Abstract
The practical requirement of pest control in agriculture is an evident application of principles of tri-trophic ecological interactions—plant (crop), herbivore (pest), and predator (natural enemy). We analyze the coffee agroecosystem and four of its main pests, the coffee berry borer, the coffee leaf miner, the green coffee scale, and the coffee leaf rust, comparing a mainland site (Mexico) and an island site (Puerto Rico). In addition to the direct trophic, competitive, and mutualistic interactions, there are a variety of indirect higher order interactions, many of which qualitatively increase the complexity of the organizational structure. We explore how the basic nodes of the natural enemy network remain relatively constant between mainland and island, but their frequency and, especially, the complications of their interconnections are dramatically different. The coffee berry borer is preyed upon by numerous species of ants at both sites with an indirect effect of larger ants interfering with smaller ants, the latter of which are able to penetrate the coffee seed through the holes the borer makes and prey on the larvae and eggs of the berry borer. While the larger ants do indeed impede the berry borer directly, their indirect effect of excluding the smaller ants counters this effect. Similarly, in Puerto Rico Anolis lizards, effective predators on the berry borer, tend to avoid areas dominated by specific species of ants, a pattern not seen at the mainland site. The coffee leaf miner is extremely common in Puerto Rico and quite rare in Mexico, for reasons unknown. Its adults are attacked by the common coqui frogs and anoline lizards, again with the indirect effect of some species of ants involved. The scale insect is common in Mexico but mainly associated with a specific species of Azteca ants, providing refuge for predatory beetle larvae and thus enhancing control of this pest across the entire landscape. In Puerto Rico the coffee leaf miner is present but only very rarely reaches pest status. The coffee leaf rust has a variety of potential biological control elements at both sites, yet only on Puerto Rico do they (the control elements) reach significant status, perhaps an element in the dramatic difference between the mainland and island site in terms of the importance of the coffee leaf rust.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The practical requirement of pest control in agriculture is perhaps the most evident application of the idea of tri-trophic ecological interactions—plant (crop), herbivore (pest), and predator (natural enemy). Detailed study with a narrow focus is made possible by the artificial (in principle, experimental) setting of both plant and herbivore, such that the complexities of the higher trophic levels can be studied in distinct, but planned, agroecological contexts. Certain cultivated plants which tend to have similar herbivores from place to place, have been effectively placed in particular sites (farms) and provide a unique scientific opportunity for the investigation of trophic interactions. They can be used effectively as replicated treatments of focal trophic modules in a variety of specified ecological contexts, structured by both management and associated biodiversity. Here we focus on the similarities and differences associated with a tropical mainland site and a tropical island site in structuring the complexity of four focal tri-trophic systems, each associated with four of the major recognized pest species of coffee.
Since the publication of island life (Wallace 1892), the science of ecology has been attendant to obvious patterns that occur when comparing the flora and fauna of islands to their counterparts on nearby mainland areas. The evident balance between arrival of species (immigration) and local extinction of species, first advocated by Levins and Heatwole (1963) and eventually formulated into an elegant graphical framework by MacArthur and Wilson (1963), in what remains a common discourse about island biogeography. Yet tropical island travelers frequently note more than just a relative paucity of species on islands. It is frequently the case that evident structural details are obvious (Vitousek et al. 2013), including structural elements of trophic connections (Gravel et al. 2011).
Our focus is on the coffee agroecosystem at a mainland site in Mexico and an island site in Puerto Rico. The coffee agroecosystem is a novel ecosystem (Hobbs et al. 2009, 2013; Morse et al. 2014; Perfecto and Vandermeer 2015; Lewis et al. 2019). In these cases, the study of community structure confronts the challenge (and opportunity) of not only explaining extant spatial and organizational patterns (e.g., species abundance distributions, species area curves, spatial clusters of species, etc.), but also the realities of recent, sometimes concurrent, interactions among the species of concern. Indeed, there has been burgeoning interest in novel ecosystems for this very reason (Solórzano et al. 2021; Teixeira et al. 2021; Ammar et al. 2021; Rinkevich 2021), and their likely volatile nature, being far from equilibrium, makes them important candidates for the study of regime change as we move from mainland to island in our focus.
In particular, the coffee agroecosystem has received considerable attention from ecologists (Vandermeer et al. 2010, 2019; Zewdie et al. 2020, 2021; Avelino et al. 2006, 2012, 2022; Philpott et al. 2007, 2008). Our work has been explicitly aimed at understanding the pest control aspects of this system from a community ecology perspective (e.g., Vandermeer et al. 2010, 2019; Vandermeer and Perfecto 2019; Perfecto et al. 2003, 2021), a subject that, we argue, has essential spatially explicit dynamics associated with its complex organizational structure. Although frequently unrecognized by practitioners, we argue it is nevertheless critical to understanding the persistence of the system, given its recent assembly (little more than two centuries old in the Neotropics), yet remarkably persistent elements (Vandermeer and Perfecto 2019; Perfecto and Vandermeer 2020).
The use of the network metaphor has become ubiquitous in ecology. In our restricted use, we consider the complications of a single trophic level, the consumers, predators, or pathogens, of the coffee pests. It is clear from previous work in both Mexico (Vandermeer et al. 2010, 2019) and (Hajian-Forooshani et al. 2020; Perfecto et al. 2021) that the natural enemies of the pests (third trophic level) exist within dynamical constraints among them. It is those dynamical constraints we suggest are highly variable and take on distinct meanings on islands as opposed to mainland cases. We herein review some of those complexities, comparing the mainland site in the southwestern Chiapas (Mexico) to the island site in the central mountains of Puerto Rico.
1 The Organizational Complexity of the System
There are at the base of the network, four evident tri-trophic systems: (1) a leaf miner (Leucoptera coffeela) and its enemies, (2) a seed predator (Hypothenemus hampei) and its natural enemies, (3) a sap sucker (Coccus viridis) and its natural enemies, and (4) a fungal pathogen (Hemileia vastatrix) and its natural enemies. The natural enemies exist at various scales of organizational complexity, forming a network containing more than just direct trophic connections, perhaps best characterized as a hypergraph (Golubski et al. 2016; González et al. 2021). Because the backbone primary producer is coffee, we can define very specific patches of vegetation in the broader landscape that contain this hypergraph. The system is a complicated spatial mosaic at various scales, from meters (Vandermeer and Perfecto 2023) (i.e., the patch scale), to the patches or spirals formed by a multi-Turing process at the level of hectares (i.e., the farm scale), to the variability from farm to farm at the level of kilometers (i.e., the landscape scale) (Perfecto and Vandermeer 2020).
A keystone functional group in this general narrative is the community of ants, nesting and foraging both arboreally (in coffee bushes and shade trees) and terrestrially (on the ground). They create a spatial “pilot pattern” (Vandermeer and Jackson 2018) into which the other natural enemies of the coffee pests are constrained. Thus, the community ecology of ants partially determines the spatial pattern of the natural enemies that impact the pests of the agroecosystem.
The simplest level of organizational complexity is the system of “guilds” or “functional groups,” in this case the herbivores and the predators (Fig. 12.1a). It includes the four main coffee pests: the coffee berry borer (CBB), the coffee leaf miner (CLM), the coffee leaf rust (CLR), and the green coffee scale (GCS), along with five functional levels of natural enemies, namely: vertebrate predators, arthropod predators/parasitoids, mycoparasites, and ants (De la Mora et al. 2008; Vandermeer et al. 2010, 2019; Gonthier et al. 2013; Pak et al. 2015; Morris et al. 2018; Hajian-Forooshani et al. 2023). As we move from the mainland situation to the island situation, the functional groups maintain much of their integrity, but their internal structure becomes significantly changed, and, especially important, the pattern of interconnections among them changes dramatically. In network science one might characterize the system as having consistent basal nodes (the state variables, pests, are the same from place to place), but variable higher trophic nodes and wiring (the manner in which the other state variables occur and are interconnected) varies dramatically. This variability in network configuration must, however, be understood under a framework of strong spatial patterning of the system, much of which is driven, directly and indirectly, by ants (Vandermeer et al. 2019; Vandermeer and Perfecto 2020).
Each of what we refer to as functional groups is composed of various species, some of which have specialized roles in the overall system, leading to the second level of organizational complexity (Fig. 12.1b). Some components of community structure fit well within the framework of functional groups (e.g., parasitoids of leaf miners), while others contain individual species that in the end engage in species-specific “functions” (as we noted above, we use the phrase functional groups only for convenience of presenting levels of organizational complexity).
In addition to the direct trophic, competitive, and mutualistic interactions (or, first order interactions, as in Fig. 12.1a and b), our research has uncovered a variety of indirect higher order interactions, many of which qualitatively increase the complexity of the organizational structure (Fig. 12.1c). For example, lizards eat CLM larvae, but their consumption rate of CLM is decreased when within spatial clusters of the electric ants (Perfecto et al. 2021), a phenomenon evident in Puerto Rico but virtually lacking entirely in Mexico. This is a second order interaction. In addition, the presence of the phorid fly parasitoids (Pseudacteon spp.) that attack some of the ant species (at least S. invicta and L. iniquum in Puerto Rico and A. seriaceasur in Mexico), in addition to having a direct interaction with the ants, also initiate a third order interaction by disrupting other interactions in the system. For example, S. invicta ants in Puerto Rico and A. seriaceasur ants in Mexico decrease the attack rate of the CBB (second order interaction), but the phorid flies interfere with this action of the ants (the phorids decrease the ants’ ability to interfere with the CBB’s ability to attack the coffee). This is a third order interaction, sometimes referred to as trait-mediated cascades (Liere and Larsen 2010; Hsieh et al. 2012, 2022; Greeney et al. 2015; Haggerty et al. 2018; Wood et al. 2020) and have been shown to have important community-level effects (Schmitz et al. 2004; Haggerty et al. 2018; Bairey et al. 2016; González et al. 2021), and also resonates in complex ways with spatial pattern formation (Seifan and Kadmon 2006).
2 The Coffee Berry Borer
Perhaps the most directly obvious of the four pests is the infamous coffee berry borer (CBB), due to its habit of drilling directly into the seed, which is the basic commodity that goes to market. It emerged as a major pest in the 1980s and is regarded as far more important, on most farms, than any of the other pests. A variety of natural enemies have been reported, including the fungus Bauvaria bassiana (De La Rosa et al. 2000), Anole lizards (Monagan Jr et al. 2017), birds and bats (Williams-Guillén et al. 2008; Karp et al. 2013), and parasitic hymenoptera (Gómez et al. 2005; Howard and Infante 1996). However, by far the most obvious natural enemies are ants.
There is now a substantial literature documenting the general category “ants” as major predators on this seed-eating herbivore (Morris and Perfecto 2016; Morris et al. 2015; 2018; Armbrecht and Gallego 2007; Larsen and Philpott 2010; Philpott and Armbrecht 2006; Philpott et al. 2008, 2012; Gonthier et al. 2013; Bustillo et al. 2002; De la Mora et al. 2015). Some rather casual observations can easily convince one that, at least in coffee farms in Mexico, the Azteca ants are major predators of (or at least antagonists to) the CBB (Perfecto and Vandermeer 2006; Gonthier et al. 2013; Pardee and Philpott 2011). It takes the CBB approximately 1–2 h to completely burrow into the fruit, which means it is unprotected and unable to escape the predacious activity of the ants during that window of time. If Azteca and some other ants encounter a berry borer trying to burrow into a seed, they grab the borer by its posterior end and pull it out of the fruit.
A number of smaller species of ants are also known predators of CBB (Gonthier et al. 2013; Morris et al. 2018). These species offer considerable regulatory potential since they can enter the coffee seed through the hole that the borer made (Larsen and Philpott 2010). One group is the twig-nesting complex, including the genus Pseudomyrmex (at least three species are common), and Procryptocerus scabriusculus, all adept at entering hollow arboreal structures since they normally nest in hollow twigs (Larsen and Philpott 2010). Other small arboreal ants capable of entering the hole made by the CBB include the arboreally nesting Solenopsis picea, which nests in superficial structures, such as moss, surrounding the branches of the coffee bushes (Morris and Perfecto 2016). In addition, a variety of ground foraging ants, small enough to enter the CBB’s hole include Pheidole protensa, and a variety of other species in that same genus. Of particular interest is the well-known Wasmannia auropunctata (the electric ant or the “little fire ant”), which nests and forages on both the ground and arboreally (Yitbarek et al. 2017a, b; Morris and Perfecto 2016). It is extremely abundant on Puerto Rican coffee farms and plays, we think, an important role not only for control of the CBB, but, as discussed below, for two of the other pests as well.
In Mexico, Azteca clearly dominates over the smaller species in the system, reducing their nest density (and thus overall population density) significantly (Jiménez-Soto et al. 2013). Although Azteca are not present in Puerto Rico, another species fills some of its role, specifically with respect to controlling the CBB, the invasive tropical fire ant Solenopsis invicta. Similar to Azteca, S. invicta is too large to enter the bored berry but acts to repel adult CBB attempting entrance to the berry. In one study, examination of the scars made by the berry borer and extrapolating as to the role of smaller ants (especially Monomorium floricola, Pheidole morens, Brachymyrmex heeri, and W. auropunctata), allowed for the separation of the CBB’s attack from its survival. Indeed, the larger S. invicta seems to reduce the attack of CBB, while the smaller ants reduce its survival (i.e., after having entered the seed, what is its reproductive success). Thus, these two ant types (large and small) had a combined effect of reducing the CBB damage, although through distinct mechanisms (Newson et al. 2021).
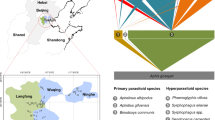
In sum, a variety of ant species are predators on the CBB, both in Mexico and Puerto Rico, suggesting that “ants” represent an excellent natural enemy to regulate the CBB. However, the foregoing natural history suggests that the system is not so simple. While several of the smaller arboreal species (Pseudomyrmex spp., S. picea, P. scabriusculus, and W. auropunctata in Mexico and M. floricola, P. morens, B. heeri, and W. auropunctata in Puerto Rico) could be effective predators on adults, larva and pupa of CBB after it burrowed in the seed, they are effectively unable to engage in such predation if Azteca (in Mexico) or S. invicta (in Puerto Rico) are around. Fruits that are not harvested tend to dry out and fall to the ground, providing a refuge for the beetles during the dry season, but also being exposed to the potential predation from the smaller ground foraging ants. Yet those smaller ants have dramatically reduced populations if they are forced to compete with larger ants. In other words, the whole system seems to be operating in a complicated fashion with potential predators interfering with one another, but perhaps acting in an “emergent” fashion to at least partially regulate this key herbivore. It is worth noting that despite the dramatic differences in the specific identity of the ant species involved, a similar biological control story involving ants seems to emerge for both Mexico and Puerto Rico. These similarities and differences are summarized in a comparison network graph in Fig. 12.2.
The regionally distinct communities associated with the coffee berry borer (CBB) in Mexico and Puerto Rico. Links between organisms denote interactions, with arrowheads (triangles) showing positive effects and circles negative effects. The interaction networks are constructed from observations by the authors in both field and laboratory settings. Dotted blue lines are trait-mediated indirect interactions. Yellow filled circles represent relatively common organisms in each agroecosystem. Solenopsis invicta, Wasmannia auropuctata, Pseudomyrmex simplex, Monorium floricola, Linepethema iniquum, Solenopsis tericola, and Azteca sericeasur are all ants. The case of L. iniquum is displayed because of its interesting interaction with W. auropunctata, which is thought to be a major predator of the CBB, yet the interaction between the two species is strongly affected by a phorid (Yitbarek et al. 2017a, b). The various species of ants tend to fall into two functional groups, S. invicta (Puerto Rico) and A. sericeasur (Mexico) are larger ants that are unable to penetrate the hole made in the coffee berry by the borer, whereas all the other species are small and can do so. While the particular species composition is sometimes the same in the two sites (e.g., W. auropunctata), oftentimes the species are distinct but their functional property of preying on larvae of the CBB is the same (e.g., L. iniquum and S. tericola, both of which are displayed here as symbolic of many other small species at each of the sites)
The system is complicated by the fact that the electric ant, W. auropunctata, is also regarded as a pest (it stings harvest workers and thus is reported to reduce harvest efficiency). It seems that the only agents with the potential to control it are other ants (Fig. 12.2), particularly the imported fire ant (S. invicta), also considered a pest, and the big-headed ant (Pheidole megacephala) (Vázquez Moreno et al. 2012; Pérez-Consuegra et al. 2018). The latter is also a non-native ant and seems to compete with S. invicta on the ground, although the relationship between these two is not well-understood. The dynamics of this subsystem have been partially documented, including, for example, the formation of spatial pattern through complex interactions between S.invicta and the W. auropunctata (Perfecto and Vandermeer 2020; Vandermeer and Perfecto 2020), the different foraging strategies of the two ants on the CBB (Newson et al. 2021), and the effect of W. auropunctata as both predator of the CBB and antagonist of other predators of the CBB, mainly lizards (Perfecto and Vandermeer 2020a; Perfecto et al. 2021; Monagan Jr et al. 2017). It is worth emphasizing the special place of W. auropunctata as the “worst pest anyone has ever seen” (personal information from several farmers) and as a demonstrated natural enemy of CBB (and the coffee leaf miner), a classic ecological contradiction that an honest management program needs to consider. It is both pest and a natural enemy of pest. Recently the importance of yet another species of ant, Monomorium floricola has been reported as forming an intransitive loop with the other two dominant species (S. invicta and W. auropunctata), complicating this story considerably (Vandermeer and Perfecto 2023).
In addition to ants, it is well known that the potential for several species of Anolis to act as an effective predator of the CBB a force in both Mexico and Puerto Rico (Monagan Jr et al. 2017), documenting what most farmers understand to be the case. At the scale of a whole farm, there is a negative relationship between W. auropunctata and Anolis (Perfecto et al. 2021), presumably due to the lizard’s avoidance of general areas where W. auropunctata are common (Perfecto and Vandermeer 2020a; Perfecto et al. 2021). Further, a spatial mosaic pattern tends to form, presumably due to the complicated Turing-like effects of the parasitic phorid flies acting on the ants, the Azteca in Mexico and the S. invicta in Puerto Rico, coupled with an intransitive structure emerging from that Turing-like effect, plus the competitive effect of the fire ants on the electric ants in Puerto Rico (Vandermeer and Perfecto 2020; 2023). Surveys on one of the farms in Puerto Rico where this effect was studied suggest that Anolis lizards avoided the patches dominated by electric ants, which is a spatial pattern that in turn emerges from the complicated dynamics involving S. invicta, W. auropunctata, Monomorium floricola, and phorid flies in Puerto Rico (Vandermeer and Perfecto 2023). Therefore, the phorid fly/S.invicta/W. auropunctata/M. floricola Anolis lizard combination has its own spatially explicit context (Vandermeer and Perfecto 2020; Vandermeer and Perfecto 2023; Perfecto et al. 2021) that will ultimately determine the biological control of the CBB, through the multiple pathways of predation on both adults (outside and inside berries) and brood (inside berries) by the two ants and the lizards. While Anoline lizards are obviously potential predators of the CBB in Mexico, the dramatic difference in population densities of lizards between Puerto Rico, where virtually every coffee bush contains a lizard, and Mexico where they are difficult to find, suggests that the lizards are a far more important predator in Puerto Rico than in Mexico (Monagan Jr et al. 2017).
3 The Leaf Miner System
The coffee leaf miner (Leucoptera coffeella, Lepidoptera: Lyonetiidae, hereafter CLM) is of main concern for coffee production in various locations. In Puerto Rico it is responsible for 20–40% loss in production and in Mexico for up to 12% (Dantas et al. 2021). It is a specialist on Coffea, and its damage significantly reduces photosynthetic rates, frequently resulting in defoliation (Guerreiro Filho 2006). The CLM adult oviposits its eggs on the surface of the coffee leaves. Seven to twelve days later the larva hatches and burrows into the coffee leaf where it starts feeding on the palisade parenchyma of the leaf (usually for about 12 days) (Pereira et al. 2007; Guerreiro Filho 2006). During this stage, larvae create the “mine,” the characteristic damage that defines this pest. During the last larval instar, the larva emerges from the mine and begins cocoon construction, a process taking about an hour, and leaving the larvae quite vulnerable to visual predators. The adult emerges from the cocoon 5 days later. The different life stages of the CLM are vulnerable to different natural enemies at each stage. The eggs are also vulnerable to generalist predators such as ants (Lomeli-Flores et al. 2009), the larvae inside the mines are susceptible to parasitoids (Gallardo Covas 1988; Lomeli-Flores et al. 2009), and the transition between larvae and pupae, where the larvae are unprotected while building their cocoon, are vulnerable to predation by lizards, ants, and frogs. The pupae may also be susceptible to predation by small ants and parasitoids. Finally, the adults are prey of lizards and frogs (Beard et al. 2021), although the apparent night flights of adults probably restricts the impact of lizards. However, the extent to which natural enemies affect each other and the impact of these potential interactions on the CLM dynamics remains enigmatic.
In Mexico, the potential pool of natural enemies is expected to be more diverse in comparison to Puerto Rico because of its mainland status. Studies on the community of natural enemies in Mexico have identified 22 species of parasitoids and another 17 predators (Lomeli-Flores et al. 2009), but this is likely an underrepresentation of the diversity of predators on the CLM (cite?). Among the natural enemies, predatory wasps have been shown to play an important role and are responsible for the mortality of up to 69% of the CLM (Lomeli-Flores et al. 2009), actively infesting both egg and larval stages. In addition, ant communities, which can be relatively diverse in coffee agroecosystems, have been shown to be one of the most important predators of this pest—e.g., twig-nesting ants prey on the eggs and pupal stages (De la Mora et al. 2008) of the CLM, and other arboreal ants have been reported to prey on eggs, larvae, and pupae of this pest (Lomeli-Flores 2009).
In comparison, the diversity of potential natural enemies in Puerto Rico is lower than in Mexico. A survey of parasitoids in 1985 showed the presence of only six parasitoids causing rates of parasitism from 19.5–23.5% (Gallardo Covas 1988). Other important predators in Puerto Rico are the native coqui frogs (Euleutherodactylus spp.) and the lizards (Anolis spp.), both known for being important generalist predators (Beard et al. 2021). Anoline lizards are especially abundant in coffee agroecosystems (Borkhataria et al. 2012). Furthermore, there is a dramatic difference in population density of these lizards, with Puerto Rico housing extremely large local densities relative to Mexico (Monagan Jr et al. 2017). Comparing lizards to frogs, the latter are believed to be important predators specifically of the adult stage of the CLM since both are nocturnal, while lizards are more likely to prey upon the larvae when they are spinning their protective cocoon, which seems to be done exclusively during the daylight hours (personal observations). Both types of vertebrate predators are seemingly more important in the island agroecosystems than on the mainland sites.
Ants are also important predators of the CLM in both mainland and island sites. The diversity of ants on coffee farms in Mexico is estimated to be well over 150 species in comparison to approximately 30 in Puerto Rico (personal observations). A recent study found that the presence of the invasive (in Puerto Rico) ant Wasmannia auropunctata is negatively related with the abundance of Anolis spp. in Puerto Rico (Perfecto and Vandermeer 2020a; Perfecto et al. 2021). Additionally, patches with high densities of W. auropunctata and low densities of Anolis spp had higher number of CLM mines, than patches with low densities of W. auropunctata and high densities of Anolis spp. (Perfecto et al. 2021). The reduced co-occurrence, of W. auropunctata and Anolis spp., suggests an antagonistic interaction between the two, where W. auropunctata may be modifying the behavior of the lizards, mainly by harassing them, causing them to flee the areas where it is dominant, consequently reducing anoline predation on the CLM (Perfecto et al. 2021). Comparative studies of this relationship are not available due to the relative rarity of both Anoline lizards and W. auropunctata in Mexico (part of its native range).
The negative effects of exotic ant species may also affect the community of native ants which plausibly could be better at the controlling of the CLM. Preliminary results of surveys on the CLM in coffee agroecosystems suggest higher damage by the CLM in plants dominated by W. auropunctata or Solenopsis invicta compared to plants dominated by native ants (personal observations). We speculate that the differing status of CLM in Mexico and Puerto Rico may be related to the diversity of predators in each system, clearly linked to well-known patterns of biodiversity on islands.
4 The Scale Insect System
Casual observations leave little doubt that the green coffee scale (GCS) has dramatically different population dynamics on the mainland versus the island. In Mexico, there are frequently significant concentrations of GCS on leaves and some bushes are completely covered with them, something very rarely seen in Puerto Rico. However, in both cases any local concentration of scale insects is associated with some species of mutualistic ants (Rivera-Salinas, unpublished data). Particularly large concentrations of scale insects are associated with the ant Azteca seriaceasur in Mexico (Perfecto and Vandermeer 2006; Vandermeer and Perfecto 2006; Livingston et al. 2008; Vandermeer and Perfecto 2019), although a variety of other species also provide mutualist havens albeit not to the same extent as A. seriaceasur.
There is a strong spatial component associated with the scale insect in Mexico which is not easily observable in Puerto Rico. In the former site, the beetle Azya orbigera is a voracious predator of both larvae and adults of the GCS. Beetle larvae produce waxy filaments that allow them to evade attack by the ants and thus find much sustenance on leaves and branches containing large numbers of GCS. Beetle adults, contrarily, have no such protection and thus have no protection against the ants as they seek out their food, (Vandermeer et al. 2010). These basic natural history observations set up a dramatic expectation regarding spatial patterns. GCS disperse by wind (the first instar larvae are known as crawlers, the main dispersal form). Consequently, some individuals find themselves at a distance far removed from the mother scale who, for the most part, must have been under the protection of one of the ant protectorates. If the distance is too great, the probability of being attacked by the adult predatory beetle is far greater than that of being discovered by an ant worker and thus coming under the protection of the ants. But the extant range of ant foraging is itself partly dependent on where the concentrations of GCS happen to be. Thus, first instar GCS, probably dispersing more or less randomly from their nascent patch. Those that disperse from within a high-density patch are not likely to encounter predatory beetles immediately, since they will most likely remain within the spatial area tended to by their ant associates and thus remain under ant protection. Contrarily, those whose dispersal is initiated from an area removed significantly from a high-density patch, perhaps from a mother GCS that has managed, by chance, to escape beetle predation even though not under ant protection, are not likely to immediately find ant protectors. This basic structure leads to the prediction that the pattern in the field should be “hysteretic,” with two critical transitions, one from very low scale populations jumping to very high populations and another from very high GCS populations jumping to very low populations, with a hysteretic zone and alternative equilibria, in between. This expected pattern is evident in Mexico associated with the ant A. sericeasur (Vandermeer and Perfecto 2019) but clearly not found associated with any of the ant species in Puerto Rico (unpublished data).
Under some circumstances ant protective effects on GCS may not have to do with predation, but with the indirect effects of removing the honeydew produced by the GCS, effectively making survival and instar metamorphosis more efficient by reducing incidence of fungi covering the adults (Jha et al. 2012). Although direct evidence is lacking, the fact that the ants (in the case of A. seriaceasur in Mexico) remove honey dew at a rate sufficient to avoid build-up of secondary fungal growth may also affect the build-up of the scale pathogen Lecanicillium lecanii. It is certainly plausible that the GCS, under protection from the ants, are “cleaned” regularly by the ants, until a critical transition is reached where the ants cannot keep up with excessive honeydew production, thus leaving some individual GCS particularly susceptible to attack by the fungal pathogen, clearly a mechanism for the spatial patterns observed (Vandermeer and Perfecto 2019). However, other studies suggest that the ants have an opposite effect by promoting to some extent the dispersal of L. lecanii conidia (Jackson et al. 2012a).
The relationships between ants, GCS, and the latter’s pathogens observed at the mainland sites appear to be considerably different in Puerto Rico. In Mexico L. lecanii attacking GCS is clearly density-dependent as would be expected for any disease (Jackson et al. 2012a). In contrast, casual observations in Puerto Rico, plus testimony from farmers, suggest that the dramatic epizootics of L.lecanii on scale insects are virtually independent of the density of the scale insects. Indeed, in Puerto Rico it is relatively common to find coffee leaves with a single GCS insect completely covered with L. lecanii, an observation rarely if ever made in Mexico. Yet, it seems that the biodiversity of sap-sucking Hemipterans is actually quite large in Puerto Rico (personal observations), even though the particular species, C. viridis, never seems to gain the prominence it does in Mexico. The potential for production of L. lecanii would thus seem larger in Puerto Rico, a point of some importance as we turn to a discussion of the coffee leaf rust system.
5 The Coffee Leaf Rust System
The coffee leaf rust (CLR) is a fungal pathogen that has followed coffee to nearly every region of cultivation, having just recently reached Hawaii, where initial reports suggest devastating impacts on production. From early epidemics of CLR in Ceylon in the late 1800s to the more recent “big rust” epidemic in Central America in 2012, the variable and often destructive dynamics of this fungal pathogen around the globe has long puzzled plant pathologists and agricultural practitioners. Understanding the underlying mechanisms driving CLR infection dynamics has proven elusive although it is an area of active research (Avelino et al. 2006, 2012; Belachew et al. 2020). While an intersection of ecological, economic, and sociological factors likely contributed to the realization of the “Big Rust” epidemic in Central America (McCook and Vandermeer 2015; McCook 2019), it has been difficult to pinpoint a singular or most dominant contributing factor. Some research suggests the potential importance of abiotic factors in structuring the dynamics of the pathogen (Avelino et al. 2015), but other research has shown that climatic factors alone are insufficient (Bebber et al. 2016). Our team’s work (Jackson et al. 2012a; Vandermeer et al. 2014, 2019; Hajian-Forooshani et al. 2016, 2020, 2023), in contrast with many phytopathologists, seeks to understand the system with a focus on the top-down components, that is, the natural enemies.
The CLR and its community of natural enemies has notably distinct dynamics on a mainland site (Chiapas, Mexico) compared to an island site (Puerto Rico) (Hajian-Forooshani et al. 2016, 2023). The two sites seem to experience dramatically differently dynamics of CLR even though they have similar timelines in terms of both the arrival of coffee and the pathogen (cite?). Southern Mexico recently experienced a devastating CLR epidemic while Puerto Rico has yet to experience an island-wide epidemic, although similar local epidemics have occurred in the past (at the level of a single farm or closely grouped farms). It has been suggested that top-down trophic control of the CLR is far more important in Puerto Rico than in Mexico (Hajian-Forooshani et al. 2016, 2023; Hajian-Forooshani and Vandermeer 2022). That is, the community of natural enemies contains the main controlling agents keeping the CLR pathogen at relatively benign levels in Puerto Rico but fails to do so in Mexico. As to why this is the case or how extensive the pattern may be, we await further research results and hope to stimulate similar research agendas in other areas of coffee cultivation.
Two of the natural enemies are shared on the mainland site and the island: Lecancillium lecanii, a generalist fungal parasite that attacks both other fungi and insects, and Mycodiplosis hemileiae, a fly larva that is a rust specialist (attack rates by L.lecanii and M. hemileiae were 60% and 32.2% in Puerto Rico but only 8.7% and 3.8% in Mexico in one study; Hajian-Forooshani et al. 2016). In some farms on Puerto Rico over 80% of the coffee trees had L. lecanii attacking CLR year-round, while in Mexico L. lecanii exhibited strongly seasonal dynamics where an upper limit of only about 20% of plants would have L. lecanii for a short period of time (Hajian-Forooshani et al. 2023). The dynamics of the natural enemy community in Puerto Rico are notably more complicated than those in Mexico, due in part to Puerto Rico having two additional common natural enemies, namely, a group of gastropods which consume CLR spores (Hajian-Forooshani et al. 2020), and a community of mites that consume and reproduce in lesions CLR spores (Hajian-Forooshani and Vandermeer 2022; Hajian-Forooshani et al. 2023).
The dramatically different dynamics of shared consumers in both regions begs the question of what factors may be driving these differences. Similar to other facets of the main-land/island coffee agroecosystems elaborated in this chapter, the context in which these tri-trophic modules are embedded impacts their dynamics. Figure 12.3 shows the hypothesized interaction networks (informed by a combination of laboratory experiments and field observations) centered on the CLR in Mexican and Puerto Rican agroecosystems.
The regionally distinct communities associated with the coffee leaf rust in Mexico and Puerto Rico. Links between organisms denote interactions, with arrowheads (triangles) showing positive effects and circles negative effects. Note that interaction networks are constructed from observations by the authors in both field and laboratory settings. Dotted blue lines are trait-mediated indirect interactions, dotted red lines represent trait-mediated indirect interaction cascades. Yellow filled circles represent relatively common organisms in each agroecosystem. Solenopsis invicta, Wasmannia auropuctata, Pseudomyrmex simplex, Monorium floricola, and Azteca sericeasur are all ants
Just as in other tri-trophic components of the coffee agroecosystems discussed in this chapter, ants play an important role in structuring the tri-trophic modules associated with CLR (see Fig. 12.1). In Mexico, Azteca sericeasur, whose spatial pattern sets the stage for a variety of other interactions in the system (Vandermeer et al. 2008, 2019), influences the dynamics of the CLR in complicated and context-dependent ways. This is illustrated by the simultaneous positive and negative forcing Azteca has on different components of the natural enemy-CLR-coffee tri-trophic interaction. On the one hand, prior work has shown how Azteca builds populations of scale insects which are alternative resources for L. lecanii, resulting in local increases in L. lecanii which attack CLR (see Vandermeer et al. 2010; Jackson et al. 2012a, b, 2016). On the other hand, preliminary surveys suggested that sites with Azteca were associated with reduced numbers of M. hemileiae (Hajian-Forooshani et al. 2016). This suggests that Azteca has both indirect positive (through promoting L. lecanii) and indirect negative (through reducing M. hemileiae) effects on coffee. Preliminary analysis suggests that the net effect of Azteca on the CLR tends to be indirectly negative on coffee—sites with Azteca are associated with higher amounts of CLR. We speculate that this may be the result of a trophic cascade whereby Azteca reduces M. hemileiae thus releasing CLR locally in space.
While Azteca is absent in Puerto Rico, other ants (especially S. invicta) fulfill similar roles ecologically, with clear implications for the dynamics of the CLR via their effects on the natural enemies. Additionally, the contexts of agroecosystem management have important implications for the dynamics of these complex interaction networks (see Fig. 12.1). One such management factor that we hypothesize is important in structuring tri-trophic dynamics is the intercropping of citrus with coffee. In large-scale surveys across the coffee producing region of Puerto Rico, it is evident that inter-cropping with citrus influences the ant communities found on coffee farms as well as the density of their Hemipteran mutualist partners. Furthermore, the CLR experiences higher levels of attack by L. lecanii when citrus is present (unpublished data). Together, results from these surveys suggest that citrus promotes elevated amounts of L. lecanii which then spill over to attack the CLR.
While multiple studies now highlight differences in the relative abundance of natural enemies when comparing Puerto Rico to Mexico, there are still gaps in our understanding of the implications of these differences for CLR dynamics. While our working hypothesis is that these differences in enemy communities drive the large-scale qualitative dynamics of the CLR, a detailed understanding of the action of natural enemies is still an area of active research. For example, the natural enemies M. hemelia, L. lecanii, and the rust mites are all associated with a reduction in spore load as the CLR progresses (unpublished data). Furthermore, multiple co-occurring natural enemies seem to have a synergistic effect in reducing CLR-spores. These observations are currently under study.
6 Conclusion
We presented a comparison of the particular case of a novel tri-trophic ecosystem as it exists at a site in southern Mexico as compared to the island site of Puerto Rico. Even though the basic elements of the system are the same (four particular pest species, which is to say the herbivore level of the tri-trophic structure plus a taxonomically diverse yet functionally similar natural enemies, which is to say the predator level of the tri-trophic structure), we see dramatic differences between regions. Although our studies have been concentrated at only two sites, our experience in other neighboring sites (Cuba in the Caribbean and other large farms in Mexico, Nicaragua and Costa Rica) suggest to us that these patterns are indeed representative of a mainland/island comparison. Using a consistent “habitat background” (the ecosystem called the coffee farm) and a stable and consistent lowest trophic level (the coffee plant), it seems that the trophic connections we have uncovered are representative of how this level of trophic complexity plays out in an island/mainland framework.
Casting these observations in a classic trophic dynamic framing, one important and highly cited ecosystem function is “control from above,” which is to say, the control of a population (its size, distribution, rate of growth, etc.) is affected from the trophic level above it—predators control prey, herbivores control plants, pathogens control hosts. In particular, the control of herbivores by the community of predators and pathogens is an essential ecosystem function and clearly an ecosystem service when dealing with agroecosystems—the biological control of pests. Furthermore, it is a striking example of how basic ecological knowledge can enrich our understanding of an applied system leading, we propose, to an enlightened approach to the fundamental problem of pest control.
References
Ammar Y, Niiranen S, Otto SA, Möllmann C, Finsinger W, Blenckner T (2021) The rise of novelty in marine ecosystems: the Baltic Sea case. Glob Chang Biol 27(7):1485–1499
Armbrecht I, Gallego MC (2007) Testing ant predation on the coffee berry borer in shaded and sun coffee plantations in Colombia. Entomol Exp Appl 124(3):261–267
Avelino J, Cristancho M, Georgiou S, Imbach P, Aguilar L, Bornemann G, Läderach P, Anzueto F, Hruska AJ, Morales C (2015) The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Security 7:303–321
Avelino J, Gagliardi S, Perfecto I, Isaac M, Liebig T, Vandermeer J, Merle I, Hajian-Forooshani Z, Motisi N (2022) Tree effects on coffee leaf rust at field and landscape scales. Plant Dis 107:247
Avelino J, Romero-Gurdián A, Cruz-Cuellar HF, Declerck FA (2012) Landscape context and scale differentially impact coffee leaf rust, coffee berry borer, and coffee root-knot nematodes. Ecol Appl 22(2):584–596
Avelino J, Zelaya H, Merlo A, Pineda A, Ordóñez M, Savary S (2006) The intensity of a coffee rust epidemic is dependent on production situations. Ecol Model 197(3–4):431–447
Bairey E, Kelsic ED, Kishony R (2016) High-order species interactions shape ecosystem diversity. Nat Commun 7(1):12285
Beard KH, Durham SL, Willig MR, Zimmerman JK (2021) Lizard and frog removal increases spider abundance but does not cascade to increase herbivory. Biotropica 53(2):681–692
Bebber DP, Castillo ÁD, Gurr SJ (2016) Modelling coffee leaf rust risk in Colombia with climate reanalysis data. Philos Trans R Soc Lond B Biol Sci 371(1709):20150458
Belachew K, Senbeta GA, Garedew W, Barreto RW, Del Ponte EM (2020) Altitude is the main driver of coffee leaf rust epidemics: a large-scale survey in Ethiopia. Trop Plant Pathol 45:511–521
Borkhataria RR, Collazo JA, Groom MJ (2012) Species abundance and potential biological control services in shade vs. sun coffee in Puerto Rico. Agric Ecosyst Environ 151:1–5
Bustillo AE, Cardenas R, Posada FJ (2002) Natural enemies and competitors of Hypothenemus hampei (Ferrari)(Coleoptera: Scolytidae) in Colombia. Neotrop Entomol 31:635–639
Dantas J, Motta IO, Vidal LA, Nascimento EF, Bilio J, Pupe JM, Veiga A, Carvalho C, Lopes RB, Rocha TL, Silva LP (2021) A comprehensive review of the coffee leaf miner leucoptera coffeella (Lepidoptera: Lyonetiidae)—a major pest for the coffee crop in Brazil and others neotropical countries. Insects 12(12):1130
De la Mora A, García-Ballinas JA, Philpott SM (2015) Local, landscape, and diversity drivers of predation services provided by ants in a coffee landscape in Chiapas, Mexico. Agric Ecosyst Environ 201:83–91
De la Mora A, Livingston G, Philpott SM (2008) Arboreal ant abundance and leaf miner damage in coffee agroecosystems in Mexico. Biotropica 40(6):742–746
De La Rosa W, Alatorre R, Barrera JF, Toriello C (2000) Effect of Beauveria bassiana and Metarhizium anisopliae (Deuteromycetes) upon the coffee berry borer (Coleoptera: Scolytidae) under field conditions. J Econ Entomol 93(5):1409–1414
Gallardo Covas F (1988) Faunal survey of the coffee leaf miner, Leucoptera coffeella, parasitoids in Puerto Rico. Journal of Agriculture of the University of Puerto Rico (Puerto Rico) 72(2):255–263
Golubski AJ, Westlund EE, Vandermeer J, Pascual M (2016) Ecological networks over the edge: hypergraph trait-mediated indirect interaction (TMII) structure. Trends Ecol Evol 31(5):344–354
Gómez J, Barrera JF, Rojas JC, Macias-Samano J, Liedo JP, Cruz-Lopez L, Badii MH (2005) Volatile compounds released by disturbed females of Cephalonomia stephanoderis (hymenoptera: Bethylidae): a parasitoid of the coffee berry borer Hypothenemus hampei (Coleoptera: Scolytidae). Fla Entomol 88(2):180–187
Gonthier DJ, Ennis KK, Philpott SM, Vandermeer J, Perfecto I (2013) Ants defend coffee from berry borer colonization. Biol Control 58:815–820
González CG, Van Cauwelaert EM, Boyer D, Perfecto I, Vandermeer J, Benítez M (2021) High-order interactions maintain or enhance structural robustness of a coffee agroecosystem network. Ecol Complex 47:100951
Gravel D, Massol F, Canard E, Mouillot D, Mouquet N (2011) Trophic theory of Island biogeography. Ecol Lett 14(10):1010–1016
Greeney HF, Meneses MR, Hamilton CE, Lichter-Marck E, Mannan RW, Snyder N, Snyder H, Wethington SM, Dyer LA (2015) Trait-mediated trophic cascade creates enemy-free space for nesting hummingbirds. Sci Adv 1(8):e1500310
Guerreiro Filho O (2006) Coffee leaf miner resistance. Braz J Plant Physiol 18:109–117
Haggerty MB, Anderson TW, Long JD (2018) Fish predators reduce kelp frond loss via a trait-mediated trophic cascade. Ecology 99(7):1574–1583
Hajian-Forooshani Z, Perfecto I, Vandermeer J (2023) Novel community assembly and the control of a fungal pathogen in coffee agroecosystems. Biol Control 177:105099
Hajian-Forooshani Z, Rivera Salinas IS, Jiménez-Soto E, Perfecto I, Vandermeer J (2016) Impact of regionally distinct agroecosystem communities on the potential for autonomous control of the coffee leaf rust. J Environ Entomol nvw125:1521
Hajian-Forooshani Z, Vandermeer J (2022) Ecological perspectives on the coffee leaf rust. In: Muschler R (ed) Climate-smart production of coffee: achieving sustainability and ecosystem services
Hajian-Forooshani Z, Vandermeer J, Perfecto I (2020) Insights from excrement: invasive gastropods shift diet to consume the coffee leaf rust and its mycoparasite. Ecology 101(5):e02966–e02966
Hobbs RJ, Higgs ES, Hall CM (2013) Defining novel ecosystems. Novel ecosystems: Intervening in the new ecological world order, pp 58–60
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 24(11):599–605
Howard RW, Infante F (1996) Cuticular hydrocarbons of the host-specific ectoparasitoid Cephalonomia stephanoderis (hymenoptera: Bethylidae) and its host the coffee berry borer (Coleoptera: Scolytidae). Ann Entomol Soc Am 89(5):700–709
Hsieh HY, Liere H, Soto EJ, Perfecto I (2012) Cascading trait-mediated interactions induced by ant pheromones. Ecol Evol 2(9):2181–2191
Hsieh HY, Vandermeer J, Perfecto I (2022) Surprising effects of cascading higher order interactions. Sci Rep 12(1):19378
Jackson D, Skillman J, Vandermeer J (2012a) Indirect biological control of the coffee leaf rust, Hemileia vastatrix, by the entomogenous fungus Lecanicillium lecanii in a complex coffee agroecosystem. Biol Control 61(1):89–97
Jackson DW, Zemenick K, Huerta G (2012b) Occurrence in the soil and dispersal of Lecanicillium lecanii, a fungal pathogen of the green coffee scale (Coccus viridis) and coffee rust (Hemileia vastatrix). Trop Subtrop Agroecosystems 15(2)
Jackson D, Zemenick AT, Malloure B, Quandt CA, James TY (2016) Fine-scale spatial genetic structure of a fungal parasite of coffee scale insects. J Invertebr Pathol 139:34–41
Jha S, Allen D, Liere H, Perfecto I, Vandermeer J (2012) Mutualisms and population regulation: mechanism matters. Bioscience 69(12):974–996
Jiménez-Soto E, Cruz-Rodríguez JA, Vandermeer J, Perfecto I (2013) Hypothenemus hampei (Coleoptera: Curculionidae) and its interactions with Azteca instabilis and Pheidole synanthropica (hymenoptera: Formicidae) in a shade coffee agroecosystem. Environ Entomol 42(5):915–924
Karp DS, Mendenhall CD, Sandí RF, Chaumont N, Ehrlich PR, Hadly EA, Daily GC (2013) Forest bolsters bird abundance, pest control and coffee yield. Ecol Lett 16(11):1339–1347
Larsen A, Philpott SM (2010) Twig-nesting ants: the hidden predators of the coffee berry borer in Chiapas, Mexico. Biotropica 42(3):342–347
Levins R, Heatwole H (1963) On the distribution of organisms on islands. Caribb J Sci 3:173–177
Lewis CL, Granek EF, Nielsen-Pincus M (2019) Assessing local attitudes and perceptions of non-native species to inform management of novel ecosystems. Biol Invasions 21(3):961–982
Liere H, Larsen A (2010) Cascading trait-mediation: disruption of a trait-mediated mutualism by parasite-induced behavioral modification. Oikos 119(9):1394–1400
Livingston GF, White AM, Kratz CJ (2008) Indirect interactions between ant-tended hemipterans, a dominant ant Azteca instabilis (hymenoptera: Formicidae), and shade trees in a tropical agroecosystem. Environ Entomol 37:734
Lomeli-Flores R (2009) Natural enemies and mortality factors of the coffee leafminer Leucoptera coffeella (Guerin-Meneville)(Lepidoptera: Lyonetiidae) in Chiapas, México (Doctoral dissertation), vol 51. Texas A & M University, p 51
Lomeli-Flores JR, Barrera JF, Bernal JS (2009) Impact of natural enemies on coffee leafminer Leucoptera coffeella (Lepidoptera: Lyonetiidae) population dynamics in Chiapas, Mexico. Biol Control 51(1):51–60
MacArthur RH, Wilson EO (1963) The theory of Island biogeography, monographs in population biology. Princeton University Press, Princeton, NJ
McCook S (2019) Coffee is not forever: a global history of the coffee leaf rust. Ohio University Press
McCook S, Vandermeer J (2015) The big rust and the red queen: Long-term perspectives on coffee rust research. Phytopathology 105(9):1164–1173
Monagan IV Jr, Morris JR, Davis Rabosky AR, Perfecto I, Vandermeer J (2017) Anolis lizards as biocontrol agents in mainland and Island agroecosystems. Ecol Evol 7(7):2193–2203
Morris, J. R., Jimenez-Soto, E., Philpott, S. M., & Perfecto, I. 2018. Ant-mediated (hymenoptera: Formicidae) biological control of the coffee berry borer: diversity, ecological complexity, and conservation biocontrol
Morris JR, Perfecto I (2016) Testing the potential for ant predation of immature coffee berry borer (Hypothenemus hampei) life stages. Agric Ecosyst Environ 233:224–228
Morris JR, Vandermeer J, Perfecto I (2015) A keystone ant species provides robust biological control of the coffee berry borer under varying pest densities. PLoS One 10(11):e0142850
Morse NB, Pellissier PA, Cianciola EN, Brereton RL, Sullivan MM, Shonka NK, Wheeler TB, McDowell WH (2014) Novel ecosystems in the Anthropocene: a revision of the novel ecosystem concept for pragmatic applications. Ecol Soc 19(2)
Newson J, Vandermeer J, Perfecto I (2021) Differential effects of ants as biological control of the coffee berry borer in Puerto Rico. Biol Control 160:104666
Pak D, Iverson AL, Ennis KK, Gonthier DJ, Vandermeer JH (2015) Parasitoid wasps benefit from shade tree size and landscape complexity in Mexican coffee agroecosystems. Agric Ecosyst Environ 206:21–32
Pardee GL, Philpott SM (2011) Cascading indirect effects in a coffee agroecosystem: effects of parasitic phorid flies on ants and the coffee berry borer in a high-shade and low-shade habitat. Environ Entomol 40(3):581–588
Pereira EJG, Picanço MC, Bacci L, Crespo ALB, Guedes RNC (2007) Seasonal mortality factors of the coffee leafminer, Leucoptera coffeella. Bull Entomol Res 97(4):421–432
Pérez-Consuegra N, Mirabal L, Jiménez LC. (2018) The role of biological control in the sustainability of the Cuban Agri-food system. Elementa: science of the Anthropocene 6
Perfecto I, Hajian-Forooshani Z, White A, Vandermeer J (2021) Ecological complexity and contingency: ants and lizards affect biological control of the coffee leaf miner in Puerto Rico. Agric Ecosyst Environ 305:107104
Perfecto I, Mas A, Dietsch T, Vandermeer J (2003) Conservation of biodiversity in coffee agroecosystems: a tri-taxa comparison in southern Mexico. Biodivers Conserv 12:1239–1252
Perfecto I, Vandermeer J (2006) The effect of an ant-hemipteran mutualism on the coffee berry borer (Hypothenemus hampei) in southern Mexico. Agric Ecosyst Environ 117(2–3):218–221
Perfecto I, Vandermeer J (2015) Structural constraints on novel ecosystems in agriculture: the rapid emergence of stereotypic modules. Perspect Plant Ecol Evol Syst 17(6):522–530
Perfecto I, Vandermeer J (2020) The assembly and importance of a novel ecosystem: the ant community of coffee farms in Puerto Rico. Ecol Evol 10(23):12650–12662
Perfecto I, Vandermeer J (2020a) Antagonism between Anolis spp. and Wasmannia auropunctata in coffee farms on Puerto Rico: potential complications of biological control of the coffee berry borer. Caribb J Sci 50(1):43–47
Philpott SM, Arendt WJ, Armbrecht I, Bichier P, Diestch TV, Gordon C, Greenberg R (2008) Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv Biol 22(5):1093–1105
Philpott SM, Armbrecht I (2006) Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol Entomol 31(4):369–377
Philpott SM, Bichier P, Rice R, Greenberg R (2007) Field-testing ecological and economic benefits of coffee certification programs. Conserv Biol 21(4):975–985
Philpott SM, Pardee GL, Gonthier DJ (2012) Cryptic biodiversity effects: importance of functional redundancy revealed through addition of food web complexity. Ecology 93(5):992–1001
Rinkevich B (2021) Ecological engineering approaches in coral reef restoration. ICES J Mar Sci 78(1):410–420
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7(2):153–163
Seifan M, Kadmon R (2006) Indirect effects of cattle grazing on shrub spatial pattern in a Mediterranean scrub community. Basic Appl Ecol 7(6):496–506
Solórzano A, Brasil-Machado A, Ribeiro de Oliveira R (2021) Land use and social-ecological legacies of Rio de Janeiro’s Atlantic urban forests: from charcoal production to novel ecosystems. R Soc Open Sci 8(6):201855
Teixeira CP, Fernandes CO, Ahern J, Honrado JP, Farinha-Marques P (2021) Urban ecological novelty assessment: implications for urban green infrastructure planning and management. Sci Total Environ 773:145121
Vandermeer J, Armbrecht I, De la Mora A, Ennis KK, Fitch G, Gonthier DJ et al (2019) The community ecology of herbivore regulation in an agroecosystem: lessons from complex systems. Bioscience 69(12):974–996
Vandermeer J, Jackson D (2018) Stabilizing intransitive loops: self-organized spatial structure and disjoint time frames in the coffee agroecosystem. Ecosphere 9(12):e02489
Vandermeer J, Jackson D, Perfecto I (2014) Qualitative dynamics of the coffee rust epidemic: educating intuition with theoretical ecology. Bioscience 64(3):210–218
Vandermeer J, Perfecto I (2006) A keystone mutualism drives pattern in a power function. Science 311(5763):1000–1002
Vandermeer J, Perfecto I (2019) Hysteresis and critical transitions in a coffee agroecosystem. Proc Natl Acad Sci 116(30):15074–15079
Vandermeer J, Perfecto I (2020) Endogenous spatial pattern formation from two intersecting ecological mechanisms: the dynamic coexistence of two noxious invasive ant species in Puerto Rico. Proc R Soc B 287(1936):20202214
Vandermeer J, Perfecto I (2023) Intransitivity as a dynamic assembly engine of competitive communities, vol 120. PNAS
Vandermeer J, Perfecto I, Philpott SM (2008) Clusters of ant colonies and robust criticality in a tropical agroecosystem. Nature 451(7177):457–459
Vandermeer J, Perfecto I, Philpott S (2010) Ecological complexity and pest control in organic coffee production: uncovering an autonomous ecosystem service. Bioscience 60(7):527–537
Vázquez Moreno LL, Alfonso Simonetti J, Ramos Y, Martínez A, Moreno Rodríguez D, Matienzo Brito Y (2012) Relaciones de hypothenemus hampei urculi (coleoptera: urculionidae: scolytinae) con el suelo del cafetal como base para su manejo agroecológico. Agroecología 7(1):2012
Vitousek P, Loope LL, Adsersen H (eds) (2013) Islands: biological diversity and ecosystem function, vol 115. Springer Science & Business Media
Wallace AR (1892) Island life, 2013th edn. University of Chicago Press, Chicago
Williams-Guillén K, Perfecto I, Vandermeer J (2008) Bats limit insects in a neotropical agroforestry system. Science 320(5872):70–70
Wood ZT, Fryxell DC, Moffett ER, Kinnison MT, Simon KS, Palkovacs EP (2020) Prey adaptation along a competition-defense tradeoff cryptically shifts trophic cascades from density-to trait-mediated. Oecologia 192:767–778
Yitbarek S, Perfecto I, Vandermeer JH, (2017a) Parasite mediated competition facilitates invasion. bioRxiv, p. 167254
Yitbarek S, Vandermeer JH, Perfecto I (2017b) From insinuator to dominator: foraging switching by an exotic ant. Divers Distrib 23(7):820–827
Zewdie B, Tack AJ, Adugna G, Nemomissa S, Hylander K (2020) Patterns and drivers of fungal disease communities on Arabica coffee along a management gradient. Basic Appl Ecol 47:95–106
Zewdie B, Tack AJ, Ayalew B, Adugna G, Nemomissa S, Hylander K (2021) Temporal dynamics and biocontrol potential of a hyperparasite on coffee leaf rust across a landscape in Arabica coffee’s native range. Agric Ecosyst Environ 311:107297
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vandermeer, J., Hajian-Forooshani, Z., Rivera-Salinas, I.S., Perfecto, I. (2024). Pest Control in Coffee: A Tri-trophic Comparison between a Mainland and an Island Agroecosystem. In: Moreira, X., Abdala-Roberts, L. (eds) Ecology and Evolution of Plant-Herbivore Interactions on Islands. Ecological Studies, vol 249. Springer, Cham. https://doi.org/10.1007/978-3-031-47814-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-47814-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47813-0
Online ISBN: 978-3-031-47814-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)