Abstract
During the last few decades, much research has been performed concerning vestibular implants. At the moment, they are mostly used to treat bilateral vestibulopathy, a very debilitating disorder for which no other effective treatment options exist. The concept is similar to that of a cochlear implant, which has already been used for many years to treat severe sensorineural hearing loss. The vestibular implant aims to artificially restore vestibular function through electrical stimulation of the vestibular nerve branches. This chapter provides an overview of current knowledge on vestibular implant candidacy, surgical techniques, device subtypes, stimulation profiles, clinical outcomes, and possible complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vestibular implant
- Vestibular prosthesis
- Bilateral vestibulopathy
- Vertigo
- Artificial vestibular function
- VI-implantation criteria
- Semicircular canal implant
- Otolith implant
- Dizziness
- Balance
Introduction
During the last few decades, much research has been performed to develop an implantable vestibular prosthesis to artificially restore vestibular function. The concept is similar to a cochlear implant (CI), which has already been used for many years for treating severe sensorineural hearing loss. The vestibular implant (VI) aims to provide the central nervous system with information about head spatial orientation and movement. The pioneering work of Bernard Cohen and Jun-Ichi Suzuki in the 1960s, who first described eye movements originating from electrically stimulating the ampullary nerves of rabbits, pigeons, cats, and monkeys [1], inspired Gong and Merfeld, who described the first vestibular prosthesis that was tested in guinea pigs [2]. Those animal experiments paved the way for human research. In 2004, the first human patients underwent vestibular stimulation before undergoing surgery for cochlear implantation or surgical labyrinthectomy [3]. In these trials, it was confirmed that electrical stimulation of the ampullary nerves could produce a nystagmic response aligned with the plane of the stimulated canal. After this proof of concept, Guyot et al. performed the first vestibular implantation in humans in 2007 [4]. This implant was a modified CI (MED-EL, Innsbruck, Austria), in which one electrode was removed from the cochlear array and implanted in the vicinity of the posterior ampullary nerve. It was demonstrated that a patient could adapt to electrical stimulation of the vestibular system without too much discomfort and that, once the adaptation was completed, the electrical stimulation could be modulated to artificially evoke smooth eye movements [5]. As these prerequisites for developing a VI were fulfilled, the implant was modified into a multichannel prosthesis and implanted for the first time in 2012 by the Geneva-Maastricht Group [6]. At the moment, two main types of VIs exist: the semicircular canal implants and the otolith implants. There are two subtypes of semicircular canal (SCC) implants: pure VIs and cochleovestibular implants, which combine a CI with a VI.
In this chapter, we provide an overview of current knowledge concerning VI candidacy, operative techniques, device programming, clinical outcomes, possible complications, and a future outlook.
Candidacy
To date, VIs are only available in a research setting, and therefore no reimbursement criteria exist. They are mainly developed to treat patients with bilateral vestibulopathy (BV). This is a very debilitating disorder that can lead to a broad spectrum of symptoms like, for example, postural instability, impairment of spatial orientation, and distorted vision in dynamic conditions (i.e., while walking), commonly known as oscillopsia. It has been conservatively estimated that more than 1.8 million people worldwide have severe bilateral vestibular loss, but this is probably an underestimation [7]. To date, BV has a poor prognosis, as more than 80% of the patients experience no significant improvement despite vestibular rehabilitation [8]. Vestibular implantation could be the solution for treating this debilitating condition.
To facilitate comparison of results from different studies, VI-implantation criteria were proposed by van de Berg et al. [9] (Table 15.1) in cooperation with all current research groups. The diagnostic criteria for BV, according to the Bárány Society, were modified and extended because they are solely based on the horizontal vestibular-ocular reflex (VOR) function. As vestibular implantation is practically irreversible and can cause a deterioration of residual vestibular functions, it was important to include the function of all three SSCs. For the otolith implants, cervical and ocular vestibular myogenic potentials (cVEMP and oVEMP) were included in the criteria as well.
Possibly, more extensive criteria, including patients with fluctuating vestibular disorders, elderly patients with presbyvestibulopathy, pediatric patients, or chronically uncompensated unilateral vestibulopathy, might be considered in the future. Additionally, bilateral VIs could also be considered. Interestingly, research has already demonstrated that VI information is able to overrule residual natural vestibular information [10, 11]. This could lead to using a VI as a “vestibular pacemaker” in cases of a fluctuation of the vestibular function and vertigo attacks in disorders like Meniere’s disease [10]. Former research, however, demonstrated a loss of residual vestibular function as a consequence of the implantation surgery [11]. Therefore, at the moment, the use of the VI as a pacemaker should be considered only in patients with no “useful” vestibular function.
SSC Implants
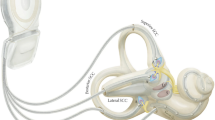
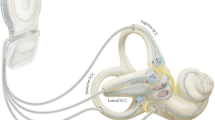
To date, most research has been done on SSC implants. Each research group has a specific subtype. The group of Geneva-Maastricht uses a vestibulocochlear implant manufactured by the company MED-EL (Innsbruck, Austria) (Fig. 15.1).
The system comprises 12 stimulation contacts. The three most basal electrodes from the cochlear array were taken out and replaced by three individual electrode branches. These three individual branches were designed to be inserted into the SSCs to allow for stimulation of the vestibular ampullary nerves. The remaining nine stimulation contacts make up the cochlear array, designed to be inserted into the cochlea like a CI [12]. At the moment, the Geneva-Maastricht Group only uses the VI on patients with severe hearing loss, as the chances of hearing loss from implanting the vestibular organs are high. All patients were candidates for CI surgery, which reduced the surgical risks and increased the benefit for the patients.
The Baltimore group uses a VI without a CI, also manufactured by the company MED-EL (Innsbruck, Austria) [13, 14]. This implant consists of three electrode arrays (each for one SSC) with three stimulating contacts per array and one reference electrode.
The implant of the Washington group is similar to the vestibulocochlear implant of the Geneva-Maastricht Group but consists of a combined 16-channel cochlear and six-channel vestibular prosthesis (two stimulating contacts in each vestibular branch) (Cochlear Ltd., Sydney, Australia) [15, 16]. The initial design did not include a cochlear array [11], which was added to the second generation of the implant.
Surgical Techniques
Two surgical techniques were developed over the past years: the extralabyrinthine and the intralabyrinthine approaches [6].
The Intralabyrinthine Approach
The first step of the intralabyrinthine approach is a cortical mastoidectomy and posterior tympanotomy. Subsequently, the anterior ends of the anterior and lateral canals are blue-lined by drilling cranially at the dome of the lateral canal and following it until a V-shape appears.
Then, the SCCs are carefully fenestrated [12, 13], and the electrodes are inserted as close as possible to the ampullary nerves (Figs. 15.2, 15.3 and 15.4).
The posterior canal is located medially to the facial nerve, at an imaginary horizontal line through the stapes footplate, between the sigmoid sinus and the facial nerve. Again, the SCC is fenestrated, and an electrode is positioned close to the ampullary nerve. Finally, the canals are closed with fascia and bone chips [13], glass ionomer (Ketac), or hydroxylapatite bone cement and fibrin sealant [12]. This technique carries a risk of sensorineural hearing loss as the inner ear is opened.
The Extralabyrinthine Approach
In the extralabyrinthine approach, the electrodes are placed outside the bony labyrinth, close to the ampullary branches of the vestibular nerves. The posterior ampullary nerve is reached by a transmeatal approach, modifying the technique of Gacek for treating benign paroxysmal positional vertigo [17]. The floor of the round window niche is drilled in its most rostral part, followed by a blue-lining of the nerve and electrode placement. The lateral and anterior ampullary nerves are reached after removal of the malleus head and the incus. The nerves can be reached after drilling ventral to the prominence of the lateral SCC, inferior to the tegmental roof, and superior to the facial canal.
Electrode fixation is extremely difficult in the extralabyrinthine technique. Also, conductive hearing loss is possible due to the removal of the malleus head and incus body, but by performing a type III ossiculoplasty (small columella) during the same surgery, a good postoperative hearing can be expected. The proximity to the facial nerve, however, increases the risk of a perioperative lesion in this extralabyrinthine technique. Additionally, the superior and lateral ampullary nerves are close to each other outside the labyrinth. Selective stimulation of both structures remains challenging. The main advantages, however, are the electrode positioning close to the ampullary nerves and the fact that this approach does not require opening the labyrinth, which reduces the risk of sensorineural hearing loss compared to the intralabyrinthine technique. Due to the greater disadvantages of the extralabyrinthine approach, most groups prefer the intralabyrinthine technique for the majority of patients. However, both techniques could be used as complementary procedures or as alternatives, depending on the specific pathology and the selected patient [6].
Intraoperative Measurements
The intralabyrinthine approach implies an almost blind insertion of the electrodes, and therefore, it remains difficult to estimate how far the electrodes should be inserted into the SCCs to be in close contact with the sensory epithelium of the ampullary nerves. Intraoperative objective measurements could thus help define the optimal electrode position.
Tonic eye movements can be evoked during surgery. If the eye movements elicited are in the plane of the stimulated canal, the electrode might be correctly positioned; if not, the position can be modified. It is necessary to lower anesthesia to measure these reflexes, especially propofol [12]. Finding the correct level of anesthesia for these corrections is difficult and time-consuming. An alternative is performing the surgery under local anesthesia, though this is very demanding for the patient.
Additionally, implant telemetry measurements can be performed, such as intraoperative electrode impedances and electrically evoked compound action potentials (eCAPs). Depending on the implant design, these measurements can be done in the same canal, between the different vestibular canals, or between the cochlea and the different canals [11, 18, 19]. eCAPs are not always present; they have many morphologic differences, and the exact meaning and long-term relevance of these eCAP measurements remain largely unknown. So although vestibular eCAPs are a good sign of stimulating the vestibular nerves, more research is necessary to implement them as a reliable clinical tool [11, 18,19,20].
Perioperative imaging techniques can also be used to define optimal electrode positioning. One of them is fluoroscopy, an imaging technique that captures moving images in real-time using X-rays. Fluoroscopy has already been useful in cochlear implantation in cases of difficult cochlear anatomy [21, 22]. Additionally, a study on cadaveric human heads demonstrated the utility of this technique in vestibular electrode insertion [23]. With fluoroscopy-guided imaging, the electrodes could be correctly inserted in 94% of the 18 SCCs, compared to 75% with blind insertion (Fig. 15.5).
Further research should investigate the value of this technique in the operating room, taking into account long-term follow-up. An intraoperative CT scan is another interesting option. Additionally, other tools for correct electrode placement should be developed and evaluated.
Stimulation Profile
In current SCC implants, a motion sensor is rigidly fixated on the patient’s head, where it measures head angular velocity in all axes of movement. The measured signals are then transformed into relevant electric signal patterns, which are delivered to the vestibular nerves by the implanted electrodes.
At the moment, vestibular reflexes are restored by implanting only one ear. This requires re-establishing a baseline electrical activity, which can be increased or decreased to allow for encoding bi-directional head movements. The most commonly used waveform is a biphasic, charge-balanced pulse train (100–400 μs/phase) presented at a rate of 200–400 pulses per second [24]. The stimulation has to be charge-balanced (the amount of charge given to the nerve is the same as the amount of charge drawn out of the nerve) because the accumulation of charge could lead to neural damage [25]. This excludes the use of monophasic stimulation, as chemical reversibility of the neural stimulation is essential [26]. The duration and amplitude of the stimulation should be high enough to sufficiently stimulate a nerve, but not too high, as this could lead to tissue damage, spurious current spread, and excessive power consumption. The phase duration has to be as short as possible, as shorter phase durations seem to facilitate broader dynamic ranges for electrical stimulation and allow faster stimulation rates. However, very short phase durations seem to be less effective for generating electrically evoked vestibulo-ocular responses. One study compared different stimulation profiles in one patient [25]. The 200 μs/phase profile presented the best balance to enhance responses at low stimulation currents while still allowing a good dynamic range [27]. The pulse frequency should be as high as possible because stimulating faster lowers stimulation thresholds (until the point of saturation is reached). A higher frequency thereby causes an increase in VOR magnitude and leads to less current spread.
In the Geneva-Maastricht Group, the amplitude of the baseline stimulation is mostly set in the middle of the dynamic range of each patient. The dynamic range is the range between the lowest perception threshold and the upper comfortable level, or the level immediately below the presentation of unwanted responses such as facial nerve activation. Sometimes a supranormal baseline is chosen to reduce the asymmetry of the electrically evoked VOR responses (the response to an inhibitory signal tends to be lower than that of an excitatory signal). However, in a study by Crétallaz et al. [25], no differences were found between a baseline of 30%, 50%, or 70% of the dynamic range.
This baseline stimulation can cause vestibular symptoms similar to those experienced by patients with sudden unilateral vestibular loss (e.g., nystagmus) that attenuate after a variable period of a maximum of 30 min (adaptation). After repeated on-off transitions, the adaptation period diminishes to only a few minutes without discomfort [5]. In response to rotation of the head, this baseline can then be up-modulated or down-modulated. Amplitude modulation, frequency modulation, or co-modulation (both amplitude and frequency) can be used. A good balance has to be found between sufficient response and the current spread. Gain is increased by a higher amplitude or a higher pulse rate. Pulse amplitude modulation is suggested to be the preferred strategy for VI modulation, as it evokes larger amplitude eye movement responses than pulse rate modulation [25]. However, higher amplitudes not only generate a higher magnitude of the VOR, but they also cause a higher amount of current spread, possibly by expanding the electrical field and therefore recruiting afferents in an adjacent canal, leading to more misalignment or even facial nerve or cochlear stimulation [28]. Therefore, co-modulation of pulse rate and amplitude also appeared to be a promising stimulation program to maximize eVOR velocities in animal studies.
The combination of a VI with a CI leads to additional challenges concerning the optimization of the stimulation profiles. When a VI is combined with a CI, concurrent stimulation could also affect vestibular-evoked responses (i.e., alter the magnitude and direction of eye movements) and/or auditory performance (i.e., perceived pitch and loudness, speech recognition). These concurrent stimulations have already been reported both with CI and VI only stimulation and in combined vestibulocochlear systems [15, 29]. So far, the results of different modulation programs also remain highly variable across patients. This could be due to the variable central compensation and distribution of regular versus irregular afferents in each patient. At the moment, the fitting of the VI needs to be done for each canal individually. This is very time-consuming. More research remains necessary to obtain an optimal stimulation profile and facilitate fitting procedures that can be generalized to the majority of patients.
Outcome
Once the proof of the feasibility of a VI in humans was achieved, many studies investigating different outcome measures followed. The VOR was the most frequently used outcome measure because the improvement of oscillopsia is one of the main objectives. As already mentioned, eye movements could be electrically evoked predominantly in the plane of all three stimulated canals [6]. This electrically evoked VOR was elicited in patients with unilateral Meniere’s disease [11, 30] and patients with BV [12]. The mean peak eye velocities were within the range of compensatory eye movements reported during important dynamic daily activities, such as walking or running (20–30°/s) [12].
Furthermore, the possibility of achieving an artificial VOR during rotatory chair testing was demonstrated [31, 32]. The artificial VOR showed the same frequency dependency characteristics as the natural VOR; in the low frequencies, the VOR was almost absent but increased at 1 and 2 Hz, similar to the natural reflex [31, 33].
To evaluate the higher frequencies of the angular VOR, one study performed the video head impulse test in patients with a VI [24]. When the VI was “on,” there was an increased gain as well as a decrease in corrective saccades. On the contrary, reversing the transfer function of the implant (i.e., inhibitory stimulation for excitatory head movement) led to a negative or reversed VOR gain (i.e., eyes moving in the same direction as the head). Additionally, there was an increase in the amplitude and number of corrective saccades. Interestingly, there was high interelectrode variability. Also, in most cases, the gain for the excitatory head impulse test (movement toward the implanted site, leading to an excitatory signal) was superior to the inhibitory impulse (movement away from the implanted site, leading to an inhibitory signal). This functional asymmetry is not surprising, as only unilateral stimulation was performed.
The electrically evoked VOR was not always precisely aligned with the stimulated canal, most likely due to current spread to the other canals and/or otolith organs [6, 12]. This misalignment could probably be diminished by an optimization of the electrode position and stimulation profile. Additionally, animal studies (in chinchillas and nonhuman primates) have shown an improvement of the misalignment after 7 days of continuous stimulation due to central compensation [34, 35].
In previous studies, each canal was activated separately. In 2019, Boutros et al. [14] evaluated the effect of targeting multiple canals simultaneously. This simultaneous stimulation evoked responses aligned with the vector sum of all individual responses.
Another interesting finding in previous studies was the fact that eye movements could be successfully evoked regardless of the etiology of the vestibular deficit or the duration of the disease. Case reports of patients who had no vestibular function for 20–50 years have been published [6, 12, 31]. This is a very important finding, as there was some concern about the degeneration of dendrites over time causing a decrease in stimulation potential. Concerning the etiology of vestibular loss, the small sample sizes of current studies impede statistical analysis. However, in the current cohort, DFNA9 patients showed the smallest responses [12]. This could be due to a severe loss of cochleovestibular nerve dendrites.
Previous results were only studied shortly after activation of the implant. In 2019, the first long-term study was performed by the Baltimore group [14]. Four human patients received continuous stimulation of all three ampullary nerves 24 h a day for 1–2 years. Although animal studies raised concerns about a degradation of the effect of the VI after prolonged stimulation, in these four human cases, the electrically evoked eye movements persisted after long-term use of the implant [14].
Next to the VOR function, Perez Fornos et al. studied the effect of the VI on the vestibulo-colic and vestibullo-spinal pathways. Electrically elicited cervical vestibular evoked myogenic potentials (ecVEMPs) with similar characteristics as the classically acoustically elicited cVEMPs could be recorded upon stimulation in five out of eight patients. Additionally, a stepping test was performed on three patients. The inhibitory conditions led to a head and thorax rotation toward the implanted side, while excitation resulted in rotations toward the nonimplanted side [36]. This was also found in a study by Phillips et al. [30]. These results prove that VIs can activate the vestibulo-colic pathway and induce controlled postural responses. This could be due to current spread to the otoliths but also due to possible ampullary projections to the vestibulo-colic and vestibulo-spinal pathways, for example, in converging vestibular nuclei neurons.
The first proof of functional rehabilitation by the VI was demonstrated by the restoration of visual acuity in dynamic situations [37]. When walking, patients with BV often suffer from a significant loss of visual acuity, presumably due to a diminished VOR function. The dynamic visual acuity during walking could be restored to close to normal values in all studied patients after turning on the VI [37].
Additionally, Chow et al. [13] reported an improvement in posture and gait after long-term continuous vestibular stimulation. Finally, a subjective improvement in quality of life was demonstrated 6 months and 1 year after vestibular implantation [13].
Otolith Implants
Evidence concerning the implantation of the SCCs is growing, yet studies concerning otolith implants remain scarce. Compared to the relatively straightforward anatomy of the SCCs, the anatomy and physiology of the otolith organs are much more complex. The hair cells and afferent nerve fibers inside the utricle and saccule have different directional sensitivities compared to the SCCs, which have a unidirectional sensitivity. Furthermore, the otolith organs comprise complex information on head translation in three dimensions and signals of head position with respect to gravity, including “Static” tilts and “dynamic” head accelerations. Moreover, otolith-ocular reflexes are small and widespread, which makes it more difficult to study the results of otolith stimulation [38]. Recently, two cases of implantation of the otolith organs in humans were reported [39]. The VI comprised a CI (CI24RE, Cochlear Ltd.) of which three electrodes were used for vestibular implantation. The implant was combined with a supplementary CI, with a full electrode array inserted into the cochlea.
The procedure started with a mastoidectomy and posterior tympanotomy, followed by regular cochlear implantation. Subsequently, the vestibule was opened using a carbondioxide laser, aiming to reach the inferior vestibular nerve afferents near the saccular macula. The three first contacts of the VIs were inserted in the vestibule, and the electrodes were fixated at the oval window, the fossa incudis, and the cortical edge of the mastoidectomy.
Outcomes
The first experiment on otolith stimulation was performed perioperatively in four patients suffering from definite unilateral Meniere’s disease. Three channels of a CI were inserted in the vestibule before the labyrinthectomy. eCAPs and oVemps could be evoked in all ears. eCAPS could be obtained in 10 out of 12 channels, and the amplitude growth function followed the same behavior as in the auditory nerve in all cases, which was considered an indication of neural viability. Thereafter, the electrodes were removed and the patients were implanted with a regular CI [40].
Subsequently, three patients were implanted with an otolith-stimulating VI. Intraoperative eCAPS were used in combination with electrically elicited oVEMPs to define the optimal electrode position. In all patients, cVEMPs were absent before surgery, but electrically evoked cVEMPs could be obtained afterward. The video head impulse test gain was found without changes in all subjects [41].
Additional tests were performed on two patients. Subjective visual vertical testing improved in one out of two patients. Further findings included improvements in computerized dynamic posturography, dynamic gait index, and Time UP and GO tests in both cases. The dizziness handicap index also improved in both patients. None of these tests were placebo-controlled [39].
Complications/Risks
The most important possible complication of vestibular implantation is hearing loss, which can vary from mild hearing loss to complete deafness. This is why most groups until now have implanted only patients with severe hearing loss, and the VI is combined with a CI. The Baltimore group is the only group that does not use a combined cochlear-vestibular procedure at the moment. They reported hearing loss in seven out of eight implanted patients (87.5%). In three patients, hearing loss was severe; the other four patients only had a modest hearing loss (3–16 dB) [13]. The group of Phillips et al. [11, 19] implanted patients with Meniere’s disease. In all patients, hearing loss deteriorated profound sensorineural hearing loss without measurable speech discrimination. Therefore, their second-generation implant was provided with a CI as well. On the contrary, animal studies and some cases have shown that it is possible to preserve hearing [42,43,44]. Possibly, hearing loss could be reduced by improving surgical techniques in addition to newer electrode designs. Also, some etiologies could be more prone to hearing loss than others.
Like hearing, residual vestibular function can also deteriorate due to implantation [11, 30]. In addition, vestibular stimulation can also cause sound, tinnitus, pressure, transient imbalance, dysgeusia, and facial twitching and tingling, but this can be diminished by reducing the stimulation current [12, 13]. These manifestations could be due to current spread to the cochlea and facial nerve, but they could also be due to saccular stimulation because of saccular projections to the cochlear nucleus.
Conclusion
The VI is an upcoming treatment modality for BV and possibly even broader indications. Current evidence already suggests the possible positive effects of the VI for vestibular patients. So far, the number of studied patients is small, however, and only a few long-term studies have been reported. At the moment, research groups are conducting studies to refine surgical techniques, stimulation paradigms, and rehabilitation procedures, as all these topics are essential for a clinically useful VI.
References
Guyot JP, Guinand N, Perez Fornos A. Tribute to Bernard Cohen - whose pioneering work made the vestibular implant possible. Front Neurol. 2020;11:452. https://doi.org/10.3389/fneur.2020.00452.
Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28(5):572. https://doi.org/10.1114/1.293.
Wall C, Kos MI, Guyot J-P. Eye movements in response to electric stimulation of the human posterior ampullary nerve. Ann Otol Rhinol Laryngol. 2007;116(5):369. https://doi.org/10.1177/000348940711600509.
Guyot JP, Perez Fornos A. Milestones in the development of a vestibular implant. Curr Opin Neurol. 2019;32(1):145–53. https://doi.org/10.1097/WCO.0000000000000639.
Guyot JP, Sigrist A, Pelizzone M, Kos MI. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otol Rhinol Laryngol. 2011;120(3):143–9. https://doi.org/10.1177/000348941112000301.
van de Berg R, Guinand N, Guyot JP, Kingma H, Stokroos RJ. The modified ampullar approach for vestibular implant surgery: feasibility and its first application in a human with a long-term vestibular loss. Front Neurol. 2012;3:18. https://doi.org/10.3389/fneur.2012.00018.
Ward BK, Agrawal Y, Hoffman HJ, Carey JP, della Santina CC. Prevalence and impact of bilateral vestibular hypofunction. JAMA Otolaryngol Head Neck Surg. 2013;139(8):803. https://doi.org/10.1001/jamaoto.2013.3913.
Zingler VC, Weintz E, Jahn K, et al. Follow-up of vestibular function in bilateral vestibulopathy. J Neurol Neurosurg Psychiatry. 2008;79(3):284. https://doi.org/10.1136/jnnp.2007.122952.
van de Berg R, Ramos A, van Rompaey V, et al. The vestibular implant: opinion statement on implantation criteria for research. J Vestib Res. 2020;30(3):213–23. https://doi.org/10.3233/ves-200701.
van de Berg R, Guinand N, Ranieri M, et al. The vestibular implant input interacts with residual natural function. Front Neurol. 2017;8:644. https://doi.org/10.3389/fneur.2017.00644.
Golub Justin S, Leo L, Nie K, et al. Prosthetic implantation of the human vestibular system. Otol Neurotol. 2014;35(1):136–47.
Guinand N, van de Berg R, Cavuscens S, et al. Vestibular implants: 8 years of experience with electrical stimulation of the vestibular nerve in 11 patients with bilateral vestibular loss. ORL J Otorhinolaryngol Relat Spec. 2015;77:227–40. https://doi.org/10.1159/000433554.
Chow MR, Ayiotis AI, Schoo DP, et al. Posture, gait, quality of life, and hearing with a vestibular implant. N Engl J Med. 2021;384(6):521–32. https://doi.org/10.1056/NEJMoa2020457.
Boutros PJ, Schoo DP, Rahman M, et al. Continuous vestibular implant stimulation partially restores eye-stabilizing reflexes. JCI Insight. 2019;4(22):e128397. https://doi.org/10.1172/jci.insight.128397.
Phillips JO, Ling L, Nowack A, Rebollar B, Rubinstein JT. Interactions between auditory and vestibular modalities during stimulation with a combined vestibular and cochlear prosthesis. Audiol Neurotol. 2020;25(Suppl):1–2. https://doi.org/10.1159/000503846.
Rubinstein JT, Ling L, Nowack A, Nie K, Phillips JO. Results from a second-generation vestibular implant in human subjects: diagnosis may impact electrical sensitivity of vestibular afferents. Otol Neurotol. 2020;41(1):68–77. https://doi.org/10.1097/MAO.0000000000002463.
Gacek RR. Transection of the posterior ampullary nerve for relief of benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1974;83:596–605.
Nguyen TAK, Cavuscens S, Ranieri M, et al. Characterization of cochlear, vestibular and cochlear-vestibular electrically evoked compound action potentials in patients with a vestibulo-cochlear implant. Front Neurosci. 2017;11:645. https://doi.org/10.3389/fnins.2017.00645.
Phillips JO, Ling L, Nie K, et al. Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol. 2015;113:3866–92. https://doi.org/10.1152/jn.00171.2013.-Animal.
Phillips C, Ling L, Oxford T, et al. Longitudinal performance of an implantable vestibular prosthesis. Hear Res. 2015;322:200–11. https://doi.org/10.1016/j.heares.2014.09.003.
Fishman AJ, Thomas Roland J, Alexiades G, Mierzwinski J, Cohen NL. Fluoroscopically assisted cochlear implantation. Otol Neurotol. 2003;24:882.
Coelho DH, Waltzman SB, Roland JT. Implanting common cavity malformations using intraoperative fluoroscopy. Otol Neurotol. 2008;29(7):914–9.
Stultiens JJA, Postma AA, Guinand N, Pérez Fornos A, Kingma H, van de Berg R. Vestibular implantation and the feasibility of fluoroscopy-guided electrode insertion. Otolaryngol Clin N Am. 2020;53(1):115–26. https://doi.org/10.1016/j.otc.2019.09.006.
Guinand N, van de Berg R, Cavuscens S, et al. The video head impulse test to assess the efficacy of vestibular implants in humans. Front Neurol. 2017;8:600. https://doi.org/10.3389/fneur.2017.00600.
Crétallaz C, Boutabla A, Cavuscens S, et al. Influence of systematic variations of the stimulation profile on responses evoked with a vestibular implant prototype in humans. J Neural Eng. 2020;17(3):036027. https://doi.org/10.1088/1741-2552/ab8342.
van de Berg R, Guinand N, Stokroos RJ, Guyot J-P, Kingma H. The vestibular implant: quo Vadis? Front Neurol. 2011;2:47. https://doi.org/10.3389/fneur.2011.00047.
Boutabla A, Cavuscens S, Ranieri M, et al. Simultaneous activation of multiple vestibular pathways upon electrical stimulation of semicircular canal afferents. J Neurol. 2020;267(S1):273–84. https://doi.org/10.1007/s00415-020-10120-1.
Nguyen TAK, Digiovanna J, Cavuscens S, et al. Characterization of pulse amplitude and pulse rate modulation for a human vestibular implant during acute electrical stimulation. J Neural Eng. 2016;13(4):046023. https://doi.org/10.1088/1741-2560/13/4/046023.
Wolter NE, Gordon KA, Campos JL, et al. BalanCI: head-referenced cochlear implant stimulation improves balance in children with bilateral cochleovestibular loss. Audiol Neurotol. 2020;25(1–2):60–71. https://doi.org/10.1159/000503135.
Phillips C, de Francisci C, Ling L, et al. Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp Brain Res. 2013;229(2):181–95. https://doi.org/10.1007/s00221-013-3604-3.
Fornos AP, Guinand N, van de Berg R, et al. Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol. 2014; https://doi.org/10.3389/fneur.2014.00066.
Pelizzone M, Fornos AP, Guinand N, et al. First functional rehabilitation via vestibular implants. Cochlear Implants Int. 2014;15(Suppl 1):S62–4. https://doi.org/10.1179/1467010014Z.000000000165.
van de Berg R, Guinand N, Khoa Nguyen TA, et al. The vestibular implant: frequency-dependency of the electrically evoked vestibulo-ocular reflex in humans. Front Syst Neurosci. 2015;8:255. https://doi.org/10.3389/fnsys.2014.00255.
Dai C, Fridman GY, Chiang B, et al. Directional plasticity rapidly improves 3D vestibulo-ocular reflex alignment in monkeys using a multichannel vestibular prosthesis. J Assoc Res Otolaryngol. 2013;14(6):863–77. https://doi.org/10.1007/s10162-013-0413-0.
Dai C, Fridman GY, Chiang B, et al. Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp Brain Res. 2011;210:595–606. https://doi.org/10.1007/s00221-011-2591-5.
Fornos AP, van de Berg R, Armand S, et al. Cervical myogenic potentials and controlled postural responses elicited by a prototype vestibular implant. J Neurol. 2019;266:33–41. https://doi.org/10.1007/s00415-019-09491-x.
Guinand N, van de Berg R, Cavuscens S, et al. Restoring visual acuity in dynamic conditions with a vestibular implant. Front Neurosci. 2016;10:577. https://doi.org/10.3389/fnins.2016.00577.
Hageman KN, Chow MR, Roberts D, et al. Binocular 3D otolith-ocular reflexes: responses of chinchillas to prosthetic electrical stimulation targeting the utricle and saccule. J Neurophysiol. 2020;123:259–76. https://doi.org/10.1152/jn.00883.2018.-From.
Macias AR, de Miguel AR, Montesdeoca IR, Barreiro SB, González JCF. Chronic electrical stimulation of the otolith organ: preliminary results in humans with bilateral vestibulopathy and sensorineural hearing loss. Audiol Neurotol. 2020;25(1–2):79–90. https://doi.org/10.1159/000503600.
Ramos de Miguel A, Falcon Gonzalez JC, Ramos Macias A. Vestibular response to electrical stimulation of the otolith organs. Implications in the development of a vestibular implant for the improvement of the sensation of gravitoinertial accelerations. J Int Adv Otol. 2017;13(2):154–61. https://doi.org/10.5152/iao.2017.4216.
Rodriguez Montesdeoca I, Ramos de Miguel A, González JCF, et al. Differences in vestibular-evoked myogenic potential responses by using cochlear implant and otolith organ direct stimulation. Front Neurol. 2021;12:663803. https://doi.org/10.3389/fneur.2021.663803.
Bierer SM, Ling L, Nie K, et al. Auditory outcomes following implantation and electrical stimulation of the semicircular canals. Hear Res. 2012;287(1–2):51–6. https://doi.org/10.1016/j.heares.2012.03.012.
Rubinstein JT, Bierer S, Kaneko C, et al. Implantation of the semicircular canals with preservation of hearing and rotational sensitivity. Otol Neurotol. 2012;33(5):789. https://doi.org/10.1097/MAO.0b013e318254ec24.
van de Berg R, Lucieer F, Guinand N, et al. The vestibular implant: hearing preservation during intralabyrinthine electrode insertion-a case report. Front Neurol. 2017;8:137. https://doi.org/10.3389/fneur.2017.00137.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Loos, E. et al. (2023). Vestibular Implants. In: Crane, B.T., Lustig, L., de Souza, C. (eds) Disorders of the Vestibular System. Springer, Cham. https://doi.org/10.1007/978-3-031-40524-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-40524-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40523-5
Online ISBN: 978-3-031-40524-2
eBook Packages: MedicineMedicine (R0)