Abstract
Aqueous and ionic liquid-based extracts of grape pomace were utilized for silver nanoparticles (AgNPs) synthesis. The ionic liquid-based extracts contained higher levels of phenolic acids and flavanols that are natural capping agents and showed higher synthesis efficiency. Three extracts of grape pomace (GPE) could produce silver nanoparticles. Specifically, syntheses silver nanoparticles by water and ionic liquid solvent GPE showed various sizes, 19–33 nm and 8–15 nm, respectively and various colloidal stability. The used DESs-based extracts lead to synthesis of silver nanoparticles with improved dispersion and colloidal stability. The effective colloid stability of AgNPs is believed to be the result of the DESs stability, used in the synthesis of colloidal system. All synthesized AgNPs by both water and DESs extracts showed activity against Escherichia coli, but the AgNPs prepared with DES have the highest antibacterial activity against microorganisms.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
1 Introduction
The search for new methods of synthesis of nanomaterials belongs to the key priorities of the development of innovative, energy- and resource-saving “green” methods of modern chemical technology for the production of nanostructured oxide materials and attracts considerable attention of researchers, as indicated by the number of publications in leading world publications [1,2,3]. The work of domestic and foreign scientists in this field relates to increasing the effectiveness of traditional, mostly well-known, chemical methods. The use of solvothermal synthesis methods allows varying the structural and sorption characteristics, and the CVD method allows obtaining powders of different morphologies. However, the above-mentioned methods require further study in order to establish the possibility of obtaining a wider list of nanosized materials with a simultaneous study of the influence of the method and the conditions of the synthesis on the properties and morphology of the obtained materials and their multifunctional properties.
Today, green/phytochemical synthesis methods, which involve the use of plant extracts, have significant popularity [4,5,6,7,8,9]. An alternative develops the green method synthesized metal nanoparticles from corresponding metal ions using the reducing and capping agents of phytochemical from agro waste extracts. Among foreign scientists, “green” nanotechnologies, which use environmentally safe chemical, technological, and production processes, have acquired rapid development. At the same time, the “green” method is understood as the use of extracts of plant raw materials and their processing waste [4], as well as the use of physical methods of exposure: ultrasonic treatment [5] or microwave irradiation [6]. The advantages of these methods are the possibility of obtaining a wide range of nanomaterial with high reactivity and pronounced polyfunctional properties. In their work, foreign scientists use extracts of plant raw materials for the synthesis of nanosized particles of metals (Ag, Fe) [10,11,12] and oxide compounds of various component composition TiO2, SnO2, CuO, Ag–SnO2 [13,14,15,16,17,18,19,20,21,22,23] and materials based on them for use as sorption-photocatalytic, sensory, antioxidant, anti-corrosion materials. The extracts play the role of both a reducing agent and a capping agent. Often, extracts are prepared with known solvents, such as water, ethanol, and mixtures thereof. The search for biodegradable and effective, “green” solvents for the extraction of natural organic compounds from plant raw materials is one of the main trends in the development of modern chemical technology and engineering. However, there are absolutely no studies on the use of plant extracts based on deep eutectic solvents for the phytochemical/biomimetic synthesis of nanomaterials [24,25,26,27,28]. For the extraction of natural secondary metabolites of plant raw materials, the overwhelming majority use toxic and aggressive organic solvents or the most common are “traditional” solvents. Deep eutectic solvent (DES) are a recently discovered category of potentially more stable alternative solvents. For the reasons set out above, the so-called low-temperature eutectic solvents, namely deep eutectic solvent are attractive, generally recognized in the world as absolutely safe, which fully comply with the principles of green chemistry and are considered 4th generation solvents for the chemical technology of the twenty-first century. These modern solvents have some advantages such as non-flammability, low toxicity, and biocompatibility. These solvents have special qualities which are not feasible with normal solvents, like lower melting mixtures, lower-transition temperature mixtures, or deep eutectic ionic liquids. Their properties can be adjusted by modifying the hydrogen bond acceptor/donor structures or by changing the molar ratio of their components. Due to its properties, the III type of DES is of the greatest interest. Therefore, there is a prospect of research and application of this type of solvents for extraction of plant raw materials and synthesis of nanomaterials. Using these solvents, it is possible to increase the yield of phenolic compounds by increasing the operating temperature without damaging the active substances, due to the increase in the solubility and diffusion coefficients of polyphenolic compounds in the solvents. Their polarity can be changed by modifying their composition, so they can be used to solubilize a wide range of biologically active compounds. The studies consider DES based on choline chloride because of its historical primacy and practical qualities (low cost, zero toxicity, extensive previous industrial experience with this material). However, it has a certain drawback: extracts obtained on its basis are limited in practical use due to the presence of chloride in its composition. To expand the scope of application, it is necessary to use a less toxic acceptor for the formation of hydrogen bonds. The literature reports that betaine and proline can be an alternative to choline chloride. It is a cheap natural resource, biodegradable, non-toxic and can be obtained from sugar beets. At the same time, DESs based on betaine and proline have a wide prospect of application. There are publications in the literature that describe the use of aqueous-ethanol extracts of grape pomace for the synthesis of silver nanoparticles [27,28,29]. A fundamental flaw in water-based extracts is the absence of the necessary viscosity to ensure the structural stability of the medium, which requires using a lot of compounds to stabilization AgNPs. Thus, given the lack of research on “green”/phytochemical synthesis based on plant extracts obtained with the latest solvents, the authors suggest conducting research in this direction.
In this work, it is proposed to compare the physicochemical properties of silver nanoparticles obtained by synthesis using a traditional aqueous (water) extract of grape pomace (W-EGP) and based on a betaine/pyrrolidine-2-carboxylic acid-based natural deep eutectic solvent (DES-EGP).
2 Comparative Characterization of Grape Pomace Extract Obtained with Water and Deep Eutectic Solvents for Nanomaterials Synthesis
2.1 DES Preparation
DES was prepared according to the procedure described in previous works [29]. Betaine and Pyrrolidine-2-carboxylic acid (Sigma Aldrich, 98.9% purity) and 2-Hydroxypropanoic acid (d,l-lactic acid, Sigma Aldrich, 91.4% purity) to prepare the DES were used. (Table 1). For this, lactic acid and betaine were mixed in sealed glass flasks with a capacity of 100 ml, taken in molar ratios, respectively. The mixture was continuously stirred at a temperature of 60 °C at a speed of 400 rpm in a magnetic stirrer until the mixture formed a clear solution.
2.2 Extraction Procedure
Aqueous and DES extractions of the grape pomace were done following the method described in previous work [29]. Prepared DESs were utilized to extract phytochemicals from grape pomace with 1:10 solid to liquid ratio, 45 min at room temperature. The suspension was then filtrated through a filter paper to obtain the extract. The macerated extracts using Whatman filter paper were filtered. The resulting extracts were named as follows: W-GPE and DES-1, DES-2.
2.3 Extract Composition
The analysis of the extracts was carried out using the methods of high-performance liquid chromatography with a diode array detector (HPLC–DAD) and high-performance liquid chromatography-mass spectrometry (HPLC–MS). The identification was confirmed by comparing the retention time, UV and mass spectra of the detected compound with the spectra of a pure standard. LC-DAD analysis was performed on an Agilent Technologies 1260 Infinity HPLC equipped with a Thermo Scientific Hypersil Gold C18 column (150 × 4.6 mm, 5 μm) and a diode array detector. The mobile phases were (A) 2.5% v/v acetic acid in water and (B) 2.5% v/v acetic acid in methanol, flow rate 1 mL/min, temperature column temperature was 25 °C, and the injection volume—10 μl. Before introducing the samples, a 5-min equilibration with 5% B was carried out. Elution was carried out according to a linear gradient with the following conditions: 0 min, 5% B; 20 min, 20% B; 15 min, 40% B; 18 min, 30% B; 28 min, 20% B; 35 min, 0% B. The run time was 35 min, and data were recorded at 325 and 354 nm for the identification of phenolic acids, flavonols, and flavonoids.
The identification was confirmed by a liquid chromatography-mass spectrometric system. LC–DAD–MS analysis was performed on a Thermo Fisher Scientific UltiMate 3000 RSLC equipped with a Thermo Scientific Hypersil Gold aQ C18 column (150 × 4.6 mm, 5 μm particle size), a diode array detector coupled to a Thermo Scientific TSQ triple quadrupole mass—Fortis spectrometer with ESI source. The mobile phases were (A) 0.1% v/v formic acid in water and (B) methanol, the flow rate was 0.6 mL/min, the column temperature was 25 °C, and the injection volume was 5 mcl. Equilibration with 10% B was carried out for 3 min before the introduction of samples. Elution was carried out according to a linear gradient with the following conditions: 0 min, 10% B; 30 min, 50% B; 45 min, 100% B; 50 min, 100% B; 60 min, 10% B. Run time was 60 min, and data were recorded at 256, 282, 325, and 354 nm to identify phenolic acids, flavonols, and flavonoids. The electrospray ionization (ESI) source was operated in negative ion mode at 4.5 kV and scanned from m/z 170 to 700.
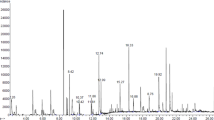
Figure 1 shows percentage (%) of the polyphenolic compounds extracted from the grape pomace extracts obtained by DESs and water (Fig. 1). The use of water made it possible to extract flavonols and hydroxycinnamic acids; therefore, it was used as a standard to evaluate the diversity of the studied extracted compounds from grape pomace. According to the present study, the main component of water grape pomace extract is hydroxycinnamic acids.
Soluble conjugated flavonoids or phenols were the main phenolic components that could be isolated from grape pomace. It was found that the phenolic acids are represented by compounds: gallic acid, protocatechuic acid, p-hydroxybenzoic acid, coutaric acid, caffeic acid, epicatechin, syringic acid, p-coumaric acid. In particular, hydroxycinnamic acids are eluted in the first 10 min of working time, while flavonoids are eluted later. In addition, hydroxycinnamic acids showed higher intensity peaks at 325 nm, while flavonoids are more easily seen at 354 nm. Preliminary identification of anthocyanins and hydroxycinnamic acids was carried out using UV spectra of each peak obtained on DAD-LC chromatograms and compared with UV spectra of standard compounds. The main component of DESs grape pomace extract is anthocyanins. It was established that the higher the content of flavonoids and anthocyanin is observed in DES solvents. Grape pomace extract mainly consists of phenolic compounds, predominantly 3,4,5-trihydroxybenzoic acid (gallic acid 10.8%) and 3-(3,4-Dihydroxyphenyl)-2-propenoic acid (caffeic acid (8.7%). When using water as a solvent, a low content of flavonoids in the extract was recorded. Because extraction with this solvent at a pH close to neutral favors the extraction of more polar biologically active compounds, such as hydroxycinnamic acids. For example, caffeic and syringic acids are more soluble in water than flavonoids. These compounds are known today for their reducing ability. The dominant anthocyanins were identified are petunidin 3-O-glucoside and delphinidin 3-O-glucoside. Comparative characteristics of the component composition of the extracts indicate that the aqueous extract contains phenolic acids to a greater extent. While the extracts obtained by DES contain anthocyanins and flavonoids to a greater extent. The analysis of the component composition the aqueous grape pomaces extract indicate that it contains a more lower content of anthocyanins and higher content of phenolic acids therefore is a probably can provide higher reducing capacity. While the extract obtained with DES, having a similar composition, but higher content of anthocyanins, flavonoids and higher viscosity and may be more effective for stabilizes colloidal stability.
2.4 Reducing Power of the Extracts
Due to high polarity of the DES are often used as solvent for extraction polyphenolic compounds. DES and water extracts were characterized for their total phenolic content (TPC), total flavonoids content (TFC), and reducing activity. In general, the higher the total phenolic contents in the extract, the greater the reducing forces. The highest content of TFC was found in the extracts obtained by DES, namely in the extracts obtained by DES-1. The extract obtained by DES contained a large amount of flavonoids compared with water extract. This could be associated with interactions that are present due to hydrogen bonds, formed among molecules of DES and flavonoids of extract. The polarity and viscosity of DES are the main properties that affect the extraction of flavonoids. The data obtained are consistent with the results of chromato-mass spectral analysis, according to which the extracts obtained by DES have a high content of anthocyanins and catechins, which belong to flavonoids. Comparing extracts of both type solvents, it was observed that DES was able to present good extraction of flavonoid content.
Figure 2 presents a bar graph representing reducing power of water grape pomace extract and DES-based extracts. The reducing ability of the aqueous extract is slightly higher than that of the extracts obtained by DES. However, the values of reducing capacity of DESs are of the same order, so it can be proposed that the reducing ability of the studied extracts is sufficient for the reduction of silver ions.
Chemical evaluation of reducing ability was compared to the electrochemical one. From electrochemical point of view, a reduction ability agrees well with the ability to donate an electron and can be estimated by the value of oxidation potential and anodic current.
The potential scan range was –1.0–1.2 V/SSCE with a scan rate of 50 mV/s. The scan started from the OCP in cathodic direction and reversed once the potential reached 1.2 V/SSCE. Totally, five cycles were measured in each solution to ensure data convergence. The electrochemical measurements for water solution were not performed, due to low width of electrochemical stability window on platinum electrodes. The Versa STAT 3 Potentiostat Galvanostat (AMETEK Scientific Instruments, USA) was used to conduct polarization measurements.
The cyclic voltammograms are shown in Fig. 3. Curves for both extracts shows to reduction peaks in the similar potential range: − 0.17 and + 0.24 V/SSCE for DES-1 and − 0.10 and + 0.39 V/SSCE for DES-2. However, the anodic current is nearly one order of magnitude higher for DES-1 (1.1 mA) comparing to DES-2 (0.17 A) meaning DES-1 would act better as a reducing agent in the synthesis of NPs. The electrochemical approach agrees well with the phosphomolybdenum method, where DES-2 also showed lower reducing capacity.
2.5 Synthesis Procedure of Silver Nanoparticles (AgNPs)
In this study, grape pomace DESs extract (DES-1 and DES-2-GPE, and water extract (W-GPE) was used to synthesize AgNPs. Green synthesis of AgNPs was synthesized from 0.01 M AgNO3 using the reducing and capping agents of water and ionic liquid-based grape pomace extracts. The equivalent volumes of the extract solution and 0.01 M AgNO3 were heated separately in the glass beakers in the water bath to the temperature of 80 °C. The success of biosynthesized of Ag NPs was confirmed by spectroscopic study.
2.6 UV–vis Characterization of AgNPs
The UV–visible spectrum of AgNPs exhibits the maximum absorption at 436 nm after 48 min, 48 h, 96 h, and 1 month after the synthesis of nanoparticles (Fig. 4).
For the reaction mixtures in which AgNO3 was mixed with only DESs was no recorded by UV–vis spectra surface plasmon resonance which indicates the no color change and formation of Ag NPs. Comparison of the UV spectra (Fig. 4) indicates that the aqueous extract has a higher reduction power than the DES extracts. Thus, UV spectra of colloidal systems W-GTE obtained using an aqueous extract (after 45 min reaction) of grapes contained more intensive peak (3.23 a.u.) confirming the formation of nanoparticles in the range 400–450 nm. Probably, after 45 min, the maximum amount of silver nitrate was reduced in the reaction mixture with water extract. Plasmon resonance centered at 430 nm which indicates that the particle size in reaction mixture is higher, and the product of synthesized AgNPs is more polydisperse. The UV–visible spectrum of AgNPs synthesized by W-GPE exhibits the maximum absorption also at 436 nm and the position of the peak remains unchanged despite of the 48–96 h of the storages time. The dispersed solutions of reaction mixtures synthesized by the extracts based on DESs have higher values of the absorption spectra after 96–120 h. The data obtained confirm that the reducing ability of the extract based on proline-lactic acid (DES-1) is higher than that of betaine-lactic acid (DES-2), as evidenced by the higher values of the absorption spectra (2.8 a.u. and 1.5 a.u., respectively) (Fig. 5). When colloid systems by uses of DES-based extracts were formed, the signal peak of absorption spectra was vitiated from 2.11 to 2.03 during the time, which was mainly due to the formation of an intermolecular hydrogen bond between organic compounds and DES. After 96 h the UV-peak in the visible spectrum for the system of nanoparticle that was synthesis by using water grape pomace extract was decreased.
Analysis of the data obtained indicates that both studied synthesized systems are stable even after a long period of time, namely 1 month. Generally speaking, the reducing of silver is mainly influenced by reducing capacity of the compounds of the extract but on the stability of system influence intermolecular interactions between the capping compounds of the extracts and DES. The UV–Vis spectra show that only DESs (Fig. 4a) used by the synthesis of AgNPs were not as efficient as DES-based extract grape pomace (Fig. 4b, c). Specifically, solution DES with silver barely displayed an SPR band after 45 min. These results indicate that a higher concentration of phenolic compounds in the aqueous extract of grape pomace results in a higher reducing capacity of the extract. Thus, it can be concluded that the higher reducing potential of the water grape pomace extract leads to the more efficient synthesis of silver nanoparticles. However, when using an aqueous extract, the presence of capping components is not completely sufficient, which leads to the formation of particles of a larger size and polydispersity. While when uses DESs extract, an additional contribution to colloidal stability is probably provided by an ionic liquid. Due to its high viscosity, DES acts as a structuring component on the stabilization process of AgNP. All synthesis colloidal systems appeared to contain AgNPs on the UV–Vis spectra. However, the intensity the absorbance of UV-peak in visible spectra for the DES-1-GPE was higher than for the DES-2 extract. Thus, the lower synthesis efficiency of AgNP by uses DESs extracts was apparently associated with lower phenolic aced and flavanols levels. It should be noted that DES-based systems are more stable. After 1 month the intensity the absorbance of the UV-peak in visible spectra for the extract DES-1 and DES-2 were higher than for the W-GPE. Through UV-analysis it was found that DESs in extracts exerted constructive effects on AgNP synthesis, although the DESs themselves cannot reduce silver ions. This suggests that these DES may be intermolecular capping compounds during the formation of AgNP.
2.7 Morphology of Silver Nanoparticles
Surface morphology of the prepared AgNPs was evaluated using scanning electron microscopy. The SEM images clearly showed a uniform spherical shape with smooth surface of the synthesized AgNPs by DES and water-based grape pomace extract. These results indicate that AgNPs mainly in the spherical shape of nanoparticles (8–15 nm) (Fig. 6). The SEM images of AgNPs synthesized by water-based grape pomace extract showed the existence of small spherical nanoparticles with a size ranged from 19 to 33 nm. Although all systems were either spherical or nearly spherical, the DES-based AgNPs displayed different sizes than the water-based AgNPs. An addition, compared with the water extract, the morphology of the AgNPs synthesized by DES-based extracts of did not change significantly depending on the type of ionic liquid, which further showed that DES has capping action. Therefore, it is supposed that DES may play role by functioning on the surface of the AgNPs to enhance their dispersion. The two systems of AgNPs (DES-1 and DES-2) displayed different stability and the same morphology. It should be noted that the type of extract also exhibited varying degrees of effects on the stability of colloidal system. However, AgNPs synthesized by the DES extract had better dispersion than from the water extract, what does a narrow and clear peak indicate on UV–vis spectra. Between the two extracts the water-based one a more showy effect on the synthesis, but DESs-based extracts additional on capping process. The positive capping effect of the DES facilitates the stability to the surfaces of the Ago, which can enhance their property in the application.
2.8 Antibacterial Properties
The formation of AgNP based on DES-based extract makes a high degree of versatility, suitable for a variety of applications. The obtained silver nanoparticles show disinfectant (antimicrobial) properties (Table 2) against pathogenic bacteria Escherichia coli ATCC 25922 [30]. Table 3 presents the results of antibacterial activity for the AgNPs. The entire colloids systems variable ZI was fixed. The result showed that the W-GPE exhibited negligible inhibition zone against strain of bacteria. A maximum of 10.2 mm diameter ZI was recorded for DES-1, followed by 8 mm against DES-2 and ≤ 7.0 mm against W-GPE.
3 Conclusions
In this study, green synthesis of Ag NPs was successfully synthesized using the reducing and capping agents of aqueous and ionic liquid-based extracts of grape pomace. During extraction with DES, it was possible to obtain a complex mixture of hydroxycinnamic acids and flavonoids, while the traditional solvent used mainly hydroxycinnamic acids. The AgNPs was characterized by UV and SEM analysis result which confirmed the spherical form of nanosilver. The ionic liquid-based GPE shows profitably effects on the synthesis efficiency and nanoparticle properties of AgNPs as compared with water-based. The results showed that DESs provide high extraction yields for grape pomace green reducing compounds which could potentially improve effectiveness process of synthesis. It was revealed that the positive effects of DESs extracts originated from their combined actions as extraction solvents and stable colloids media. AgNPs exhibited antimicrobial activity against for Gram-negative (E. coli) bacteria. This work broadens the understanding influence of DES relatively of the improving the stability colloid systems for their next industrial application.
References
M. Rafique, I. Sadaf, M.S. Rafique et al., A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 45(7), 1272–1291 (2017). https://doi.org/10.1080/21691401.2016.1241792
P. Phanjom, G. Ahmed, Effect of different physicochemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae (MTCC No. 1846) and their antibacterial effect. Adv. Nat. Sci. Nanosci. Nanotechnol. 8 (2017). https://doi.org/10.1088/2043-6254/aa92bc
S. Iravani, H. Korbekandi, S.V. Mirmohammadi et al., Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 9, 385–406 (2014)
V.K. Sharma, C.M. Sayes, B. Guo et al., Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: a review. Sci. Total Environ. 653, 1042–1051 (2019). https://doi.org/10.1016/j.scitotenv.2018.10.411
A.A. Ramanathan, M.W. Aqra, An overview of the green road to the synthesis of nanoparticles. J. Mater. Sci. Res. Rev. 2(3), 1–11 (2019). https://doi.org/10.9734/JMSRR/2019/46014
G. Vasyliev, V. Vorobyova, M. Skiba, L. Khrokalo, Green synthesis of silver nanoparticles using waste products (apricot and black currant pomace) aqueous extracts and their characterization. Adv. Mater. Sci. Eng. 2020, 4505787 (2020). https://doi.org/10.1155/2020/4505787
G. Vasyliev, V. Vorobyova, Valorization of food waste to produce eco-friendly means of corrosion protection and green synthesis of nanoparticles. Adv. Mater. Sci. Eng. 2020, 6615118 (2020)
K. Mcnamara, S.A.M. Tofail, K. Mcnamara et al., Advances in physics: X nanoparticles in biomedical applications. Adv. Phys. X 2, 1–35 (2017). https://doi.org/10.1080/23746149.2016.1254570
O.A. Pivovarov, M.I. Skiba, A.К Makarova et al., One-pot synthesis of silver nanoparticles using nonequilibrium low temperature plasma in the presence of polyvinyl alcohol. Voprosy khimii i khimicheskoi tekhnologii. 3, 113–120 (2018)
M.I. Skiba, A.A. Pivovarov, A.K. Makarova, et al., Plasma-chemical synthesis of silver nanoparticles in the presence of citrate. Chem. J. Moldova. 13(1), 7–14 (2018). https://doi.org/10.19261/cjm.2018.475
K.M.M. Abou El-Nour, A. Eftaiha, A. Al-Warthan et al., Synthesis and applications of silver nanoparticles. Arab. J. Chem. 3, 135–140 (2010). https://doi.org/10.1016/j.arabjc.2010.04.008
S. Ahmed, M. Ahmad, B.L. Swami, S. Ikram, A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. Int. J. Adv. Eng. Technol. 7, 17–28 (2016). https://doi.org/10.1016/j.jare.2015.02.007
V. Vorobyova, G. Vasyliev, M. Skiba, Eco-friendly “green” synthesis of silver nanoparticles with the black currant pomace extract and its antibacterial, electrochemical, and antioxidant activity. Appl. Nanosci. 10, 4523–4534 (2020). https://doi.org/10.1007/s13204-020-01369-z
N. Tarannum, Y.K. Gautam, Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv. 9(60), 34926–34948 (2019). https://doi.org/10.1039/C9RA04164H
J. Singh, T. Dutta, K.H. Kim et al., Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Environ. Nanotechnol. 16(1), 84 (2018). https://doi.org/10.1186/s12951-018-0408-4
S. Ghotekar, T. Pagar, S. Pansambal, et al., A review on green synthesis of sulfur nanoparticles via plant extract, characterization and its applications. Adv. J. Chem. Sect. B. 2(3), 128–143 (2020). https://doi.org/10.33945/SAMI/AJCB.2020.3.5
S. Li, B. Zhou, B. Ren et al., Preparation of MgO nanomaterials by microemulsion-based oil/water interface precipitation. Mater. Lett. 171, 204–207 (2016). https://doi.org/10.1016/j.matlet.2016.02.048
V. Pillai, P. Kumar, M.J. Hou, P. Ayyub et al., Preparation of nanoparticles of silver halides, superconductors and magnetic materials using water-in-oil microemulsions as nano-reactors. Adv. Coll. Interface Sci. 55, 241–269 (1995). https://doi.org/10.1016/0001-8686(94)00227-4
R.D. Rivera-Rangel, M.P. González-Muñoz, M. Avila-Rodriguez et al., Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf., A 536, 60–67 (2018)
A. Abo-Hamad, M. Hayyan, Abdul Hakim, M. AlSaadi et al., Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 273, 551–567 (2015).https://doi.org/10.1016/j.cej.2015.03.091
J.-S. Lee, Deep eutectic solvents as versatile media for the synthesis of noble metal nanomaterials. Nanotechnol. Rev. 6(3) (2017). https://doi.org/10.1515/ntrev-2016-0106
A. Safavi, M. Shekarnoush, M. Ajamian, High-yield synthesis, characterization, self-assembly of extremely thin gold nanosheets in sugar based deep eutectic solvents and their high electrocatalytic activity. J. Mol. Liq. 279, 208–223 (2019). https://doi.org/10.1016/j.molliq.2019.01.111
A. Söldner, J. Zach, M. Iwanow et al., Preparation of magnesium, cobalt and nickel ferrite nanoparticles from metal oxides using deep eutectic solvents. Chem. Eur. J. 22(37), 13108–13113 (2016). https://doi.org/10.1002/chem.201602821
D.O. Oseguera-Galindo, R. Machorro-Mejia, N. Bogdanchikova et al., Silver nanoparticles synthesized by laser ablation confined in urea choline chloride deep-eutectic solvent. Colloid Interface Sci. Commun. 12, 1–4 (2016). https://doi.org/10.1016/j.colcom.2016.03.004
W. Da Silva, C.M.A. Brett, Novel biosensor for acetylcholine based on acetylcholinesterase/poly(neutral red)—deep eutectic solvent/Fe2O3 nanoparticle modified electrode. J. Electroanal. Chem. 872, 114050 (2020). https://doi.org/10.1016/j.jelechem.2020.114050
F. Aghazadeh, M. Aghazadeh, Effects of deep eutectic solvents in prearation of nanoparticles TiO2. Int. J. Bio-Inorg. Hybr. Nanomater. 6(4), 215–220 (2017). http://ijbihn.iauvaramin.ac.ir/article_660057_33411bbb370d1c83fba283a2ab449298.pdf
G.D. Saratale, R.G. Saratale, K. Dong-Su et al., Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens. Novel Approach Nanomater. 10(8), 1457–1472 (2020). https://doi.org/10.3390/nano10081457
M. Skiba, V. Vorobyova, Green synthesis of silver nanoparticles using grape pomace extract prepared by plasma-chemical assisted extraction method. Mol. Cryst. Liq. Cryst. 674(1), 142–151 (2018). https://doi.org/10.1080/15421406.2019.1578520
G. Vasyliev, K. Lyudmyla, K. Hladun et al., Valorization of tomato pomace: extraction of value-added components by deep eutectic solvents and their application in the formulation of cosmetic emulsions. Biomass Convers. Biorefinery. 12, 95–111 (2022). https://doi.org/10.1007/s13399-022-02337-z
N. El-Desouky, K. Shoueir, I. El-Mehasseb et al., Synthesis of silver nanoparticles using bio valorization coffee waste extract: photocatalytic flow-rate performance, antibacterial activity, and electrochemical investigation. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-021-02256-5
Acknowledgements
The authors thank students of National Technical University of Ukraine «Igor Sikorsky Kyiv Polytechnic Institute» for actively participating in the discussion on the study topic.
Funding Statement
This work was supported by the Ministry of Education and Science of Ukraine [registration no. 0121U100409, 2021].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Vorobyova, V., Skiba, M., Kotyk, M., Vasyliev, G. (2023). Synthesis of Silver Nanoparticles Using Ionic Liquid Solvent-Based Grape Pomace Extracts. In: Fesenko, O., Yatsenko, L. (eds) Nanoelectronics, Nanooptics, Nanochemistry and Nanobiotechnology, and Their Applications . NANO 2022. Springer Proceedings in Physics, vol 297. Springer, Cham. https://doi.org/10.1007/978-3-031-42708-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-42708-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42707-7
Online ISBN: 978-3-031-42708-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)