Abstract
Most recent vertebrate extinctions have happened on islands, often associated with the introduction of non-native species. This has led to drastic changes in island community compositions and their ecological functions, with unknown consequences for island ecosystems. Species interactions have been particularly strongly impacted, such as interactions between plants and the animals that eat fruits and thereby disperse seeds. Loss of seed dispersal may limit plant movement and recruitment success, increasing the risk of associated secondary extinctions of fleshy fruited plants. However, plants differ in the degree of their dependency on interactions with animals and there is very little direct empirical evidence that co-extinction has happened. Here, we provide an overview of what we know, and do not know, about the consequences of losing frugivore seed dispersers for island plants and discuss the main challenges for quantifying the problem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Insular
- Co-extinction
- Functional extinction
- Non-native
- Invasive
- Frugivory
- Seed dispersal
- Recruitment success
- Movement
- Data collection
1 Introduction
Islands provide unique opportunities for studying the structure and function of ecological communities. Because islands are isolated from immigration, and often small, their communities tend to be ecologically less complex than those of mainland ecosystems (Whittaker et al. 2017). Within these simpler communities, ecological release from predation and competition has enabled rapid evolution of a wide variety of extraordinary species, such as giant tortoises and flightless birds. As a consequence, islands are biodiversity hotspots that harbour 15–20% of all terrestrial species, many of which are endemic to islands, in spite of constituting only 3.5% of the earth’s land surface (Whittaker et al. 2017). At the same time, they are hotspots of extinction, with 74% of all recently extinct vertebrates having gone extinct on islands (Tershy et al. 2015). Of these island extinctions, 86% are associated with the introduction of non-native species (Bellard et al. 2016), such as rats, cats, goats, and pigs, that predate on some native species and compete with others. The introduction of predators and competitors has eroded the benevolent conditions that characterized these native insular communities, with profound consequences for island biota (Whittaker and Fernandez-Palacios 2007; Drake and Hunt 2009).
The combination of species extinctions and introductions of both animals and plants have had secondary effects on the remnant communities, in many cases due to restructuring of species interaction networks. As a case in point, up to 90% of woody plants in the (sub-)tropics interact with animal species (mostly birds, mammals, reptiles, and invertebrates) that disperse their seeds by swallowing or carrying their fruits and dropping them elsewhere. This facilitates plant movement and may also directly improve seed germination through mechanical pulp removal and through chemical effects during gut passage (Fleming et al. 1987; Fleming and Kress 2013; Traveset 1998; Traveset et al. 2007; Valido and Olesen 2019; Falcón et al. 2020; Lim et al. 2020; Kissling et al. 2009). Loss of frugivores may thus impede the dispersal and reproduction of most island plants, raising the concern that this may lead to plant population declines and ultimately co-extinctions (Bond 1994; Brodie et al. 2014; Heinen et al. 2020; Fadini et al. 2009; Meyer and Butaud 2009).
Though potentially highly important, these secondary dynamics remain mostly conjecture, and a number of analytical and conceptual challenges make it difficult to empirically estimate the actual magnitude of the threat from loss of ecological interactions such as frugivory. Here, we provide an overview of what we know, and do not know, about the consequences for island plants of losing frugivore seed dispersers, and discuss the main challenges for quantifying the problem.
2 Vulnerability of Plant-Frugivore Interactions on Islands
By dispersing seeds, animals increase plant recruitment success and drive movement dynamics in plant populations, making plants less vulnerable to disturbances such as habitat loss and fragmentation (Fleming and Kress 2013). However, the many anthropogenic threats to both animals and plants combined can easily disrupt frugivory and seed dispersal interactions (Fleming and Kress 2013; Kaiser-Bunbury et al. 2010). We argue here that island communities are particularly vulnerable to plant-frugivore interaction loss, exacerbating the pressure on the integrity of island ecosystems.
On islands, the narrower breadth of available resources (e.g. different food sources or suitable habitat) has in many locations caused a wider range of organisms to supplement their diet with fruits (Kaiser-Bunbury et al. 2010; Traveset and Richardson 2014), increasing the prevalence of frugivory. Some of these animals destroy the seeds, but many others end up playing a key ecological role as seed dispersers (Kaiser-Bunbury et al. 2010; Carpenter et al. 2020). One example is that of lizards, which, in contrast to the mainland, incorporate a sizable portion of fruit into their diets on many islands worldwide (Valido and Olesen 2019), including, for example, several geckos and skinks (e.g. Phelsuma spp. and Leiolopisma spp.) on the Indian Ocean island of Mauritius (Cheke and Hume 2008). Island communities may thus be characterized by many plant-animal interactions.
At the same time, island communities have relatively low species numbers, which also makes island plants particularly vulnerable to the effects of interaction loss (Kaiser-Bunbury et al. 2010; McConkey and O’Farrill 2016). In the larger interaction networks of mainland communities, the loss of any one species may be compensated for by interactions with other species, whereas in island communities, lost species often have few or no replacements (Fleming and Kress 2013; Kaiser-Bunbury et al. 2010; Fricke et al. 2018). Even small reductions in the absolute number of interactions may have important consequences, and a scenario where a plant species loses all possible seed dispersers is substantially more likely (Fleming and Kress 2013; Kaiser-Bunbury et al. 2010; McConkey and O’Farrill 2016). In addition, global extinctions are more common on islands relative to mainland (Tershy et al. 2015), even under population pressures that cause range reductions, which means that interaction loss in mainland systems is more often likely to be temporary.
Plants lose seed dispersal already before their seed dispersers go globally extinct, and even a moderate decline in the population size of a key seed disperser may negatively impact plant recruitment success and movement potential (Fleming and Kress 2013; McConkey and O’Farrill 2016). This may be particularly pronounced for island populations, characterized by low population sizes and limited distributions, and on many islands, hunting, habitat destruction, and competition and predation by introduced species have severely reduced the abundance of vertebrate seed dispersers (Heinen et al. 2018; Tershy et al. 2015), more so than in mainland ecosystems (Tershy et al. 2015). Such marked declines in disperser populations may eventually lead to “functional” extinction, in which a frugivore species persists, but no longer disperses seeds to an ecologically relevant degree; that is frugivore-mediated seed dispersal occurs so rarely that it does not affect plant population dynamics (McConkey and O’Farrill 2016).
More subtle and harder to detect pressures on plant-frugivore interactions are animal behavioural responses to ecosystem changes (Heinen et al. 2020, 2023; McConkey and O’Farrill 2016). As the population sizes of plants decrease rapidly on islands, some of the animals that depend on fruits may have to shift their diets to more common plants, resulting in a negative feedback cycle as already declining plant species lose reproductive potential (Fricke et al. 2018; Oleksy et al. 2021). In addition to abundance declines, interaction behaviour on islands can also be altered by introduced species (Heinen et al. 2023; Oleksy et al. 2021; Reinegger et al. 2021) because of their competitive advantage in ecosystems characterized by evolution under low competition (Whittaker and Fernandez-Palacios 2007). Many introduced fruiting plants offer attractive rewards (e.g. sweet, pulp-rich mango) that outcompete native plants for attracting frugivores (Linnebjerg et al. 2009; Oleksy et al. 2021). Such dietary shifts towards introduced plant species have already been described for Mauritius and Hawaii (Linnebjerg et al. 2009; Oleksy et al. 2021; Vizentin-Bugoni et al. 2019) and have the potential to reduce native plant seed dispersal and shift the ecological balance towards introduced plants, even in the absence of extinctions. Introduced non-native frugivores may also be sustained by non-native plants, further facilitating their invasion (Linnebjerg et al. 2009; Traveset and Richardson 2014) and driving a cascade of ecological effects that erode island ecosystems.
3 The Extent of Frugivore Change on Islands Globally and in Mauritius
Losses of vertebrate frugivores characterize many islands globally, although the extent differs geographically among islands, and has been particularly high on those islands that are isolated from the mainland, have small area sizes, and a higher maximum elevation (Heinen et al. 2018; Fig. 3.1). The pattern of preferential extinction of larger frugivores is globally consistent (Heinen et al. 2018). The same is true for frugivores that cannot fly (Fig. 3.2) (Heinen et al. 2018). The loss of many large frugivores from island communities has decreased their community-level mean body mass by 37% and maximum mass by 51% (Heinen et al. 2018), leaving only the smaller frugivores that likely cannot swallow and disperse the largest seeds (Fig. 3.3).
Frugivore species richness (birds, mammals, reptiles) prior to human arrival and the proportion of this that has gone extinct on 74 islands within 20 archipelagos worldwide (Heinen et al. 2018)
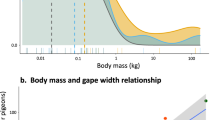
Generalized linear mixed-effects model of extinction probability of insular frugivorous birds, mammals, and reptiles in relation to their body mass and ability to fly (adapted from Heinen et al. 2018)
Though extinctions and their local effect are relatively well-described, there is no similar global consensus on the effect of introductions of non-native species of frugivorous birds, mammals, and reptiles. However, a number of individual systems have been the focus of concerted research effort, and we thus here pay particular attention to case studies on a single well-studied island, where the local-scale effects of frugivore extinctions and introductions have been studied intensively: the Western Indian Ocean island of Mauritius (Cheke and Hume 2008). Mauritius was one of the last islands in the world to be colonized by humans (in AD 1638), which means that its colonization history has been documented exceptionally well (Cheke and Hume 2008). The island is also severely affected by human activities, causing the loss of almost all of its native vegetation, the introduction of many non-native species, and many extinctions, including frugivores such as the iconic Dodo (Raphus cucullatus), giant skinks (Leiolopisma mauritiana), as well as an entire genus of endemic giant tortoises (Cylindraspis spp.) (Cheke and Hume 2008).
The frugivore community on Mauritius was substantially changed by extinctions and introductions (Box 3.1, Heinen et al. 2018, 2023). After human arrival, only 15 of the 26 known native frugivorous birds, mammals, and reptiles survived and 14 non-native frugivores established (Cheke and Hume 2008; Heinen et al. 2023). In particular, the extinction of many large Mauritian frugivores left only small native frugivores in the community. Large animals are particularly vulnerable to hunting and habitat loss because of their generally smaller population sizes and larger range sizes (Cardillo 2003; Dirzo et al. 2014). The remaining small-bodied native frugivores are unlikely to swallow and thus disperse the largest seeds (Heinen et al. 2018, 2023). This increases the risk that some Mauritian plant species may lose most or all of their native dispersers (Heinen et al. 2018, 2023), potentially putting them at risk of secondary extinction (Heinen et al. 2020). Indeed, a recent empirical study confirmed that the recruitment success of many native plants in one of the best-preserved forests (Brise Fer) is now very low (Albert et al. 2021). The many introduced frugivorous animals likely cannot replace extinct species as seed dispersers, as they frequently destroy the native seeds (Heinen et al. 2023).
In addition to being affected by disperser loss, many native plants suffer from competition for nutrients and water with introduced plants, lowering their fruit production (Bissessur et al. 2023; Monty et al. 2013). Intensive conservation efforts have reduced invasive species abundance and managed to bring back several critically endangered species from the brink of extinction and improved their IUCN status (e.g. Mauritius kestrel, pink pigeon, echo parakeet, Telfair’s skink) (Cheke and Hume 2008). However, nearly all native frugivores remain rare and many seed dispersal interactions with native plants are likely functionally extinct.
4 Consequences of Seed Disperser Loss for Island Plants
The effects of loss of frugivore-mediated seed dispersal for island plants can be divided into two categories: those that affect plant recruitment success (e.g. seed handling and short-distance dispersal) and those that affect the population-level movement of plants (e.g. long-distance dispersal). There are three stages at which these processes are affected: functional limitation of the interaction, functional extinction of the interaction, and ultimately frugivore extinction.
The primary effect of disperser loss is on the recruitment success of plants (Fig. 3.4) (Fleming and Kress 2013). The very act of handling fruits by frugivores may be beneficial for seed germination. Seed handling by frugivorous vertebrates generally leads to removal of fruit pulp from the seeds by means of chewing, pecking, or ripping (Traveset 1998; Traveset et al. 2007). This in itself has been shown to increase seed germination because it can remove germination inhibitors present in the pulp and prevents seed destruction by insects, bacteria, and fungi attracted to the rotting pulp (Traveset 1998; Traveset et al. 2007). In addition to that, seeds that are swallowed can be abraded mechanically and/or chemically by passing through the intestinal system of animals, in some cases increasing germination success (Traveset 1998; Traveset et al. 2007). Finally, the faecal matter or oral ejecta pellet (Fig. 3.5) in which the seeds end up can provide a beneficial depositional environment for emerging seedlings (Traveset 1998; Traveset et al. 2007). Island and mainland frugivores both provide these services to plants. However, islands with lower species richness than mainland potentially provide plants with lower functional redundancy in these services.
Conceptual explanation of the effects of frugivores on plant recruitment. Frugivores can increase germination success by removing pulp from seeds or by passing seeds through their gut. Frugivores also contribute to the Janzen-Connell effect (Janzen 1970; Connell 1971), whereby plants that are dispersed further away from the mother plant suffer less predation and competition and therefore have higher chances of survival and reproduction. The young trees that are shown are similar in age, but one is suffering from competition near the mother tree which stunted growth and hampered fruit production
Evidence of plant-frugivore interactions between flying foxes (Pteropus niger) and endemic plants on Mauritius. (a) Mashed-pulp ejecta in the shape of the upper palate on the left and fruit with teeth marks on the right (Mimusops maxima). (b) Seed germinating while still stuck in the ejecta (Labourdonnaisia glauca). Both photos by Julia Heinen
Plant recruitment potential on islands has been changed by a shift in the types of frugivores (e.g. morphology, behaviour, ecological function) that are able to eat different fruits and handle them differently (Heinen et al. 2018, 2023). The over-representation of large-bodied frugivores (e.g. various species of giant tortoise) among island extinctions (Fig. 3.2; Heinen et al. 2018, 2023) has left mostly small frugivores to disperse native seeds. These species may not be able to swallow (Heinen et al. 2023) or carry larger seeds (though they may be able to handle and depulp them), and they generally have smaller guts that can fit fewer seeds (Wotton and Kelly 2011, 2012). Introduced non-native frugivores may potentially take over the role of seed dispersers (Griffiths et al. 2011; Rodriguez 2006; Vizentin-Bugoni et al. 2019), but may handle seeds differently or predate on them (Carpenter et al. 2020; Heinen et al. 2023; McConkey and O’Farrill 2016), and may facilitate the spread of invasive non-native plants as well (Traveset and Richardson 2014; Linnebjerg et al. 2009). The reduction or loss of (mechanical) seed handling by native frugivores can therefore reduce the number of new plants in the population (McConkey and O’Farrill 2016), and for plants that strongly depend on frugivores for their population survival, this has the potential to result in plant co-extinctions (Fleming and Kress 2013).
In addition to the beneficial effects of mechanical seed handling (e.g. depulping, gut abrasion), the primary effect of frugivory is to move seeds away from the mother plant (Fleming and Kress 2013). Even short-distance movements of seeds have been shown to reduce the impact of host-specific parasites, diseases, and predators on seeds and seedlings, as well as reducing sibling competition associated with vicinity to the mother plant (Fig. 3.4, the Janzen-Connell effect, Janzen 1970; Connell 1971). The types of seed and seedling predators (including parasites and diseases) can be divided into “distance-responsive-predators” that kill seeds and seedlings near the mother tree (these may specialize on that particular species) and “density-dependent-predators” that kill them where they are most abundant (can be generalists) (Janzen 1970; Connell 1971). The mix of predator types contributes to a mixed distribution of plant species over the landscape, because each species is able to escape their own host-specific predators and diseases, and species that become common will suffer more host-specific predation (Janzen 1970; Connell 1971). The effects are predicted to be strongest in tropical and wet habitats due to higher pathogen abundance and diversity (Janzen 1970; Connell 1971), which is also where frugivore-mediated seed dispersal is most common (Fleming et al. 1987; Fleming and Kress 2013; Kissling et al. 2009). Additionally, most islands of oceanic origin are (sub)tropical and characterized by many frugivore species (Heinen et al. 2018).
On islands, where communities are generally less diverse than on the mainland, seed predators (and pathogens) may be opportunistic generalists more often than specialists on the seeds of a single plant species, potentially making the density-dependent Janzen-Connell effect less strong in insular environments (Hansen et al. 2008). If abundance of seed predators is too low on islands, as is the case for both generalist and specialist frugivorous weevils in New Guinea lowland rainforest, they may be below a critical threshold to cause Janzen-Connell effects (Ctvrtecka et al. 2014). On the other hand, island plants that do rely on escape from density-dependent predation and competition may be more vulnerable than they would be on the mainland because the remaining habitats are too small to support viable populations (Hansen et al. 2008). On Mauritius, an experimental study of the Janzen-Connell effect for Syzygium mamillatum (Myrtaceae) showed strong negative distance-dependent effects on survival and growth of seedlings when they were close to their mother trees (Hansen et al. 2008). This was mainly due to fungi and insect damage, and it is unknown whether these are generalists or specialists (Hansen et al. 2008).
Though most seeds are dispersed over relatively short distances, frugivory occasionally leads to long-distance movements with population-level effects (Fig. 3.6) (Fleming et al. 1987; Fleming and Kress 2013). Sometimes plant seeds are moved far enough to colonize new islands, such as when birds and flying foxes transport ingested seeds of fleshy fruited plants from Sumatra or Java to the Krakatau islands (Whittaker and Jones 1994). These islands were completely sterilized by a volcanic eruption in 1880 (and again in 2019), but the native forests were quickly rebuilt, in part driven by frugivore-mediated dispersal from nearby forests (Whittaker and Jones 1994). Some island frugivores, especially those that can fly, occasionally travel to other nearby islands or have home ranges that extend across several islands, connecting multiple island populations (Zann 1992; Vidal et al. 2014).
Conceptual explanation of the effects of frugivores (blue) on plant movement at population level. Frugivores can spread seeds to new areas, assisting their colonization, or connect existing populations, facilitating migration, gene flow (green and orange genotypes), and metapopulation dynamics, or provide rescue effects and source-sink dynamics to locally declining plant populations
Long-distance dispersal events (Fig. 3.6) contribute to island metapopulation dynamics and enable source-sink dynamics and rescue effects for connected locally declining sub-populations of plants (Amarasekare 2004; Nathan and Muller-Landau 2000; Nathan 2006; Rogers et al. 2021; Vidal et al. 2014). Reductions in seed dispersal would impede metapopulation dynamics, leading to reduced levels of gene flow and over time eroding genetic diversity (Fleming and Kress 2013; Moreno-Mateos et al. 2020; Rogers et al. 2021). This might result in negative eco-evolutionary feedback loops (Moreno-Mateos et al. 2020), with lower genetic diversity decreasing resilience to disturbances and reducing the adaptation to new environments (Fricke et al. 2022; Rogers et al. 2021). Therefore, the reduced movement potential of plants might limit their resilience to the effects of anthropogenic disturbances in the present and future and can affect the plant’s ability to track environmental changes, such as global climate change (Fricke et al. 2022; Rogers et al. 2021).
The distance over which plants can now be dispersed is also affected by the loss of many large frugivores on islands (Heinen et al. 2018), because the remaining small frugivores can generally travel less far and have shorter gut retention times (Vidal et al. 2014; Wotton and Kelly 2011, 2012). Vertebrate seed dispersers that were unable to fly have been more vulnerable to extinction in the past, such as island birds that lost the ability to fly (Heinen et al. 2018), further exacerbating the reduction in plant movement potential. Some introduced non-native frugivores may be able to provide long-distance seed dispersal, but, as discussed before, they may also do so for invasive plants (Traveset and Richardson 2014).
The complete loss of frugivore-mediated seed dispersal following frugivore extinction could in theory lead to plant co-extinction (Fleming et al. 1987; Fleming and Kress 2013; Rogers et al. 2021). This is most likely for plants that depend on this for their reproduction, without alternative strategies or new interaction partners (Heinen et al. 2020). In turn, the loss of these plants could then cause further co-extinctions of other species that depend on them for their survival, with the potential to trigger extinction cascades and ecological collapse or shifts to alternative stable states (Fleming et al. 1987; Fleming and Kress 2013; Rogers et al. 2021).
5 Challenges in Identifying Effects of Disperser Loss on Plants
It is clear that the loss of frugivore seed dispersers may affect plant populations negatively, in particular in island communities. However, there are a number of challenges for identifying the exact extent of dispersal loss effects on islands. First, it is difficult to assess with high certainty whether species have gone extinct, and even more difficult to assess whether interactions are fully functionally extinct. Second, seed dispersal interactions are not always obligate and plants may have alternative strategies for reproduction or dispersal, making frugivore extinctions less problematic than we may assume. And, third, it is challenging to obtain suitable data for assessing the ecological consequences of disperser loss in-depth. Below, we review each of these three challenges in turn and they are summarized in Table 3.1.
5.1 Determining Species Extinctions, Functional Extinctions, and Co-extinctions
True extinction of plants and animals is difficult to determine with certainty because it requires the entire island to have been searched thoroughly for any remaining individuals, by those who have the skills to correctly identify them. Because of this, there are still people undertaking targeted searches for species such as the Tasmanian tiger (Thylacinus cynocephalus) and in some cases they are successful, such as with the recent rediscovery of the presumed extinct Fernandina island Galapagos tortoise (Chelonoidis phantasticus) (Jensen et al. 2022). The same regularly happens for plants, for example, through drone surveys of inaccessible mountain slopes in Hawaii (La Vigne et al. 2022). The motivation and funding necessary to do this may be higher for some species than others, leaving us with a broad range of confidence in the robustness of extinction status assessments. This in turn makes it difficult to determine whether plants have truly lost their frugivore seed dispersers, have been negatively impacted by this, or have suffered co-extinction as a consequence themselves.
Co-extinction of plants is often mentioned as an important consequence of losing frugivore seed dispersers, though we are not aware of any conclusive empirical evidence for this. Most of the known co-extinctions refer to parasites of which the hosts have gone extinct due to very strong obligate interactions, such as the two species of feather lice of the passenger pigeon (Dunn 2009). The idea of co-extinctions driven by disperser losses has caused concern for island plants with fruits characteristic of dispersal by extinct animals, such as fleshy fruits with large seeds that can be reached by ground-dwelling large animals (Guimarães Jr et al. 2008). These plants are believed to be on their way to co-extinction with a delayed effect.
A well-known, but untrue, story about disperser loss co-extinction comes from Mauritius. The extinction of the famous Dodo (about 360 years ago) was initially thought to have left the “Dodo tree” (tambalacoque, Sideroxylon grandiflorum, Sapotaceae) without a seed disperser, predicting its inevitable co-extinction in the near future (disputed by Baider and Florens 2006). This was because it has an exceptionally thick (~6 mm) woody endocarp around its seed that was incorrectly thought to need abrasion in the Dodo’s strong, stone-filled crop to allow the seed to break through and germinate (Baider and Florens 2006). However, this appears not to have been the case as seeds can still be found germinating on the forest floor in absence of the Dodo (Fig. 3.7), although the tree is increasingly rare (possible causes discussed in Baider and Florens 2006).
There are several other rare plants in Mauritius that possess traits that suggest that their seeds used to be dispersed by frugivores that no longer exist. For example, the palm Hyophorbe vaughanii (Arecaceae) has very large, single seeds (2 cm diameter) embedded in large fruits (4 cm diameter) that have an inconspicuous brown colour until they fall to the ground where they ripen and break open to reveal their attractive bright orange pulp which seems to invite large ground-dwelling frugivores (e.g. giant tortoises or Dodos) that are no longer present to eat them (Fig. 3.8, see top middle). Co-extinction risk may be even harder to detect in plants that used to be dispersed by small frugivores and have no particular traits that indicate disperser loss. Disperser loss co-extinction is a particularly poignant example of the difficulty in obtaining convincing empirical evidence, as the effects of losing seed dispersers are hard to disentangle from other extinction drivers, and long generation times of plants may cause a delay in secondary extinctions.
Functional extinction may also be difficult to detect. In particular, functional extinction may be the result of, for example, a drastic reduction of fruit production, removing the potential for interaction even when both species are still abundant (Heinen et al. 2018; McConkey and O’Farrill 2016). This has been observed for several plants on Mauritius, which reduced fruit set as a response to intensified nutrient competition in environments heavily invaded by non-native plants (Monty et al. 2013). Control of invasive non-native plants may therefore be more important to prevent functional extinction of plants than restoring seed dispersal interactions (Bissessur et al. 2023). Destruction of flowers and unripe fruits by introduced animals (e.g. by macaques on Mauritius) may also cause functional extinction of seed dispersal interactions without initial loss of interaction partners (Reinegger et al. 2021). When seeds are dispersed by frugivores very rarely but successfully, this may still contribute to the reproduction and dispersal of plants often enough to affect their population dynamics, so that the ecological function is still maintained (Heinen et al. 2020; McConkey and O’Farrill 2016). Important questions for directing conservation efforts are “how often do seeds need to be dispersed to prevent plant extinction?”, “how often do frugivores need to interact with the plants to achieve this?”, and “how many frugivores are needed for functional seed dispersal interactions?”. However, these are difficult to answer because they each require a lot of observations and in-depth studies that are very hard to do, such as on seed handling, gut passage, and germination, especially if fruits and frugivores are already rare. A clear overview of the factors that determine interaction functionality is thereby essential for determining whether functional extinction has taken place.
The Seed Dispersal Effectiveness (SDE) framework (Box 3.2) quantifies and qualifies many factors that are important in determining the contribution of individual seed dispersers to plant fitness (Schupp 1993; Schupp et al. 2010). This is a comprehensive framework that requires in-depth investigations into many different aspects of plant and frugivore morphology, behaviour, and ecology, such as germination experiments and gut passage timing. It is therefore difficult to collect enough information to use it for whole communities, even simplified island communities that provide ideal model systems.
Another simplified approach is that interaction functionality may be restored by increasing distributional range overlap between the interacting species, and by preventing disruptions of encounter rates between the species and success rates of interactions that do take place (Heinen et al. 2020). It is also important to be aware that to prevent or overcome functional extinction, general conservation management efforts may not always be adequate, such as fencing a habitat, and may require additional specific management efforts (Heinen et al. 2020). Obligate seed dispersal interactions are thereby a priority for restoration of functionality, because a lack of alternative dispersal strategies is most likely to cause negative secondary effects on plant populations (Heinen et al. 2020).
5.2 Alternative Modes of Reproduction and Dispersal
Not all plants that offer fleshy fruits or arils to frugivores depend on animals as their only means of dispersal, making it challenging to identify which interactions are obligate and/or at risk of negative consequences of disperser loss (Heinen et al. 2020; Rogers et al. 2021). There are at least two ways for plants to disperse to new sites. Their seeds may have multiple dispersal strategies that do not only make use of frugivores (diplochory), such as by wind, water, gravity, or external attachment to animals (Vargas et al. 2015). Alternatively, some plants can reproduce and spread vegetatively (e.g. root stems) in addition to producing fruits and can either switch between strategies as needed or rarely do so (Vargas et al. 2015). Functional traits of the plants can be used to help identify which plant-frugivore interactions are obligate without alternatives (e.g. presence of root stems or floating seeds) (Bond 1994; Heinen et al. 2020), but this is not always possible. At the same time, many frugivorous island animals appear to be opportunists and generalists that feed on many different plant species due to the scarcity of resources and strong dependency on what is available on their island, making many fruit-feeding interactions non-obligate from their point of view. It is challenging but important to find out whether frugivore-mediated seed dispersal is obligate, and if not, what alternative dispersal strategies are being used to evaluate whether plant populations are potentially negatively affected by frugivore extinctions (Heinen et al. 2020). Alternative strategies may not be sufficient in the long run because alternative seed dispersal will maintain the genetic recombination benefits of sexual reproduction, but vegetative reproduction will not (Fleming et al. 1987; Fleming and Kress 2013; Rogers et al. 2021). High genetic diversity can increase island plant resilience to the many threats on islands, such as changing climate, habitat loss from sea level rise, and human disturbance. Additionally, long-distance dispersal, such as colonization of islands, may be harder without animal seed dispersers (Rogers et al. 2021) like flying foxes that transport seeds to the Krakatau islands (Whittaker and Jones 1994). Such negative effects may take too long to detect due to the long generation time of plants. Many island plants have particularly slow generation times due to the lack of competition during their evolution (Robertson et al. 2006; Whittaker and Fernandez-Palacios 2007).
Another way for plants to overcome disperser loss is to gain new interactions with introduced frugivores (Heinen et al. 2023; Kaiser-Bunbury et al. 2010; Vizentin-Bugoni et al. 2019). These have in some cases been able to take over the role of extinct seed dispersers (Griffiths et al. 2011), but it is difficult to determine the full extent of the ecological impact of these species (Nogués-Bravo et al. 2016). It requires many thorough studies that take years to complete into whether they, for example, outcompete native frugivores, spread non-native plants, predate on seeds, or affect other native species on the island. This is not feasible at all. In the case of deliberately introducing non-native species to take over the role of extinct seed dispersers and restore island ecosystems, acting fast with the risk of potential unknown negative consequences is sometimes prioritized over doing nothing at all (Griffiths et al. 2011; Nogués-Bravo et al. 2016). For example, Aldabra giant tortoises Aldabrachelys gigantea are used to replace extinct giant tortoises Cylindraspis spp. in Mauritius and extinct ducks in Hawaii.
5.3 Obtaining Comprehensive Data
An important challenge is that it can take a lot of time to run experiments and collect the necessary comprehensive data to investigate the full extent of consequences of losing seed dispersers for island plants. Slow plant life history, as is characteristic for many island plants, can also add to the time required to obtain data. For example, it can take several years before dormant seeds germinate and for new plants to produce seeds of their own, limiting the feasibility of experiments on plant recruitment success and risking incorrect results (Robertson et al. 2006). The longer it takes to run germination experiments, the longer the exposure is to factors that risk its failure, such as fungal infections, drought, weeds, and ants or rats removing seeds. Collection of observational data is not only time-consuming but often physically challenging, walking through difficult terrain trying to find plants and animals and observe their behaviour. Reaching remote islands over rough oceans can be very challenging, dangerous (e.g. high wave boat landing on rocky shores of Round island in Mauritius), costly (e.g. helicopters), and time-consuming, with few opportunities to return which requires commitment to long stays (e.g. Aldabra in Seychelles). There is also a strong element of chance involved in obtaining observational data. The observer needs to spend enough time in the right place at the right moment to, for example, find plants with ripe fruits and to see a lizard quickly swallow one of them in the distance.
Then there is the challenge of correctly identifying both the animal and plant involved in the interaction, which requires a clear view of the species and proper training, experience, and access to literature, especially for plants. Evidence of interactions can be obtained through direct observations, where it may be hard to identify which fruit is eaten (e.g. trees may cross branches) and whether it is truly the fruit that was eaten (e.g. not the insect on it or leaf next to it, not only tasting and rejecting the fruit), or through indirect observations (Fig. 3.5a) of teeth markings on fruit or ejecta of hard parts of fruit pulp and seeds from the mouth (may be similar among species). In some cases, indirect evidence of successful seed dispersal can be clear, such as the germination of seeds embedded in ejecta of the only frugivorous bat on Mauritius (Fig. 3.5b). Determining the outcome of different seed handling events based on (field) observations, such as whether the seed is destroyed or dispersed viably, is challenging because it may be difficult to see or track and may vary depending on chance (e.g. chewing) and the context of the environment (e.g. whether there are other ripe fruits nearby). Rare interactions or dispersal events are especially hard to capture, risking a bias in any observations towards more common interactions (Magurran and McGill 2010). The lack of observational data is not a reliable way to exclude that an event occurs, and observation time influences the number of possible observations (Magurran and McGill 2010). The limited number of species-level interactions within the relatively smaller communities on islands is therefore perhaps more feasible to identify than those on the mainland.
Data collection is especially challenging for researchers with very limited access to funding because they do not always have money to travel to and access specimen collections and herbaria or to conduct fieldwork, especially if this takes a lot of time, forcing prioritization (Ramírez-Castañeda et al. 2022). Many island nations have limited funding opportunities. Field-based data collected by local people is often used by researchers from other regions, not always benefitting those who collected the data, which poses ethical challenges (Ramírez-Castañeda et al. 2022).
The collection of plant and animal morphometrics and functional traits comes with challenges related to misclassification, oversimplification, or inappropriate generalization. This is especially the case when data is collected that represents whole species rather than individuals. Animal morphometrics, such as gape size (e.g. bill width) to determine the potential size of seeds that can be swallowed, are usually taken from adult individuals even though in nature there is usually a mix of animals with different ages and morphometrics. For example, adult Aldabra giant tortoise beak width may not be representative for the size of fruits that can be swallowed by the many smaller juveniles present on Aldabra Island. Strong sexual dimorphism can also make it challenging to come up with one measurement to represent a characteristic at the species level. Slight differences in measuring techniques and equipment used by different researchers can influence the accuracy of the data obtained (Magurran and McGill 2010). Under ideal circumstances, average morphometrics are based on hundreds of specimens to make them as representative as possible. However, these may not be easily accessible because they are spread out over collections worldwide that are not digitized, or they may be so rare that only one specimen is available. This can create variation in representativeness of data at species level depending on the amount of intraspecific variation within the characteristic, which may be more problematic for some types of studies than for others. For example, adult bird bill sizes might not vary as much within the species, but fruit size and shape can vary a lot within the species, even if they come from the same tree (Fig. 3.9). Comparing the same trait across different species can be difficult when species vary a lot in appearance, making it hard to identify what needs to be measured, such as fruit lengths for several native Mauritian plants (Fig. 3.8).
Categorical data can oversimplify characteristics and may be classified differently by different researchers. Fruit colour is an example of a plant trait that is often used in frugivory research which seems straightforward to collect but is challenging in reality. Many fruits have several colours (e.g. red with white dots, or berries that are either purple or white), change colour during their ripening process (e.g. green to red), or have colours that are difficult to define (e.g. turquoise). Proposed solutions are to reduce fruit colours to single basic colour terms (Dominy et al. 2003), assign primary and secondary colours, or use broader colour classifications such as “conspicuous” and “non-conspicuous” (Onstein et al. 2020; Schmidt et al. 2004). However, these colours are still assigned by researchers based on their own human vision, which may be less relevant for interactions with animals that have different types of colour vision (e.g. UV vision in birds) (Honkavaara et al. 2002). The choice of data to collect needs to be representative of the factors that influence plant-frugivore interactions. Some of these factors are very difficult or impossible to take into account, such as the smell of fruits.
Collecting data for extinct species is especially challenging because specimens and information about past interactions are often limited. For some islands, such as Mauritius, there are several historical drawings and descriptions of animals and what they ate (Box 3.1) (Cheke and Hume 2008). Reliability of these sources varies because many stories and drawings are based on second-hand accounts by people who were able to write and draw, and basic biological understanding was limited. In some cases, detailed scaled botanical drawings of fruits can be used to estimate fruit measurements for extinct plants or rare ones that do not produce ripe fruits anymore. Even behavioural information on interactions can sometimes still be inferred. For example, on Mauritius where specific berries were found in a pigeon crop and extinct giant tortoises were described eating “fallen apple-like fruits”, which narrows down the possible plants (Hume and Winters 2016). In cases where only some parts of the animals remain, partial specimens or subfossils, some morphometrics that are available can be converted with calculations to others, such as lizard snout-vent length (Pough 1980; Meiri 2010) or tortoise curved carapace length to body mass (Heinen et al. 2018), or newly calculated based on large available datasets. Scientific articles that describe newly discovered extinct animals rarely have more than a few subfossil bones to work with and provide only measurements relevant to species identification instead of those that are relevant to their ecological roles (e.g. bill width), but often provide scaled images that can be used to derive them and compare the species to its closest relative. If no other information is available for extinct species, data from a morphologically similar, close taxonomically related species can be used as a substitute. The measurements can be either used directly for one substitute species or averaged across several species, or adjusted for described differences with the extinct species (e.g. 30% larger) (Heinen et al. 2018). There are many creative ways of overcoming challenges with data collection that need to be carefully considered because they may work better for answering some research questions than for others.
6 Conclusion
To conclude, the interactions between species, specifically frugivore-mediated seed dispersal, have been altered on islands worldwide due to extinctions and introductions of animals and plants. These changes can negatively impact the recruitment success and dispersal of island plants, which are particularly vulnerable due to limited alternative interaction partners in simple island communities. Extinctions of large, flightless frugivorous birds, mammals, and reptiles are most common on isolated and small islands, like Mauritius, where introduced frugivores are likely not good at replacing extinct species as seed dispersers and often destroy seeds. Before extinctions happen, declines in animal seed disperser abundance can already lead to “functional” extinction, where frugivores persist but do not disperse seeds enough to affect plant population dynamics. In addition to extinctions and functional extinctions, shifting animal behaviour, such as dietary changes towards common or non-native fruits, poses a threat to native plant-frugivore interactions that is difficult to detect.
The consequences of frugivore-mediated seed dispersal loss on island plants can be categorized into two main aspects: its impact on plant recruitment success involving seed handling (e.g. pulp removal and gut abrasion) and short-distance dispersal, and its influence on population-level plant movement encompassing long-distance dispersal (e.g. colonization and metapopulation dynamics).
However, there are several challenges in identifying the consequences of disperser loss on plants. Determining species extinctions, functional extinctions, and co-extinctions is difficult, and conclusive evidence is often lacking. Detecting functional extinction is particularly challenging without fully understanding the factors determining interaction functionality, for which a clear overview is lacking. Identifying which interactions are obligate and at risk from seed disperser loss is also complex. Furthermore, assessing dependency on animal seed dispersal is complicated by alternative dispersal strategies of plants, such as vegetative growth or seeds that can also be dispersed via wind, water, gravity, or attachment to animals.
Comprehensive data collection on plant-animal interactions presents challenges, including the time required, limited funding, and difficulties identifying species. Collecting data for extinct species is especially challenging due to limited specimens and interaction information. Overcoming the challenges researchers face in determining the causes, the extent, and the consequences of frugivore-mediated seed dispersal loss is crucial for gaining fundamental understanding of plant-animal interactions and for effective conservation and management strategies in island ecosystems. Furthermore, these challenges underscore the need for collaborative research, adaptive conservation strategies, and ongoing monitoring to address the impact of disperser loss on fragile island ecosystems.
Box 3.1 Extinctions and Introductions in the Mascarenes
When early mariners first arrived on remote oceanic islands such as Mauritius and Rodrigues, after months at sea and an odious diet of dried or salted food, they encountered an unspoilt ecosystem full of animals, the like of which they had never seen before. Birds, bats, and reptiles abounded, and there were so many giant tortoises that one could walk a hundred paces solely on their backs (Leguat 1708). The skies were full of flying foxes, giant lizards crawled on the ground and climbed the trees, and a variety of tame and colourful birds, several unable to fly, such as the famous Dodo, inhabited the forests (Cheke and Hume 2008; Hume 2017). Many of these species were described and illustrated, albeit somewhat poorly, with more emphasis placed on their culinary qualities than anything else (Leguat 1708; Cheke and Hume 2008). Hungry sailors easily caught these island inhabitants, since they had evolved in isolation from mammalian predators, and had no fear of humans (Hume 2017; Steadman 2006). The sailors did not arrive alone, and introduced livestock, which included the accidental introduction of black rats. Rats quickly established on the island along with cats, and goats, pigs, and exotic plants were left behind as a source of food for crews of the next visiting ships (Hume 2017; Steadman 2006). As island species have generally evolved in isolation from competition, they were unable to compete with the introduced rapidly spreading, opportunistic invasives, causing many of the endemic species to go extinct (Blackburn et al. 2004, 2009; Whittaker and Fernandez-Palacios 2007). Because of overwhelming human interference, island ecosystems on a global scale have suffered the same consequences as those in Mauritius and Rodrigues, and have been irreversibly damaged.

Parrot hunting on Mauritius (de Bry 1601)
Box 3.2 Seed Dispersal Effectiveness (SDE)
The Seed Dispersal Effectiveness (SDE) framework for quantifying and qualifying seed dispersal effectiveness outlines many factors that are important in determining how individual seed dispersers contribute to plant fitness (Schupp 1993; Schupp et al. 2010). Its purpose is to provide an organizing framework for the study of ecological and evolutionary consequences of seed dispersal rather than identifying functional extinction, but can be of use in this context to evaluate single plant-frugivore interactions. It states that the quantitative component of seed dispersal (number of seeds dispersed) depends on (1) the number of visits, influenced by frugivore local abundance and degree of frugivory, and (2) the number of seeds dispersed per visit, influenced by the number of fruits handled per visit, the handling behaviour and the frugivore body size (Schupp et al. 2010). The qualitative component of seed dispersal (probability of seed producing adult) depends on (1) the quality of seed treatment in mouth and gut, influenced by seed breakage and digestion, and the altered germinability; and (2) the quality of seed deposition, influenced by seed survival (herbivory and pathogens), seedling emergence (germination requirements), and the subsequent survival and growth (herbivory, pathogens, competition, and physiological requirements) (Schupp et al. 2010).
References
Albert S, Flores O, Baider C, Florens FV, Strasberg D (2021) Differing severity of frugivore loss contrasts the fate of native forests on the land of the Dodo (Mascarene archipelago). Biol Conserv 257:109131
Amarasekare P (2004) Spatial dynamics of mutualistic interactions. J Anim Ecol 73(1):128–142
Baider C, Florens FBV (2006) Current decline of the “Dodo Tree”: a case of broken-down interactions with extinct species or the result of new interactions with alien invaders. Emerging Threats to Tropical Forests. Chicago University Press, Chicago, pp 199–214
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12(2):20150623
Bissessur P, Reinegger RD, Baider C, Mamoodee R, Florens FBV (2023) Invasive alien plant control: the priority to save one of the most rapidly declining island-endemic plant species worldwide. J Nat Conserv 126417
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305(5692):1955–1958
Blackburn TM, Lockwood JL, Cassey P (2009) Avian invasions: the ecology and evolution of exotic birds, vol 1. Oxford University Press
Bond WJ (1994) Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc Lond B Biol Sci 344(1307):83–90
Brodie JF, Aslan CE, Rogers HS, Redford KH, Maron JL, Bronstein JL, Groves CR (2014) Secondary extinctions of biodiversity. Trends Ecol Evol 29(12):664–672
Cardillo M (2003) Biological determinants of extinction risk: why are smaller species less vulnerable? Anim Conserv Forum 6(1):63–69
Carpenter JK, Wilmshurst JM, McConkey KR, Hume JP, Wotton DM, Shiels AB et al (2020) The forgotten fauna: native vertebrate seed predators on islands. Funct Ecol 34(9):1802–1813
Cheke AS, Hume JP (2008) Lost land of the Dodo: the ecological history of the Mascarene Islands. T and AD Poyser, London
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dyn Popul 298:312
Ctvrtecka R, Sam K, Brus E, Weiblen GD, Novotny V (2014) Frugivorous weevils are too rare to cause Janzen–Connell effects in New Guinea lowland rain forest. J Trop Ecol 30(6):521–535
de Bry, J.T. (1601) India Orientalis: Fünfter theil der Orientalischen Indien. Durch Matthes Becker, Frankfurt.
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014) Defaunation in the Anthropocene. Science 345(6195):401–406
Dominy NJ, Svenning JC, Li WH (2003) Historical contingency in the evolution of primate color vision. J Hum Evol 44(1):25–45
Drake DR, Hunt TL (2009) Invasive rodents on islands: integrating historical and contemporary ecology. Biol Invasions 11:1483–1487
Dunn RR (2009) Coextinction: anecdotes, models, and speculation. Holocene Extinct:167–180
Fadini RF, Fleury M, Donatti CI, Galetti M (2009) Effects of frugivore impoverishment and seed predators on the recruitment of a keystone palm. Acta Oecol 35(2):188–196
Falcón W, Moll D, Hansen DM (2020) Frugivory and seed dispersal by chelonians: a review and synthesis. Biol Rev 95(1):142–166
Fleming TH, Breitwisch R, Whitesides GH (1987) Patterns of tropical vertebrate frugivore diversity. Annu Rev Ecol Syst 18(1):91–109
Fleming TH, Kress WJ (2013) The ornaments of life. University of Chicago Press
Fricke EC, Ordonez A, Rogers HS, Svenning JC (2022) The effects of defaunation on plants’ capacity to track climate change. Science 375(6577):210–214
Fricke EC, Tewksbury JJ, Rogers HS (2018) Defaunation leads to interaction deficits, not interaction compensation, in an island seed dispersal network. Glob Chang Biol 24(1):e190–e200
Griffiths CJ, Hansen DM, Jones CG, Zuël N, Harris S (2011) Resurrecting extinct interactions with extant substitutes. Curr Biol 21(9):762–765
Guimarães PR Jr, Galetti M, Jordano P (2008) Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS One 3(3):e1745
Hansen DM, Kaiser CN, Müller CB (2008) Seed dispersal and establishment of endangered plants on oceanic islands: the Janzen-Connell model, and the use of ecological analogues. PLoS One 3(5):e2111
Heinen JH, Florens FV, Baider C, Hume JP, Kissling WD, Whittaker RJ, Rahbek C, Borregaard MK (2023) Novel plant–frugivore network on Mauritius is unlikely to compensate for the extinction of seed dispersers. Nat Commun 14(1):1019
Heinen JH, Rahbek C, Borregaard MK (2020) Conservation of species interactions to achieve self-sustaining ecosystems. Ecography 43(11):1603–1611
Heinen JH, van Loon EE, Hansen DM, Kissling WD (2018) Extinction-driven changes in frugivore communities on oceanic islands. Ecography 41(8):1245–1255
Honkavaara J, Koivula M, Korpimäki E, Siitari H, Viitala J (2002) Ultraviolet vision and foraging in terrestrial vertebrates. Oikos 98(3):505–511
Hume JP (2017) Extinct birds, 2nd edn. Bloomsbury
Hume JP, Winters R (2016) Captive birds on Dutch Mauritius: bad-tempered parrots, warty pigeons and notes on other native animals. Hist Biol 28(6):812–822
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104(940):501–528
Jensen EL, Gaughran SJ, Fusco NA, Poulakakis N, Tapia W, Sevilla C, Málaga J, Mariani C, Gibbs JP, Caccone A (2022) The Galapagos giant tortoise Chelonoidis phantasticus is not extinct. Commun Biol 5(1):1–8
Kaiser-Bunbury CN, Traveset A, Hansen DM (2010) Conservation and restoration of plant–animal mutualisms on oceanic islands. Perspect Plant Ecol Evol Systemat 12(2):131–143
Kissling WD, Böhning-Gaese K, Jetz W (2009) The global distribution of frugivory in birds. Glob Ecol Biogeogr 18(2):150–162
La Vigne H, Charron G, Rachiele-Tremblay J, Rancourt D, Nyberg B, Lussier Desbiens A (2022) Collecting critically endangered cliff plants using a drone-based sampling manipulator. Sci Rep 12(1):1–11
Leguat F (1708) Voyage et avantures de François Leguat et de ses compagnons en deux isles désertes des Indes Orientales. (1689–1698). J.J. de Lorme, Amsterdam. 2 vols
Lim JY, Svenning JC, Göldel B, Faurby S, Kissling WD (2020) Frugivore-fruit size relationships between palms and mammals reveal past and future defaunation impacts. Nat Commun 11(1):1–13
Linnebjerg JF, Hansen DM, Olesen JM (2009) Gut passage effect of the introduced red-whiskered bulbul (Pycnonotus jocosus) on germination of invasive plant species in Mauritius. Austral Ecol 34(3):272–277
Magurran AE, McGill BJ (eds) (2010) Biological diversity: frontiers in measurement and assessment. OUP, Oxford
McConkey KR, O'Farrill G (2016) Loss of seed dispersal before the loss of seed dispersers. Biol Conserv 201:38–49
Meiri S (2010) Length–weight allometries in lizards. J Zool 281(3):218–226
Meyer JY, Butaud JF (2009) The impacts of rats on the endangered native flora of French Polynesia (Pacific Islands): drivers of plant extinction or coup de grâce species? Biol Invasions 11(7):1569–1585
Monty MF, Florens FV, Baider C (2013) Invasive alien plants elicit reduced production of flowers and fruits in various native forest species on the tropical island of Mauritius (Mascarenes, Indian Ocean). Trop Conserv Sci 6(1):35–49
Moreno-Mateos D, Alberdi A, Morriën E, van der Putten WH, Rodríguez-Uña A, Montoya D (2020) The long-term restoration of ecosystem complexity. Nat Ecol Evol 4(5):676–685
Nathan R (2006) Long-distance dispersal of plants. Science 313(5788):786–788
Nathan R, Muller-Landau HC (2000) Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15(7):278–285
Nogués-Bravo D, Simberloff D, Rahbek C, Sanders NJ (2016) Rewilding is the new Pandora’s box in conservation. Curr Biol 26(3):R87–R91
Oleksy RZ, Ayady CL, Tatayah V, Jones C, Froidevaux JS, Racey PA, Jones G (2021) The impact of the Endangered Mauritian flying fox Pteropus niger on commercial fruit farms and the efficacy of mitigation. Oryx 55(1):114–121
Onstein RE, Vink DN, Veen J, Barratt CD, Flantua SG, Wich SA, Kissling WD (2020) Palm fruit colours are linked to the broad-scale distribution and diversification of primate colour vision systems. Proc R Soc B 287(1921):20192731
Pough FH (1980) The advantages of ectothermy for tetrapods. Am Nat 115(1):92–112
Ramírez-Castañeda V, Westeen EP, Frederick J, Amini S, Wait DR, Achmadi AS, Andayani N et al (2022) A set of principles and practical suggestions for equitable fieldwork in biology. Proc Natl Acad Sci 119(34):e2122667119
Reinegger RD, Oleksy RZ, Bissessur P, Naujeer H, Jones G (2021) First come, first served: fruit availability to keystone bat species is potentially reduced by invasive macaques. J Mammal
Robertson AW, Trass A, Ladley JJ, Kelly D (2006) Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Funct Ecol:58–66
Rodriguez LF (2006) Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions 8(4):927–939
Rogers HS, Donoso I, Traveset A, Fricke EC (2021) Cascading impacts of seed disperser loss on plant communities and ecosystems. Annu Rev Ecol Evol Syst 52:641–666
Schmidt V, Martin Schaefer H, Winkler H (2004) Conspicuousness, not colour as foraging cue in plant–animal signalling. Oikos 106(3):551–557
Schupp EW (1993) Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 107(1):15–29
Schupp EW, Jordano P, Gómez JM (2010) Seed dispersal effectiveness revisited: a conceptual review. New Phytol 188(2):333–353
Steadman DW (2006) Extinction and biogeography of tropical Pacific birds. University of Chicago Press
Tershy BR, Shen KW, Newton KM, Holmes ND, Croll DA (2015) The importance of islands for the protection of biological and linguistic diversity. Bioscience 65(6):592–597
Traveset A (1998) Effect of seed passage through vertebrate frugivores’ guts on germination: a review. Perspect Plant Ecol Evol Systemat 1(2):151–190
Traveset A, Richardson DM (2014) Mutualistic interactions and biological invasions. Annu Rev Ecol Evol Syst 45:89–113
Traveset AAJA, Robertson AW, Rodríguez-Pérez J (2007) A review on the role of endozoochory in seed germination. Seed dispersal: theory and its application in a changing world:78–103
Valido A, Olesen JM (2019) Frugivory and seed dispersal by lizards: a global review. Front Ecol Evol 7:49
Vargas P, Arjona Y, Nogales M, Heleno RH (2015) Long-distance dispersal to oceanic islands: success of plants with multiple diaspore specializations. AoB Plants 7
Vidal MM, Hasui E, Pizo MA, Tamashiro JY, Silva WR, Guimarães PR Jr (2014) Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95(12):3440–3447
Vizentin-Bugoni J, Tarwater CE, Foster JT, Drake DR, Gleditsch JM et al (2019) Structure, spatial dynamics, and stability of novel seed dispersal mutualistic networks in Hawai’i. Science 364:78–82
Whittaker RJ, Fernández-Palacios JM (2007) Island biogeography: ecology, evolution, and conservation. Oxford University Press
Whittaker RJ, Jones SH (1994) The role of frugivorous bats and birds in the rebuilding of a tropical forest ecosystem, Krakatau, Indonesia. J Biogeogr:245–258
Whittaker RJ, Fernández-Palacios JM, Matthews TJ, Borregaard MK, Triantis KA (2017) Island biogeography: taking the long view of nature’s laboratories. Science 357(6354)
Wotton DM, Kelly D (2011) Frugivore loss limits recruitment of large-seeded trees. Proc R Soc B Biol Sci 278(1723):3345–3354
Wotton DM, Kelly D (2012) Do larger frugivores move seeds further? Body size, seed dispersal distance, and a case study of a large, sedentary pigeon. J Biogeogr 39(11):1973–1983
Zann RA (1992) The birds of Anak Krakatau: the assembly of an avian community. GeoJournal 28(2):261–270
Acknowledgements
Funding was provided by Carlsberg grant no. CF19-0695.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Heinen, J.H., Borregaard, M.K. (2024). The Consequences of Species Extinctions and Introductions for Plant-Frugivore Interactions on Islands. In: Moreira, X., Abdala-Roberts, L. (eds) Ecology and Evolution of Plant-Herbivore Interactions on Islands. Ecological Studies, vol 249. Springer, Cham. https://doi.org/10.1007/978-3-031-47814-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-47814-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47813-0
Online ISBN: 978-3-031-47814-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)