Abstract
Undoubtedly, polyvinyl chloride (PVC) is one of the most produced synthetic polymers globally and is used in all areas of life. Its general structure consists of hydrocarbon and chloride as well known. The main reasons for its widespread use in our life are low production cost, high mechanical strength, and chemical stability. The PVCs have significant problems such as low thermal resistance or weak impact strength. Thus, nowadays, the current studies are noteworthy on the PVC-matrix composites reinforced with micro-/nano-based fillers. The primary purpose of this studies improves the mechanical, physical, or chemical properties of PVC. Of course, the essential feature of a composite structure is the matrix/reinforcement interface and its interactions. In addition to the production method, the selection of matric and reinforcement fillers is the main factor affecting the adhesion and interactions between the interface. In this chapter, an overview of the possible interaction of PVC with micro- and nano-fillers is presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Polyvinyl chloride (PVC), composed of 42% hydrocarbon and 58% chloride, was first discovered by the scientist Regnault in 1835. After polyethylene (PE) and polypropylene (PP), the PVC is one of the thermoplastic polymers we use primarily in everyday life, such as construction, packaging, medical, or clothing. Some application areas of PVC can be listed as pipes, electric wires, window profiles, credit cards or bags, and tubing for blood transfusion [46]. More recently, wood fiber PVC has become more popular, with good mechanical properties, moisture, fungal resistance, long life, wood-like surface performance, and recyclability [16, 46].
The primary reasons for PVC’s widespread use are its low cost, high mechanical strength, and high chemical resistance [5, 76, 78]. Moreover, good insulation and fire resistance may be considered as their other vital features [5]. However, the PVCs have major problems such as low thermal stability, low impact strength, and low strength [83]. Current studies in this field have focused mainly on the addition of impact regulators and coupling agents to improve composite properties [46]. Many researchers have conducted numerous studies on PVC matrix composites and/or nanocomposites to enhance PVCs’ properties [16, 78].
The fillers reinforced into the PVC matrix can be divided into PVC matrix composites and PVC matrix nanocomposites. However, different fillers are reinforced into the PVC matrix beyond size. These can generally be categorized as organic and inorganic fillers, but this classification is very generic. To make an understandable classification for fillers reinforced into the PVC matrix, these fillers can be classified as follows: (i) carbon-based fillers, (ii) natural fillers, (iii) mineral and metal oxide fillers [24]. Carbon-based fillers reinforced into the PVC matrix include carbon nanotubes (CNTs), graphene oxide (GO), carbon black, graphite, etc. Natural fillers include rice husk, bamboo fiber, coconut shell, banana pseudostem, etc. Even though plenty of metal oxide fillers is used in the PVC matrix, the most commonly used ones are SiO2, TiO2, ZnO, MgO, etc. The most common mineral clays are bentonite, kaolinite, talc, etc. [24, 35, 78] (Fig. 1).
The type and size of fillers used to improve the properties of the PVC matrix are essential. However, another equally important parameter is figuring out and intervening with matrix/filler interaction. Interfacial interaction of PVC matrix/fillers can be enhanced so that an effective improvement can be seen in many properties of composites, particularly the mechanical properties [87, 88], as well as the wettability and adhesion between PVC matrix/fillers can be developed with the surface modification of fillers [29].
Filler materials can be used as particle or fiber reinforcements. Filler materials are particles when geometric axes have a similar order of magnitude in two or three dimensions, depending on the Aspect ratio (for example, a spherical or discoid shape). The aspect ratio of particle filler plays an important role. The higher the aspect ratio, the higher the strength of the composite. The aspect ratio must be obtained above the critical value for better stress transfer [50]. Fibers can be defined when one dimension is considerably larger than the other two dimensions (e.g., carbon nanotubes have approximately 1000 nm in length only compared to ~ 1 nm diameter). Fiber-reinforced composites have been widely used in applications such as construction for many years, and their market share is increasing in many other industries. Fiber-reinforced composites consist of fibers that act as reinforcements or fillers and a polymer matrix. In fiber-reinforced composites, the matrix protects fibers against external environmental harmful impacts [9].
As stated above, the bonding formation in the composite structure is explained with the concept of “wettability” or “interface adhesion” [26]. Interface adhesion is the other important parameter that should be considered after selecting the matrix and filler elements, which are the main elements of the composite that will produce the desired properties. Because in the absence of a good interface adhesion, the charge from matrix phase-to-filler phase is not effectively transferred on composite materials [25].
Polymer matrix composites (PMCs) may be exposed to stresses in operating conditions. Stress concentration can be caused in the composite for two reasons (i) thermal processing due to different thermal expansion coefficients for fiber and matrix and (ii) mechanical stresses due to different strength properties of both materials. When an adhesive with a low interface is subjected to composite loads, micro-cracks develop on the interface and spread to the matrix. Consequently, the composite suffers damage in the form of stripping or fracture [52].
Although composite materials are produced by joining macro grade matrices and fillers in different ways, they are expected to act as a single material when subjected to physical, chemical, or mechanical impact. In this respect, the value of the bond strength or adhesion on the interface determines the behavior of the composite [99]. Mechanical interlocking, electrostatic interaction, molecular interaction, and chemical bonding are all bonding methods at the interface [29].
However, the high adhesive strength at the interface does not certainly lead to optimal composite properties. It is crucial to figure out the role of physicochemical interactions at the fiber-matrix interface and to establish quantitative correlations between the nature and level of those interactions and the ultimate mechanical behavior of composite materials [37]. In other words, the effect of interface adhesion (W) on the magnitude of stress transfer capacity from fiber to matrix must be analyzed [91]. This interface stress transfer capability is measured through a fragmentation test on single-fiber composites and is defined as shear strength, τ, in the interface. τ is expressed as follow (Eq. 1) [49].
where d is the fiber diameter, \({l}_{c}\) is the critical length of the fiber part at the end of the decomposition process, and \({\sigma }_{f\left({l}_{c}\right)}\) defines the fiber stress strength at the length of the measurement equal to \({l}_{c}\). The extent of theoretical and experimental approaches leading to the establishment of such relationships is discussed [49]. Layers that exhibit physical and mechanical properties different from mass, i.e., the presence of the interface, affect the results. Therefore, it is of primary importance to comprehend the role of physicochemical interactions at the fiber-matrix interface and to establish quantitative relationships between the nature and level of those interactions and the ultimate mechanical behavior of composite materials.
The physical attraction between electrically neutral bodies is best described as the wetting of solid surfaces by liquids. Wetting-related bonding involves very short-range interactions of atom-scale electrons that develop only when the atoms of the components approach or come in contact with each other within a few atomic diameters [73]. For continuous fiber composites, in particular, weak adhesion may result from insufficient physical contact between the resin and the fiber due to incomplete wetting. Contact between a liquid and a solid can be established provided that the liquid is not too viscous and a thermodynamic driving force is present. Therefore, wetting must be done for any adhesion to occur. A high value for a liquid surface free energy prevents the spread of a liquid droplet from covering the surface. The wetting or contact angle \((\theta )\) is achieved by a balancing force (Young equation). Most of polymeric surfaces can be described as low energy while fillers surface is described as high energy. The total surface energy of a material is \({(\gamma }_{i}\). It can be considered that this value consists of two parts; Lifshitz-van der Waals \(({\gamma }_{i}^{LW})\) and acid–base component \(({\gamma }_{i}^{AB})\) [17]. The first section represents long-distance dispersion forces, orientation (Keesom), and induction (Debye), while the second section represents short-distance H-bond or Lewis’s acid–base interactions. Ideally, it is necessary to characterize the solid resin (polymer) and filler (fiber) surface energies with proper probe fluids for LW and acid–base components and to calculate adhesion for any resin-solid system.
Consequently, methods such as roughening the matrix-reinforcement interface and modifying the matrix and fillers may be employed to ensure good wettability in the interface. These surface modification effects are attributed to thermodynamic driving forces and have been shown to affect polymer adhesion and other characteristics depending on the surface and interface forces.
2 PVC Matrix Composites Reinforced with Micro/Nano-fillers
In micro filler/polymer composites, surface modification of particles has already been a broadly applicable technique for minimizing particle/particle interaction and increasing particle/matrix interaction. Along with the latest advances in nanoscience and nanotechnology, the correlation between polymer nanocomposites’ properties and nanoparticles’ surface modification has also become a significant interest [74].
Polymer matrix nanocomposites (PNCs) represent a radical alternative to the conventional filler polymers or polymer mixtures, which are the fiber of the modern plastic industry. Unlike the traditional composites where the reinforcement is at the micron level, PNCs are sampled with separate components at several nanometres levels [96]. In recent years, it has been observed that the addition of the low content of these nano-filler agents to the polymer may lead to improvements in the mechanical, thermal barrier, and flammability characteristics without affecting their machinability.
PNCs are classified by the size of nano-filler agents. One-dimensional nano-fillers are: plates, laminas, and/or shells; two-dimensional nano-fillers are in the form of nanotube and nano-fibers that are smaller than 0.1 in diameter, and three-dimensional nano-fillers are isodimensional nanoparticles such as nanometric silica [56].
In a PVC matrix, the capabilities of the interfaces stand out to transfer stresses from matrix to fiber in the interaction of nano-filler. Theoretically, the composite’s strength increases as the filler agent's particle size reduction for a given particle volume fraction. A smaller particle size with a higher surface area yields higher strength by transferring more efficient stress [87, 88]. The matrix can completely cover the filler agent surface under optimum particle loading, while the small particles lead to good distribution and strong particle–matrix interaction [50].
As a result, interface control is critical to obtaining a good bond between the inorganic filler and the polymer. The ratio of particle volume to interface volume changes drastically in favor of interfaces with decreasing filler size (Fig. 2). The emergence of new properties with nano−, meso− and micro-material interactions will also be the subject of recent studies.
Schematic representation of the ratio particles/interfaces changes with the size of the filler [71]
3 Interaction of PVC Matrix with Organic Fillers
3.1 Interaction with Carbon-Based Fillers
In recent years, the addition of carbon-based nanofillers into PVC, such as graphite, graphene (Gr), graphene oxide (GO), and carbon nanotube (CNT), is very frequently referred to in the literature [4, 5, 68]. In general, the approach for obtaining nanocomposites is primarily based on generating a large interface between the nanosized building blocks and the polymer matrix. However, this approach alone is not sufficient. Improvement in the physical and chemical properties of the PVC nanocomposites with carbon-based fillers mostly depends on the components’ characteristics like their composition, concentration orientation, and structure. The interfacial interactions between the composite matrix and the filler are of prime importance in this respect that characterizes the interphase [30, 101]. The interphase is the immobilized polymer layer that covers the surface of the filler particles. Therefore, the effect of the interphase is directly related to its homogeneity, the quantity of material used, and the characteristics of the mixing components, particularly particle sizes. The influence of the interphase can be insignificant for larger particle sizes due to their lower surface areas to allow significant amounts of interphase to form. However, nanoparticles have very large surface areas that can make up interphase of a substantial volume fraction of the total composite. For that reason, while using nanoparticles, the composite properties are determined by the interphase. Both the amount of material attached to the interphase and the interaction resistance due to mixing can affect the properties of the nanocomposites significantly [74].
In some cases, they use appropriate surface modifications when necessary, resulting in good interfacial adhesion and leading to the success of developing new nanocomposites. As a matter of fact, many of the distinctive features of the nanocomposites that high modulus of elasticity, high yield strength, low permeability, etc., are influenced mainly by the interphase. This part discusses the interactions of the carbon-based fillers such as graphite, Gr, GO, and CNT with the PVC matrix.
As it is known, graphite comes from the Greek word graphein, which means to write. Graphite can be seen in powder, flake-like, flat, fibrous, and spherical forms with various particle sizes. Flake-like graphite consists of a thin layer less than 100 nm in thickness. Carbon atoms in graphite are arranged in hexagonal sheets, and the distances between the two carbon atoms of each sheet’s hexagon are 1.42 Å [5]. Carbon atoms are linked to each other by a covalent bond in these layers. In graphite, each layer of the hexagonal sheet of carbon is connected by Van der Walls forces.
Another carbon product, graphene, was obtained more than a decade ago by graphite exfoliation by Andre Geim and Konstantin Noholev. Then its synthesis techniques, properties, and potential applications were investigated worldwide [70]. Graphene is known for its superior properties. It is considered two-dimensional because it is only one atom thin and formed by the perfect arrangement of covalently bonded carbon atoms in a hexagonal honeycomb lattice [30]. The carbon–carbon bond length in graphene is 0.142 nm. Graphene has attracted a lot of interest owing to its lightweight, high young modulus (~1.100 GPa), high electron mobility at the room temperature (250.000 cm2·V−1·s−1), high thermal conductivity (5000 W·m−1·K−1), large specific surface areas (2630 m2·g−1), and good compatibility with polymers [8, 51, 106]. In addition, it has also been used as a high-performance lubricant in various regions due to its flexible graphitic layers, high strength, and atomically smooth surface [97].
Graphene oxide (GO) is generally synthesized by Hummers, Staudenmaier, and Brodie methods by the oxidation of natural graphite flake to various levels [5, 106]. Strong acids and oxidants are used for the oxidation of graphite. However, oxidation levels of the graphite are related to graphite precursor, reaction conditions, and method used. Oxidation, epoxide, hydroxyl groups, and the carboxylate of some polar groups are linked with each layer. While carbonyl groups are placed near the edges, other groups stay above and below of each graphene layer [5].
Carbon nanotubes (CNTs) are known for their unique properties such as thermal, optical, and mechanical properties. Various methods that are widely used for the synthesis of carbon nanotubes are laser ablation, arc-discharge, thermal chemical vapor deposition, chemical vapor deposition (CVD), plasma-enhanced chemical vapor deposition, and chemical vapor deposition with microwave plasma, hydrothermal synthesis, solar oven and electrolysis method, etc. [67]. In CNTs structures, atoms form hexagonal geometry, and atoms bond with each other by planar sp2 configuration (as in graphite plate). Carbon nanotubes can be of single-layer (single-walled) or multi-layered (multi-walled). Single-walled nanotubes are formed by one-layer cylinders of graphite, while multi-walled tubes are composed of around 50 [1]. In short, a single-layer carbon nanotube can be described as a form of graphite wrapped in a cylinder of the atomic plane resembling a honeycomb without creating any defects. Despite their very thin structures, they are very strong and rigid and can be used as excellent conductors if properly fabricated. Diameters of carbon nanotubes can be in the order of nanometres and lengths of micrometres. In multi-walled tubes, the distance between the two tubes is usually greater than the bond distance between the carbon atoms forming the tube. In nested tubes, if the distance between the walls of the tubes is small enough (0.15 nm) to allow the carbon atoms to bond, the carbon atoms bond tetrahedrally by sp3 configuration. In other words, each carbon atom has four tetrahedrally bonded neighbors. The rods consist of completely hollow or partially filled tubular structures. The flexibility of these structures is less than the tubes; they also show different mechanical and electronic properties than single-walled tubes [62].
Graphite enhances the multifunctional features of nanocomposites like thermal, mechanical, electrical, and lubricating properties while in use as fillers due to its sigma bond, delocalized pi bond, and Vander Waals forces between graphite sheets. Addition of graphite filler into the PVC composites with conventional polymer processing methods like compression and injection molding are known. The properties of the PVC/graphite composites depend on various parameters such as the composition of the PVC and its production method, the structure of the graphite, and interactions and level of dispersion in the polymer matrix [5]. Polymers are used as insulators in the electrical industry due to their high specific electrical resistance. If electroconductivity is desired, the conductive fillers are added to the polymer matrix. With electroconductive properties, nanocomposite materials are widely used in the electronics field, particularly in power for electrical-conductivity, conductive adhesives, electromagnetic interference shielding, conductive paints, gas and pressure sensor [6, 65].
Other than graphite, the addition of carbon black has also been studied. It was reported that carbon black with a high surface area improved the electrical current percolation at lower concentrations due to a conductive network. Still, its weak interactions and porous structure decreased the mechanical properties [65]. However, the advantage of the addition of graphite is its good conductivity and its lubricating effect in the melt. In some cases, the graphite is coated with some elements such as Cu. It was found that the metalized graphite significantly increases the viscosity of compositions. For that matter, the compositions exhibit superior heat transfer characteristics due to the strong adsorption interaction of the binding agent to the filler surface. The use of more conductive copper-plated graphite makes it possible to create electroconductive polymer composites with high adhesive strengths [10, 64, 65]. The effects of the graphite-filler on the PVC/styrene-acrylonitrile composites, in respect of electrical, morphological, and hardness, were investigated. Analysis of the composites by microscopy revealed the formation of a conduction network at the interfacial regions in a well-dispersed graphite-polymer matrix. Improvement in conductivity and hardness even at a very low filler loading was reported by Sachdev [77]. Graphite is used as electronically conducting filler in various proportions to polyethylene oxide (PEO)/PVC nanocomposites using the solution casting technique. It was noted that the increase in filler loading increased the electrical conductivity. But, increasing the amount of graphite also impaired the young modules and tensile strength of the nanocomposites. 5 wt% of graphite loading was found to be adequate for that matter, whereas its higher level of usage between 15–25 wt% caused agglomerations [32]. To enhance the electrical conductivity of the PEO/PVC, the addition of graphite together with FeCl3 in PEO/PVC by solution casting method has been found beneficial. As per the method described elsewhere [85], graphite and 6 wt% FeCl3 were mixed in toluene with stirring and then washed and dried for 5 h in an oven at 60 ˚C. The FeCl3 doped ground graphite powder was then blended with the tetrahydrofuran (THF) solution of PEO/PVC and casted on a glass mould and dried at room temperature. It was seen that electrical conductivity performance was enhanced with addition of fillers however, it adversely effected the tensile strength of the nanocomposite films. The THF blend of the sonicated dispersion of graphite in N-methyl-2-pyrrolidinone (NMP) was also used as fillers for the PVC composites by solution blending method. With this method, the uniform distribution and interaction of graphite nanosheets and polymer increased Young’s modulus by 63%, and it also increased the tensile strength by 19% [63]. The addition of graphite nanosheets into the PVC matrix improves the electrically conductive of the nanocomposites. It also increases the thermal stability, dielectric constant, and absorption of the magnetic waves at microwave frequency [6].
Graphene/PVC nanocomposites possess very high elasticity modules, and graphene addition to polymers improves the thermal, mechanical, and electrical properties of the nanocomposites [45]. The superior properties of the graphene doped polymer matrix, such as strength and toughness, were due to the prevention of the propagation of microcracks [97]. As a result, the mechanical and wear resistance of the nanocomposites increases significantly. The synthesis method of graphene/PVC nanocomposites is based on the solvent evaporation method. Tetrahydrofuran (THF) is generally used as a solvent for PVC that forms thin polymer films (foils) and fibers. The advantage of this method is the dispersion of nanoparticles in the solvent can be enhanced by sonication. However, secondary aggregation of the nanofiller due to solvent evaporation should be considered. A method such as mixing with molten polymer and in situ polymerization is also used for the graphene/PVC nanocomposites [101]. The homogenous dispersion of the graphene filler into the PVC was investigated by using various dispersants such as oleic acid, polysorbate 80, and Curcuma longa L. rhizome. The improvement in dispersion stability and disaggregation of the filler was observed with Curcuma longa L. rhizome [101]. Wang et al. [98] studied the synthesis of the multi-layer graphene (MLG)/PVC composites by using melt-mixing technics.
The improvement in mechanical properties was attributed to the uniform-homogenous dispersion and interactions of the MLG and PVC. The interactions and reinforcing mechanisms of MLG/PVC composites are shown in Fig. 3. It can be noticed how the strong interactions between entangled PVC chains enhance the mechanical strength and tensile modules. With the use of plasticizers, e.g., DOP (dioctyl phthalate), the distance between PVC chains gets enlarged, weakening the interactions between the polarized groups resulting in the decrease in mechanical properties. However, when MLG added, the interaction between components and the mobility of the PVC chains decreases. Owing to high structural integrity and low surface energy of MLG, DOP molecules absorbed easily improving the compatibility of the MLG and PVC matrix. The uniform dispersion and effective stress-transfer among the components cause to enhance the tensile modules of the nanocomposites.
Structural schematic of MLG/PVC composites a neat PVC, b soft PVC containing DOP plasticizer, c MLG/DOP/PVC composites (Reprinted with permission from ref. [98], Copyright 2015, Elsevier)
Graphene oxide (GO) is attracting researchers worldwide due to its exceptional properties in various areas such as chemical sensors, composite materials, energy storage, and optical and electrical devices. However, to exploit the structure and physical properties of the carbon materials derived from graphite such as graphene, GO, chemically reduced GO (CRGO) or thermally reduced GO (TRGO), or functionalized graphene sheets (FGS), the degree of dispersion, orientation and interfacial adhesion are very important for the manufacturing of polymer nanocomposites. Various surface-modified functionalized GO of all allotropic modifications can be developed with its functional groups. These functionalized GO sheets show strong hydrophilic properties and easily disperse in water or other aqueous media to form stable colloids. Due to its hydrophilic nature, GO sheets can only be distributed in aqueous media, which is incompatible with organic polymers [19]. In the Hummers method, the graphite is oxidized by potassium permanganate (KMnO4) and sulfuric acid (H2SO4), while in Brodie and Staudenmaier’s method, the combination of potassium chlorate (KClO3) with nitric acid (HNO3) is used to oxidize graphite [106]. The use of GO as a filler in thermoplastics and the importance of its dispersion in a polymer matrix, and interfacial interactions between the filler and polymeric matrix are briefly discussed as follows. To improve its homogenous dispersion in the PVC matrix, GO are functionalized. Thus, strong hydrogen-bonding interaction between the PVC chain and hydroxyl, carboxyl and epoxy groups of GO sheets can be obtained [5]. In studies by Li et al. [55], enhancement of the interfacial interaction of the PVC and reduced graphene oxide (rGO) was achieved by hybridizing rGO with zinc oxide (ZnO) nanoparticles. Synthesis of the rGO filled with ZnO nanoparticles (rGO-ZnO) by one-pot chemical route was done by mixing GO and zinc nitrate (Zn(NO3)2) in water and adding sodium hydroxide and hydrazine hydrate. TEM, SEM, and XRD methods analyzed the obtained rGO-ZnO hybrid. rGO-ZnO/PVC composites were obtained by a simple solution mixing and drop-casting. Tensile strength, interfacial tension, and glass transition data evaluated interfacial interaction between PVC and rGO-ZnO. It was found that the ZnO nanoparticles acted as a bridge to connect PVC via electrostatic attraction and hydrogen bonding linking rGO by a p–∏ stacking/electrostatic interaction. Significant enhancement in mechanical properties and glass transition temperature was observed due to very strong interfacial interaction between PVC and nanofillers.
In another report, the synthesis of GO reinforced PVC-waterborne castor alkyd (WCA) nano-composites (PVC/WCA/GO) films via solution blending technique was discussed. For the GO synthesis, the natural graphite and NaNO3 were dissolved in H2SO4, and then KMnO4 was gradually added into this suspension as an oxidation agent. The suspension was filtered and washed with a warm HCL solution. In the last stage, it was filtered and dried. The obtained GO was then added to PVC/WCA using the solution blending method. It was noted that the samples with proper dispersion in the composite matrix showed significant improvement in tensile strength and elastic modulus of the nanocomposite. 260 and 185% improvements in tensile strength and elastic modulus were achieved by adding 0.5 wt% of GO compared to the neat polymer [102]. GO reinforced PVC nanocomposites can be obtained by the colloidal blending method too. Likewise, GO powder synthesized via the Hummers method is also used in this method. Before preparing PVC/GO composites, GO was separately dispersed in THF and sonicated. Then the mixture was sonicated for homogenous dispersion. Significant improvements in mechanical strength, thermal stability, and electrical properties were observed due to the homogeneity of the nanocomposites and their strong interactions between covalently bonded PVC matrix and GO [18]. Lee et al. [53] studied the synthesis of flexible PVC nanocomposites with fillers of hyperbranched polyglycerol (HPG) with functional graphene oxide (HGO). The production of the HGO via ring-opening polymerization of glycidol was seen in Fig. 4.
Synthesis of HGO by Surface-initiated polymerization of Glycidol followed by esterification with butyric anhydride (Reprinted with permission from [53], Copyright 2017, American Chemical Society)
GO was first obtained with Hummers method, treated with glycidol mixture, and yielded HPG-grafted GO. Then, the hydroxyl groups were esterified by using butyric anhydride. It was indicated that the hyperbranched polyglycerol (HPG) and functionalized graphene oxide (HGO) caused uniform dispersion of the fillers and resulted in strong interactions between the HGO and PVC [53]. In another work, a significant improvement in mechanical properties of the PVC nanocomposites was reported using various GO derivatives. Firstly GO, poly (methyl methacrylate) (PMMA) grafted GO, polydopamine coated GO (rGO@PDA), reduced by dopamine (rGO@PDA-g-PMMA) were synthesized by Hummers method and then blended with PVC to form nanocomposites. Superior mechanical and thermal properties were reported for the nanocomposite thus formed, and this was due to the homogenous distribution and interfacial interactions of the fillers in the PVC matrix [36].
The effect of both multi and single-walled CNTs on the PVC matrix was also discussed in the literature. Especially nowadays, CNTs have become one of the most attractive materials in nanocomposites due to their properties such as high aspect ratio, low density, and high electrical conductivity with excellent mechanical and thermal properties. Even using a very small amount of CNTs in the polymer matrix provides very good mechanical strength and electrical conductivity of the composite material compared to the conventional fillers. The CNTs-based nanocomposites are being used in various areas such as field emission, electromagnetic interference shielding, and actuators. Manufacturing CNT-based nanocomposites are difficult due to the Van der Waals interactions between CNTs, hindering effective unbundling. As a result, inhomogeneous dispersion of CNTs due to segregation adversely affects the properties of the nanocomposites. The successful fabrication of CNTs based nanocomposites depends on the high degree of dispersion of CNT in the main polymer matrix. Since the carbon nanotubes tend to self-associate into microscale aggregates, the surfactants such as sodium dodecyl sulfate, sodium dodecylbenzene sulfonate, and N-methyl-2-pyrrolidone are used to make a uniform dispersion of CNT in polymer matrix [57, 95]. Interfacial interactions can be maximized by chemical modification of CNTs to disperse or to make them compatible with the various polymer matrices. A synergy between the solvent and polymer was reported due to the high solubility of the long alkyl-chain modified multiwalled carbon nanotubes (MWNTs) and PVC in cyclohexane. The increase in thermal stability, conductivity, and improved young modules was attributed to the modification of CNTs and cooperative effects between solvent/polymer/CNTs [79]. The effects of the various amounts of single wall carbon nanotubes (SWCNT) in PVC composites revealed improved mechanical and thermal properties. The homogenous dispersion and interfacial adhesion between the PVC/SWCNT matrix consist of 0.25–0.75 wt% of fillers, resulting in increases in Young’s modulus, tensile strength, elongation to break, and toughness. In composites including 1 wt% of the nanotubes agglomerated, decreases were observed in mechanical properties due to agglomeration acting as stress concentrators during the stress loading [105].
4 Interaction with Natural-Based Fillers
Synthetic fiber-reinforced polymer composites are widely used in high-performance applications such as the aerospace and automotive industries due to their superior mechanical properties and lightweight. However, their common usage can cause major ecological problems if they are not adequately recycled [50]. These reasons have led researchers to investigate using natural fibers as polymer reinforcements. PVC is a well-known contaminant due to the release of harmful substances into the atmosphere, such as hydrogen chloride and dioxins, during processing or decomposition. The combination of PVC and natural fibers is an exciting alternative as natural fibers are ‘ecologically friendly’ [13].
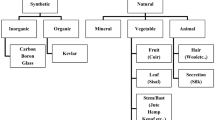
PVC composites made of natural fibers are preferred because of their low cost, renewability, recyclability, biodegradability, corrosion resistance, thermal and electrical insulation, and good mechanical properties [9, 40, 61]. Given the relatively low impact resistance of the PVC matrix, however, it is more practical to develop impact strength. Current studies on PVC/natural fiber composites have focused mainly on the addition of impact regulators and the use of coupling agents to improve composite characteristics [46]. Natural fibers may originate from animal wool, hair, silk, and plant (wheat, hemp, sisal, jute, kenaf, coconut) or less used mineral origin [9, 13]. Figure 5 shows the classification of the natural filler agents [13].
The vegetable fibers are natural compounds made from cellulose, bonded to a hemicellulose and lignin matrix. Lignin-rich fibers (e.g., coir fiber, kenaf) are flexible and have high deformation [23]. Cellulose-rich fibers (e.g., banana, pineapple leaves, rice husk, potato peel, and canola straw) are used as fillers for high-performance materials [90]. Stalk fiber is obtained from the straw of cultivated plants, such as wheat, oat, maize, barley, or rape [7]. Stalk fiber and straw improve the strength of PVC composites without increasing their weight [12, 58]. Kenaf Hibiscus cannabinus is a plant known as deccan hemp and java jute from the Malvaceae family. Kenaf is one of the allied fibers of jute and displays similar characteristics. Jute is a bast fiber obtained from the trunk stripes of Corchorus species plants. Jute fibers have good mechanical properties, such as high tensile strength and thermal/insulating properties [75].
Sisal fibers are obtained from the leaves of the sisal plant. Sisal fibers are complicated and have a rough texture. Gomuti fibers are usually black and stiff fibers. Arenga is obtained from the sugar palm tree. Hemp and jute fibers have a thinner texture and a smaller diameter than coir, gomuti, and sisal [93]. Reinforcing kenaf, jute, and sisal fibers into the PVC matrix improves its mechanical properties [2, 20, 47]. Bamboo is an essential source of forest that grows abundantly in many tropical and subtropical regions, particularly in Asia. It has easy machinability with fast growth, high strength, and surface hardness. Thus, PVC/Bamboo composites are widely used in furniture production and construction materials [72]. Around 2000 different plant fibers are used in various applications, including composite [14]. The physical and mechanical properties of some of these fibers are given in Table 1.
The natural fiber structurally contains cellulose, hemicellulose, lignin, pectin, and wax materials [69]. The plant fiber (Fig. 6) wall consists of three main layers: middle lamella (lumen) and primary and secondary walls (PW, SW1, SW2) [21, 52]. The middle lamella connects the single fibers as the fiber bundle. Therefore, extraction processes can separate single fibers from fibers. In-plant fiber, the S2 layer is the part that determines the longitudinal mechanical properties. The helical structure of long cellulose chains makes up most of the microfibrils. Amorphous regions composed of lignin and hemicellulose are packed with cellulose chains. Microfibrils have a diameter of 10−30 nm [69].
A high aspect ratio (length/width) in cellulose-based fiber composites is very important, indicating potential strength properties. Continuous fibers have a high aspect ratio and provide the composite with good strength properties. Low aspect ratio discontinuous fibers are suitable for complex parts and large-scale production [21].
Natural fibers are inherently hydrophilic, poorly resistant to moisture, and incompatible with the hydrophobic PVC matrix [40]. The incompatibility of natural fibers results in a weak fiber/matrix interface leading to a decrease in the mechanical properties of the composites. Reinforcing natural hydrophilic fibers in the PVC matrix leads to a heterogeneous system with lower properties due to poor fiber-matrix adhesion. Because of the cellulose and lignin hydroxyl (OH) groups found in the structure of natural fibers, these fibers have a good potential for chemical treatment. -OH group’s reaction can alter the surface energy and polarity of natural fibers [52]. Chemical treatment of fibers or surface modification improves fiber and matrix adhesion, which is critical in developing further composites.
Fiber modification may be alkali, silane, acetylation, benzoylation, acylation and acrylonitrile grafting, malleated coupling agents, permanganate, peroxide, isocyanate, stearic acid, sodium chlorite, triazine, fatty acid derivate (oleoyl chloride) and fungal, etc. [92]. Additionally, as an alternative coating technique, plasma surface treatment and plasma polymerization were used mainly for the surface modification of fibers [86]. Various coupling agents used are titanate, silane, betaine, malleated PP (MAPP)]; compatibilizer, for example, polypropylene-grafted-maleic anhydride (PP-g-MAH), maleic anhydride; and surface modifier usages such as stearate, stearic acid, [11]. As a result, adhesive strength determines the effectiveness of stress transfer between PVC and filler materials. These processes have improved homogeneity, and as a result, the distribution of the particles has improved to provide a better bond between the fillers and the matrix.
Alkali or mercerization treatment is one of the standard chemical treatment methods applied to natural fibers to strengthen the thermoplastic PVC matrix, which removes certain amounts of wax, oil, and lignite covering the outer surface of the cell wall [22]. Also, alkali treatment is cheaper than other chemical treatment methods. Upon alkali treatment, the crystallinity of Cellulose increases, and the impurities and swelling decrease in wood fiber, resulting in a smaller number of -OH groups [42].
If it is looked at binding agents and surface modification in natural fillers, we can see that they increase the cross-bonding rating. It has been reported that the tensile strength of PVC reinforced with cellulosic fibers (wood pulp) decreases with the use of untreated fibers, whereas the strength of the composite increases by 51% with the wood pulp reinforcement developed with silane. The PVC/ sawdust composite study with three different silane agents revealed enhanced mechanical properties compared to untreated composites [46]. This is because the silane is a dispersion agent. The titanate and aluminate coupling agents (ACAs) are improving interface compatibility and enhancing the mechanical properties of WPC. In this case, the effect of the aluminate is weak, and the effect of the titanate is apparent [54]. Adding up to 7% lignin as a bonding agent in PVC/rice straw composite enhanced its tensile strength and reduced the weight gain percent and the swelling. The main reasons behind these trends were the binding of hydrophobic lignin to the hydroxy surface of lignocellulose materials, improvement in the bonding between rice straw particles, and the reduction of gap content [46].
Strong interface adhesion between PVC and amino groups containing chlorine on the WF (Wood filler) surface is encountered, resulting in significant increases in the mechanical properties of composites [82]. The properties of PVC matrix natural fillers composites depend on removing non-cellulose components from the fiber surface and forming a clean and rough surface for better interface adhesion [38]. Coupling agents or compatibilizers are used to promote the bonding of functional groups to enhance interface adhesion binding between fiber/matrix [104]. The studies have also revealed that reduced fiber hydrophilic ability and polarity increase the angle of contact with water, but vice versa for fluids without polar [84]. Samajpati and Sengupta [80] investigated the wettability of five plant fibers on different polymer matrices. Finally, he found that fiber/matrix wettability is governed by the physical and chemical properties of the fiber surface and polymer surface tension. Consequently, the classic contact angle method plays an informative role in wettability and interface adhesion.
5 Interaction of PVC Matrix with Inorganic Fillers
5.1 Minerals and Metal Oxide Fillers
Good modulus, strength, rigidity, durability, hardness, and particularly low-price composites have been obtained by adding mineral nano-fillers to the PVC matrix [94]. Mineral fillers that are frequently used in PVC matrix are mineral clays such as montmorillonite (MMT), sodium montmorillonite (NaMMT), organophilic montmorillonite (oMMT), other mineral clays such as laponite, bentonite, hectorite, kaolinite, and halloysite, mica, vermiculite (a similar mineral), calcium carbonate, CaCO3-silica, talc-layered double hydroxides (LDHs) [24].
It is available in two crystal forms: Calcium carbonate (CaCO3), calcite (rhombohedral), and aragonite (orthorhombic) [35]. Calcium carbonates are mainly used along with polyvinyl chloride (PVC) for their hydrophobic character. Nanosized calcium carbonate and silica filler improve toughness, electrical properties, heat resistance, radiation resistance, and other properties in the matrix and reduce the cost of composites [87, 88]. Talcs are alumina silicates. They are usually reinforced into the PVC matrix with CaCo3. Bentonite, a form of montmorillonite clay, is a mono aluminum layer consisting of an octagonal plate inserted between two layers of silicone and tetrahedral plates [103]. Many researchers have studied the reinforcement of bentonite and derivative clay minerals to develop thermal, mechanical, and flame retardant features in a PVC matrix [33, 43]. Also, montmorillonite and organophilic montmorillonite are reinforcing elements frequently used in PVC matrix. These clays are added in different morphologies from micro to nano levels as aggregated, intercalated, and exfoliated in the matrix [15]. In the aggregated structure, clay tactoids are well dispersed in the polymer matrix, but single clay layers are not delaminated. In the Intercalated structure, clay tactoids exhibit delamination to a certain degree. Therefore, polymer chains may diffuse into the galleries between them. In the exfoliated structure, clay tactoids are completely decomposed apart into single layers that are homogeneously distributed in the matrix [89]. In particular, exfoliation structures are preferred for storage modulus, increased tensile and flexural properties, heat distortion temperature, decrease in gas permeability, and unique properties [27]. Mica is aluminum silicate in terms of chemical composition. It can also contain Al, Si, K, Mg, and Li elements. They are also called as potassium, magnesium and lithium mica when compared to other elements. Mica is used as filler to improve Young’s modulus, hardness and dielectric properties of some polymeric materials, including PVC. Silica (SiO2) is preferred in a PVC composite material as it is fuel efficiency, dielectric properties, improvement of mechanical properties, and resistance to heat and moisture [35].
Metal oxides are another group of fillers that are reinforced with PVC polymers. These reinforcements are used to prevent thermal degradation of PVC in particular [31]. Zinc oxide is a white pigment with a UV barrier, providing good electrical conductivity and high heat resistance to the resins. The addition of metal oxide in PVC, such as ZnO particles, was preferred as ZnO particles strongly interact with PVC chains resulting in mechanical improvement [55]. Furthermore, the nanosized ZnO stands out in improving PVC's structural, optical, and thermal properties [59]. Titanium dioxide is a white pigment that enhances the UV barrier and provides aging, water, and heat resistance. Titanium dioxide is primarily used in thermoplastics and unsaturated polyesters [35]. The addition of TiO2 into a polymer may significantly affect the polymer's electrical, optical, and photocatalytic properties. It is anticipated that it will be one of a wide range of technological applications in the future as a nanocomposite [59].
Magnesium oxide (MgO) increases polymer resins’ strength, creep resistance, and thermal conductivity. Today, ceramics such as alumina and silicon oxide are widely used in many engineering applications such as automotive, electronics, and aerospace. Among these ceramics, alumina or aluminum oxide (Al2O3) at various purity levels is one of the most cost-effective materials in the engineering ceramics family due to several desirable properties, such as excellent hardness and abrasion resistance good dielectric properties. It also has good acid resistance and the resistance to alkali attack at high temperatures and high thermal conductivity, providing benefits in PVC micro and nanocomposites in particular. In the polymer, beryllium oxide was used as microspheres. It was determined that the electrical and thermal conductivities of the polymer have increased [35].
The interface interaction of PVC/mineral fillers and their effect on the performance of polymer nanocomposites are affected by four factors. These factors are the properties of the components, the composition, the structure, and the interface interactions. In Fig. 7, the formation of a surface layer of varying thickness, different from the matrix, is schematized due to the adsorption interaction of the polymer with the solid surface. In this case, the interaction of polymeric molecules with a solid surface should lead to a redistribution of intermolecular bonds at the surface or transition layer and the formation of new physical junctions in the physical network.
The scheme of types of filled systems (redrawn with permission from ref [44], Copyright 1995, Elsevier)
The effect of the interphase depends on the amount and properties of the interphase. This is why nano-fillers stand out. This is since their interactions in the matrix are only atomic in places. However, nanoparticles often have issues with homogenous dispersion, which means that the large surface area expected of them is not always achieved. The conditions of interface interaction between the matrix and fillers determine the free surface energy of the filler agent and the ratio between the filler and the matrix. These materials are divided into two groups high surface energy (metals, oxides, and other inorganic materials) or low surface energies (polymers, organic compounds) [44].
PVC polymers have acidic qualities and may interact with fillers with basic properties (SiO2, CaCO, mineral, etc.). If there is no special interaction at the phase boundary, the thermodynamic adhesion work is determined by the phase's thermodynamic cohesion work, which has lower cohesion energy. In this case, the adhesion can be improved by boosting the polymer cohesion strength. The surface is the simplest way to alter interface interactions. For this purpose, the surface should have interaction of fillers with each other and matrix-filler agent interaction. Agglomeration refers to the interaction of the filler agents with one another, and when the size shrinks, agglomeration becomes simpler [60]. PVC mineral matrix is supplied by interface development modifiers (coupling agents, surfactant, etc.) in composites, and these modifiers can be selected based on the composition of the components. Coating the surface of filler agents with a low molecular weight organic compound is the oldest and most used modification. Amphoteric surfactants are generally used with one or more polar groups and a long aliphatic chain. The surface treatment of CaCO3 with stearic acid is a typical example [74].
The principle of the process is the preferential adsorption of the polar group of the surfactant on the surface of the fillers. High-energy surfaces of inorganic fillers are usually able to interact mainly with the polar group of the surfactant. Reactive surface treatment assumes that the coupling agent undergoes a chemical reaction with both components. However, coupling agents are considerably more complex than non-reactive agents; the coupling agent polymerization makes the development of chemically bonded and physisorbed layers difficult to define surface chemistry, characterize the interlayer and optimize the operation. Mica, silicate, CaCO3, and Mg(OH)2 fillers are examples of fillers and reinforcements that contain reactive OH groups on their surface and have been effectively employed with silane coupling agents [39, 60]. Natural bentonite is considered a hydrophilic agent. Therefore, it is incompatible with organic compounds like polymers and plastics. It may be converted into organo clay and hydrophobic by employing quaternary ammonium, a nitrogen-containing surfactant [28]. Figure 8 shows the modified clay and its incorporation into the polymer.
Schematic diagram showing clay modification and intercalation of polymer to form polymer nanocomposites (Reprinted with permission from ref [48], Copyright 2015, Elsevier)
The surface of metal oxide NPs can be modified using physicochemical interactions between the NPs and the modifiers. The matrix can occur through a combination of different mechanisms in the material, such as the distribution of metal or metal oxides, complexation/chelation, electrostatic interactions, and precipitation or reduction reactions. The parameters that control the nature of the nanostructured metal oxide/polymer composite are (i) the nature of the functional polymer, (ii) the type of nanoparticle precursor, (iii) the reaction that forms nanoparticles, and (iv) the composition of the metal and metal oxide nanoparticles [81].
The modifiers have weak van der Waals forces or hydrogen bonds on the NP surface in the physical modification approach. Recently, surface modification of NPs such as TiO2, Fe2O3, Al2O3, and Al2O3-CuO has been done using surfactants. Chemical surface modification is an effective method to improve nanomaterial surface properties. This technique is based on the covalent bond between the modifier and the NP surface. Various coupling agents, Thiols, amines, organophosphorus molecules, carboxylic acids, polymers, and silanes have improved NP dispersion in various media [3]. The behavior of treated and untreated nanoparticles in the composite varies under operating conditions. When the nanocomposite is subjected to mechanical stresses, a stress concentration appears around it. Cavitation (voids) occurs at the polymer/nanoparticle interface in unmodified/uncoated particles. Surface coating of nanoparticles with terminal alkyl groups causes cavitation to occur at higher stresses due to the high interfacial adhesion achieved by increasing the adhesion work and the interfacial surface area [34].
Consequently, the interaction of polymeric molecules with a solid surface (including the polymeric one) should result in a redistribution of intermolecular bonds on the surface or in the transition layer and the formation of additional physical joints on the physical network [44].
References
Abdelrazek, E.M., Elashmawi, I.S., Hezma, A.M., et al.: Effect of an encapsulate carbon nanotubes (CNTs) on structural and electrical properties of PU/PVC nanocomposites. Physica B 502, 48–55 (2016). https://doi.org/10.1016/j.physb.2016.08.040
Abdrahman, M.F., Zainudin, E.S.: Properties of kenaf filled unplasticized polyvinyl chloride composites. Key Eng. Mater. 471–472, 507–512 (2011). https://doi.org/10.4028/www.scientific.net/KEM.471-472.507
Ahangaran, F., Navarchian, A.H.: Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Coll. Interface. Sci. 286, 102298 (2020). https://doi.org/10.1016/j.cis.2020.102298
Ahlatcioglu Ozerol, E., Bozlar, M., Bulent Ustundag, C., et al.: Latex-based carbon nanotube composites. In: Abraham, J., Thomas, S., Kalarikkal, N, (eds.) Handbook of carbon nanotubes. Springer International Publishing, Cham, pp 1–24 (2021). https://doi.org/10.1007/978-3-319-70614-6_9-1
Ahmad, N., Kausar, A., Muhammad, B.: Perspectives on polyvinyl chloride and carbon nanofiller composite: A review. Polym.-Plast. Technol. Eng. 55(10), 1076–1098 (2016). https://doi.org/10.1080/03602559.2016.1163587
Al-Hartomy, O.A., Al-Salamy, F., Al-Ghamdi, A.A., et al.: Influence of graphite nanosheets on the structure and properties of PVC-based nanocomposites. J. Appl. Polym. Sci. 120(6), 3628–3634 (2011). https://doi.org/10.1002/app.33547
Alther, G.R.: Removing oils from water with organoclays. J. Awwa 94(7), 115–121 (2002)
Bayer, J., Granda, L.A., A. JM, et al.: Cellulose polymer composites (WPC). In: Fan, M., Fu, F. (eds.) Advanced high strength natural fibre composites in construction, pp. 115–139. Woodhead Publishing (2017)
Bolotin, K.I., Sikes, K.J., Jiang, Z., et al.: Ultrahigh electron mobility in suspended graphene. Solid State Commun. 146(9–10), 351–355 (2008). https://doi.org/10.1016/j.ssc.2008.02.024
Brebu, M.: Environmental degradation of plastic composites with natural fillers—A review. Polymers 12(1), 166 (2020). https://doi.org/10.3390/polym12010166
Budash, Y., Novak, D., Plavan, V.: Structural and morphological characteristics of polyethylene composites with different conductive fillers. Materiale Plastice 53(4), 693–698 (2016)
Chan, J.X., Wong, J.F., Hassan, A., et al.: Mechanical properties of wollastonite reinforced thermoplastic composites: A review. Polym. Compos. 41(2), 395–429 (2020). https://doi.org/10.1002/pc.25403
Chand, N., Fahim, M., Sharma, P., et al.: Influence of foaming agent on wear and mechanical properties of surface modified rice husk filled polyvinylchloride. Wear 278–279, 83–86 (2012). https://doi.org/10.1016/j.wear.2012.01.002
Chauhan, V., Kärki, T., Varis, J.: Review of natural fiber-reinforced engineering plastic composites, their applications in the transportation sector and processing techniques. J. Thermoplast. Compos. Mater., 089270571988909. https://doi.org/10.1177/0892705719889095
Chirayil, C.J., Mathew, L., Thomas, S.: Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 37(1–2), 20–28 (2014)
Dantas de Oliveira, A., Augusto Gonçalves Beatrice, C.: Polymer nanocomposites with different types of nanofiller. In: Nanocomposites−Recent evolutions. IntechOpen. (2019). https://doi.org/10.5772/intechopen.81329
Darus SAAZBM, Shamsudin, E.B., Samsuri, M.B.: A short review on natural fiber reinforced polyvinyl chrolide and its application. In: 2019, pp. 82–88. E-Proceeding of Greentech‘19. (2019)
Della Volpe, C., Siboni, S.: From van der Waals equation to acid-base theory of surfaces: A chemical-mathematical journey. Rev. Adhes. Adhes. 10(1), 47–97 (2022). https://doi.org/10.47750/RAA/10.1.02
Deshmukh, K., Joshi, G.M.: Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym. Testing 34, 211–219 (2014). https://doi.org/10.1016/j.polymertesting.2014.01.015
Deshmukh, K., Khatake, S.M., Joshi, G.M.: Surface properties of graphene oxide reinforced polyvinyl chloride nanocomposites. J. Polym. Res. 20(11), 286 (2013). https://doi.org/10.1007/s10965-013-0286-2
Djidjelli, H., Boukerrou, A., Founas, R., et al.: Preparation and characterization of poly(vinyl chloride)/virgin and treated sisal fiber composites. J. Appl. Polym. Sci. 103(6), 3630–3636 (2007). https://doi.org/10.1002/app.25502
El Messiry, M., El Deeb, R.: Analysis of the wheat straw/flax fiber reinforced polymer hybrid composites. J. Appl. Mech. Eng. 05(06). https://doi.org/10.4172/2168-9873.1000240
Faiad, A., Alsmari, M., Ahmed, M.M.Z., et al.: Date palm tree waste recycling: Treatment and processing for potential engineering applications. Sustainability 14(3), 1134 (2022). https://doi.org/10.3390/su14031134
Faruk, O., Bledzki, A.K., Fink, H.-P., et al.: Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 37(11), 1552–1596 (2012). https://doi.org/10.1016/j.progpolymsci.2012.04.003
Faruk, O., Bledzki, A.K., Fink, H.-P., et al.: Progress report on natural fiber reinforced composites. Macromol. Mater. Eng. 299(1), 9–26 (2014). https://doi.org/10.1002/mame.201300008
Feldman, D.: Poly(vinyl chloride) Nanocomposites. J. Macromol. Sci., Part A 51(8), 659–667 (2014). https://doi.org/10.1080/10601325.2014.925265
Fu, S.-Y., Feng, X.-Q., Lauke, B., et al.: Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. B Eng. 39(6), 933–961 (2008). https://doi.org/10.1016/j.compositesb.2008.01.002
Fuentes, C.A., Guo, H., Zhang, Y. et al.: Wettability and interphase adhesion of molten thermoplastics on glass fibres. In: International glass fiber symposium, 2016. (2016)
Gacitua, W., Ballerini, A., Zhang, J.: Polymer nanocomposites: Synthetic and natural fillers a review. Maderas. Cienc. Y Tecnol. 7(3). https://doi.org/10.4067/S0718-221X2005000300002
Guchait, A., Saxena, A., Chattopadhyay, S., et al.: Influence of nanofillers on adhesion properties of polymeric composites. ACS Omega 7(5), 3844–3859 (2022). https://doi.org/10.1021/acsomega.1c05448
Guler, S.H., Guler, O., Dikici, B.: Structure–property relationships in polymer nanocomposites. In: Handbook of carbon nanotubes. Springer International Publishing, Cham, pp 1–27. https://doi.org/10.1007/978-3-319-70614-6_1-1
Gupta, M.C., Viswanath, S.G.: Role of metal oxides in the thermal degradation of poly(vinyl chloride). Ind. Eng. Chem. Res. 37(7), 2707–2712 (1998). https://doi.org/10.1021/ie9700167
Hajar, M.D.S., Supri, A.G., Hanif, M.P.M., et al.: Effect of graphite loading on the electrical and mechanical properties of Poly (Ethylene Oxide)/Poly (Vinyl Chloride) polymer films. J. Phys. Conf. Ser. 908, 012020 (2017). https://doi.org/10.1088/1742-6596/908/1/012020
Hashim, F.S.: Enhancement of mechanical properties for reinforced Iraqi bentonite clay polyvinyl chloride composite using ultrasonic technique. 2(6), 24–32 (2012)
Hounsham, I.D., Titow, W.V.: Fillers in PVC. PVC Technol., 215–254 (1984). https://doi.org/10.1007/978-94-009-5614-8-8
Hsissou, R., Seghiri, R., Benzekri, Z., et al.: Polymer composite materials: A comprehensive review. Compos. Struct. 262, 113640 (2021). https://doi.org/10.1016/j.compstruct.2021.113640
Hu, J., Jia, X., Li, C., et al.: Effect of interfacial interaction between graphene oxide derivatives and poly(vinyl chloride) upon the mechanical properties of their nanocomposites. J. Mater. Sci. 49(7), 2943–2951 (2014). https://doi.org/10.1007/s10853-013-8006-1
Huang, S., Fu, Q., Yan, L., et al.: Characterization of interfacial properties between fibre and polymer matrix in composite materials – A critical review. J. Market. Res. 13, 1441–1484 (2021). https://doi.org/10.1016/j.jmrt.2021.05.076
Huang, Z., Wang, N., Zhang, Y., et al.: Effect of mechanical activation pretreatment on the properties of sugarcane bagasse/poly(vinyl chloride) composites. Compos. A Appl. Sci. Manuf. 43(1), 114–120 (2012). https://doi.org/10.1016/j.compositesa.2011.09.025
Jancar, J., Fekete, E., Hornsby, P.R. et al. (eds).: Mineral fillers in thermoplastics I. Advances in polymer science. Springer Berlin Heidelberg, Berlin, Heidelberg. https://doi.org/10.1007/3-540-69220-7
Jawaid, M., Abdul Khalil, H.P.S.: Cellulosic/synthetic fibre reinforced polymer hybrid composites: A review. Carbohyd. Polym. 86(1), 1–18 (2011). https://doi.org/10.1016/j.carbpol.2011.04.043
Kabir, M.M., Wang, H., Lau, K.T., et al.: Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos. B Eng. 43(7), 2883–2892 (2012). https://doi.org/10.1016/j.compositesb.2012.04.053
Kallakas, H., Shamim, M.A., Olutubo, T., et al.: Effect of chemical modification of wood flour on the mechanical properties of wood-plastic composites. Agron. Res. 13(3), 639–653 (2015)
Karakus, S., Budinski-Simendic, J., Korugic-Karasz, L., et al.: Characterization of poly(vinyl chloride)/bentonite nanocomposite prepared via melt blending method: Nanocomposites via melt blending. ACS Symp. Ser. 1061, 103–113 (2010). https://doi.org/10.1021/bk-2010-1061.ch008
Karger-Kocsis, J.: Polymer reinforcement. (1996). https://doi.org/10.1016/1359-835x(96)90021-4
Khaleghi, M., Didehban, K., Shabanian, M.: Effect of new melamine-terephthaldehyde resin modified graphene oxide on thermal and mechanical properties of PVC. Polym. Testing 63, 382–391 (2017). https://doi.org/10.1016/j.polymertesting.2017.08.018
Khalil, H.A., Tehrani, M., Davoudpour, Y., et al.: Natural fiber reinforced poly(vinyl chloride) composites: A review. J. Reinf. Plast. Compos. 32(5), 330–356 (2013). https://doi.org/10.1177/0731684412458553
Khan, R.A., Khan, M.A., Zaman, H.U., et al.: Fabrication and characterization of jute fabric-reinforced PVC-based composite. J. Thermoplast. Compos. Mater. 25(1), 45–58 (2012). https://doi.org/10.1177/0892705711404726
Kotal, M., Bhowmick, A.K.: Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 51, 127–187 (2015). https://doi.org/10.1016/j.progpolymsci.2015.10.001
Krishnan, P.: Evaluation and methods of interfacial properties in fiber-reinforced composites. In: Mechanical and physical testing of biocomposites, fibre-reinforced composites and hybrid composites. Elsevier, pp. 343–385. https://doi.org/10.1016/B978-0-08-102292-4.00018-7
Kuan, H.T.N., Tan, M.Y., Shen, Y., et al.: Mechanical properties of particulate organic natural filler-reinforced polymer composite: A review. Compos. Adv. Mater. 30, 263498332110075 (2021). https://doi.org/10.1177/26349833211007502
Lee, C., Wei, X., Kysar, J.W., et al.: Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887), 385–388 (2008). https://doi.org/10.1126/science.1157996
Lee, C.H., Khalina, A., Lee, S.H.: Importance of interfacial adhesion condition on characterization of plant-fiber-reinforced polymer composites: A review. Polymers 13(3), 438 (2021). https://doi.org/10.3390/polym13030438
Lee, K.W., Chung, J.W., Kwak, S.-Y.: Flexible Poly(vinyl chloride) nanocomposites reinforced with hyperbranched polyglycerol-functionalized graphene oxide for enhanced gas barrier performance. ACS Appl. Mater. Interfaces 9(38), 33149–33158 (2017). https://doi.org/10.1021/acsami.7b10257
Li, H., Huneault, M.A.: Crystallization of PLA/Thermoplastic starch blends. Int. Polym. Proc. 23(5), 412–418 (2008). https://doi.org/10.3139/217.2185
Li, P., Chen, X., Zeng, J.-B., et al.: Enhancement of the interfacial interaction between poly(vinyl chloride) and zinc oxide modified reduced graphene oxide. RSC Adv. 6(7), 5784–5791 (2016). https://doi.org/10.1039/C5RA20893A
Marquis, D.M., Guillaume, E., Chivas-Joly, C.: Properties of nanofillers in polymer. In: Nanocomposites and polymers with analytical methods. InTech. https://doi.org/10.5772/21694
Mallakpour, S., Rashidimoghadam, S.: Investigation on morphology, properties, and applications of hybrid poly(vinyl chloride)/metal oxide composites. In: Hybrid polymer composite materials. Elsevier, pp. 343–377. https://doi.org/10.1016/B978-0-08-100787-7.00014-7
Móczó, J., Pukánszky, B.: Particulate fillers in thermoplastics. In: Polymers and polymeric composites: A reference series. Springer Berlin Heidelberg, pp. 1–43. https://doi.org/10.1007/978-3-642-37179-0_7-2
Mohammed, L., Ansari, M.N.M., Pua, G., et al.: A Review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 1–15 (2015). https://doi.org/10.1155/2015/243947
Monthioux, M., Serp, P., Flahaut, E, et al.: Introduction to Carbon Nanotubes. In: Springer Handbook of Nanotechnology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 43–112. https://doi.org/10.1007/978-3-540-29857-1_3
Nawaz, K., Ayub, M., Ul-Haq, N., et al.: The effect of graphene nanosheets on the mechanical properties of polyvinylchloride. Polym. Compos. 37(5), 1572–1576 (2016). https://doi.org/10.1002/pc.23328
Novak, D., Budash, Y., Bereznenko, N.: Graphic modeling of conductive filler spatial distribution in polymer matrix. Vlakna a Textil 23(2), 37–42 (2016)
Novak, D., Bereznenko, N., Smernytskyi, D., et al.: Electrical, rheological and mechanical properties of polyvinyl chloride/copper plated graphite composites. Vlakna a Textil 26(2), 43–47 (2019)
Nurazzi, N.M., Asyraf, M.R.M., Rayung, M., et al.: Thermogravimetric analysis properties of cellulosic natural fiber polymer composites: A review on influence of chemical treatments. Polymers 13(16), 2710 (2021). https://doi.org/10.3390/polym13162710
Paradise, M., Goswami, T.: Carbon nanotubes–Production and industrial applications. Mater. Des. 28(5), 1477–1489 (2007). https://doi.org/10.1016/j.matdes.2006.03.008
Pekturk, H.Y., Elitas, M., Goktas, M., et al.: Evaluation of the effect of MWCNT amount and dispersion on bending fatigue properties of non-crimp CFRP composites. Eng. Sci. Technol., Int. J. 34, 101081 (2022). https://doi.org/10.1016/j.jestch.2021.101081
Pereira, P.H.F., de Rosa, M.F., Cioffi, M.O.H, et al.: Vegetal fibers in polymeric composites: a review. Polímeros 25(1), 9–22 (2015). https://doi.org/10.1590/0104-1428.1722
Phiri, J., Gane, P., Maloney, T.C.: General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 215, 9–28 (2017). https://doi.org/10.1016/j.mseb.2016.10.004
Pleşa, I., Noţingher, P., Schlögl, S., et al.: Properties of polymer composites used in high-voltage applications. Polymers 8(5), 173 (2016). https://doi.org/10.3390/polym8050173
Qian, S., Wang, H., Zarei, E., et al.: Effect of hydrothermal pretreatment on the properties of moso bamboo particles reinforced polyvinyl chloride composites. Compos. B Eng. 82, 23–29 (2015). https://doi.org/10.1016/j.compositesb.2015.08.007
Ramanathan, T., Bismarck, A., Schulz, E., et al.: Investigation of the influence of acidic and basic surface groups on carbon fibres on the interfacial shear strength in an epoxy matrix by means of single-fibre pull-out test. Compos. Sci. Technol. 61(4), 599–605 (2001). https://doi.org/10.1016/S0266-3538(00)00239-6
Rong, M.Z., Zhang, M.Q., Ruan, W.H.: Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: a review. Mater. Sci. Technol. 22(7), 787–796 (2006). https://doi.org/10.1179/174328406X101247
Roul C (2009) The international jute commodity system. Northern Book Centre, New Delhi
Saad, A.L.G., Sayed, W.M., Ahmed, M.G.M., et al.: Preparation and properties of some filled poly(vinyl chloride) compositions. J. Appl. Polym. Sci. 73(13), 2657–2670 (1999). https://doi.org/10.1002/(SICI)1097-4628(19990923)73:13%3c2657::AID-APP14%3e3.0.CO;2-7
Sachdev, V.K., Srivastava, N.K., Panwar, V, et al.: Electrical conductivity of prelocalized graphite-filled blend of polyvinyl chloride/styrene acrylonitrile composites in relationship to morphology and hardness. Phys Status Solidi (A) 203(15), 3754–3761. https://doi.org/10.1002/pssa.200521424
Safarpour, M., Safikhani, A., Vatanpour, V.: Polyvinyl chloride-based membranes: A review on fabrication techniques, applications and future perspectives. Sep. Purif. Technol. 279, 119678 (2021). https://doi.org/10.1016/j.seppur.2021.119678
Salavagione, H.J., Martínez, G., Marco, C.: A polymer/solvent synergetic effect to improve the solubility of modified multi-walled carbon nanotubes. J. Mater. Chem. 22(14), 7020 (2012). https://doi.org/10.1039/c2jm16113c
Samajpati, S., Sengupta, S.: Wetting characteristics of long vegetable fibres. Indian J. Fibre Text. Res. 31(2), 262–266 (2006)
Sarkar, S., Guibal, E., Quignard, F., et al.: Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. J. Nanopart. Res. 14(2), 715 (2012). https://doi.org/10.1007/s11051-011-0715-2
Shah, B.L., Matuana, L.M., Heiden, P.A.: Novel coupling agents for PVC/wood-flour composites. J. Vinyl Add. Tech. 11(4), 160–165 (2005). https://doi.org/10.1002/vnl.20056
Shah, R., Kausar, A., Muhammad, B., et al.: Progression from graphene and graphene oxide to high performance Polymer-based nanocomposite: A review. Polym.-Plast. Technol. Eng. 54(2), 173–183 (2015). https://doi.org/10.1080/03602559.2014.955202
Sinha, E., Panigrahi, S.: Effect of plasma treatment on structure, wettability of jute fiber and flexural strength of its composite. J. Compos. Mater. 43(17), 1791–1802 (2009). https://doi.org/10.1177/0021998309338078
Siti Hajar, M.D., Teh, P.L., Kahar, A.W.M., et al.: Effect of Graphite Loading and Addition of Ferric Chloride (FeCl3) on the Properties of Poly (Vinyl Chloride)/Poly (Ethylene Oxide)/Graphite Polymer Films. IOP Conference Series: Materials Science and Engineering 429, 012036 (2018). https://doi.org/10.1088/1757-899X/429/1/012036
Sun, D.: Surface modification of natural fibers using plasma treatment. In: Biodegradable green composites. Hoboken, NJ: John Wiley & Sons, Inc, pp. 18–39. https://doi.org/10.1002/9781118911068.ch2
Sun, S., Li, C., Zhang, L., Du, H.L., et al.: Effects of surface modification of fumed silica on interfacial structures and mechanical properties of poly(vinyl chloride) composites. Eur. Polymer J. 42(7), 1643–1652 (2006). https://doi.org/10.1016/j.eurpolymj.2006.01.012
Sun, S., Li, C., Zhang, L., Du, H., et al.: Interfacial structures and mechanical properties of PVC composites reinforced by CaCO3 with different particle sizes and surface treatments. Polym. Int. 55(2), 158–164 (2006). https://doi.org/10.1002/pi.1932
Tan, B., Thomas, N.L.: A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 514, 595–612 (2016). https://doi.org/10.1016/j.memsci.2016.05.026
Tanpichai, S., Witayakran, S.: All-cellulose composites from pineapple leaf microfibers: Structural, thermal, and mechanical properties. Polym. Compos. 39(3), 895–903 (2018). https://doi.org/10.1002/pc.24015
Teklal, F., Djebbar, A., Allaoui, S., et al.: A review of analytical models to describe pull-out behavior–Fiber/matrix adhesion. Compos. Struct. (2018). https://doi.org/10.1016/j.compstruct.2018.06.091
Thyavihalli Girijappa, Y.G., Mavinkere Rangappa, S., Parameswaranpillai, J., et al.: Natural fibers as sustainable and renewable resource for development of Eco-friendly composites: A comprehensive review. Front. Mater. 6. https://doi.org/10.3389/fmats.2019.00226
Ticoalu, A., Aravinthan, T., Cardona, F.: A review of current development in natural fiber composites for structural and infrastructure applications. In: Southern Region Engineering Conference 2010, SREC 2010—Incorporating the 17th annual international conference on mechatronics and machine vision in practice, M2VIP 2010 (November), pp. 113–117
Tuen, B.S., Hassan, A., Abu Bakar, A.: Mechanical properties of talc-and (calcium carbonate)-filled poly(vinyl chloride) hybrid composites. J. Vinyl Add. Tech. 18(2), 76–86 (2012). https://doi.org/10.1002/vnl.20280
Vaisman, L., Wagner, H.D., Marom, G.: The role of surfactants in dispersion of carbon nanotubes. Adv. Coll. Interface. Sci. 128–130, 37–46 (2006). https://doi.org/10.1016/j.cis.2006.11.007
Vera, M., Mella, C., Urbano, B.F.: Smart polymer nanocomposites: Recent advances and perspectives. J. Chil. Chem. Soc. 65(4), 4973–4981 (2020). https://doi.org/10.4067/S0717-97072020000404973
Wang, H., Xie, G., Zhu, Z., et al.: Enhanced tribological performance of the multi-layer graphene filled poly(vinyl chloride) composites. Compos. A Appl. Sci. Manuf. 67, 268–273 (2014). https://doi.org/10.1016/j.compositesa.2014.09.011
Wang, H., Xie, G., Ying, Z., et al.: Enhanced mechanical properties of multi-layer graphene filled Poly(vinyl chloride) composite films. J. Mater. Sci. Technol. 31(4), 340–344 (2015). https://doi.org/10.1016/j.jmst.2014.09.009
Wang, M., Zhao, Q.: Biomedical composites. In: Encyclopedia of biomedical engineering. Elsevier, pp. 34–52 (2019). https://doi.org/10.1016/B978-0-12-801238-3.99868-4
Wilczewski, S., Skórczewska, K., Tomaszewska, J., Lewandowski, K., et al.: Manufacturing homogenous PVC/graphene nanocomposites using a novel dispersion agent. Polym. Testing 91, 106868 (2020). https://doi.org/10.1016/j.polymertesting.2020.106868
Vasanthkumar, M.S., Bhatia, R., Arya, V.P. et al.: Characterization, charge transport and magnetic properties of multi-walled carbon nanotube–polyvinyl chloride nanocomposites. Phys. E: Low-Dimens. Syst. Nanostructures 56, 10–16 (2014). https://doi.org/10.1016/j.physe.2013.08.010
Wilczewski, S., Skórczewska, K., Tomaszewska, J., Lewandowski, K.: Structure and properties of poly(vinyl chloride)/graphene nanocomposites. Polym. Testing 81, 106282 (2020). https://doi.org/10.1016/j.polymertesting.2019.106282
Yadav, M., Ahmad, S., Chiu, F.-C.: Graphene oxide dispersed polyvinyl chloride/alkyd green nanocomposite film: Processing and physico-mechanical properties. J. Ind. Eng. Chem. 68, 246–256 (2018). https://doi.org/10.1016/j.jiec.2018.07.051
Yalcin, B., Cakmak, M.: The role of plasticizer on the exfoliation and dispersion and fracture behavior of clay particles in PVC matrix: a comprehensive morphological study. Polymer 45(19), 6623–6638 (2004). https://doi.org/10.1016/j.polymer.2004.06.061
Yue, X., Chen, F., Zhou, X.: Improved interfacial bonding of PVC/wood-flour composites by lignin amine modification. BioResources 6(2), 2022–2034 (2011). https://doi.org/10.15376/biores.6.2.2022-2034
Zanjanijam, A.R., Bahrami, M., Hajian, M.: Poly(vinyl chloride)/single wall carbon nanotubes composites: Investigation of mechanical and thermal characteristics. J. Vinyl Add. Tech. 22(2), 128–133 (2016). https://doi.org/10.1002/vnl.21413
Zhu, Y., Murali, S., Cai, W., et al.: Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 22(35), 3906–3924 (2010). https://doi.org/10.1002/adma.201001068
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guler, S.H., Simsek, T., Guler, O., Dikici, B. (2024). Possible Interaction of PVC with Micro-and Nano-fillers. In: H, A., Sabu, T. (eds) Poly(Vinyl Chloride) Based Composites and Nanocomposites. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-45375-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-45375-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-45374-8
Online ISBN: 978-3-031-45375-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)