Abstract
Portal vein thrombosis (PVT) is a condition characterized by narrowing or occlusion of portal vein that is usually seen in patients with hepatic cirrhosis or malignancies, but presentation in healthy young patients is rare. In a non-cirrhotic liver, primary myeloproliferative disorders (MPD) are the most common procoagulant state associated with PVT. Non-cirrhotic nonmalignant acute PVT usually presents with abdominal pain (91%), fever (53%), and ascites (38%). Extension of portal vein thrombus into a superior mesenteric vein may lead to intestinal ischemia, bowel infarction, and sepsis and is responsible for high mortality in this subset of patients. We herein report a relatively rare presentation of myeloproliferative disease in a 42-year-old woman who sought medical attention for fever and abdominal pain associated with high levels of CA-125. Standard diagnostic workup did not reveal clear PVT signs. The thrombosis progressively involved splenic and superior mesenteric veins leading to bowel perforation and septic shock. Recovery was slow and optimal long-term management with anticoagulant is still under question. Despite a wide range of therapeutic options for the management of non-cirrhotic PVT are described in the literature, the efficacy of those available therapies in advanced and complicated cases like this is not well established.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Portal vein thrombosis
- Mesenteric vein thrombosis
- Procoagulant state
- Myeloproliferative disease
- Case report
- Anchoring bias
- Medical education
-
Consider procoagulant states in patients with abdominal thrombosis and no risk factors for hepatopathies or malignancies.
-
Increase awareness of the complexity associated with portal vein thrombosis among the medical community.
-
Management of splanchnic vein thrombosis and prehepatic hypertension with discussion of the benefits and risks of anticoagulant medications.
-
The importance of continuous reassessment of patients without making judgment based on previous information to avoid potentially dangerous anchoring biases.
1 Introduction
Portal vein thrombosis (PVT) is a condition, usually associated with hepatic cirrhosis or hypercoagulable syndromes, in which thrombi are formed within portal venous system and can extend to intrahepatic portal vein branches or to splenic and superior mesenteric veins, but it is only rarely recognized in otherwise healthy patients [1]. Many local and systemic factors, including intra-abdominal inflammation, liver tumors or cirrhosis, viral infections, congenital or acquired prothrombotic mutations, and myeloproliferative disorders, can be associated with splanchnic venous system thrombosis (SVT). We present a case of a 42-year-old lady, whose medical history was unremarkable, who has recently come to our attention for fever, ascites, abdominal pain, and hepatosplenomegaly associated with undiagnosed splanchnic venous thrombosis that evolved to intestinal perforation and septic shock. This case shows how misleading clinical presentation of a relatively uncommon disorder has determined delay in the diagnosis and therefore has led to an extremely severe septic shock sustained by multiresistant germs.
Case Presentation
A 42-year-old white Caucasian woman presented to our Emergency Department (ED) with fever up to 39 °C and abdominal pain. Her previous medical history was unremarkable, except for microcytic anemia, Gilbert disease, and previous Epstein Barr Virus (EBV) infection in February 2022. She was also vaccinated against SARS-CoV2 with two jabs, the last administered in December 2021. Two days before ED admission, the patient got an abdomen computed tomography (CT) scan prescribed by her gynecologist because he detected free fluid in the Douglas pouch and high levels of CA-125 during a routine gynecological examination. The CT scan did not show any findings suggestive of genital malignancy but revealed instead a mild chronic hepatopathy with ascites and splenomegaly. On admission to the ED, the patient was febrile (38.5 °C), hemodynamically stable, GCS 15, mild pain on deep and superficial abdomen palpation. Blood specimens for generic blood tests and culture were withdrawn and sent to the laboratory. Results are reported in ◘ Table 3.1.
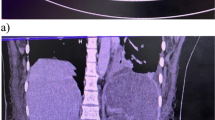
She was therefore admitted to the Internal Medicine ward and antibiotic therapy with piperacillin/tazobactam was started empirically. On the third day of hospitalization, her white blood cells (WBC) count increased, and a new CT scan showed complete thrombosis of the portal vein, partial recent thrombosis of the splenic vein, and severe distension of intestinal loops (◘ Fig. 3.1). The patient was therefore started on enoxaparin, parenteral nutrition, and diuretics. Afterwards, the patient’s clinical condition progressively deteriorated since she developed bilateral pleural effusion with progressive hypoxemic respiratory failure and severe diarrhea that led to hemodynamic instability and Intensive Care Unit (ICU) admission for severe hypovolemic shock.
She underwent emergency laparotomy with drainage of six liters of peritoneal fluid, bowel manipulation with lysis of adherences and section of a necrotic, perforated, intestinal loop. Because of the severe inflammation and massive bleeding, abdomen was temporarily closed with a Bogota-bag and, after two abdominal cavity revisions for bleeding control, vacuum-assisted abdominal closure was adopted. After surgery, the patient’s condition was still critical, and she remained on high-dose vasopressors (noradrenaline and terlipressin) and put on antibiotic therapy with vancomycin, meropenem, and caspofungin. Progressively, the patient’s condition improved and after five days of ICU stay, she was successfully weaned off mechanical ventilation and put on high flow nasal cannula. Abdominal cavity revisions were therefore performed after 48 and 96 h and during the last surgery for abdominal wall closure, hepatic biopsy was obtained. Meanwhile, our Hematology consultant recommended the following laboratory and genetic tests: reticulocytes count, schistocytes count, FVIII and FVIII inhibitors, C and S proteins, LAC (lupus anticoagulant), JAK2 (Janus Kinase 2), c-mpl (thrombopoietin receptor), CALR (calreticulin), and PNH (paroxysmal nocturnal hemoglobinuria) clone.
Blood cultures at ICU admission resulted positive for Candida glabrata and Vancomycin-Resistant Enterococcus faecium, and antimicrobial therapy was therefore modified with tedizolid instead of vancomycin. A peritoneal sample also tested positive for E. faecium XDR (extensively drug resistant) and so tigecycline was added to antimicrobial therapy. She progressively recovered from septic shock and, after sixteen days, was discharged from ICU and admitted to the Gastroenterology Department.
2 Investigations
PVT in healthy patients is usually associated with various systemic prothrombotic states or specific hepatic conditions, such as cirrhosis or hepatocarcinoma, but also with other malignancies. In this case, the first CT scan promptly excluded solid tumors but showed a chronic liver disorder of unknown etiology. Clinicians thus focused their attention on the chronic hepatopathy per se. On admission, an extensive serology screening for Hepatitis A Virus, Hepatitis B Virus, Hepatitis C Virus, Citomegalovirus, and Epstein Barr Virus (HAV, HBV, HCV, CMV, and EBV) was performed to rule out possible viral etiology, and microbial cultures and paracentesis were also withdrawn. The gastroscopy showed esophageal varices with no signs of active bleeding, and band ligation of varices was therefore scheduled on elective regimen of treatment. Serology was negative for viral hepatitis, and hepatic biopsy only showed an inflammatory, but not cirrhotic, hepatopathy. Hematologic laboratory tests and genetic screening (mentioned above) were diriment for diagnosis. In fact, the genetic screening revealed JAK2 V617F mutation and so the patient was diagnosed with a myeloproliferative disease. However, septic shock, recurrent surgeries, and hepatosplenomegaly condition did not allow the distinction between polycythemia vera, essential thrombocythemia, and myelofibrosis and, consequently, osteomedullar biopsy and bone marrow aspiration were scheduled. On an elective regimen, she underwent an osteomedullar biopsy of the iliac crest that revealed primary myelofibrosis with megakaryocytes activation.
3 Differential Diagnosis
On admission to the ED, the patient presented with fever and a specific abdominal symptoms, and signs of prehepatic portal hypertension were not clear. Laboratory tests showed neutrophil leukocytosis, normal platelets count, normal first-line coagulation tests, liver function tests (with the only exception of mild hyperbilirubinemia), and high levels of C-reactive protein. Moreover, porto-spleno-mesenteric venous thrombosis and hepatic cavernomatosis were not clearly evident on the first CT scan, and the first diagnostic hypothesis included hepatitis of viral, metabolic, or autoimmune etiology and occult gynecological malignancy. Coagulopathy and other hematologic disorders were not considered at the beginning. Thrombophilic screening was carried out only after ICU admission and showed decreased levels of C and S proteins, LAC 1.65 (mild positive), ANA (anti-nuclear antibodies) 1:160, ACA (anti-centromere antibodies) IgM positive, slightly increased FVIII and absent FVIII inhibitor, and positive JAK2.
4 Treatment
The management of this patient has been quite challenging under different points of view. First, the antibiotic therapy required particular attention and remodulation because we faced multiresistant germs. When she was first admitted to hospital care, she was put on piperacillin/tazobactam (4.5 g every 6 h) on an empirical basis but, progressively, her conditions got worse and so piperacillin/tazobactam was switched to meropenem (2 g every 8 h). Subsequently, she was affected by a severe septic shock managed with the adoption of vancomycin (1 g bolus and 2 g/24 h continuously infused), meropenem, and caspofungin (70 mg bolus and 50 mg daily) which in turn have been modified with the addition of tedizolid (200 mg/day) and tigecycline (100 mg bolus and then 50 mg twice a day), while vancomycin was discontinued (see above for other details).
Also, the surgical management was not easy because she first underwent emergency laparotomy with drainage of six liters of peritoneal fluid, bowel manipulation with lysis of adherences and section of a necrotic, perforated, intestinal loop, and because of the severe inflammation and massive bleeding, abdomen was left open in anticipation of a follow-up procedure. She therefore underwent two abdominal cavity revisions in the next 24 h for bleeding control, and the abdominal wall was closed with a temporary abdominal dressing that works with negative pressure allowing edema reduction, fast re-entry, and protection of abdominal content. Moreover, revisions were scheduled every 48 h until the improvement of patient conditions made the definitive closure safe. A savage treatment, called Meso-Rex bypass, was not possible because mesenteric vein was thrombosed as well.
A crucial aspect in the management of PVT was anticoagulation. She was first started on enoxaparin 4000UI twice a day when PVT was diagnosed. Afterward, in ICU, we put her on continuous infusion of unfractioned heparin (UFH) with aPTT target of 1.5 times greater than normal. When septic shock was solved, UFH was discontinued, and enoxaparin was started again. The efficacy of anticoagulation in patients with evidence of acute PVT has been reported in literature and may result in recanalization of thrombi but, unfortunately, we have not seen it in our patient. Thrombolytic therapy, despite its effectiveness, was not considered in this patient because it was too hazardous [2]. She also received numerous blood transfusions (including erythrocytes, platelets, and plasma) but her laboratory tests showed a picture of pancytopenia even at the discharge from the Gastroenterology department.
5 Evolution, Outcome, and Follow-up
The patient’s condition gradually improved in ICU, so she was discharged from the ICU after two weeks and admitted to the Gastroenterology Department. Despite the resolution of the shock, she remained septic and on antibiotics and her general condition was mediocre (she lost more than 20 kg of weight and was not able to take care of herself). A new abdomen CT scan confirmed SVT without recanalization. She also underwent a medullary needle aspiration that revealed an increased count of plasma cells and an osteomedullar biopsy of the iliac crest that revealed primary myelofibrosis with megakaryocytes activation. She was definitively discharged after two months of hospital stay and she is now on a follow-up program with hepatologists and hematologists, but therapeutic strategies have not been defined yet.
6 Discussion
PVT in healthy young patients usually could represent the first manifestation of a myeloproliferative disorder in about 22–48% of patients; an overt MPD might be diagnosed subsequently in 51% of patients [1]. Its onset may be both acute and chronic, with different clinical presentations. When not adequately recognized and treated, SVT can lead to portal hypertension with subsequent occult bleeding and hypersplenism with pancytopenia that make myeloproliferative disease difficult to diagnose. In our patient, thrombosis also involved splenic and superior mesenteric veins that have been considered only at a later time, and this may have led, as a consequence, to intestinal ischemia and worsening of septic condition [3, 4].
This case is relevant to clinical practice because it highlights how important it is to not focus our attention on one specific problem (even when the diagnosis seems clear) because this may lead to anchoring bias errors. In fact, at the beginning, the SVT was misdiagnosed, and its relevance was underestimated because our colleagues focused their attention on gynecological malignancy and chronic liver disease of suspected viral etiology, and this has led to a delay in the diagnosis of the prothrombotic condition which in turn has determined a rapid progression toward extremely critical conditions on ICU admission. In fact, despite a negative comprehensive workup, in young patients with atypical abdominal thrombosis without risk factors for hepatopathies, there should be a high suspicion of prothrombotic and myeloproliferative disorders that must be included in the differential diagnostic algorithm of PVT/SVT [1]. SVT in the absence of cirrhosis and hepatocarcinoma is a finding that, despite being uncommon, should be considered because it is easy to diagnose and needs prompt treatment with anticoagulant and/or thrombolytic therapy [2, 4]. Early diagnosis is the only way to prevent late complications of SVT (esophageal varices bleeding and intestinal ischemia) that could be life threatening.
Take-Home Messages
-
Despite being rare, portal vein thrombosis can be the first presentation of a myeloproliferative disease.
-
A high degree of suspicion is required for a prompt diagnosis of splanchnic vein thrombosis and acute mesenteric ischemia due to the nonspecific symptoms of these conditions.
-
Due to improvements in imaging, the diagnosis of both acute mesenteric ischemia and splanchnic venous thrombosis has become timelier and more accurate.
-
Early diagnosis and anticoagulant management are essential to stop thrombus extension and obtain recanalization.
-
Consequences of misdiagnosed portal and mesenteric vein thrombosis may be life threatening leading to bowel perforation and sepsis with septic shock.
-
In patients with unclear clinical presentation, it is important to be aware of anchoring bias to avoid missing rare diagnoses.
Summary
Portal vein thrombosis (PVT) is a condition characterized by narrowing or occlusion of portal vein that is usually seen in patients with hepatic cirrhosis or malignancies, but presentation in otherwise healthy young patients is rare. We report a case of a 42-year-old lady, whose medical history was unremarkable, who has recently sought medical attention for fever, ascites, abdominal pain, and hepatosplenomegaly associated with high levels of CA-125. Few days prior hospitalization, she had taken a computed tomography that did not show clear signs of PVT. A standard comprehensive workup did not show any overt hepatic disease or malignancy but, despite these initial results, other causal factors were not investigated, and CT scan was not repeated on hospital admission. The diagnosis of splanchnic vein thrombosis sustained by a procoagulant disorder was made only after admission to ICU for an extremely severe septic shock caused by bowel perforation. This case shows how aspecific and misleading clinical presentation of a relatively uncommon disorder has determined delay in the diagnosis and therefore has led to an extremely severe septic shock sustained by multiresistant germs. Moreover, this case highlights the intersection of several of the most important aspects of mesenteric ischemia of venous origin, including the high index of suspicion required to diagnose the patient before progression to transmural necrosis, improved early diagnosis with contrast-enhanced CT, and both the surgical and medical treatment of this condition.
References
Ponziani FR, Zocco MA, Campanale C, Rinninella E, Tortora A, Di Maurizio L, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. 2010;16(2):143–55.
Mansour N, Öcal O, Gerwing M, Köhler M, Deniz S, Heinzow H, et al. Interventional recanalization therapy in patients with non-cirrhotic, non-malignant portal vein thrombosis: comparison between transjugular versus transhepatic access. Abdom Radiol (NY). 2022;47(3):1177–86.
Splanchnic VD, Thrombosis V. Splanchnic vein thrombosis. Semin Thromb Hemost. 2015;41(5):494–502.
Cohen R, Mallet T, Gale M, Soltys R, Loarte P. Portal vein thrombosis. Case Rep Vasc Med. 2015;2015:823063.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Caporale, M., Piervincenzi, E. (2023). Intraabdominal Sepsis: Portal and Mesenteric Vein Thrombosis as First Presentation of Myeloproliferative Disease in a Young Woman. In: Pérez-Torres, D., Martínez-Martínez, M., Schaller, S.J. (eds) Best 2022 Clinical Cases in Intensive Care Medicine. Lessons from the ICU. Springer, Cham. https://doi.org/10.1007/978-3-031-36398-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-36398-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36397-9
Online ISBN: 978-3-031-36398-6
eBook Packages: MedicineMedicine (R0)