Abstract
In this chapter, an experimental investigation is carried out to study the characteristics of the pressure-composition isotherms (P-C-T) of the LaNi4Mn0.5Co0.5 alloy. These isotherms provided important information about the phase transitions, thermodynamic properties and stability of the alloy under different conditions. The effects of feed pressure and cooling, as well as heating temperature on the rate of hydrogen uptake/desorption by the alloy were analyzed. The effects of the partial substitution of Ni by the elements Mn and Co on the phase structure and hydrogen storage properties of LaNi4Mn0.5Co0.5 alloy are studied. It is known that the Mn, Co elements decrease the plateau pressure of hydrogen absorption and desorption of LaNi4Mn0.5Co0.5 alloy. For that, the substitution of these elements improve kinetic velocity of hydrogen storage capacity. The LaNi4Mn0.5Co0.5 alloy also shows excellent plateau performance with very small hysteresis and sloping which is attributed to the interstitial size effect.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The world is currently experiencing an increase in demand for energy due to population growth, urbanization and industrialization. Fossil fuel consumption has been linked to a range of environmental issues, including air and water pollution, deforestation, and climate change. Burning fossil fuels like coal, oil and gas releases carbon dioxide and other greenhouse gases into the atmosphere, which trap heat and contribute to global warming [1, 2]. In this case, the transition to non-carbon and renewable energy sources is essential for reducing our impact on the environment and ensuring a sustainable energy future. Renewable energy sources include solar, wind, hydropower, geothermal and biomass are replenished naturally and don’t produce harmful emissions like fossil fuels do. These sources of energy have the potential to provide a significant amount of the world’s energy needs while reducing greenhouse gas emissions and environmental impacts [3, 4]. Hydrogen is an energy carrier that can be produced from various renewable sources, including wind, solar, and hydroelectric power. Furthermore, hydrogen can be stored and transported easily and can be used to power a range of applications, including transportation, electricity generation, and industrial processes. As a result, hydrogen has the potential to play a significant role in a sustainable and low-carbon energy system. However, it is worth noting that the production and use of hydrogen currently face some challenges, such as high production costs and the need for specialized infrastructure. Despite these challenges, there is still significant interest in hydrogen as a clean and versatile fuel. Research and development efforts are ongoing to improve the efficiency and cost-effectiveness of hydrogen production methods, as well as to develop the necessary infrastructure to support its use [5, 6].
Hydrogen storage is an important aspect of using hydrogen as an energy source. It has a very low volumetric energy density, which means that it requires a large volume of space to store. There are several methods for storing hydrogen, including compressed gas storage, liquid hydrogen storage, and solid-state hydrogen storage. Compressed gas storage involves storing hydrogen under high pressure, typically in tanks made of lightweight, high-strength materials like carbon fiber. Liquid hydrogen storage involves cooling hydrogen to extremely low temperatures, at which point it becomes a liquid that can be stored in specialized tanks. Solid-state hydrogen storage involves storing hydrogen in materials that can absorb and release it, such as metal hydrides, chemical hydrides, and carbon-based materials [6,7,8].
Compared to other materials, the storage of hydrogen by the LaNi5 alloys is considered one of the most promising because of their large volumetric absorption capacity of hydrogen, better cycling performance and absorption-desorption kinetics at low pressure and temperature. On the other hand, these types of metals have a low thermal conductivity which defines one of the major obstacles to hydrogen storage [9,10,11]. To address this challenge, researchers are exploring various approaches to improve the thermal conductivity of metal hydrides, such as adding high-conductivity materials like carbon nanotubes or graphene to the material. Another approach that has been explored is the use of heat exchangers to remove excess (provided) heat generated (needed) during hydrogen absorption (desorption).
Researchers have explored the use of LaNi5-based alloys in which the nickel element is replaced with other elements, such as iron (Fe), cobalt (Co), aluminum (Al), manganese (Mn), and others. These alloys have improved thermal conductivity and mechanical properties, which can enhance the performance and durability of the material during hydrogen absorption and desorption. Additionally, the substitution of different elements into the LaNi5 compound can change the crystal structure and other properties, which can affect its performance and stability [12,13,14]. However, further research is needed to optimize the properties of these alloys and to understand their behavior under different conditions, such as temperature and pressure, in order to fully evaluate their potential for hydrogen storage.
The LaNi4Mn0.5Co0.5 alloy is a modified version of LaNi5, where the nickel atoms are replaced with manganese and cobalt atoms. The substitution of cobalt for nickel enhances the alloy’s mechanical properties, making it more resistant to fracture and deformation during hydrogen absorption and desorption. Meanwhile, the addition of manganese improves the alloy's hydrogen storage capacity and kinetics, as well as its resistance to oxidation and other forms of degradation [15]. This work describes the study on hydrogen storage properties of a LaNi4Mn0.5Co0.5 alloy. In this study we have investigated the amount of hydrogen that can be absorbed and released by the alloy, as well as its kinetics and stability under different conditions. Additionally, we have looked at the effect of nickel substitution on the isotherm properties of the alloy.
2 Experimental Procedures
The preparation of the LaNi4Mn0.5Co0.5 sample was done through the melting and casting processes. In this process, the elements of the alloy are first mixed together in a ball mill to form a homogeneous powder. Then the powder is put under high pressure and high temperature to form a dense and solid material. This solid alloy is crushed with the help of a very fine-grain powder ball crusher. The obtained sample was characterized by XRD techniques, then it was tested to determine its hydrogen storage properties, such as its hydrogen storage capacity and kinetics. These properties are essential for evaluating the suitability of the material for use in hydrogen storage applications.
The metal powder sample was prepared from primary metals (La, Ni, Mn, and Co, with 99.97% purity) in an argon atmosphere. Before studying the LaNi4Mn0.5Co0.5 alloy, it was ground to very fine powder grains using a mechanical grinder.
Activation of the sample was carried out by several cycles of absorption/desorption of pure hydrogen (99.99%) at a Temperature Tfluid = 298 K and under a pressure of 15 bar for the absorption process and Tfluid = 333 K and under a pressure of 2 mbar for the desorption process (Fig. 1). Once the maximum amount of hydrogen readout of saturation achieved, then the sample is activated [13, 14, 17].
After activation, measurements of pressure-composition-temperature (P-C-T) isotherms were made at a temperature range of T = 298 K to T = 333 K.
3 Results and Discussion
3.1 Characterization Techniques
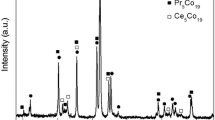
We first tried to see the morphology of the developed sample to explain the substitution effect of the atom Ni by the elements Co and Mn on the hydrogenation kinetics and the stability of the formed hydride.
The morphological structure of the LaNi4Mn0.5Co0.5 compound was determined by an X-ray diffraction analysis. Figure 2 represents the XRD spectra of the alloy before hydrogenation. The diffract grams show that the structural phases of the alloy network are of hexagonal CaCu5 type, corresponding to the compound LaNi5.
The structure of the LaNi4Mn0.5Co0.5 alloy is hexagonal with a P6/mmm space group and lattice parameters of a = b = 5.005 Å and c = 8.141 Å. These lattice parameters are slightly different from those of pure LaNi5, which has lattice parameters of a = b = 4.992 Å and c = 8.127 Å [13]. Therefore, the substitution of nickel with manganese and cobalt in the LaNi4Mn0.5Co0.5 structure can lead to changes in lattice parameters and crystal structure compared to pure LaNi5. According to this result, the new sample can have better reaction kinetics and the mid-plateau equilibrium pressure would be lower. Hence, we proposed to study experimentally reaction kinetics and stability to confirm this result.
3.2 Hydrogen Storage Property
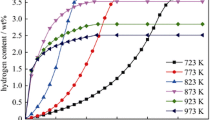
Figure 3 shows the effect of the temperature on the amount of hydrogen absorbed and desorbed by the LaNi4Mn0.5Co0.5 alloy. It is expected that at low temperatures, the amount of hydrogen desorbed by the LaNi4Mn0.5Co0.5 alloy is generally lower than at higher temperatures and vice versa. This is due to the fact that the diffusion of hydrogen in the lattice of the alloy is limited by the low mobility of hydrogen atoms at low temperatures. However, the amount of hydrogen absorbed by the alloy at low temperatures can still be significant, depending on the hydrogen pressure and the microstructure of the alloy.
At temperatures T = 283 K and 308 K, the LaNi4Mn0.5Co0.5 alloy exhibits maximum hydrogen storage capacity, with a balance between strong hydrogen absorption and reasonable desorption kinetics, respectively. At these temperatures, the diffusion of hydrogen into the alloy lattice is faster and the activation energy for hydrogen desorption is lower, allowing an optimal balance between the storage capacity of hydrogen and kinetics. The higher the temperatures, greater is the amount of hydrogen desorbed by the LaNi4Mn0.5Co0.5 alloy than at lower temperatures, due to the mobility of the hydrogen atoms. However, the LaNi4Mn0.5Co0.5 alloy can also experience significant degradation and reduction in its hydrogen storage capacity at these temperatures, due to the formation of unwanted phases or structural changes.
The substitution of nickel for manganese and cobalt has an effect on the properties of metal hydride materials, including the kinetics of hydrogen absorption. These elements lead to changes in the crystalline structure of the material, changing the size and distribution of the pores, as well as the interactions between hydrogen atoms and the crystalline network.
The substitution of nickel (Ni) for manganese (Mn) and cobalt (Co) in the LaNi5 alloy led to an improvement in the kinetics of hydrogen absorption at the same operating temperature and pressure (Fig. 4). It is important to note that the specific effects of Ni substitution by Mn and Co on the kinetics of hydrogen absorption can vary depending on the composition and microstructure of the alloy.
To study the effect of the substitution of Nickel by the elements Mn and Co on the properties of the absorption/desorption kinetics of hydrogen, the pressure-composition isotherms (P–C–T) of LaNi4Mn0.5Co0.5 were measured at different temperatures. Figure 5a and b shows these curves P-C-T at T = 298 K, T = 303 K, and T = 313 K. It is clear that the increase in temperature affects the pressure of absorbed or desorbed hydrogen at equilibrium.
The mid-plateau equilibrium pressures show a low inclination in the P-C-T curves in Fig. 5. The rate of increase in absorption plate pressure and desorption depends on the increase in temperature. In addition, the hydrogen storage capacity of the LaNi4Mn0.5Co0.5 alloy decreases with increasing temperature.
Figure 6 shows the effect of the substitution of Ni by the elements Mn and Co on the balance pressure in the middle of the tray. It can be seen that the pressure of the plate between hydrogen absorption and desorption decreases in comparison with the isotherms of the LaNi5 alloy. By referring to the cell volumes found in the RDX analyses (see Fig. 2), it is possible to see that a large volume of cells corresponds to a low-pressure plate. It is believed that cell volume expansion leads to a decrease in hydride plateau pressure.
The evolution of pressure in the middle of the plateau followed the law of Van’t Hoff. The application of the Van’t Hoff equation (1) makes it possible to calculate the values of enthalpy “ΔH” and entropy “ΔS” of the reaction in the processes of H2 absorption-desorption by the LaNi4Mn0.5Co0.5 alloy.
where:
-
T (K): experimental temperature
-
Peq (bar): equilibrium pressure
-
R = 8.314 Jmol−1K−1: universal gas constant
-
ΔS (Jmol−1): reaction entropy
-
ΔH (Jmol−1): reaction enthalpy.
Figure 7 represents the variation of absorption/desorption plateau pressure by the LaNi4Mn0.5Co0.5 alloy. This curve shows the evolution of plateau pressure (Peq) as a function of the inverse temperature. Values of ΔH and ΔS are determined from Fig. 7. The values of ΔH and ΔS are: ΔH = 22225 ± 5 Jmol−1 and ΔS = 71 ± 1 Jmol−1. The value of the hybrid formation enthalpy of the alloy is lower than the value of the formation enthalpy of the LaNi5 alloy (ΔHLaNi5 = 30.1 KJmol−1) [13, 16]. This is explained by the effect of the substitution of the Nickel Ni element by the Mn and Co elements on the improvement of the pressure of the hydrogen absorption-desorption plate and the reduction of the hysteresis of the hydrogen absorption-desorption plate.
According to the Van’t Hoff equation, decreasing plateau pressure for absorption/desorption processes means rapid hydrogen recovery.
4 Conclusion
A morphological study of the LaNi4Mn0.5Co0.5 samples was carried out by X-ray diffraction. The behavior of hydrogen absorption and desorption isotherms for the LaNi4Mn0.5Co0.5 powder sample was investigated experimentally.
The values of enthalpy (ΔH) and entropy (ΔS) of the hydride have been determined experimentally by means of the Van’t Hoff equation.
The experimental results suggest that the partial substitution of Ni of Mn and Co elements has an important effect on the properties of hydrogen absorption/desorption kinetics by the LaNi4Mn0.5Co0.5 alloy.
References
Johnston, B., Michael, C., Mayo Anshuman, K.: Hydrogen: the energy source for the 21st century. Technovation 25(6), 569–585 (2005)
Dantzer, P.: Metal-hydride technology: a critical review. In: Wipf, H. (ed.) Hydrogen in Metals III. Properties and Applications. Top. Appl. Phys. 73, 279–340 (1997)
Andreas, Z.: Materials for hydrogen storage. Mater. Today 6(9), 24–33 (2003)
Volodymyr, A.Y., Mykhaylo, V.L.: Laves type intermetallic compounds as hydrogen storage materials: a review. J. Alloy. Compd. 916, 165219 (2022)
Yongyan, X., Yuan, D., Wei, L., Xin, Z., Jin, X., Zeming, Y.: Research progress of hydrogen energy and metal hydrogen storage materials. Sustain. Energy Technol. Assess. 55, 102974 (2023)
Dantzer, P.: Static, dynamic and cycling studies on hydrogen in the intermetallics LaNi5 and LaNi4.77Al0.22. J. Less Common Metals 131(1–2), 349–363 (1987)
Sulaiman, N.N., Ismail, M.: Effects of TiF3 addition on the hydrogen storage properties of 4MgH2 + Cd composite. Int. J. Hydrogen Energy 44(58), 30574–30582 (2019)
Martínez-Amariz, A., Bellon, D.: Effect of adding ZrM (M=Fe, Ni) intermetallic compounds on the hydrogen absorption/desorption properties of TiCr1.1V0.9 alloy. Heliyon 8(3), 09042 (2022)
Zhu, Z., et al.: Stability of LaNi5−xCox alloys cycled in hydrogen—Part 1 evolution in gaseous hydrogen storage performance. Int. J. Hydrogen Energy 44(29), 15159–15172 (2019)
Liu, J., et al.: New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5−xAlx alloys. Int. J. Hydrogen Energy 42(39), 24904–24914 (2017)
Singh, B.K., Cho, S.W., Bartwal, K.S.: Effect on structure and hydrogen storage characteristics of composite alloys Ti0.32Cr0.43V0.25 with LaNi5 and rare-earth elements La, Ce, Y. J. Alloys Compd. 478(1–2), 785–788 (2009)
Bajahzar, A., Bouzid, M., Briki, C., Nasri, F., Belmabrouk, H., Jemni, A.: Experimental and numerical study of the isotherms and determination of physicochemical parameters of the hydrogen absorption/desorption process by the metal hydrides. Int. J. Hydrogen Energy 45(30), 15281–15293 (2020)
Briki, C., Bouzid, M., Dhaou, M.H., Jemni, A., Lamine, A.B.: Experimental and theoretical study of hydrogen absorption by LaNi3.6Mn0.3Al0.4Co0.7 alloy using statistical physics modeling. Int. J. Hydrogen Energy 43(20), 9722–9732 (2018)
Yang, H., Chen, Y., Tao, M., Wu, C.: Microstructure and electrochemical properties of LaNi4–xFeMnx (x = 0–0.8) hydrogen storage alloys. J. Rare Earths 27(5), 853–857 (2009)
Liu, J., Cheng, H., Han, S., Liu, H., Huot, J.: Hydrogen storage properties and cycling degradation of single-phase La0.60R0.15Mg0.25Ni3.45 alloys with A2B7-type superlattice structure. Energy 192, 116617 (2020)
Kuang, G., Li, Y., Ren, F., Hu, M., Lei, L.: The effect of surface modification of LaNi5 hydrogen storage alloy with CuCl on its electrochemical performances. J. Alloy. Compd. 605, 51–55 (2014)
Wjihi, S., Briki, C., Sellaoui, L., Jemni, A., Ben Lamine, A.: Theoretical study of hydrogen desorption on Mg50Ni50 using statistical physics treatment. Int. J. Hydrogen Energy 42(13), 8733–8743 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Briki, C., Belkhiria, S., Jemni, A. (2024). Experimental Study of Hydrogen Storage at Low-Pressure and Low Temperatures Using Metal Hydrides. In: Ali-Toudert, F., Draoui, A., Halouani, K., Hasnaoui, M., Jemni, A., Tadrist, L. (eds) Advances in Thermal Science and Energy. JITH 2022. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-43934-6_31
Download citation
DOI: https://doi.org/10.1007/978-3-031-43934-6_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43933-9
Online ISBN: 978-3-031-43934-6
eBook Packages: EngineeringEngineering (R0)