Abstract
ZnO-Alg/CS composites doped with Ca2+, Zn2+, Cu2+, and Ag+ ions were synthesized for potential application in the treatment of damaged areas of the skin. Polyelectrolyte binding of oppositely charged polymer chains of Alginate (Alg) and Chitosan (CS), as well as additional “cross-linking” with metal ions, ensure the composite’s stability in a physiological environment and provide antimicrobial properties. Loading of composites with metal ions provides enhanced antimicrobial properties and increases the degree of porosity, which positively affects the ability of materials to absorb liquid and exudate and accelerate wound healing. It has been experimentally proven that the Gram-negative E. coli ATCC 25922 is more sensitive to the action of composites than the Gram-positive S. Aureus ATCC 25923. At the same time, Cu-doped samples exert an antimicrobial effect on both bacteria. The effect of Ca2+ ions in the composition of the composite material is noteworthy. Calcium by itself is not an antibacterial agent, but in a complex with CS, it shows antimicrobial activity. The integrated indicator of antimicrobial activity for samples containing Ca2+, Zn2+, Cu2+, and Ag+ ions is close to or slightly higher than for commercial pharmaceutical agents Chlorhexidine and Metrogyl Denta.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Porous biomaterials based on polymers are widely used in the treatment of purulent wounds and infected ulcers. Such materials include a highly absorbent material capable of removing and storing a large amount of exudate, have high flexibility, adhere well to the wound surface, and minimize pain [7]. Rapid wound closure is the first goal of treatment for acute or chronic wounds that are difficult to heal due to infections that can delay the healing process. Wound infection is caused by local microflora or the environment growing directly in the wound area. Therefore, antibacterial agents play an important role in preventing wound infection. Historically, the resistance of microorganisms to antimicrobial drugs is the reason for their excessive and long-term use. As microbes are exposed to therapeutic agents, they develop resistance to antimicrobial agents through their rapid reproduction and genetic diversity. Thus, there is a need to develop safe, non-toxic compositions that prevent microbial proliferation [8].
Among biopolymers, natural CS and Alg have great scientific appeal in the fields of medicine, biotechnology, pharmacy, and cosmetics due to their biocompatibility with native tissues of the macroorganism, biodegradation, and antimicrobial activity. CS derivatives are characterized by wound-healing properties, as they exhibit greater antimicrobial activity compared to pure CS [4]. Chitosan is a polysaccharide component of crustacean shells, one of the few natural cationites. In the structure, CS has three types of reactive functional groups that ensure its chemical interactions, namely amino groups in the C-2 position, as well as primary and secondary hydroxyl groups in the C-6 and C-3 positions, respectively. This gives chitosan such properties as biocompatibility with native tissues of the macroorganism, biodegradation, and antimicrobial activity [3]. Glucosamine links in the chitosan structure are places of attachment to its molecular chain. Thus, amino groups in the protonated form allow attachment through electrostatic interaction. The nucleophilic properties of amines allow attachment by covalent bonds because amines have an active ionic pair of electrons on the electronegative nitrogen atom. Metal ions bind to chitosan through complexation mechanisms. The above properties of chitosan are widely used in practice in combination with growth factors and antibacterial agents [15].
Another polysaccharide of natural origin is alginate, which is a component of brown seaweed and is often used in tissue engineering due to its high biocompatibility and ability to easily and quickly form a gel in very mild conditions [5]. Modification of biomedical materials with metal ions and bioactive substances to provide additional properties is one of the modern approaches in medical materials science. A key property of functional polymers is their ability to form complexes with various metal ions in solutions. The addition of divalent cations such as calcium, zinc, and copper in an aqueous solution forms crosslinks between Alg and CS molecules, which leads to the formation of hydrogels—a three-dimensional network that stabilizes polymers [2].
ZnO nanoparticles are used as fillers for the polymer matrix due to their excellent antibacterial activity. Moreover, they do not show any negative effect on normal cells when used in appropriate concentrations [13]. The skin is the largest organ of the body. One of the serious problems in wound healing is the infection of the damaged part of the skin with microorganisms, such as bacteria. Infection delays skin recovery and can lead to further problems [4].
Therefore, wound dressings with antimicrobial activity are useful in minimizing microbial wound infections. In addition, porosity is a necessary and important characteristic of the absorbing biomaterial, which ensures interaction with exudate and its adsorption, and also ensures the necessary degree of water vapor penetration (water vapor transition). At the same time, the degree of porosity should provide the necessary mechanical stability to maintain the structural integrity of the biomaterial. Thus, the goal of this project is to create a composite material doped with metal ions and to study the effect of metal ions on the degree of porosity and antimicrobial properties.
2 Experiment Details
2.1 Materials
The following materials and chemicals were used in the course of work: alginate (Alg, E401) with a molecular weight of 15 kDa, chitosan (CS, Mm 300 kDa, Acros organics, USA), zinc oxide ZnO (own synthesized), zinc nitrate Zn(NO3)2, polyethylene glycol (PEG-400), TWEEN-80, (China production).
2.2 Composite Preparation
The basis was a 3% solution of sodium alginate. Fine powders (≤ 63 μm) of ZnO were mixed with 3% Alg aqueous solution in the ratio Alg: (ZnO) = 2:1 in terms of dry substances. The 0.5% of TWIN-80 тa 5% polyethylene glycol was added to the above mixture, followed by sonification. The resulting colloidal suspension was poured into the mold 5 mm thick and lyophilized (− 55 °C) for 24 h. After lyophilization, the composite was first immersed for 4 h in a 1% solution of CS for polyelectrolyte interaction of CS and Alg functional groups. Additional ionic cross-linking of CS macromolecules was carried out in 0.25 M solutions of CaCl2, ZnSO4, CuSO4, and AgNO3 for 40 min. The samples were then immersed in ethyl alcohol (50% solution), washed thoroughly with deionized water, and dried at 37 °C under pressure.
According to our protocol, ZnO was synthesized in the presence of Alg [9]. Briefly, 4 ml of a 3 wt% solution of sodium alginate was added to 200 ml of a 0.2 M zinc nitrate hexahydrate solution. The formation of the ZnO compound started after the addition of 15 ml of a 25 wt% ammonia solution. Then, the entire volume of the solution was additionally heated to a temperature of 80 °C with magnetic stirring to complete the formation of ZnO. After cooling, the sample was repeatedly rinsed with distilled water until neutral, and ZnO fraction was separated by centrifugation. The product dried at 37 °C was crushed to a powder with a dispersion of < 63 µm.
The obtained ZnO-Alg/CS composite subsequently served as a control (Control). Since the contents of Alg, CS, and ZnO are constant in all samples, these components are not reflected in the sample’s name. According to the type of metal ion present, the composites were named Ca, Zn, Cu, and Ag.
2.3 Research Methods
The surface morphology investigation was carried out using an SEM FEI Inspect S 50. Porosity (P) determination of the experimental composites was carried out using the earlier described method [14].
The elemental composition of synthesized samples was studied by an X-ray fluorescence (XRF) analysis using an ElvaX Light SDD spectrometer (www.elvatech.com). It is capable of identifying elements from Na (Z = 11) to U (Z = 92). A rhodium anode tube is used to obtain X-ray radiation. The voltage of the X-ray tube was 12 kV. The current was selected automatically to provide a sufficient load of simultaneously registered characteristic photons of ~ 50,000 counts. The registration time was the 30 s.
The antimicrobial activity of Me-ions doped biopolymer composites was studied against Gram-negative Escherichia coli ATCC 25922, and Gram-positive Staphylococcus aureus ATCC 25923 bacteria test cultures. The Agar diffusion methods carried out for the antimicrobial assessment. For this, the sterile Müller-Hinton nutrient medium was poured into sterile Petri dishes with a 4 mm thick layer. The plates were left at room temperature to solidify. Then, a suspension of the test microorganisms (inoculum) was prepared. A pure daily culture grown on a solid nutrient medium was used for that purpose. Identical, clearly isolated colonies were selected. The loopful of cells from a single colony was transferred to a test tube with a sterile saline solution. The inoculum turbidity was adjusted to a McFarland standard of 0.5, corresponding to 1.5 × 108 CFU (colony forming units) in 1 ml. The 2 ml inoculum pipetted on the surface of the nutrient medium in a Petri dish was evenly spread over the agar surface by shaking, and then the excess liquid was removed. The opened cups were left at room temperature for 10 min for drying. The 6 mm disks were cut from experimental samples preliminarily moistened in sterile water and placed on the surface of the nutrient. After the sample’s application, the Petri dishes were incubated at 37 °C for 24 h in a thermostat, and then the growth inhibition zone (ZOI) of the target microorganism was measured.
Integral index of antibacterial activity (A) is expected according to the methodology [12] by the following formula:
where
an—the proportion of patients with selected pathogenic microorganisms in a particular disease (range 0–1); Dn—the average value of the diameter of zones of inhibition growth of the studied test strains of microorganisms; A—integral index of the antimicrobial drug activity; 25—constant. Ranges of the efficiency indicator: 1.0–1.5—the drug shows a weak antimicrobial activity; 1.5–2.5—drug shows a mean antimicrobial activity; more than 2.5 shows strong antimicrobial activity.
Statistical data was processed using the program Excel (VS Office 2003) with geometric mean and the possibility of discrepancies (p) indicators.
3 Results and Discussion
The ZnO polymer scaffold is a macroporous three-dimensional network formed by the polyelectrolyte interaction between macromolecules of natural polymers Alg and CS, in the pores of which are immobilized particles of zinc oxide (ZnO). Polyelectrolyte binding of polymers ensures the stability of the composites in a physiological environment. To provide antimicrobial properties and increase mechanical stability, the synthesized materials were additionally cross-linked with metal ions from 0.25 M solutions of CaCl2, ZnSO4, CuSO4, and AgNO3. Previous in vitro studies regarding samples’ structural integrity in the buffer solution SBF (simulated body fluid) proved that the composite is a three-layer structure, which has two surface layers doped with metal ions Ca2+, Zn2+, Cu2+, Ag+ and an inner porous layer, represented by a grid of lyophilized Alg fibrils with immobilized ZnO particles. The surface layers were resistant to degradation, dispersion, and swelling, while the inner layer swelled much faster and acquired the consistency of a gel after 3 days of being in the SBF. CS macromolecules were first of all bound to Alg macromolecules located on the surface, forming a compacted film. In turn, metal ions bound to CS macromolecules form bridges between Alg and CS and also adhere to the surface of inorganic ZnO microparticles. Figure 1 schematically shows the composite components’ interaction and the material’s structure.
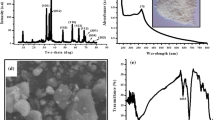
Figure 2 shows the XRF spectra of experimental samples, which confirm the content of metal ions in the composite structure.
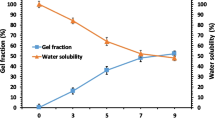
As already mentioned, the degree of porosity is of great importance for biomaterials that can potentially be used as wound dressings. Studies have proven the influence of metal ions (Ca, Zn, Cu, Ag) on the porosity of the composite material (Fig. 3).
Doping composites with metal ions increases the degree of porosity compared to the control sample. The change in porosity is influenced by the state of the dispersed system caused by the action of adsorbed metal ions, namely the balance of the energies of electrostatic attraction and repulsion between charged particles. The predominance of the repulsive energy of similarly charged adsorbed ions leads to the formation of a positive “wedging” pressure, which causes the repulsion of surfaces and, in turn, affects porosity.
In electrolyte solutions, Cu2+, Zn2+, Ca2+, and Ag+ ions are adsorbed on the particles of the dispersed phase of the samples, giving them a positive charge. As a result, repulsive forces arise, which, according to the theory of DLFO (Deryagin–Landau–Fairway–Overbeek), are electrostatic in nature. A positive wedging pressure is formed, which leads to an increase in the distance between macromolecules. This is the reason for the increase in the porosity of samples containing metal ions.
The morphology of the cross-sections of the experimental samples is shown in Fig. 4. The images confirm the porous structure of the samples with a highly developed surface, which facilitates the absorption and retention of fluid and exudate when used as wound dressings.
Macromolecules of CS form with metal ions chelate complexes, which are able to exert an enhanced antimicrobial effect [11]. In vitro studies proved that the outer layers of the formed scaffold doped with metal ions showed increased mechanical stability in SBF.
Figure 5 shows the antimicrobial effect of metal ions containing composites in the form of zones of growth inhibition (ZOI) of Gram-positive S. Aureus ATCC 25923 and Gram-negative E. coli ATCC 25922 microorganisms. Quantitative values of ZOI are given in Table 1.
As the experiment shows, E. coli ATCC 25922 is more sensitive to the action of composite materials. At the same time, Cu-doped samples have a good antimicrobial effect against both Gram-negative E. coli ATCC 25922 and Gram-positive S. Aureus ATCC 25923 bacteria. The action of calcium ions in the composite material is remarkable. Calcium by itself is not an antibacterial agent, but when combined with chitosan, it has an antimicrobial effect. The antimicrobial activity of samples containing Ag+ ions is surprisingly low. This happens as a result of the interaction of silver ions with the components of the reaction mixture with the formation of poorly soluble compounds and, as a result, weak diffusion of Ag+ ions. So, for example, the formation of poorly soluble silver carbonate in the solution is likely: 2Ag+ + CO32− → Ag2CO3↓.
For the comparative characterization of the experimental samples’ antimicrobial ability against the investigated test strains, the calculation of the integral indicator (A) of antimicrobial activity was carried out in relation to the commercial Chlorhexidine and Metrogyl Denta, which are used as antimicrobials in dentistry. The applied vector theory made it possible to represent A as a vector in n-dimensional space with coordinates in the form of a growth inhibition zone for each test microorganism. The results showed that the value of A for samples containing Ca2+, Zn2+, Cu2+, and Ag+ ions is close to or higher than for the above pharmaceuticals. The smallest A value is for the Control. According to methodological recommendations, the samples are classified as having medium antimicrobial activity, except for Control and Ag, whose antimicrobial activity is weak.
Scientific sources provide two main mechanisms of antimicrobial action: (a) the toxic effect of metal ions on the cell membrane of bacteria; (b) the toxicity of ROS (reactive oxygen particles), formed with the participation of ZnO, on the components of the bacterial cell. Antibacterial activity is the result of the formation of such ROS as hydrogen peroxide (H2O2), peroxide anion (O2−), and hydroxyl radicals (OH−). These particles damage cellular components such as DNA, lipids, and proteins [1]. Positively charged metal ions can also directly interact with negatively charged components of the bacterial wall [10]. Composites containing ZnO disrupt the integrity of the cell membrane, which leads to damage to membrane proteins and the lipid layer [6]. In addition, it is shown that chelate complexes of chitosan with metal ions exhibit greater antimicrobial activity compared to pure chitosan [11]. Positively charged sites of CS, joining the negatively charged surface of a microbial cell, disrupt its metabolism.
4 Conclusions
ZnO-Alg/CS composites doped with Ca2+, Zn2+, Cu2+, and Ag+ ions were created for potential application in the treatment of damaged areas of the skin. Polyelectrolyte binding of oppositely charged polymer chains Alg and CS and additional cross-linking with metal ions ensure the stability of composites in a physiological environment and provide antimicrobial properties. Doping composites with metal ions increase the degree of porosity compared to the control sample, which positively affects the ability of the material to absorb liquid and exudate. The advantage of the repulsive energy of the same charged metal ions adsorbed on inorganic particles leads to the formation of a positive “wedging” pressure, which causes the repulsion of the surfaces and, in turn, affects the porosity.
It was experimentally proven that the Gram-negative microorganism E. coli ATCC 25922 is more sensitive to the action of composites than the Gram-positive S. Aureus ATCC 25923. At the same time, Cu-doped samples exert an antimicrobial effect on both bacteria. The effect of Ca2+ ions in the composite is remarkable. Calcium is not an antibacterial agent, but it has antimicrobial activity in a complex with CS. The integrated indicator of antimicrobial activity for samples containing Ca2+, Zn2+, Cu2+, and Ag+ ions is close to or slightly higher than for commercial pharmaceutical agents Chlorhexidine and Metrogyl Denta.
References
L.C. Ann, S. Mahmud, S.K.M. Bakhori et al., Antibacterial responses of zinc oxide structures against Staphylococcus aureus, pseudomonas aeruginosa and streptococcus pyogenes. Ceram Int. 40, 2993 (2014). https://doi.org/10.1016/j.ceramint.2013.10.008
S. Blatt, B. Al-Nawas, A systematic review of latest evidence for antibiotic prophylaxis and therapy in oral and maxillofacial surgery. Infection 47, 519–555 (2019). https://doi.org/10.1007/s15010-019-01303-8
E.M. Costa, S. Silva, F.K. Tavaria, M.M. Pintado, Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe 20, 27–31 (2013). https://doi.org/10.1016/j.anaerobe.2013.02.002
S. Koosehgol, M. Ebrahimian-Hosseinabadi, M. Alizadeh, A. Zamanian, Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater Sci. Eng. C 79, 66–75 (2017). https://doi.org/10.1016/j.msec.2017.05.001
T. Kumar Giri, D. Thakur, Ajazuddin, et al., Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr. Drug Deliv. 9, 539–555 (2012).https://doi.org/10.2174/156720112803529800
N. Padmavathy, R. Vijayaraghavan, Interaction of ZnO Nanoparticles with microbes—a physio and biochemical assay. J. Biomed. Nanotechnol. 7, 813–822 (2011). https://doi.org/10.1166/jbn.2011.1343
W. Paul, C.P. Sharma, Advances in Wound Healing Materials: Science and Skin Engineering (Smit.RT Ltd., Shropshire, UK, 2015)
L. Pinheiro, C.I. Brito, V.C. Pereira et al., Susceptibility profile of Staphylococcus epidermidis and Staphylococcus haemolyticus isolated from blood cultures to vancomycin and novel antimicrobial drugs over a period of 12 years. Microb. Drug Resist. 22, 283–293 (2016). https://doi.org/10.1089/mdr.2015.0064
A. Pogrebnjak, L. Sukhodub, L. Sukhodub et al., Composite material with nanoscale architecture based on bioapatite, sodium alginate and ZnO microparticles. Ceram Int (2019). https://doi.org/10.1016/j.ceramint.2019.01.043
R. Sinha, R. Karan, A. Sinha, S.K. Khare, Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 102, 1516 (2011). https://doi.org/10.1016/j.biortech.2010.07.117
L. Sukhodub, Metal ions doped chitosan nanoparticles. J. Nano-Electron Phys. 6 (2014)
L.B. Sukhodub, G.E. Khrystian, L.F. Sukhodub et al., Composite materials based on zinc sulfide and zinc oxide: structural and biocidal properties. Ann. Mechnikov Inst. 4, 34–39 (2016)
L.B. Sukhodub, M. Kumeda, V. Bielai, L.F. Sukhodub, Hydroxyapatite-biopolymers-ZnO composite with sustained ceftriaxone release as a drainage system for treatment of purulent cavities. Carbohydr. Polym. 266, 118137 (2021). https://doi.org/10.1016/j.carbpol.2021.118137
L.F. Sukhodub, L.B. Sukhodub, O. Litsis, Y. Prylutskyy, Synthesis and characterization of hydroxyapatite-alginate nanostructured composites for the controlled drug release. Mater Chem. Phys. 217, 228–234 (2018). https://doi.org/10.1016/j.matchemphys.2018.06.071
B. Sultankulov, D. Berillo, K. Sultankulova et al., Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 9, 470 (2019). https://doi.org/10.3390/biom9090470
Acknowledgements
The National Research Foundation of Ukraine financially supported the research within the framework of the program Science for Security and Sustainable Development of Ukraine 0122U001154 and the Ministry of Science of Ukraine in the frame of a research topic 0122U000775.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Sukhodub, L.B., Kumeda, M.O., Sukhodub, L.F. (2023). Effect of Metal Ions on the Porosity and Antimicrobial Properties of ZnO-Alginate-Chitosan Composites. In: Fesenko, O., Yatsenko, L. (eds) Nanoelectronics, Nanooptics, Nanochemistry and Nanobiotechnology, and Their Applications . NANO 2022. Springer Proceedings in Physics, vol 297. Springer, Cham. https://doi.org/10.1007/978-3-031-42708-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-42708-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42707-7
Online ISBN: 978-3-031-42708-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)