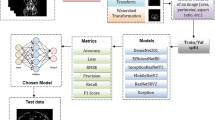
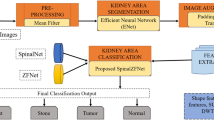
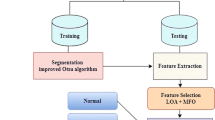
Abstract
Kidney diseases are the major reason for renal failure. Ranging from calcium deposits, stones, and to the maximum extent of chronic kidney disease, there are multiple classifications of that which may cause renal failure and lead to a large proportion of mortality. Qualitative Ultrasound images are usually preferred as the ground for examining the kidney in medical contexts. In recent times Computer-Aided Diagnosis of kidney health analysis has paved the way for the effective detection of diseases at early stages by employing convolutional Neural Networks and their allied versions of deep learning technologies. The availability of these algorithms in a simulated environment yields better results when compared to images taken in real-time cases. The performance of these algorithms is confined within a limited level of performance metrics such as accuracy and sensitivity. To address these issues, we have focussed on building an automated diagnosis of kidney diseases and classifying it according to their features illustrated in the QUS images. The anticipated methodology in this work merges the texture, statistical and histogram-based features (TSH) which are discriminative when compared with other features exhibited by the QUS, then these TSH features are employed in ResNet architecture for successful recognition of kidney diseases. The observance in the reduction of accuracy due to the improper training of the hyperparameters such as momentum and learning rate of CNN is obliterated with the usage of the position-based optimization algorithm, namely the Tree Seed Algorithm. The output of the classification was analysed through the performance analysis for the optimization-tuned kidney image standard dataset. The results from the ResNet model with TSA optimization show quite good efficiency of using an algorithmic approach in tuning deep learning architectures. Further exploration of the momentum and learning rate of the Resnet architecture makes the proposed TSH-TSA-Resnet architecture outperform the existing method and provide a classification accuracy of 98.9%.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Edvardsson, V. O., Indridason, O. S., Haraldsson, G., Kjartansson, O., & Palsson, R. (2013). Temporal trends in the incidence of kidney stone disease. Kidney International, 83(1), 146–152.
Kumar, K., & Abhishek, B. (2012). Artificial neural networks for diagnosis of kidney stones disease (Vol. 10). GRIN Verlag.
Serrat, J., Lumbreras, F., Blanco, F., Valiente, M., & López-Mesas, M. (2017). myStone: A system for automatic kidney stone classification. Expert Systems with Applications, 89, 41–51.
Howles, S. A., & Thakker, R. V. (2020). Genetics of kidney stone disease. Nature Reviews Urology, 17(7), 407–421.
Schaeffer, A. J., Feng, Z., Trock, B. J., Mathews, R. I., Neu, A. M., Gearhart, J. P., & Matlaga, B. R. (2011). Medical comorbidities associated with pediatric kidney stone disease. Urology, 77(1), 195–199.
Praveen, S. P., Srinivasu, P. N., Shafi, J., Wozniak, M., & Ijaz, M. F. (2022). ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Scientific Reports, 12, 20804. https://doi.org/10.1038/s41598-022-25089-2
Whitehurst, L., Jones, P., & Somani, B. K. (2019). Mortality from kidney stone disease (KSD) as reported in the literature over the last two decades: A systematic review. World Journal of Urology, 37(5), 759–776.
Kazemi, Y., & Mirroshandel, S. A. (2018). A novel method for predicting kidney stone type using ensemble learning. Artificial Intelligence in Medicine, 84, 117–126.
Novak, T. E., Lakshmanan, Y., Trock, B. J., Gearhart, J. P., & Matlaga, B. R. (2009). Sex prevalence of pediatric kidney stone disease in the United States: An epidemiologic investigation. Urology, 74(1), 104–107.
Ahmed, S., Srinivasu, P. N., Alhumam, A., & Alarfaj, M. (2022). AAL and internet of medical things for monitoring type-2 diabetic patients. Diagnostics, 12, 2739. https://doi.org/10.3390/diagnostics12112739
Sood, A., Sarangi, S., Pandey, A., & Murugiah, K. (2011). YouTube as a source of information on kidney stone disease. Urology, 77(3), 558–562.
Matlaga, B. R., Schaeffer, A. J., Novak, T. E., & Trock, B. J. (2010). Epidemiologic insights into pediatric kidney stone disease. Urological Research, 38(6), 453–457.
Scherer, K., Braig, E., Willer, K., Willner, M., Fingerle, A. A., Chabior, M., Herzen, J., Eiber, M., Haller, B., Straub, M., Schneider, H., & Pfeiffer, F. (2015). Non-invasive differentiation of kidney stone types using X-ray dark-field radiography. Scientific Reports, 5(1), 1–7.
Kahani, M., Tabrizi, S. H., Kamali-Asl, A., & Hashemi, S. (2020). A novel approach to classify urinary stones using dual-energy kidney, ureter and bladder (DEKUB) X-ray imaging. Applied Radiation and Isotopes, 164, 109267.
Thongprayoon, C., Krambeck, A. E., & Rule, A. D. (2020). Determining the true burden of kidney stone disease. Nature Reviews Nephrology, 16(12), 736–746.
Duan, X., Qu, M., Wang, J., Trevathan, J., Vrtiska, T., Williams, J. C., Krambeck, A., Lieske, J., & McCollough, C. (2013). Differentiation of calcium oxalate monohydrate and calcium oxalate dihydrate stones using quantitative morphological information from micro-computerized and clinical computerized tomography. The Journal of Urology, 189(6), 2350–2356.
Singh, P., Enders, F. T., Vaughan, L. E., Bergstralh, E. J., Knoedler, J. J., Krambeck, A. E., Lieske, J. C., & Rule, A. D. (2015, October). Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clinic Proceedings, 90(10), 1356–1365. Elsevier.
Motamedinia, P., Okhunov, Z., Okeke, Z., & Smith, A. D. (2015). Contemporary assessment of renal stone complexity using cross-sectional imaging. Current Urology Reports, 16(4), 1–7.
Caroli, A., Remuzzi, A., & Lerman, L. O. (2021). Basic principles and new advances in kidney imaging. Kidney International, 100(5), 1001–1011.
D’costa, M. R., Haley, W. E., Mara, K. C., Enders, F. T., Vrtiska, T. J., Pais, V. M., Jacobsen, S. J., McCollough, C. H., Lieske, J. C., & Rule, A. D. (2019). Symptomatic and radiographic manifestations of kidney stone recurrence and their prediction by risk factors: A prospective cohort study. Journal of the American Society of Nephrology, 30(7), 1251–1260.
Nestler, T., Haneder, S., & Hokamp, N. G. (2019). Modern imaging techniques in urinary stone disease. Current Opinion in Urology, 29(2), 81–88.
Cui, X., Zhao, Z., Zhang, G., Chen, S., Zhao, Y., & Lu, J. (2018). Analysis and classification of kidney stones based on Raman spectroscopy. Biomedical Optics Express, 9(9), 4175–4183.
Jendeberg, J., Thunberg, P., & Lidén, M. (2021). Differentiation of distal ureteral stones and pelvic phleboliths using a convolutional neural network. Urolithiasis, 49(1), 41–49.
Wang, R. C., Rodriguez, R. M., Moghadassi, M., Noble, V., Bailitz, J., Mallin, M., Carbo, J., Kang, T. L., Chu, P., Shiboski, S., & Smith-Bindman, R. (2016). External validation of the STONE score, a clinical prediction rule for ureteral stone: An observational multi-institutional study. Annals of Emergency Medicine, 67(4), 423–432.
Schütz, J., Miernik, A., Brandenburg, A., & Schlager, D. (2019). Experimental evaluation of human kidney stone spectra for intraoperative stone-tissue-instrument analysis using autofluorescence. The Journal of Urology, 201(1), 182–188.
Kavoussi, N. L., Floyd, C., Abraham, A., Sui, W., Bejan, C., Capra, J. A., & Hsi, R. (2022). Machine learning models to predict 24 hour urinary abnormalities for kidney stone disease. Urology, 169, 52–57.
Han, H., Mutter, W. P., & Nasser, S. (Eds.). (2019). Nutritional and medical management of kidney stones. Springer International Publishing.
Williams, J. C., Gambaro, G., Rodgers, A., Asplin, J., Bonny, O., Costa-Bauzá, A., Ferraro, P. M., Fogazzi, G., Fuster, D. G., Goldfarb, B. S., Grases, F., & Robertson, W. G. (2021). Urine and stone analysis for the investigation of the renal stone former: A consensus conference. Urolithiasis, 49(1), 1–16.
Deng, Y., Yang, B. R., Luo, J. W., Du, G. X., & Luo, L. P. (2020). DTI-based radiomics signature for the detection of early diabetic kidney damage. Abdominal Radiology, 45(8), 2526–2531.
Singla, R., Ringstrom, C., Hu, G., Lessoway, V., Reid, J., Nguan, C., & Rohling, R. (2022). The open kidney ultrasound data set. arXiv preprint arXiv:2206.06657.
Kuo, C. C., Chang, C. M., Liu, K. T., Lin, W. K., Chiang, H. Y., Chung, C. W., Ho, M. R., Sun, P. R., Yang, R. L., & Chen, K. T. (2019). Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digital Medicine, 2(1), 29.
Sudharson, S., & Kokil, P. (2020). An ensemble of deep neural networks for kidney ultrasound image classification. Computer Methods and Programs in Biomedicine, 197, 105709.
Verma, J., Nath, M., Tripathi, P., & Saini, K. K. (2017). Analysis and identification of kidney stone using K th nearest neighbour (KNN) and support vector machine (SVM) classification techniques. Pattern Recognition and Image Analysis, 27, 574–580.
Selvarani, S., & Rajendran, P. (2019). Detection of renal calculi in ultrasound image using meta-heuristic support vector machine. Journal of Medical Systems, 43(9), 300.
Kokil, P., & Sudharson, S. (2019). Automatic detection of renal abnormalities by Off-the-shelf CNN features. IETE Journal of Education, 60(1), 14–23.
Acknowledgements
All the authors would like to thank both the Department of Computer Science and Engineering and the Department of Electronics and Communication Engineering, at Kalasalingam Academy of Research and Education (Deemed to be University) for permission to conduct the research and provide computational facilities in the analysis of the images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nagaraj, P., Muneeswaran, V., Jeyanathan, J.S., Panda, B., Bhoi, A.K. (2023). Optimized TSA ResNet Architecture with TSH—Discriminatory Features for Kidney Stone Classification from QUS Images. In: Barsocchi, P., Parvathaneni, N.S., Garg, A., Bhoi, A.K., Palumbo, F. (eds) Enabling Person-Centric Healthcare Using Ambient Assistive Technology. Studies in Computational Intelligence, vol 1108. Springer, Cham. https://doi.org/10.1007/978-3-031-38281-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-38281-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-38280-2
Online ISBN: 978-3-031-38281-9
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)