Abstract
Chronic kidney disease (CKD) is a rapidly rising global health burden and a major risk factor for both ischaemic and haemorrhagic stroke as well as subclinical cerebrovascular disease. Several pathophysiological mechanisms have been proposed to underlie the kidney–brain axis including shared traditional risk factors such as hypertension and diabetes mellitus, uraemia-related non-traditional risk factors such as chronic inflammation and abnormal mineral-bone metabolism, and dialysis-specific factors such as cerebral hypoperfusion and intradialytic cerebral ischaemia. CKD frequently complicates routine stroke risk prediction, diagnosis, management, and prevention. It is also associated with greater stroke severity, worse functional outcomes and higher stroke mortality. Here we present an evidence-based summary of the epidemiology, pathophysiology, diagnosis, prevention, and treatment of cerebrovascular disease in CKD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebrovascular disease
- Stroke
- Ischaemic stroke
- Haemorrhagic stroke
- Transient ischemic attack (TIA)
- Chronic kidney disease
- Renal dysfunction
- Kidney impairment
- End-stage renal disease (ESRD)
- Glomerular filtration rate (GFR)
- Proteinuria
- Albuminuria
- Hypertension
- Cardiovascular disease
- Atherosclerosis
- Arteriosclerosis
- Vascular disease
- Cerebral ischemia
- Renovascular disease
- Renal artery stenosis
- Kidney and brain interactions
- Cerebrorenal syndrome
- Renal microvascular disease
- Cerebral small vessel disease
- White matter lesions
- Blood–brain barrier dysfunction
- Neurological complications in kidney disease
- Cognitive impairment in chronic kidney disease
- Neurovascular unit
- Stroke risk in CKD patients
-
Patients with low glomerular filtration rate (GFR) and/or albuminuria are at risk for both ischaemic and haemorrhagic stroke subtypes. Patients are at particularly high risk of cardioembolic and large artery stroke.
-
Hypertension, diabetes mellitus, atrial fibrillation, and accelerated atherosclerosis are major contributing risk factors but chronic inflammation and genetic factors are also beginning to emerge as important mechanisms.
-
Due to their bleeding diathesis, patients with CKD tend to have a higher rate of complications with acute stroke therapies including thrombolysis and mechanical thrombectomy.
-
Patients with CKD derive similar benefits from standard stroke preventative therapies including antiplatelet, lipid-lowering, antihypertensive therapies, and anticoagulation but their benefit is attenuated or unclear for dialysis-dependent patients.
14.1 Introduction
Chronic kidney disease (CKD) is predicted to be the fifth leading cause of death worldwide by 2040 [1]. The rise in the prevalence of CKD can be partly attributed to the rise in risk factors such as obesity and diabetes but also as a result of our increasingly elderly population with one third of people over the age of 75 being affected by CKD [2]. CKD has been established as a risk factor for cardiovascular disease [3] and in particular cerebrovascular disease (CVD), encompassing stroke and its various subsets, as well as vascular cognitive impairment and dementia [4, 5]. Compared to the general population, those with CKD have a higher incidence of the risk factors that we traditionally associate with stroke, including hypertension, diabetes mellitus, and atrial fibrillation [6]. However, there are other non-traditional risk factors purported to be as a result of kidney dysfunction including endothelial dysfunction, chronic inflammation, uraemic toxins, anaemia, mineral-bone abnormalities, and dialysis related risk factors that are associated with an increased risk of CVD [5, 7]. This chapter aims to explore the relationship between CKD and CVD via various mechanisms and also the complexities and barriers to the investigation and management of CVD in this context. In doing so we hope to provide practical guidance on the management of these patients going forward.
14.2 Epidemiology
Stroke risk when assessed by kidney function, as measured by estimated glomerular filtration rate (eGFR), demonstrated an inverse relationship with a stepwise increase in risk compared to the general population [8]. Those patients with end stage kidney disease (ESKD) receiving dialysis were at highest risk of stroke (7.1-fold increased risk). CKD staging no longer accounts for eGFR alone but also acknowledges proteinuria as an important marker of kidney dysfunction and a risk of progression to ESKD [9]. Proteinuria has also been established as a risk factor for stroke with a dose-response relationship between level of proteinuria and increasing risk of stroke [10].
When we consider the traditional stroke risk factors; hypertension, diabetes mellitus, dyslipidaemia, and atrial fibrillation, and we consider our CKD population, it is clear that there may be a confounding relationship between certain of these comorbidities and increased stroke risk in CKD [11, 12]. In particular, hypertension occurs in the majority of patients with CKD (67–92%) and is considered a major confounder when assessing the relationship between stroke and CKD [4]. Atrial fibrillation (AF) is one of the most frequently diagnosed cardiac arrhythmias found in the general population and has been found to have a bidirectional relationship with CKD in a cause and effect loop and is therefore expectedly seen with increasing frequency in those with more advanced CKD and contributes to the increasing risk of stroke in CKD patients [13]. Patients with CKD who are diagnosed with atrial fibrillation carry a poor prognosis and have been found to be at higher risk for heart failure, myocardial infarction, and all-cause mortality [14], and in studies that adjusted for age, hypertension, and cardiac disease demonstrated a higher risk of stroke and death (HR 2.00, 95%CI 1.88 to 2.14 and HR 1.76, 95% CI 1.71 to 1.82, respectively) [4].
The period surrounding initiation of renal replacement therapy (30-day period before and after) has been found to be a particularly high-risk time period for the development of stroke and transient ischaemia attack (TIA) (threefold risk) [15]. When comparing renal replacement therapy modalities, haemodialysis is most strongly associated with stroke risk [7]. However, this is likely confounded by the reasons for choosing this treatment modality (for example, they may have failed peritoneal dialysis as CKD progressed with reduced urine output). Intermittent in-centre haemodialysis remains the most commonly prescribed form of dialysis, with patients normally attending three times a week with a period of prolonged interdialytic gap towards the close of the week- the time following this gap has been associated with an increased risk of stroke [16].
Stroke is an umbrella term encompassing a multitude of intracranial pathologies of varying aetiologies and pathophysiology. ESKD is associated with a sevenfold increased risk of ischaemic stroke and a ninefold increased risk of haemorrhagic stroke, with as high as one third of patients presenting with intracranial haemorrhage (ICH) having CKD [4, 17]. CKD has been shown to increase the risk of all stroke subtypes [12] but delineating the varied risk by subtype in the CKD population is an area that requires further study.
The increased risk of stroke in this already vulnerable population confers a higher risk of disability or poor functional outcomes post stroke (25% risk 95% CI 5–48% of modified rankin score ≥ 2 at discharge), increased morbidity and mortality (138% risk of in-hospital mortality, 95% CI 61% to 257%) compared to the general population post stroke and overall they suffer from more severe strokes at time of presentation (higher National Institutes of Health Stroke Scale NIHSS) [18].
14.3 Pathophysiology and Risk Factors
In order to discuss the pathophysiology of stroke in CKD patients, one must examine a multitude of risk factors which can be categorised as traditional, non-traditional, and dialysis related risk factors. In patients with CKD, the presence of these risk factors culminates in a pro-thrombotic milieu in accordance with Virchow’s triad of vessel wall damage, stasis of blood flow and hypercoagulability [19]. In contrast to this pro-thrombotic state that we associate with ischaemic stroke, it has also been suggested that the clot formed in patients with CKD is atypical and may confer an increased risk of bleeding secondary to platelet dysfunction in the setting of uraemia and anaemia of CKD, particularly in the context of albuminuria [20].
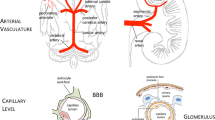
Renal and cerebral perfusion are governed by auto-regulatory mechanisms mediated by the surrounding rich capillary networks at both sites (glomeruli and blood brain barrier respectively) [21]. This shared pathophysiology may account for the susceptibility of both sites to damage via the traditional “vascular” risk factors.
Traditional risk factors include hypertension, atrial fibrillation, diabetes, carotid artery disease, obesity, and dyslipidaemia. As discussed above, these conditions often present as comorbid diagnoses in the presence of CKD and can significantly confound the risk of stroke in this population.
Hypertensive vascular damage or “strain vessel hypothesis” has been proposed as a mechanism linking CKD and stroke, with exposure of the juxtamedullary afferent arterioles and the deep perforating arteries to chronic hypertension resulting in these “strain” vessels developing hyaline arteriolosclerosis and impaired autoregulation resulting in glomerular hypertension and sclerosis and thus a decline in renal function and worsening systemic hypertension [22]. The deep perforating arteries of the brain develop a similar lipohyalinosis that also results in impaired autoregulation and the development of reduced cerebral blood flow and consequently increased ischaemic and haemorrhagic events in the areas supplies by these strain vessels [23]. Although hypertension is a major confounding factor in the relationship between CKD and stroke, the relationship is still seen in models when adjusted for hypertension [4]. Thus, this is unlikely the sole contributing mechanism for this relationship.
Non-traditional risk factors occur as a direct consequence of CKD [5]. Those with CKD are considered to be in a state of chronic inflammation contributing to endothelial damage, a hypercoagulable state and the generation of reactive oxygen species. Another hypothesis for the relationship between CKD and stroke also focuses on their shared anatomy and auto-regulatory function but identifies albuminuria as a marker for more generalised endothelial dysfunction leading to an increased risk of vascular events, the “Steno Hypothesis” [24]. Uraemia/uraemic toxins are associated with increased atherosclerosis and dyslipidaemia [25] but also platelet dysfunction increasing both the thrombotic and haemorrhagic risk in CKD [20]. CKD mineral-bone disease, and more specifically hyperphosphataemia, are associated with arterial medial calcification and potentiate vascular stiffness that can contribute to LVH and increase the risk of poor cardiovascular outcomes [26].
Haemodialysis confers its own independent risk factors for stroke mainly due to blood pressure variability, intermittent episodes of cerebral hypoperfusion which lead to chronic white matter changes, and vascular remodelling with increased arterial stiffness secondary to long-term dialysis [27]. It is likely that the period following the long interdialytic break is the time in which dialysis patients are most vulnerable to cerebral events due to haemodynamic variability. Following the prolonged interdialytic gap, dialysis patients are increasingly volume overloaded and hypertensive and more susceptible to intradialytic haemodynamic instability secondary to abnormal autonomic function [28].
14.4 Investigations
The main premise of stroke care and investigation remains based on the overarching principle that “time is brain” [29]. To reduce the risk of time delays in accessing necessary interventions, the assessment and investigation of stroke is generally a strictly protocolled practice in most centres. The protocol or pathway usually includes an initial rapid history assessment to establish risk factors, timelines, and contraindications to thrombolysis, a clinical exam using the international National Institutes of Health Stroke Scale (NIHSS) assessment as a diagnostic and prognostic tool and CT brain imaging including both non-contrast, contrast angiography and perfusion imaging.
The challenge that presents in the CKD cohort is accessing timely investigation and diagnosis due to clinical concerns regarding contrast-induced nephropathy [30]. It is important to recognise that the theoretical risk of contrast-induced nephropathy has not been demonstrated in a recent meta-analysis which examined 14 studies with 5725 patients undergoing CT angiography and perfusion and 981 patients undergoing noncontrast CT. The risk of acute kidney injury was lower in patients who received a contrast load compared with those who did not [31]. Additionally, comparing those who had prior diagnoses of CKD with those who did not, there was no significant difference in risk of acute kidney injury.
MRI is another imaging modality of import in stroke. MRI can be under-utilised in CKD patients due to concerns regarding gadolinium exposure leading to nephrogenic systemic fibrosis [32]. Current MRI protocols in stroke investigation focus on diffusion weighted imaging or susceptibility weighted imaging/gradient echo and fluid-attenuated inversion recovery (FLAIR) sequences and are in fact gadolinium free.
Based on the current evidence, one should advocate for CKD patients to receive the standardised investigations including contrast angiography and stroke-protocol MRI [33].
14.5 Acute Management
Disparities exist between the provision of stroke management in the general population versus the CKD patient cohort [34, 35], in particular with regard to access to intravenous thrombolysis with reports of significant delays in administration and also under-utilisation of this treatment method in the CKD cohort [30]. This deviation from the provision of evidence-based medicine in the CKD cohort spans across the initial stroke intervention chosen, the use of antiplatelet agents, the care of patients in a formal stroke unit and preventative interventions such as smoking cessation and statin therapy [34, 35]. The failure to provide evidence-based care in CKD is likely owing to the current lack of evidence in this field and the concerns regarding CKD/dialysis patient frailty and the increased risk of bleeding reported in this population [36].
-
1.
Thrombolysis:
Current best practice guidelines recommend the use of intravenous thrombolysis (IVT) in acute ischaemic stroke management. Better functional outcomes have been reported in patients who received IVT within 4.5 h of stroke onset [37] but lately this timeline has been expanded up to 9 h in specially selected patients, normally based on the findings of CT perfusion imaging and evidence of salvageable ischaemic brain tissue (Penumbra) versus truly infarcted tissue (Core) [38]. To date, most randomised control trials using IVT have failed to include patients with advanced CKD or, if included, failed to report stratified CKD outcomes. Studies to date in this area including a meta-analysis of seven observational studies, a post hoc analysis of the Enhanced Control of Hypertension and Thrombolysis Stroke Study and a U.S. based registry study all demonstrated increased mortality in those with CKD receiving IVT [39,40,41]. However, they failed to establish this increased mortality risk as being secondary to intracerebral haemorrhage (ICH) but instead found that these multi-morbid patients were at a higher risk of hospital acquired complications such as infections and deep venous thrombosis. Based on current evidence available, with a clearly established benefit to receiving IVT in the general population, it is proposed that IVT should be used in eligible patients with CKD and in those on dialysis once a normal activated partial thromboplastic time (APTT) has been resulted [33].
-
2.
Endovascular and Surgical Intervention.
There is a similar paucity of studies in the area of thrombectomy or endovascular clot retrieval in patients with CKD. In the absence of any clear evidence against the use of this intervention in CKD patients, we advocate for its use in suitable cases regardless of CKD stage or dialysis status [33]. Dialysis patients likely present a therapeutic challenge in terms of endovascular access and the increased bleeding risk but to date an analysis of 915 dialysis patients post thrombectomy showed lower in hospital mortality and moderate- severe disability compared with no treatment in this cohort [42]. Intervention with thrombectomy in posterior circulation stroke appears to be associated with increased ICH risk in the presence of CKD [43], but the use of thrombectomy in posterior circulation stroke remains an early and evolving intervention with benefits and risks still being established [44]. Surgical intervention such as decompressive hemi-craniectomy lacks specific evidence in those with CKD but should be offered to those who would otherwise be eligible for intervention.
-
3.
Stroke unit.
In acute stroke, admission to a dedicated stroke unit has shown both a mortality and morbidity benefit with reduced rates of post stroke dependency in the general population, with a number needed to benefit of 6 [45]. Patients with CKD, and in particular those on dialysis, are often cohorted to a renal ward regardless of reason for presentation due to nursing familiarity with this complex patient cohort. However, the benefit of acute stroke care in a specialised unit is maintained from the general population into those with established CKD and should be encouraged [35].
-
4.
Dialysis considerations.
Management of intermittent dialysis in the post stroke period presents a number of clinical challenges managing intracranial pressure, cerebral perfusion and anticoagulation [33]. Studies have shown that during intermittent haemodialysis subclinical cerebral oedema can occur [46]. In patients who have acquired an acute brain injury post stroke, an increase in intracranial pressure and increasing oedema may prove deleterious. Intracranial pressure may also be affected by changing osmolality during dialysis [47] and another factor to consider is intradialytic blood pressure and volume changes that may result in cerebral hypoperfusion and with it extension of the penumbra [27, 48]. The use of systemic anticoagulation in the acute post stroke period increases the risk of haemorrhagic transformation in the case of ischaemic stroke but also ICH extension and potential progression to herniation.
Current practice recommendations come from expert opinion based reviews and aim to avoid further intracranial insults via the above mechanisms [49, 50]. Continuous renal replacement therapy strategies have been shown to reduce the risk of cerebral oedema and hypoperfusion and thus it is recommended for use in the post-stroke period particularly in the case of patients with large infarcts, with ICH or in those who have blood pressure dependent infarcts (secondary to large vessel stenosis) [51]. Something that requires consideration in the case of continuous renal replacement therapy is the need for anticoagulation within the circuit. In this instance, regional anticoagulation with citrate is most appropriate due to its selective block of the haemostatic cascade within the circuit without effecting the circulating patient’s blood [52].
Given the risk of worsening oedema and herniation syndromes in ICH, it is recommended that dialysis should be delayed if appropriate until the patient has stabilised [53].
In those who are felt to be safe to proceed to intermittent haemodialysis (a decision made on a case-to-case basis by clinicians), there have been suggestions of using shorter dialysis times to limit changes in osmolality and of utilising additional osmoles such as mannitol or hypertonic saline. Additionally, it has been suggested that using a cooler dialysate during this acute stage would limit cerebral hypoperfusion by inducing vascocontriction and therefore avoiding intradialytic hypotension [54]. The recent MYTEMP trial has reported discordant results compared to previous studies that supported this hypothesis and calls into question the efficacy of this therapeutic intervention [55]. However, alternate studies focusing on MRI brain findings demonstrated a reduction in white matter changes when dialysis was performed at 0.5° below core body temperature compared to a standard 37°, making this an intervention that should be considered at an individual level [54].
Peritoneal dialysis may be superior to intermittent haemodialysis during this period but we would still recommend avoiding large volume, high glucose exchanges if possible to reduce the risk of osmotic changes [56].
Importantly, when considering the post-stroke period, one wants to optimise the patients’ ability to engage with the multi-disciplinary rehabilitation team and the timing and form of dialysis should take this into consideration where possible.
14.6 Preventative Therapies
14.6.1 Lifestyle Modifications
Although specific data on stroke risk reduction in this group is lacking, lifestyle modifications such as salt restriction [57], weight management [58], regular exercise [59], and smoking cessation [60] have been shown to improve intermediate outcomes associated with vascular risk such as blood pressure, lipid profiles, insulin resistance, and proteinuria, and are therefore, strongly encouraged in CKD.
14.6.2 Antiplatelet Therapies
Unfortunately, patients with moderate-to-severe CKD were excluded from most clinical trials evaluating efficacy and safety of antiplatelet agents so there is little evidence to inform guidelines in this area, particularly for primary prevention [61]. In a meta-analysis of three trials (HOT, Heart and Renal Protection [HARP], Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes [JPAD] trial) that studied the effect of antiplatelet therapy for primary prevention in CKD, there was no statistically significant reduction in major cardiovascular events including stroke (RR = 0.92, 0.49–1.73, p = 0.79) or in mortality (RR = 0.74, 95% CI 0.55 to 1.00, p = 0.05) [62]. However, there was an increase in major bleeding events (RR = 1.98, 95% CI 1.11 to 3.52, p = 0.02). The Aspirin to Target Arterial events in Chronic Kidney Disease (ATTACK) trial (NCT03796156) is an open-label, multi-centre primary prevention trial of aspirin in CKD currently underway that may help clarify the role (or lack thereof) of aspirin in this setting. There is somewhat better evidence to support the use of antiplatelet therapy in secondary vascular prevention in CKD. In a large Cochrane review of 50 RCTs (27,139 participants), antiplatelet agents reduced the risk of myocardial infarction (RR = 0.87, 95% CI 0.76–0.99), but not all-cause mortality (RR = 0.93, 0.8–1.06), cardiovascular mortality (RR = 0.89, 0.70–1.12) or specifically stroke (RR = 1.00, 0.58–1.72) [63]. However, it is unlikely that the large benefits of aspirin as demonstrated in the general population [64] would be completely nullified in patients with CKD and the guidelines consistently recommend its use for secondary prevention in this setting [61, 65, 66].
14.6.3 Anticoagulation
Similar to antiplatelet therapy, anticoagulation is highly effective in the general population [67] but tends to be underused in the renal population owing to bleeding or vascular calcification concerns, and uncertain benefit in the dialysis population [68]. However, there is clear, consistent evidence of the efficacy of warfarin for the prevention of stroke in patients with CKD albeit with a more variable effect on bleeding events [69, 70]. Novel oral anticoagulants (NOACs) appear to even more effective in CKD, as highlighted by a recent, large systematic review and meta-analysis of 11 trials (16, 787 participants) where they were associated with a lower risk of stroke or systemic embolism (RR = 0.79, 0.66 to 0.93), haemorrhagic stroke (RR = 0.48, 0.30 to 0.76), and all-cause death (RR = 0.88, 0.78 to 0.99) when compared with vitamin K antagonists [71]. There was no difference in the risk of bleeding though and this meta-analysis was limited only to patients with a creatinine clearance >25 mL/min. Reassuringly, reversal agents such as idarucizumab appear to be safe and effective in CKD [72].
Anticoagulation use in dialysis patients is more problematic. Multiple meta-analyses do not support a protective effect for warfarin in the prevention of ischaemic stroke and suggest that it is associated with increased risk of major bleeding [70, 73]. However, these have been based solely on observational cohort studies as there are no trials that have addressed this question. Furthermore, many of the included studies do not report time in the therapeutic range (TTR) which may confound some of the risk estimates. In a Danish registry study of 10,423 warfarin-treated AF patients, a TTR < 70% was associated with a higher risk of stroke/thromboembolism (HR = 1.39, 1.20–1.60) and bleeding (HR = 1.22, 1.05–1.42) among patients with eGFR of 30–59 mL/min/1.73 m2, suggesting that the quality of warfarin monitoring and management may similarly influence the efficacy and safety of warfarin in dialysis patients [74].
Vitamin K antagonists such as warfarin have also implicated in the progression of vascular calcification in these patients due to inhibition of the enzyme matrix gamma-carboxyglutamate Gla protein that scavenges calcium phosphate in tissues [75]. A recent multi-centre RCT investigated the impact of vitamin K status on vascular calcification in 132 patients on haemodialysis with AF [76]. Patients were randomised to vitamin K antagonists, rivaroxaban, or rivaroxaban plus vitamin K2 supplementation. Changes in coronary artery, thoracic aorta, and cardiac valve calcium scores and pulse wave velocity, as used to measure vascular calcification progression, were not significantly different among the treatment arms. There was also no difference in all-cause death, stroke, and cardiovascular event rates between the groups. The ongoing trial (AVKDIAL [NCT02886962]) will compare vitamin K antagonists with no anticoagulation in dialysis-dependent patients with AF may help definitively answer the question of risk:benefit ratio of warfarin in ESKD.
There is some promising observational data on NOAC use in dialysis patients. A retrospective cohort study based on United States Renal Data System (USRDS) data compared warfarin versus apixaban in 25,523 dialysis patients with AF [77]. Although there was no overall difference in the risks of stroke/systemic embolism between apixaban and warfarin (HR = 0.88, 0.69–1.12; P = 0.29), apixaban was associated with a lower risk of major bleeding (HR = 0.72, 0.59–0.87; P < 0.001). However, standard-dose apixaban was associated with lower risks of stroke/systemic embolism and death when compared with lower-dose apixaban and warfarin. The RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation (RENAL-AF) trial was unfortunately terminated early due to loss of funding, and thus, only recruited 154 patients that were followed-up for 1 year [78]. Aprixaban resulted in similar rates of bleeding and strokes as warfarin among patients with ESKD on haemodialysis. TTR with warfarin was only approximately 44%. The Edoxaban Low-Dose for EldeR CARE AF patients (ELDERCARE-AF) study is another multi-centre, ongoing RCT that will compare the safety and efficacy of once daily edoxaban versus placebo in Japanese AF patients ≥80 years of age who are considered ineligible for standard oral anticoagulant therapy [79]. This group will include those with advanced CKD or who are dialysis-dependent. There is clearly a need for further dedicated dialysis trials of DOAC versus placebo.
Left atrial appendage occlusion devices, used to lower the thromboembolic risk in those with absolute or relative contraindications to long-term oral anticoagulation, appear to be equally effective in those with CKD with similar procedural safety [80]. Those with an eGFR <30 mL/min/1.73m2 had a lower overall survival rate but the rate of non-fatal major adverse events during follow-up (stroke, TIA, and major bleeding) was not higher among patients with ESKD. However, an important limitation of this analysis was that it was a comparison based on expected event rates as opposed to trial-based evidence. There is also a temporary requirement for anticoagulation in the periprocedural period which may not be possible in a high-risk group.
14.6.4 Dual Blockade
A secondary analysis of the COMPASS (Cardiovascular OutcoMes for People using Anticoagulation StrategieS) trial revealed promising results for patients with CKD [81]. The COMPASS trial was a double-blind, double-dummy, randomised trial using a 3-by-2 partial factorial design conducted at 602 centres in 33 countries. In one randomised comparison, rivaroxaban with or without aspirin was compared with aspirin alone in patients with a history of stable atherosclerotic vascular disease (chronic coronary or peripheral artery disease). The other randomised comparison compares pantoprazole use with placebo and is still ongoing. The study, unlike many cardiovascular trials, was deliberately enriched with CKD patients, who accounted for 6276 patients out of 27,387 in total. The primary composite outcome of cardiovascular death, myocardial infarction, or stroke was reduced with rivaroxaban 2.5 mg BD plus aspirin in those with CKD (HR: 0.75; 95% CI: 0.60 to 0.94). Stroke as an individual endpoint was particularly reduced with dual blockade therapy (HR = 0.42, 0.25–0.70; p = 0.0007), and there was no excess bleeding in those with CKD as compared to those without. However, those with an eGFR <15 mL/min/1.73 m2 were excluded from the trial and there was only approximately 150 people with an eGFR 15–29 mL/min/1.73 m2 which may limit some of the generalisability of these results to all patients with CKD. In addition, those patients with a history of stroke in the preceding year were excluded, and only 5.2% of the included CKD patients had any prior history of cerebrovascular disease. Nonetheless, based on this trial, we would recommend considering low-dose rivaroxaban and aspirin for the prevention of stroke in those with an eGFR 30–59 mL/min/1.73 m2 and a prior history of coronary artery or peripheral artery disease. Dual blockade may also have a role in secondary stroke prevention though further evidence is required. The Treatment of Cardiovascular Disease with Low-Dose Rivaroxaban in Advanced Chronic Kidney Disease (TRACK) trial (NCT03969953) may help answer this question as it will randomise high-risk advanced CKD patients including those with a history of coronary artery disease, peripheral artery disease, non-haemorrhagic non-lacunar stroke, diabetes mellitus, or those ≥65 years, to low-dose rivaroxaban or placebo.
14.6.5 Lipid-Lowering Therapy
The efficacy of statin therapy for the primary prevention of stroke in CKD patients was clearly demonstrated in the landmark Study of Heart and Renal Protection (SHARP) trial, in which 9270 CKD patients with CKD without pre-existing vascular disease were randomly assigned to placebo or to the combination of simvastatin 20 mg daily plus ezetimibe 10 mg daily [82]. There was a 25% reduction in ischaemic stroke in the treatment arm. In meta-analyses of trials of statins in patients with established cardiovascular disease, there was about a 40% reduction in the risk of stroke in patients with CKD as per the general population [83, 84]. High-intensity therapy (e.g., atorvastatin 80 mg or rosuvastatin 20 mg once daily) has also been shown to be safe and effective in this group [85]. According to KDIGO guidelines [66], all CKD patients over 50 years of age should therefore be started on statin plus/minus ezetimibe therapy. The American College of Cardiology (ACC) has additionally recommended the addition of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (or ezetimibe) to maximally tolerated statin therapy in high-risk patients with atherosclerotic cardiovascular disease and CKD where less than 50% LDL-C reduction has been achieved with statins, including high-intensity statins [86].
There appears to be a “statin resistance” in the dialysis population, possibly related to a heightened role of non-traditional risk factors (e.g., mineral and bone abnormalities, uraemia) [87], additional lipid abnormalities (e.g., lipoproteins rendered highly atherogenic by oxidation or carbamylation), or intracellular cholesterol synthesis activated by inflammatory stress [88], and its pro-calcifying effects [89]. Multiple randomised trials [90, 91] including SHARP [82] did not find any benefit for statins in this population, with the exception of those with very high serum LDL-cholesterol levels (such as >145 mg/dL [3.8 mmol/L]) in a posthoc analysis of the 4D study (Die Deutsche Diabetes Dialyse Studie) [90]. For this reason, KDIGO guidelines, do not recommend starting statins de novo in dialysis patients [66].
14.6.6 Antihypertensive Therapy
Unfortunately, there has never been a dedicated blood pressure RCT in the CKD population for the prevention of stroke and most of the existing evidence has been derived from posthoc or subgroup analysis. The KDIGO 2020 Clinical Practice Guideline on the Management of Blood Pressure in CKD recommend a blood pressure of less than 120/80 mmHg in CKD for both primary and secondary prevention in patients where this level can be feasibly tolerated. This recommendation has been heavily influenced by subgroup analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) in which targeting a systolic blood pressure (SBP) <120 mmHg compared with <140 mmHg reduced rates of major cardiovascular events and all-cause death in patients with CKD [92]. The risk of stroke was similar in both treatment groups (HR = 0.99, 0.57–1.70; P = 0.96) but the trial was stopped early (median follow-up 3.3 years) so follow-up may have been too short to see a cerebrovascular protective effect. The generalisability of the results may also be limited as people with diabetes, proteinuria >1000 mg/g or prior stroke were excluded. However, specific stroke benefits associated with more intensive BP control have been seen in other trials such as the China Stroke Primary Prevention Trial (CSPPT) [93]. In this posthoc analysis of 3230 hypertensive patients with eGFR 30–60 mL/min/1.73 m2 and/or proteinuria, a time-averaged SBP of ≤135 mmHg was associated with lower risk of total first stroke compared to a time-averaged on-treatment SBP of 135 to ≤140 mmHg, (1.7% vs. 3.3%; HR = 0.51, 0.26–0.99).
As acknowledged by another recent KDIGO controversies conference [94], there is not much evidence to guide BP target thresholds in a secondary prevention setting, and the previous 2012 BP guidelines did not specifically address this group. A posthoc analysis of the Perindopril Protection against Recurrent Stroke Study (PROGRESS) showed that perindopril was associated with a 35% reduction in the risk of stroke CKD patients with a history of recently symptomatic cerebrovascular [95]. Perindopril prevented one stroke or other cardiovascular event among every 11 patients with CKD treated over 5 years, although it was unclear what the achieved blood pressure or level of urine albumin were in either arm of the trial. The Secondary Prevention of Small Subcortical Strokes (SPS3) study, in which patients with a history of lacunar stroke were randomised to a lower (<130 mmHg) versus higher (130–149 mmHg) target SBP included 474 patients with CKD [96]. Intensive BP control resulted in a statistically nonsignificant reduction in the cardiovascular composite outcome in CKD but with greater risk of kidney function decline.
The ideal BP target in dialysis patients for stroke prevention is evenly less clear with evidence of a U-shaped associations between change in SBP, all-cause mortality and cardiovascular mortality, whereby post-dialytic drops in SBP of up to 30 mmHg are associated with greater survival, but larger decreases of SBP are associated with greater mortality [97].
There is clearly a need for dedicated RCTs in CKD and dialysis patients to better establish BP targets for people with and without prior stroke.
14.6.7 Carotid Interventions
The North American Symptomatic Carotid Endarterectomy Trial (NASCET) was the only large randomised trial of carotid interventions that reported results according to kidney function [98]. Surgery was highly effective for CKD patients with symptomatic high-grade stenosis resulting in a RR reduction of 82.3% (95% CI 54.5–93.1%) compared to 50.8% (95% CI 12.6–72.3%) for patients without CKD. The number needed to treat by surgery to prevent one ipsilateral stroke within 2 years was only four for patients with CKD. Rates of perioperative cardiac complications (myocardial infarction, congestive heart failure, and arrhythmias) were higher in the CKD group though perioperative death rates were similar between groups.
However, the majority of CKD patients included in the NASCET analysis had CKD stage 3a with a mean eGFR of 49 mL/min/1.73 m2. In an analysis of the Vascular Study Group of New England database, 30-day mortality appears to increase with worsening kidney function, from 0.4% in mild CKD to 0.9% in severe CKD (defined as an eGFR <30 mL/min/1.73 m2; P = 0.01) [99]. However, in a multi-variate regression model, CKD status did not predict 30-day stroke or death, and even in patients with severe CKD, there was an overall 5-year survival rate of 71%, contrasting with the bleaker outcomes for severe CKD with PVD whose 5-year survival rate is only 21% irrespective of intervention [100]. We would therefore agree with guidance from the Society for Vascular Surgery who recommend carotid endarterectomy for symptomatic CKD patients with moderate-severe stenosis [101]. However, careful perioperative assessment and management is essential given their higher rate of periprocedural complications.
Unfortunately, the perioperative and long-term outcomes after carotid endarterectomy in dialysis patients appear to be quite poor. In a retrospective analysis of 5142 dialysis patients in the US Renal Disease System-Medicare-matched database, there was a high rate of 30-day complications including stroke, MI, and mortality for both asymptomatic and symptomatic patients (2.7% vs. 5.2% [P = 0.001], 4.6% vs. 5.0% [P = 0 0.69], and 2.6% vs. 2.9% [P = 0.61], respectively) [102]. The overall 3-year survival was also only 46% and 42% in the asymptomatic and symptomatic cohorts respectively. We would therefore recommend carotid intervention in only a select group of high-risk, symptomatic dialysis patients. There is currently insufficient evidence to recommend stenting over carotid endarterectomy in either CKD or dialysis patients. The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2; NCT02089217) is an ongoing set of trials, one of which will randomise patients in a 1:1 ratio to endarterectomy versus no endarterectomy and another will randomise patients in a 1:1 ratio to carotid stenting with embolic protection versus no stenting. This will include patients with an eGFR >30 mL/min/1.73 m2 and may therefore provide further information to inform best clinical practice in this area. However, a dedicated trial of carotid interventions in symptomatic patients with high-grade stenosis who have advanced CKD or who are dialysis-dependent is clearly required.
14.6.8 SGLT-2 Inhibitors
Sodium-glucose co-transporter-2 (SGLT-2) inhibitors appear to have promising vascular benefits in CKD patients with type 2 diabetes mellitus as demonstrated by recent large placebo-controlled outcome trials [103,104,105]. However, their potential benefit for stroke prevention in the general population or this specific group is less clear. In an analysis of the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial which randomly assigned randomly assigned 10,142 participants with type 2 diabetes mellitus and high cardiovascular risk to canagliflozin or placebo, there was no significant difference in event rates between groups (HR = 0.87; 95% CI, 0.69–1.09), though there may have been too few events overall to detect a significant benefit [106]. However, a meta-analysis of 32 trials with 75,540 participants also did not find a class or individual effect for any of the 3 SGLT-2 inhibitors therapy for stroke prevention [107].
Before You Finish: Practice Pearls for the Clinician
-
Stroke symptoms may be subtle in haemodialysis patients and therefore easily missed.
-
Admission to the stroke unit is associated with reduced mortality for patients with CKD including for those who are dialysis dependent.
-
In the absence of definitive trial evidence, the decision to anticoagulate and the choice of agent should be individualised in the haemodialysis population.
References
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–90.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–72.
van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52.
Kelly D, Rothwell PM. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J Neurol Neurosurg Psychiatry. 2020;91:88–97.
Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823–33.
Doehner W, Ural D, Haeusler KG, Čelutkienė J, Bestetti R, Cavusoglu Y, et al. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the study group on heart and brain interaction of the heart failure association. Eur J Heart Fail. 2018;20:199–215.
Findlay M, MacIsaac R, MacLeod MJ, Metcalfe W, Traynor JP, Dawson J, et al. Renal replacement modality and stroke risk in end-stage renal disease—a national registry study. Nephrol Dial Transplant. 2018;33:1564–71.
System USRD. Usrds 2009 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States; 2009.
Hallan SIRE, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77.
Lee M, Saver JL, Chang KH, Ovbiagele B. Level of albuminuria and risk of stroke: systematic review and meta-analysis. Cerebrovasc Dis. 2010;30:464–9.
Kelly DM, Rothwell PM. Does chronic kidney disease predict stroke risk independent of blood pressure?: a systematic review and meta-regression. Stroke. 2019;50:3085–92.
Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. 2019;50:229–39.
Mace-Brickman T, Eddeen AB, Carrero JJ, Mark PB, Molnar AO, Lam NN, et al. The risk of stroke and stroke type in patients with atrial fibrillation and chronic kidney disease. Can J Kidney Health Dis. 2019;6:2054358119892372.
Massicotte-Azarniouch DKJ, Carrero JJ, Lam NN, Molnar AO, Zimmerman D, McCallum MK, Garg AX, Sood MM. Incident atrial fibrillation and the risk of congestive heart failure, myocardial infarction, end-stage kidney disease, and mortality among patients with a decreased estimated GFR. Am J Kidney Dis. 2018;71:191–9.
Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24:1166–73.
Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–107.
Ovbiagele B, Schwamm LH, Smith EE, Grau-Sepulveda MV, Saver JL, Bhatt DL, et al. Hospitalized hemorrhagic stroke patients with renal insufficiency: clinical characteristics, care patterns, and outcomes. J Stroke Cerebrovasc Dis. 2014;23:2265–73.
Kumai Y, Kamouchi M, Hata J, Ago T, Kitayama J, Nakane H, et al. Proteinuria and clinical outcomes after ischemic stroke. Neurology. 2012;78:1909–15.
Kumar DRHE, Glurich I, Mazza JJ, Yale SH. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010;8:168–72.
Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–22.
Freeman WD, Wadei HM. A brain–kidney connection: the delicate interplay of brain and kidney physiology. Neurocrit Care. 2015;22:173–5.
Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–21.
Bernbaum M, Menon BK, Fick G, Smith EE, Goyal M, Frayne R, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab. 2015;35:1610–5.
Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The steno hypothesis. Diabetologia. 1989;32:219–26.
Apostolov EO, Ok E, Burns S, Nawaz S, Savenka A, Shah S, et al. Carbamylated-oxidized LDL: proatherosclerotic effects on endothelial cells and macrophages. J Atheroscler Thromb. 2013;20:878–92.
Lau WL, Ix JH. Clinical detection, risk factors, and cardiovascular consequences of medial arterial calcification: a pattern of vascular injury associated with aberrant mineral metabolism. Semin Nephrol. 2013;33:93–105.
MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol. 2017;28:2511–20.
Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20:1140–7.
Saver JL. Time is brain—quantified. Stroke. 2006;37:263–6.
Newsome BB, Warnock DG, Kiefe CI, Weissman NW, Houston TK, Centor RM, et al. Delay in time to receipt of thrombolytic medication among medicare patients with kidney disease. Am J Kidney Dis. 2005;46:595–602.
Brinjikji W, Demchuk AM, Murad MH, Rabinstein AA, McDonald RJ, McDonald JS, et al. Neurons over nephrons: systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke. 2017;48:1862–8.
Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology. 2007;245:168–75.
Kelly DM, Ademi Z, Doehner W, Lip GYH, Mark P, Toyoda K, et al. Chronic kidney disease and cerebrovascular disease: consensus and guidance from a KDIGO controversies conference. Stroke. 2021;52:e328–46.
Ovbiagele B, Schwamm LH, Smith EE, Grau-Sepulveda MV, Saver JL, Bhatt DL, et al. Patterns of care quality and prognosis among hospitalized ischemic stroke patients with chronic kidney disease. J Am Heart Assoc. 2014;3:e000905.
Findlay MD, Dawson J, MacIsaac R, Jardine AG, MacLeod MJ, Metcalfe W, et al. Inequality in care and differences in outcome following stroke in people with ESRD. Kidney Int Rep. 2018;3:1064–76.
Molnar AO, Bota SE, Garg AX, Harel Z, Lam N, McArthur E, et al. The risk of major hemorrhage with CKD. J Am Soc Nephrol. 2016;27:2825–32.
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35.
Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–803.
Jung JMKH, Ahn H, Ahn IM, Do Y, Choi JY, Seo WK, Oh K, Cho KH, Yu S. Chronic kidney disease and intravenous thrombolysis in acute stroke: a systematic review and meta-analysis. J Neurol Sci. 2015;358:345–50.
Carr SJ, Wang X, Olavarria VV, Lavados PM, Rodriguez JA, Kim JS, et al. Influence of renal impairment on outcome for thrombolysis-treated acute ischemic stroke: enchanted (enhanced control of hypertension and thrombolysis stroke study) post hoc analysis. Stroke. 2017;48:2605–9.
Tariq N, Adil MM, Saeed F, Chaudhry SA, Qureshi AI. Outcomes of thrombolytic treatment for acute ischemic stroke in dialysis-dependent patients in the United States. J Stroke Cerebrovasc Dis. 2013;22:e354–9.
Saeed F, Adil MM, Piracha BH, Qureshi AI. Outcomes of endovascular versus intravenous thrombolytic treatment for acute ischemic stroke in dialysis patients. Int J Artif Organs. 2014;37:727–33.
Laible M, Jenetzky E, Mohlenbruch MA, Neuberger U, Bendszus M, Ringleb PA, et al. Renal impairment is associated with intracerebral hemorrhage after mechanical thrombectomy in vertebrobasilar stroke. Cerebrovasc Dis. 2019;47:48–56.
Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke. 2006;37:922–8.
Stroke Unit Trialists Collaboration. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ. 1997;314:1151–9.
Winney RJ, Kean DM, Best JJ, Smith MA. Changes in brain water with haemodialysis. Lancet. 1986;2:1107–8.
Galons JP, Trouard T, Gmitro AF, Lien YH. Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest. 1996;98:750–5.
Polinder-Bos HA, Garcia DV, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP, et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol. 2018;29:1317–25.
Kumar A, Cage A, Dhar R. Dialysis-induced worsening of cerebral edema in intracranial hemorrhage: a case series and clinical perspective. Neurocrit Care. 2015;22:283–7.
Davenport A. Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int. 2008;12:307–12.
Davenport A. Changing the hemodialysis prescription for hemodialysis patients with subdural and intracranial hemorrhage. Hemodial Int. 2013;17(Suppl 1):S22–7.
Davenport A, Will EJ, Davison AM. Early changes in intracranial pressure during haemofiltration treatment in patients with grade 4 hepatic encephalopathy and acute oliguric renal failure. Nephrol Dial Transplant. 1990;5:192–8.
Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E. Regional citrate anticoagulation for RRTS in critically ill patients with Aki. Clin J Am Soc Nephrol. 2014;9:2173–88.
Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–65.
Committee MW. Personalised cooler dialysate for patients receiving maintenance haemodialysis (mytemp): a pragmatic, cluster-randomised trial. Lancet. 2022;400:1693–703.
Krane NK. Intracranial pressure measurement in a patient undergoing hemodialysis and peritoneal dialysis. Am J Kidney Dis. 1989;13:336–9.
Wanner C, Tonelli M. Kdigo clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–9.
Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82–98.
Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–93.
Chase HP, Garg SK, Marshall G, Berg CL, Harris S, Jackson WE, et al. Cigarette smoking increases the risk of albuminuria among subjects with type i diabetes. JAMA. 1991;265:614–7.
Conditions tCCfC. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. Royal College of Physicians; 2008.
Major RW, Oozeerally I, Dawson S, Riddleston H, Gray LJ, Brunskill NJ. Aspirin and cardiovascular primary prevention in non-endstage chronic kidney disease: a meta-analysis. Atherosclerosis. 2016;251:177–82.
Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013;(2):CD008834.
Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365–75.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
Buckley LF, Rybak E, Aldemerdash A, Cheng JW, Fanikos J. Direct oral anticoagulants in patients with atrial fibrillation and renal impairment, extremes in weight, or advanced age. Clin Cardiol. 2017;40:46–52.
Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–35.
Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016;149:951–9.
Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171:181.
Glund S, Stangier J, van Ryn J, Schmohl M, Moschetti V, Haazen W, et al. Effect of age and renal function on idarucizumab pharmacokinetics and idarucizumab-mediated reversal of dabigatran anticoagulant activity in a randomized, double-blind, crossover phase ib study. Clin Pharmacokinet. 2017;56:41–54.
Harel Z, Chertow GM, Shah PS, Harel S, Dorian P, Yan AT, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta-analysis. Can J Cardiol. 2017;33:737–46.
Bonde AN, Lip GYH, Kamper AL, Staerk L, Torp-Pedersen C, Gislason G, et al. Renal function, time in therapeutic range and outcomes in warfarin-treated atrial fibrillation patients: a retrospective analysis of nationwide registries. Thromb Haemost. 2017;117:2291–9.
Poterucha TJ, Goldhaber SZ. Warfarin and vascular calcification. Am J Med. 2016;129(635):e631–4.
De Vriese AS, Caluwe R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin k antagonist replacement by rivaroxaban with or without vitamin k2 in hemodialysis patients with atrial fibrillation: the Valkyrie study. J Am Soc Nephrol. 2020;31:186–96.
Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519–29.
Pokorney S. Renal hemodialysis patients allocated apixaban versus warfarin in atrial fibrillation—RENAL-AF. American Heart Association Annual Scientific Sessions (AHA 2019); 2019.
Okumura K, Lip GYH, Akao M, Tanizawa K, Fukuzawa M, Abe K, et al. Edoxaban for the management of elderly Japanese patients with atrial fibrillation ineligible for standard oral anticoagulant therapies: rationale and design of the Eldercare-AF study. Am Heart J. 2017;194:99–106.
Kefer J, Tzikas A, Freixa X, Shakir S, Gafoor S, Nielsen-Kudsk JE, et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int J Cardiol. 2016;207:335–40.
Fox KAA, Eikelboom JW, Shestakovska O, Connolly SJ, Metsarinne KP, Yusuf S. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: from the compass trial. J Am Coll Cardiol. 2019;73:2243–50.
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering ldl cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92.
Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157:263–75.
Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;(5):CD007784.
Yan YL, Qiu B, Wang J, Deng SB, Wu L, Jing XD, et al. High-intensity statin therapy in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ Open. 2015;5:e006886.
Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:1785–822.
Kassimatis TI, Goldsmith DJ. Statins in chronic kidney disease and kidney transplantation. Pharmacol Res. 2014;88:62–73.
Chen Y, Ku H, Zhao L, Wheeler DC, Li LC, Li Q, et al. Inflammatory stress induces statin resistance by disrupting 3-hydroxy-3-methylglutaryl-coa reductase feedback regulation. Arterioscler Thromb Vasc Biol. 2014;34:365–76.
Chen Z, Qureshi AR, Parini P, Hurt-Camejo E, Ripsweden J, Brismar TB, et al. Does statins promote vascular calcification in chronic kidney disease? Eur J Clin Invest. 2017;47:137–48.
Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48.
Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407.
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87.
Li Y, Liang M, Jiang C, Wang G, Li J, Zhang Y, et al. Impact of achieved blood pressure on renal function decline and first stroke in hypertensive patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33:409–17.
Cheung AK, Chang TI, Cushman WC, Furth SL, Ix JH, Pecoits-Filho R, et al. Blood pressure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:1027–36.
Perkovic V, Ninomiya T, Arima H, Gallagher M, Jardine M, Cass A, et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the progress study. J Am Soc Nephrol. 2007;18:2766–72.
Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (sps3) randomized trial. Circulation. 2016;133:584–91.
Park J, Rhee CM, Sim JJ, Kim YL, Ricks J, Streja E, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013;84:795–802.
Mathew A, Eliasziw M, Devereaux PJ, Merino JG, Barnett HJ, Garg AX. Carotid endarterectomy benefits patients with CKD and symptomatic high-grade stenosis. J Am Soc Nephrol. 2010;21:145–52.
Klarin D, Lancaster RT, Ergul E, Bertges D, Goodney P, Schermerhorn ML, et al. Perioperative and long-term impact of chronic kidney disease on carotid artery interventions. J Vasc Surg. 2016;64:1295–302.
Fallon JM, Goodney PP, Stone DH, Patel VI, Nolan BW, Kalish JA, et al. Outcomes of lower extremity revascularization among the hemodialysis-dependent. J Vasc Surg. 2015;62:1183–1191.e1181.
Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated society for vascular surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31.
Cooper M, Arhuidese IJ, Obeid T, Hicks CW, Canner J, Malas MB. Perioperative and long-term outcomes after carotid endarterectomy in hemodialysis patients. JAMA Surg. 2016;151:947–52.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295.
Zhou Z, Lindley RI, Radholm K, Jenkins B, Watson J, Perkovic V, et al. Canagliflozin and stroke in type 2 diabetes mellitus. Stroke. 2019;50:396–404.
Guo M, Ding J, Li J, Wang J, Zhang T, Liu C, et al. Sglt2 inhibitors and risk of stroke in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1977–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cathain, D.N., Kelly, D.M. (2023). Cerebrovascular Disease and Chronic Kidney Disease. In: Arıcı, M. (eds) Management of Chronic Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-42045-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-42045-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42044-3
Online ISBN: 978-3-031-42045-0
eBook Packages: MedicineMedicine (R0)