each anion influencing secondary nucleation rates, crystal growth, and agglomeration processes under Bayer DSP precipitation conditions.
Conclusions and Outlook
This review summarizes both chemical and physical properties of DSPs and reviewed key issues relating to current challenges faced by the alumina industries for silica contamination in the Bayer liquor, thermodynamics and solubility of DSPs, phase transformation, and crystallization enlargement of DSPs during Bayer process that may affect the refinery aluminium grade or subsequent separation of DSP with other minerals in bauxite residue.
The concentration of NaOH and NaAl(OH)4 is a primary factor that affects the concentration of silicate in Bayer liquor. The solubility of silicate in aluminate-free solutions is higher than in aluminate-bearing solutions under Bayer liquor conditions, and the relationship between silicate concentration and caustic concentration is positively correlated while the equilibrium silicate concentration decreases with decreasing aluminate concentration. The temperature has minimal impact on solubility within a range of 30–75 °C but has been shown to intensify at Bayer digestion temperatures. The crystalline phase also affects silica levels in the Bayer liquor, with the early amorphous phase formed during the Bayer process contributing to higher silica concentration.
For mineral phase transformation during the Bayer process, sodalite and cancrinite are common types of DSPs (digestion-soluble products) formed during the process from kaolinite. These DSPs are known to form because of solution-mediated silica-rich phase transformations, and the crystallization pathway occurs via the Ostwald successive transformation step rule. Anion impurities present in Bayer liquors can impact the phase transformation rates and trace amounts of impurities can cause significant effects on the crystal formation process. Future studies need to be done to clarify the chemical composition and mineral structures of DSPs formed during Bayer process in the presence of anion impurities.
References
Smith, P., The processing of high silica bauxites—review of existing and potential processes. Hydrometallurgy, 2009. 98(1): p. 162–176.
Misra, C. and C. Misra, Industrial alumina chemicals. Vol. 17. 1986: American Chemical Society Washington, DC.
Peng, H. and J. Vaughan. In-Situ XRD Investigation of Bauxite Dehydroxylation. in TMS Annual Meeting & Exhibition. 2018. Springer.
Faulstich, F.R.L., et al., Raman spectroscopic analysis of real samples: Brazilian bauxite mineralogy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2011. 80(1): p. 102–105.
Laws, M.P., Reactions of Kaolinite in the Predesilication Processing of Bauxite Ores. 2005: University of Melbourne, School of Chemistry.
Lowe, J.L. and C.U.o.T.D.o.A. Chemistry, DSP in the Bayer Process: A Fundamental Study of Its Precipitation and Role in Impurity Removal. 2007: Curtin University of Technology.
Breuer, R., L. Barsotti, and A. Kelly, Behavior of silica in sodium aluminate solutions. Extractive Metallurgy of Aluminum, 1963. 1: p. 133–157.
Oku, T. and K. Yamada, The dissolution rate of quartz and the rate of desilication in the Bayer liquor. Essential Readings in Light Metals: Alumina and Bauxite, Volume 1, 1971: p. 247–254.
Gilkes, R., et al. Caustic insoluble aluminium containing nanominerals in bauxite from South Western Australia. in Proceedings of the 9th International Alumina Quality Workshop. 2012.
Jamialahmadi, M. and H. Müller-Steinhagen, Thermodynamic relationships for the solubility of silica in Bayer process liquor. Aluminium (Forschung), 1992. 68(3): p. 230–234.
Peng, H., et al., The effect of leaching temperature on kaolinite and meta-kaolin dissolution and zeolite re-precipitation. Minerals Engineering, 2021. 170: p. 107071.
Peng, H., J. Vaughan, and J. Vogrin, The effect of thermal activation of kaolinite on its dissolution and re-precipitation as zeolites in alkaline aluminate solution. Applied Clay Science, 2018. 157: p. 189–197.
Whittington, B., B. Fletcher, and C. Talbot, The effect of reaction conditions on the composition of desilication product (DSP) formed under simulated Bayer conditions. Hydrometallurgy, 1998. 49(1): p. 1–22.
Barnes, M.C., J. Addai-Mensah, and A.R. Gerson, The solubility of sodalite and cancrinite in synthetic spent Bayer liquor. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999. 157(1): p. 101–116.
Müller-Steinhagen, H., Determining silica solubility in Bayer process liquor. JOM, 1998. 50(11): p. 44–49.
Komada, N., et al., Thermodynamic properties of sodalite at temperatures from 15 K to 1000 K. The Journal of Chemical Thermodynamics, 1995. 27(10): p. 1119–1132.
Mostafa, A.G., et al., Prediction of heat capacities of solid inorganic salts from group contributions. Industrial & Engineering Chemistry Research, 1996. 35(1): p. 343–348.
Mostafa, A.G., J.M. Eakman, and S.L. Yarbro, Prediction of standard heats and Gibbs free energies of formation of solid inorganic salts from group contributions. Industrial & Engineering Chemistry Research, 1995. 34(12): p. 4577–4582.
Park, H. and P. Englezos, Thermodynamic modeling of sodium aluminosilicate formation in aqueous alkaline solutions. Industrial & Engineering Chemistry Research, 1999. 38(12): p. 4959–4965.
Moloy, E.C., Q. Liu, and A. Navrotsky, Formation and hydration enthalpies of the hydrosodalite family of materials. Microporous and Mesoporous Materials, 2006. 88(1): p. 283–292.
Zeng, L. and Z. Li, Solubility and Modeling of Sodium Aluminosilicate in NaOH–NaAl (OH)4 Solutions and Its Application to Desilication. Industrial & Engineering Chemistry Research, 2012. 51(46): p. 15193–15206.
Kurdakova, S., et al., Thermodynamic properties of synthetic calcium-free carbonate cancrinite. Physics and Chemistry of Minerals, 2014. 41(1): p. 75–83.
Liu, Q., H. Xu, and A. Navrotsky, Nitrate cancrinite: Synthesis, characterization, and determination of the enthalpy of formation. Microporous and Mesoporous Materials, 2005. 87(2): p. 146–152.
Ogorodova, L., et al., Cancrinite and cancrisilite in the Khibina-Lovozero alkaline complex: Thermochemical and thermal data. Geochemistry International, 2009. 47(3): p. 260–267.
LaMacchia, R., Toward a better understanding of desilication product (DSP) precipitation kinetics, in Ninth International Alumina Quality Workshop. 2012, AQW Incorporated: Perth, Western Australia. p. 214–218.
Gasteiger, H.A., W.J. Frederick, and R.C. Streisel, Solubility of aluminosilicates in alkaline solutions and a thermodynamic equilibrium model. Industrial & Engineering Chemistry Research, 1992. 31(4): p. 1183–1190.
Staker, W., Internal Communication. 2017.
Königsberger, E., P.M. May, and G. Hefter, A comprehensive physicochemical model of synthetic Bayer liquors, in Seventh International Alumina Quality Workshop. 2005, AQW Incorporated: Perth, Western Australia. p. 74–77.
Adamson, A.N., E.J. Bloore, and A.R. Carr, Basic principles of Bayer process design, in Extractive Metallurgy of Aluminum, G. Gerard and P.T. Stroup, Editors. 1963, Interscience: NY. p. 23–58.
Smirnov, M.N., The metastable solubility of silica in aluminate solutions. The Soviet Journal of Non-Ferrous Metals, 1964. 37(1): p. 16–22.
Leiteizen, M.G., Kinetics of converting bauxite silica to sodium aluminosilicate. The Soviet Journal of Non-Ferrous Metals, 1972. 13(5): p. 37–40.
Cresswell, P., Factors affecting desilication of bayer process liquors. Proceedings of Australian Chemical Engineering Annual Conference, Chemeca 84, 1984. 12: p. 285–292.
Hewett, K.J., A.J. White, and G.I.D. Roach, Silica solubility in plant liquors, in AJW23/MDR (Confidential Report). 1987.
Smith, P.G., B.L. Fletcher, and C.C. M., Silica equilibrium in caustic aluminate solutions, in CSIRO Minerals Products Communication MPC/P-019. 1992.
Sizgek, G.D. and D.D. Aguila, Silica equilibrium equation for a full range of QAL plant liquors, in Queensland Alumina Limited. 1994.
Sipos, P., The structure of Al(III) in strongly alkaline aluminate solutions - A review. Journal of Molecular Liquids, 2009. 146(1): p. 1–14.
Anderson, G.M. and D.A. Crerar, Thermodynamics in geochemistry: the equilibrium model. 1993: Oxford University Press, USA.
Gout, R., et al., Raman spectroscopic study of aluminum silicate complexes at 20°C in basic solutions. Journal of Solution Chemistry, 2000. 29(12): p. 1173–1186.
Peng, H., J. Vaughan, and M. Zieba, The thermodynamic approach to predicting silicate solubility. 2015.
Peng, H. and J. Vaughan, Aluminate effect on desilication product phase transformation. Journal of Crystal Growth, 2018. 492: p. 84–91.
Pokrovski, G., et al., Structure and stability of aluminium-silica complexes in neutral to basic solutions: Experimental study and molecular orbital calculations. Mineralogical Magazine A, 1998. 62: p. 1194–1195.
Zeng, L. and Z. Li, Dissolution Behavior of Al, Si and Fe of Diaspore Concentrate in NaOH−NaAl (OH) 4 Solutions at Elevated Temperature. Industrial & Engineering Chemistry Research, 2013.
Gout, R., et al., Raman spectroscopic study of aluminum silicate complexes at 20 C in basic solutions. Journal of Solution Chemistry, 2000. 29(12): p. 1173–1186.
Agnew, S.F. and C.T. Johnston, Aluminum solubility in complex electrolytes, in WM2013: Waste Management Conference: International collaboration and continuous improvement. 2013: United States.
Subotic, B. and J. Bronic, Theoretical and practical aspects of zeolite crystal growth. Handbook of Zeolite Science and Technology, Marcel Dekker Inc., New York–Basel, 2003: p. 129.
Duecker, H.C., A. Weiss, and C.R. Guerra, Synthetic crystalline zeolite. 1971, Google Patents.
Ruan, S., et al., Desilication of hematite, goethite and iron powder seeded low alumina to caustic liquors. Hydrometallurgy, 2017. 169: p. 297–305.
Ruan, S., The Mechanism and Kinetics of Sodium Aluminosilicate Crystallisation in Synthetic Bayer Spent Liquor. 2015, University of South Australia.
Teas, E.B. and J.J. Kotte. The effect of impurities on process efficiency and methods for impurity control and removal. in Bauxite Symposium IV, Kingston. 1980.
Smith, P., et al. Understanding growth of DSP in the presence of inorganic ions. in Proceedings of the 6th International Alumina Quality Workshop. 2002. Brisbane Convention & Exhibition Centre Queensland, Australia.
Vogrin, J., et al., Influence of chloride on sodium aluminosilicate solubility in Bayer liquor. Microporous and Mesoporous Materials, 2020. 299: p. 110086.
Vogrin, J., et al., The anion effect on sodium aluminosilicates formed under Bayer process digestion conditions. Hydrometallurgy, 2020. 192: p. 105236.
Xu, B. and P. Smith, The effect of iron sources on caustic and alumina recovery from synthetic bayer DSP (sodalite). Hydrometallurgy, 2012. 129: p. 26–29.
Lowe, J., et al., Incorporation of impurity anions into DSP: insights into structure and stability from computer modelling. Molecular Simulation, 2006. 32(01): p. 35–44.
Lowe, J., et al. Morphology and crystallinity: Insights into the mechanism of growth of DSP. in 7th International alumina quality workshop. 2005.
Whittington, B. and T. Fallows, Formation of lime-containing desilication product (DSP) in the Bayer process: factors influencing the laboratory modelling of DSP formation. Hydrometallurgy, 1997. 45(3): p. 289–303.
Barnes, M.C., J. Addai-Mensah, and A.R. Gerson, The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous and Mesoporous Materials, 1999. 31(3): p. 287–302.
Barnes, M.C., J. Addai-Mensah, and A.R. Gerson, The kinetics of desilication of synthetic spent Bayer liquor and sodalite crystal growth. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999. 147(3): p. 283–295.
Subotić, B., et al., Transformation of zeolite A into hydroxysodalite: I. An approach to the mechanism of transformation and its experimental evaluation. Journal of Crystal Growth, 1980. 50(2): p. 498–508.
Peng, H., D. Seneviratne, and J. Vaughan, Role of the amorphous phase during sodium aluminosilicate precipitation. Industrial & Engineering Chemistry Research 2018. 57(5): p. 1408–1416.
Peng, H., M. Ding, and J. Vaughan, The Anion Effect on Zeolite Linde Type A to Sodalite Phase Transformation. Industrial & Engineering Chemistry Research, 2018. 57(31): p. 10292–10302.
Reyes, C.A.R., C. Williams, and O.M.C. Alarcón, Nucleation and growth process of sodalite and cancrinite from kaolinite-rich clay under low-temperature hydrothermal conditions. Materials Research, 2013. 16(2): p. 424–438.
Shi, L., et al., Desilication of low alumina to caustic liquor seeded with sodalite or cancrinite. Hydrometallurgy, 2017. 170: p. 5–15.
Radomirovic, T., et al., Crystallization of sodalite particles under Bayer-type conditions. Hydrometallurgy, 2013. 137: p. 84–91.
Vogrin, J., et al., Synthesis of zeolites using kaolin in concentrated sodium hydroxide-aluminate solutions. Applied Clay Science, 2023. 244: p. 107106.
Baur, W.H. and R.X. Fischer, LTN-type zeolite framework as an interpenetrating net of KFI-and SOD-type parts homeomorphic to cuprite, Cu2O. Acta Crystallographica Section B: Structural Science, 2007. 63(2): p. 229–234.
Abdullahi, T., Z. Harun, and M.H.D. Othman, A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Advanced Powder Technology, 2017. 28(8): p. 1827–1840.
Johnson, E. and S.E. Arshad, Hydrothermally synthesized zeolites based on kaolinite: a review. Applied Clay Science, 2014. 97: p. 215–221.
Zheng, K., et al., The influence of sodium carbonate on sodium aluminosilicate crystallisation and solubility in sodium aluminate solutions. Journal of Crystal Growth, 1997. 171(1): p. 197–208.
Bosnar, S., et al., Influence of anions on the kinetics of zeolite A crystallization:: a population balance analysis. Journal of crystal growth, 2004. 267(1–2): p. 270–282.
Wang, S., et al., Revealing the effect of anions on the formation and transformation of zeolite LTA in caustic solutions: an in-situ synchrotron PXRD study. Crystal Growth & Design, 2023. 23(5).
Riley, G., et al., Plant impurity balances and inclusion in DSP, in Fifth International Alumina Quality Workshop. 1999, AQW Incorporated: Bunbury, Western Australia. p. 404–414.
Lowe, J.L., DSP in the Bayer process: A fundamental study of its precipitation and role in impurity removal, in Department of Applied Chemistry. 2007, Curtin University of Technology.
Flint, E.P., L. Shartsis, and L.S. Wells, Method of reducing the concentration of silica in sodium aluminate solutions (US2519362 A). 1950.
Feher, I., et al., Reducing or compensating for sodium hydroxide loss produced during alumina manufacture. Fr Demande, 1973. 2: p. 166–188.
Teas, E.B. and J.J. Kotte, The effect of impurities on process efficiency and methods for impurity control and removal, in Proceedings of Bauxite Symposium, No. IV. 1980, Journal of the Geographic Society of Jamaica. p. 100–129.
Whittington, B.I., B.L. Fletcher, and C. Talbot, The effect of reaction conditions on the composition of desilication product (DSP) formed under simulated bayer conditions. Hydrometallurgy, 1998. 49(1–2): p. 1–22.
Seimiya, S. and M. Shietoshi, Studies on sodalite compounds in the Bayer process (2nd report). Journal of Japan Institute of Light Metals (Keikinzoku), 1962. 12(5): p. 286–291.
Seimiya, S. and S. Mori, Study on sodalite compounds in the bayer process (3rd Report): Dissolution phenomenon of sodalite compounds in sodium aluminate solution. Journal of Japan Institute of Light Metals, 1962. 12(6): p. 351–354.
Seimiya, S., Some properties of sodalite in red mud, in Extractive Metallurgy or Aluminum, G. Gerard and P.T. Stroup, Editors. 1963, Interscience: NY. p. 115–132.
Smith, P.G., et al., The composition of DSP formed under predesilication and high temperature Bayer digestion conditions. Light Metals, 2001. 1: p. 5–11.
Vogrin, J., et al., The influence of sodium sulphate on sodium aluminosilicate solubility in Bayer liquor aiding the desilication process. Hydrometallurgy, 2023. 219: p. 106079.
Acknowledgements
The authors gratefully acknowledge the financial support from Rio Tinto and the Alumina Workshop Scholarship for this project. We acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Peng, H., Vaughan, J., Wang, S., Vogrin, J., Seneviratne, D. (2024). Chemical Thermodynamics and Reaction Kinetics of Bayer Process Desilication. In: Wagstaff, S. (eds) Light Metals 2024. TMS 2024. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50308-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-50308-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50307-8
Online ISBN: 978-3-031-50308-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)