Abstract
DSM-TACE is certainly one of the least widespread TACE techniques. Nevertheless, it is the only temporary embolization technique established in clinical routine to date. Despite increasing evidence, DSM-TACE is mainly indicated for liver tumors, primarily HCC and advanced stages of disease, in which drug therapy has also made great progress in recent years. In the following book chapter, DSM-TACE is discussed in detail. To this end, the historical origins, pharmacokinetics, and basic mechanisms of DSM-TACE are first discussed. Subsequently, the current implementation in clinical routine as well as the results and safety are discussed. To the end, an overview of the current literature is then given, divided according to the individual entities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Since its introduction more than 30 years ago, transarterial chemoembolization (TACE) has become a widely accepted locoregional therapy for the palliative treatment of liver cancer. TACE can be performed with a variety of embolic and chemotherapeutic agents, and despite widespread clinical use, there is an ongoing debate about different TACE protocols and how best to deliver the therapy. Currently, two TACE techniques are mainly used in clinical routine: conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE). In cTACE, the chemotherapeutic drug is administered intra-arterially using iodized oil as a drug carrier, and the vessels supplying the tumor are subsequently mechanically embolized with spherical or nonspherical embolic agents. Conversely, DEB-TACE relies on the intra-arterial injection of drug-loaded microspheres that slowly release the cytotoxic drug into the tumor while at the same time embolizing its feeding vessels permanently. A less well-known TACE regimen is degradable starch microsphere (DSM) transarterial chemoembolization (DSM-TACE), a technique based on the use of degradable microspheres produced from hydrolyzed potato starch as a temporary embolic agent. What distinguishes DSM-TACE from other TACE techniques is the fact that as DSM are enzymatically degraded by blood amylases after a half-life of about 40 min (for microspheres 50 μm in diameter), the vascular occlusion obtained with this agent is transient. DSM-TACE thus combines high local drug delivery with a reduced risk of systemic toxicity while increasing the safety profile of the procedure by a short ischemia time and low vessel occlusion rate. Hence, DSM-TACE can be administered more unselectively than cTACE or DEB-TACE, making it especially suitable as a therapeutic alternative for patients with multifocal hepatic disease [1,2,3,4,5].
Historical Notes and Pharmacokinetics of DSM
Originally developed for scintigraphic imaging of pulmonary emboli in the early 1970s, degradable starch microspheres were found to be well tolerated when administered intravenously. Available toxicological data suggest that DSM do not directly induce any adverse reaction per se. The toxicity observed after coadministration with cytostatic agents like cisplatin, doxorubicin, or mitomycin is similar to that encountered when applying the chemotherapeutic agent alone, with the exception of enhanced liver toxicity, which is part of the desired therapeutic effect.
DSM are produced by means of polymerization from hydrolyzed potato starch, cross-linked, and substituted with glycerol ether groups. This process results in a polymerized matrix, which has no chemical name of its own but has a complex structure well suited for transient embolization of arterial blood flow. DSM are degraded enzymatically by the body’s own serum alpha-amylase. The total time of occlusion is hence indirectly proportional to the individual amount of alpha-amylase in plasma [6]. The half-life under physiological conditions at 37 °C and pH 7 ranges between 35 and 50 min. After approximately 2 h, particles are completely dissolved. The accumulating glucose monomers are dismantled and eliminated by the body’s reticulocytic and excretory systems. Commercially available DSM (e.g., EmboCept® S, PharmaCept, Berlin, Germany) come in 20-ml bottles containing a sterile and clear solution consisting of 450 mg Amilomer, DSM 35/50, and 7.5 ml sodium chloride (60 mg/ml). DSM particles have a mean diameter of 50 μm with 75% of microspheres ranging in diameter from 20 to 200 μm.
8.2 Pharmacodynamics and Mechanism of Action of DSM
DSM are used to achieve blood flow reduction and transient vascular occlusion in the peritumoral blood vessels during coadministration of cytotoxic drugs. The effects of intra-arterial administration of DSM on blood flow have been investigated in numerous preclinical and clinical studies. Furthermore, several authors have explored how the altered blood flow induced by DSM affects the regional and systemic delivery of the co-injected drug.
8.2.1 DSM-Induced Blood Flow Reduction and Transient Vascular Embolization
One of the first studies investigating intra-arterial DSM was published in 1978 by Forsberg and colleagues. Forsberg et al. studied the effects of intra-arterial DSM injection into the femoral and superior mesenteric artery on blood flow in the hind feet and intestine of rats. Using tracer microspheres and electromagnetic flow measurement, they demonstrated that after DSM infusion, blood flow in the hind feet and small intestine rapidly declined to near zero and later returned to levels in control animals [6]. Thulin et al. performed preoperative electromagnetic measurement of hepatic arterial flow in 10 patients with primary or secondary liver and demonstrated that hepatic arterial blood flow could be reduced in a dose-dependent manner by a mean maximum of 67% following DSM injection [7]. Wiggermann et al. explored the transient embolizing effect of DSM-TACE using contrast-enhanced ultrasound quantitative perfusion analysis in six patients with hepatocellular carcinoma (HCC). Compared to baseline parameters, they observed a significant reduction in peak regional blood volume and regional blood flow immediately after DSM-TACE in all cases. Over time, a stepwise revascularization of the treated lesions was documented: 90 min after embolization, perfusion parameters were not significantly different from pre-embolization values [8].
A recent study addressed a central question concerning the therapeutic effect of the transient embolization achievable with DSM: Is a transient embolization sufficient to induce necrosis of the target tumor and thus inhibit its growth? Ziemann and colleagues analyzed the effect of three different agents routinely used in the setting of TACE on tumor growth in a rat model of colorectal liver metastases. The rats were randomized into four groups treated with intra-arterial infusion of either DSM (size: 35–70 μm; dose: 6.43 mg/kg body weight), PVA microspheres (size: 70–150 μm; dose: 0.14 ml/kg body weight), iodized oil (size: 2–3 μm; dose: 0.15 ml/kg body weight), or saline (control group). Tumor growth was assessed by three-dimensional ultrasound on days 8 and 11, followed by histological and immunohistochemical analysis of tumor necrosis on day 11. In this experiment, both PVA microspheres and DSM completely inhibited tumor progression while iodized oil did not significantly affect tumor growth. Immunohistochemical analysis revealed significantly larger necrotic areas within the tumors after administration of DSM and PVA microspheres compared with iodized oil [9].
8.2.2 Regional and Systemic Delivery of the co-Injected Drug
Teder et al. investigated the effects of DSM on hepatic arterial blood flow in rats by means of a regionally injected marker, 99mTC-methylene diphosphonate (99mTc-MDP), and showed that compared to injections of pertechnetate only, the integrated exposure of the liver to pertechnetate was increased by a factor of 1.4–2.4 when microspheres were added [10]. In a clinical study published in 1982, Dakhil and colleagues injected DSM into the hepatic artery of patients with primary and metastatic liver cancer to assess whether transient vascular occlusion at the level of the arteriolar capillary bed would enhance regional uptake and decrease systemic exposure to simultaneously administered hepatic arterial bischlorethylnitrosourea (BCNU). Following intra-arterial DSM injection, hepatic arterial flow was transiently substantially reduced or stopped in all patients. When BCNU was given with microspheres, there was a 30–90% reduction in systemic nitrosourea exposure and in peak levels. Dakhil et al. thus demonstrated that concurrent intra-arterial injection of DSM and BCNU might have the potential to enhance selective regional drug effects while at the same time markedly reducing systemic toxicity [11]. Along this line, several authors have explored the use of DSM to increase the effectiveness of intra-arterial chemotherapy. The results of these studies show that in the presence of drastic blood flow reduction and transient vascular occlusion induced by DSM, the dwell times of co-injected drugs are prolonged within the injected area, creating a steep drug concentration gradient between the arteries and the target tissue with a selectively increased uptake of the co-injected drug into liver tumors compared with normal liver tissue [12].
8.2.3 Further Effects of DSM-Induced Transient Embolization: TACE and VEGF Expression
The combination of targeted administration of a chemotherapeutic drug at high concentration and induction of ischemia by embolization of tumor-feeding vessels is the key mechanism of TACE [13]. Local tumor hypoxia induced by TACE leads to a sequence of adaptive changes in the transcription and expression of hypoxia response genes in tumor cells aimed at counteracting or reversing hypoxia [14]. Hypoxia response genes are primarily regulated by hypoxia-inducible factor-1α (HIF-1α), which triggers expression of vascular endothelial growth factor (VEGF) and promotes neovascularization [15, 16]. Newly formed aberrant tumor blood vessels, characterized by structural and functional defects, further aggravate hypoxia and thereby form a vicious cycle thought to play a key role in tumor growth and metastatic seeding [16,17,18]. In the context of TACE, these biological countermeasures have the potential to interfere with the ultimate intention of anticancer treatment. In fact, studies have shown that the transient overexpression of VEGF after a single session of TACE is associated with future distant metastases, especially located in the lung or bones, and consequently with a shorter progression-free survival (PFS) [16, 19,20,21,22]. Furthermore, the peak of serum VEGF after TACE has been shown to be an independent prognostic factor of progression-fee survival (PFS) in HCC [23]. Schicho and colleagues compared serum VEGF levels in response to TACE with different embolic agents in 22 patients with HCC. In this prospective study, patients were assigned to one of three different TACE regimens (cTACE, DEB-TACE, and DSM-TACE), all performed with the same cytostatic drug (50 mg doxorubicin/m2 body surface area (BSA)). Serum VEGF levels were assessed before TACE treatment as well as 24 h and 4 weeks after treatment. Compared to baseline, a marked increase in VEGF levels was observed 24 h after cTACE (164% of baseline level) and at 4-week follow-up (170% of baseline level). Increases in serum VEGF levels were 114% and 123% following DEB-TACE and 121% and 124% for DSM, respectively. The authors conclude that conventional TACE using Lipiodol is associated with a marked increase in blood levels of the proangiogenic factor VEGF while DEB-TACE and DSM-TACE induce only a moderate VEGF response [16].
8.3 Patients and Technique
8.3.1 Patient Selection
DSM are intended for combined use with chemotherapeutic agents to escalate effectivity by increasing the retention time of the co-injected agents in the targeted liver. Like other TACE techniques, DSM-TACE can be used in patients with primary or metastatic liver cancer not amenable to curative treatments. Regarding the treatment of hepatocellular carcinoma, guidelines from all over the world (AASLD, EASL, APASL, and ESMO) endorse the use of TACE for HCC in patients with intermediate-stage disease, defined as multinodular disease confined to the liver in asymptomatic patients (performance status of 0), Child–Pugh class A or class B cirrhosis with no decompensation, and absence of portal vein invasion or extrahepatic spread [24,25,26,27]. Absolute and relative contraindications are similar to those of cTACE and DEB-TACE and include impaired liver function, uncorrectable coagulopathies (INR >1.5; aPTT >50 s; <50,000 thrombocytes), and renal failure (serum creatinine >2 mg/dl). Relative contraindications for DSM-TACE include chronic cardiac insufficiency (NYHA III–IV), acute coronary syndrome (ACS), and exophytically growing tumors. Partial or complete thrombosis of the main portal vein is often classified as an exclusion criterion for chemoembolization. Portal vein thrombosis appears to be less of a concern for DSM-TACE due to the transient embolization and the shorter ischemia time [28].
8.3.2 DSM-TACE Technique
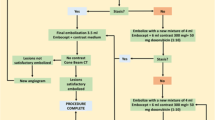
The goal of DSM-TACE is to achieve blood flow reduction and transient vascular occlusion in the peritumoral blood vessels to minimize systemic exposure to the co-injected cytotoxic drug. Due to the wide variation in number, size, and vascularity of liver tumors from one patient to the next, the dose of DSM has to be adapted individually. Complete stasis of blood flow should be avoided as this carries an increased risk of backflow into extrahepatic vascular territories. Hence, to perform DSM-TACE safely, angiographic monitoring during drug administration is crucial.
Angiographic techniques vary among institutions. Herein, we describe the angiographic technique as performed at our institution. In most patients, vascular access is gained through puncture of the common femoral artery in the right or left groin; however, alternative vascular access through the left radial or brachial artery may be used if this makes catheterization of the hepatic artery easier. Following arterial puncture using the Seldinger technique, a 4−/5-F angiographic sheath is introduced. After catheterization of the celiac trunk and the superior mesenteric artery using a diagnostic catheter, selective angiography of the aforementioned vessels is performed to accurately characterize the vessels supplying the liver. Subsequently, a coaxial microcatheter system is advanced beyond the branching of the gastroduodenal artery and positioned in the tumor-supplying branches of the hepatic artery. Unlike the classic TACE procedures, where the embolic agent has to be delivered as close as possible to the tumor (superselective embolization), DSM-TACE can be performed more unselectively in a lobar fashion way by placing the microcatheter in either the left or right hepatic branch. However, even for lobar administration, the use of a microcatheter is preferred to reduce the risk of vasospasm and arterial dissection. Once correct positioning of the microcatheter has been confirmed, the mixture of chemotherapeutic agent, DSM, and contrast agent is administered under constant angiographic control.
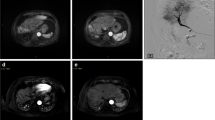
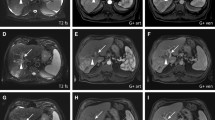
Degradable starch microspheres should be mixed with a ready-to-use solution of active substance and contrast agent. In HCC patients, DSM-TACE is performed using a mixture of 50 mg doxorubicin diluted in a saline solution with a total volume of 25 m the interval between injections to optimize the administered dose. Even if complete vascular occlusion occurs, treatment should not be discontinued, due to the risk of backflow and nontarget embolization. When DSM-TACE is performed less selectively (treatment of entire lobar or major segments), the endpoint of embolization should be a “tree-in-the-winter” appearance with occlusion of small tumor-feeding vessels but preservation of flow in the major lobar and segmental arteries. After therapy delivery, a digital subtraction angiography (DSA) is performed to confirm devascularization of target lesions and patency of the large vessels in the treated area. DSM-TACE is most effective when the procedure is performed in repeated treatment sessions. At our institution, DSM-TACE is repeated at 4- to 6-week intervals until complete disappearance of arterial enhancement is seen. In case of bilobar tumor spread in patients in whom deterioration of hepatic function is feared, the lobe with higher tumor burden is treated first, followed by the contralateral lobe. In the latter case, it is preferable to reduce the interval between the two sessions to 2 weeks if possible. Follow-up imaging is routinely performed using contrast-enhanced magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) in patients with contraindications to MRI.
8.4 Clinical Results and Safety
Recent experience suggests that this technique is particularly suitable for treating patients with more advanced HCC or with multifocal disease not amenable to superselective treatment [1,2,3,4,5]. DSM-TACE has also been tested for the palliative treatment of patients with unresectable or recurrent intrahepatic cholangiocarcinoma (iCCA) as well as for patients with chemotherapy-refractory liver metastases from colorectal cancer [29,30,31]. Alongside these studies, there are also reports in the literature on the use of DSM-TACE in rare entities like liver metastases from uveal melanoma, breast cancer, renal cell carcinoma, and neuroendocrine tumors [32,33,34,35].
8.4.1 Primary Liver Cancer
8.4.1.1 HCC
As already mentioned, different guidelines endorse the use of TACE in patients with intermediate-stage disease while especially DSM-TACE may also be an option in more advanced disease [3, 5, 24,25,26,27]. A recently published study by Orlacchio and colleagues evaluated the safety and efficacy of DSM-TACE in a large clinical population of 137 patients with unresectable HCC who underwent a total of 267 DSM-TACE procedures. Major complications occurred in only 6.8% of all procedures. According to mRECIST (modified Response Evaluation Criteria in Solid Tumors), a high objective response rate was obtained (84.3% of patients showed complete or partial responses), and the median time to progression was 12 months with an OS of 36 months. Of note, 20 patients in the study had BCLC stage C HCC [36]. In another recently published study, Iezzi and colleagues investigated DSM-TACE treatment in 40 consecutive BCLC stage B or C patients with intermediate or locally advanced stage HCC who dismissed or were ineligible for sorafenib. While technical success was achieved in all patients, no liver failure or systemic toxicity was reported. Progression-free survival (PFS) was 6.4 months and the median OS was 11.3 months [3]. These results have been corroborated in a study of Haubold and colleagues, who investigated DSM-TACE in non-resectable HCC patients ineligible for other systemic or locoregional therapies. DSM-TACE was performed successfully in 28 patients. At control imaging after three DSM-TACE procedures, the rates of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were 14.3%, 25%, 39.3%, and 21.4%, respectively. With a good OS of 682 days, the authors also conclude that DSM-TACE is a safe and promising treatment alternative. A selection of further studies in line with the results just outlined is provided in the table below (Table 8.1) [1,2,3,4,5, 36,37,38,39,40,41].
8.4.1.2 Intrahepatic Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver malignancy after hepatocellular carcinoma, and its incidence and mortality have risen globally in the past decades [42]. Despite substantial advances in diagnosis and treatment, the prognosis of patients with iCCA remains poor [42]. This is mainly due to the fact that at the time of initial diagnosis, only about 20% of patients are amenable to surgical resection, the only potentially curative treatment option for iCCA [43]. Despite a low level of evidence, locoregional therapies have shown promising results in the treatment of selected patients with unresectable or recurrent disease [44]. TACE of iCCA has been investigated in several, mostly retrospective, studies using various chemotherapeutic agents. In a recently published systematic meta-analysis of 13 studies (906 patients), pooled median survival after TACE was 14.2 months [45].
Two studies on the use of DSM-TACE in patients with unresectable iCCA have been published. Georg et al. reported their experience with a multi-agent (cisplatin/doxorubicin/mitomycin C) DSM-TACE protocol in a population of 21 patients with either unresectable iCCA progressive under systemic chemotherapy or unresectable intrahepatic tumor recurrence after prior major liver resection. The reported objective response rate was 61.1% and the reported disease control rate 100%. Median overall survival was 13.3 months with similar results in patients with primary unresectable, therapy-refractory disease (13.2 months) and patients with intrahepatic recurrence after prior liver resection (12.5 months) (Table 8.2) [30].
8.4.2 Secondary Liver Cancer
8.4.2.1 Colorectal Cancer Liver Metastasis
Colorectal cancer (CRC) is one of the most common cancers worldwide, ranking third in terms of incidence and second in terms of cancer-related death [46]. The liver is the most common site of colorectal cancer metastasis. Approximately 20% of patients with CRC present with concomitant liver metastasis at initial diagnosis while another 50% develop liver metastasis within the first 3 years after diagnosis [47]. Surgery offers the best chance of cure for patients with colorectal liver metastases (CRLM); however, only 10–25% of all patients with CRLM are amenable to surgical resection [48]. Locoregional treatments have proven to be a useful therapeutic strategy in patients with unresectable CRLM. TACE has been identified as a suitable treatment option for patients with CRLM in a neoadjuvant, symptomatic, or palliative setting [31, 49,50,51,52,53]. One of the first studies investigating the use of DSM-TACE in the management of CRLM was published in 2013 by Nishiofuku and colleagues. The authors reported the results of a phase I/II trial of DSM-TACE with cisplatin powder in 24 patients with unresectable CRLM progressing after FOLFOX (5-flourouracil, leucovorin plus oxaliplatin) chemotherapy. In phase II, the tumor response rate was 61.1%, and median overall survival was 21.1 months [54]. In a recent prospective, randomized, single-center trial, Vogl et al. compared the therapy response of third-line cTACE (n = 13) versus DSM-TACE (n = 18) in 31 patients with CRLM. In this first prospective study directly comparing cTACE and DSM-TACE in patients with CRLM, DSM-TACE showed significantly better results in terms of tumor volume reduction (p = 0.006) and tumor response according to RECIST 1.1 (p = 0.047) compared with cTACE [31]. Please find a list of studies in Table 8.3 [31, 49, 54].
Other Entities
Experience with DSM-TACE in the treatment of other entities is based on relatively limited single-center experiences; a selection of studies is compiled in Table 8.4 [33, 55,56,57,58,59].
8.5 Conclusions
-
DSM is a short-acting, nonpermanent embolic agent and therefore can be administered unselectively.
-
DSM-TACE is a safe and easy to perform interventional procedure and a useful therapeutic alternative for patients with multifocal HCC.
-
Other indications for DSM-TACE are neoadjuvant, symptomatic, and palliative treatment of locally extensive or disseminated ICC or CRLM.
-
While DSM-TACE should be suitable for nearly any patient with disseminated metastatic liver disease, evidence is still limited, except for CRLM, and published data are mostly based on monocentric studies with limited patient numbers.
References
Auer TA, Jonczyk M, Collettini F, Marth A, Wieners G, Hamm B, et al. Trans-arterial chemoembolization with degradable starch microspheres (DSM-TACE) versus selective internal radiation therapy (SIRT) in multifocal hepatocellular carcinoma. Acta Radiol. 2021;62(3):313–21.
Haubold J, Reinboldt MP, Wetter A, Li Y, Ludwig JM, Lange C, et al. DSM-TACE of HCC: evaluation of tumor response in patients ineligible for other systemic or loco-regional therapies. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2020;192(9):862–9.
Iezzi R, Pompili M, Rinninella E, Annicchiarico E, Garcovich M, Cerrito L, et al. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur Radiol. 2019;29(3):1285–92.
Schicho A, Pereira PL, Haimerl M, Niessen C, Michalik K, Beyer LP, et al. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget. 2017;8(42):72613–20.
Gross A, Albrecht T. Transarterial chemoembolisation (TACE) with degradable starch microspheres (DSM) and anthracycline in patients with locally extensive hepatocellular carcinoma (HCC): safety and efficacy. Cardiovasc Intervent Radiol. 2020;43(3):402–10.
Forsberg JO. Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres. Experiments on rats. Acta Chir Scand. 1978;144(5):275–81.
Thulin L, Tyden G, Nyberg B, Calissendorff B, Hultcrantz R. Reduction of hepatic arterial flow by degradable microspheres in patients with liver tumor. Acta Chir Scand. 1986;152:447–51.
Wiggermann P, Wohlgemuth WA, Heibl M, Vasilj A, Loss M, Schreyer AG, et al. Dynamic evaluation and quantification of microvascularization during degradable starch microspheres transarterial chemoembolisation (DSM-TACE) of HCC lesions using contrast enhanced ultrasound (CEUS): a feasibility study. Clin Hemorheol Microcirc. 2013;53(4):337–48.
Ziemann C, Roller J, Malter MM, Keller K, Kollmar O, Glanemann M, et al. Intra-arterial EmboCept S(R) and DC bead(R) effectively inhibit tumor growth of colorectal rat liver metastases. BMC Cancer. 2019;19(1):938.
Teder H, Nilsson M, Aronsen KF, Erichsen C, Jonsson PE. Influence of degradable starch microspheres (Spherex) on the retention of pertechnetate in a solitary rat liver tumor. European J Surg Oncol. 1988;14(4):327–33.
Dakhil S, Ensminger W, Cho K, Niederhuber J, Doan K, Wheeler R. Improved regional selectivity of hepatic arterial BCNU with degradable microspheres. Cancer. 1982;50(4):631–5.
Civalleri D, Esposito M, Fulco RA, Vannozzi M, Balletto N, De Cian F, et al. Liver and tumor uptake and plasma pharmacokinetic of arterial cisplatin administered with and without starch microspheres in patients with liver metastases. Cancer. 1991;68(5):988–94.
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6.
Span PN, Bussink J. Biology of hypoxia. Seminars in nuclear medicine. Elsevier; 2015.
Schicho A, Hellerbrand C, Kruger K, Beyer LP, Wohlgemuth W, Niessen C, et al. Impact of different embolic agents for Transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels. J Clin Transl Hepatol. 2016;4(4):288–92.
Miura H, Miyazaki T, Kuroda M, Oka T, Machinami R, Kodama T, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997;27(5):854–61.
Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28(1):68–77.
Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–82.
Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11(5):1077–84.
Xiong ZP, Yang SR, Liang ZY, Xiao EH, Yu XP, Zhou SK, et al. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2004;3(3):386–90.
Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–21.
Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99(10):2037–44.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and Management of Hepatocellular Carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50.
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv55.
Lucatelli P, Burrel M, Guiu B, de Rubeis G, van Delden O, Helmberger T. CIRSE standards of practice on hepatic Transarterial chemoembolisation. Cardiovasc Intervent Radiol. 2021;44(12):1851–67.
Schicho A, Pereira PL, Putzler M, Michalik K, Albrecht T, Nolte-Ernsting C, et al. Degradable starch microspheres Transcatheter arterial chemoembolization (DSM-TACE) in intrahepatic Cholangiocellular carcinoma (ICC): results from a National Multi-Center Study on safety and efficacy. Med Sci Monit. 2017;23:796–800.
Goerg F, Zimmermann M, Bruners P, Neumann U, Luedde T, Kuhl C. Chemoembolization with degradable starch microspheres for treatment of patients with primary or recurrent Unresectable, locally advanced intrahepatic cholangiocarcinoma: a pilot study. Cardiovasc Intervent Radiol. 2019;42(12):1709–17.
Vogl TJ, Marko C, Langenbach MC, Naguib NNN, Filmann N, Hammerstingl R, et al. Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial. Eur Radiol. 2021;31(4):2242–51.
Schuster R, Lindner M, Wacker F, Krossin M, Bechrakis N, Foerster MH, et al. Transarterial chemoembolization of liver metastases from uveal melanoma after failure of systemic therapy: toxicity and outcome. Melanoma Res. 2010;20(3):191–6.
Nabil M, Gruber T, Yakoub D, Ackermann H, Zangos S, Vogl TJ. Repetitive transarterial chemoembolization (TACE) of liver metastases from renal cell carcinoma: local control and survival results. Eur Radiol. 2008;18(7):1456–63.
Vogl TJ, Gruber T, Naguib NN, Hammerstingl R, Nour-Eldin NE. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol. 2009;193(4):941–7.
Schneider P, Foitzik T, Pohlen U, Golder W, Buhr HJ. Temporary unilateral microembolization of the lung-a new approach to regional chemotherapy for pulmonary metastases. J Surg Res. 2002;107(2):159–66.
Orlacchio A, Chegai F, Roma S, Merolla S, Bosa A, Francioso S. Degradable starch microspheres transarterial chemoembolization (DSMs-TACE) in patients with unresectable hepatocellular carcinoma (HCC): long-term results from a single-center 137-patient cohort prospective study. Radiol Med. 2020;125(1):98–106.
Orlacchio A, Chegai F, Francioso S, Merolla S, Monti S, Angelico M, et al. Repeated Transarterial chemoembolization with degradable starch microspheres (DSMs-TACE) of Unresectable hepatocellular carcinoma: a prospective pilot study. Curr Med Imaging Rev. 2018;14(4):637–45.
Niessen C, Unterpaintner E, Goessmann H, Schlitt HJ, Mueller-Schilling M, Wohlgemuth WA, et al. Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25(2):240–7.
Vogl TJ, Langenbach MC, Hammerstingl R, Albrecht MH, Chatterjee AR, Gruber-Rouh T. Evaluation of two different transarterial chemoembolization protocols using Lipiodol and degradable starch microspheres in therapy of hepatocellular carcinoma: a prospective trial. Hepatol Int. 2021;15:685.
Gruber-Rouh T, Schmitt C, Naguib NNN, Nour-Eldin NA, Eichler K, Beeres M, et al. Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: comparative study. BMC Cancer. 2018;18(1):188.
Minici R, Ammendola M, Manti F, Siciliano MA, Giglio E, Minici M, et al. Safety and efficacy of degradable starch microspheres Transcatheter arterial chemoembolization as a bridging therapy in patients with early stage hepatocellular carcinoma and child-Pugh stage B eligible for liver transplant. Front Pharmacol. 2021;12:634084.
Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–44.
Ebata T, Ercolani G, Alvaro D, Ribero D, Di Tommaso L, Valle JW. Current status on cholangiocarcinoma and gallbladder cancer. Liver Cancer. 2016;6(1):59–65.
Fabritius MP, Ben Khaled N, Kunz WG, Ricke J, Seidensticker M. Image-guided local treatment for Unresectable intrahepatic cholangiocarcinoma-role of interventional radiology. J Clin Med. 2021;10(23)
Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, et al. Transarterial chemoembolization and Radioembolization for Unresectable intrahepatic cholangiocarcinoma—a systemic review and meta-analysis. Cardiovasc Intervent Radiol. 2021;1-11:728.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Tasoudis PT, Ziogas IA, Alexopoulos SP, Fung JJ, Tsoulfas G. Role of liver transplantation in the management of colorectal liver metastases: challenges and opportunities. World J Clin Oncol. 2021;12(12):1193–201.
Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol. 2002;29(2):107–18.
Gruber-Rouh T, Naguib NN, Eichler K, Ackermann H, Zangos S, Trojan J, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014;134(5):1225–31.
Mahnken AH, Pereira PL, de Baere T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266(2):407–30.
Clark TW. Chemoembolization for colorectal liver metastases after FOLFOX failure. J Vasc Interv Radiol. 2013;24(1):66–7.
Gruber-Rouh T, Marko C, Thalhammer A, Nour-Eldin NE, Langenbach M, Beeres M, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol. 2016;89(1064):20151060.
Wasser K, Giebel F, Fischbach R, Tesch H, Landwehr P. Transarterial chemoembolization of liver metastases of colorectal carcinoma using degradable starch microspheres (Spherex): personal investigations and review of the literature. Radiologe. 2005;45(7):633–43.
Nishiofuku H, Tanaka T, Matsuoka M, Otsuji T, Anai H, Sueyoshi S, et al. Transcatheter arterial chemoembolization using cisplatin powder mixed with degradable starch microspheres for colorectal liver metastases after FOLFOX failure: results of a phase I/II study. J Vasc Interv Radiol. 2013;24(1):56–65.
Lindgaard SC, Brinch CM, Jensen BK, Norgaard HH, Hermann KL, Theile S, et al. Hepatic arterial therapy with oxaliplatin and systemic capecitabine for patients with liver metastases from breast cancer. Breast. 2019;43:113–9.
Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl TJ, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: phase II study in patients with liver metastases of breast cancer. Eur J Radiol. 2013;82(12):e816–22.
Vogl TJ, Gruber-Rouh T, Eichler K, Nour-Eldin NE, Trojan J, Zangos S, et al. Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: local control and survival results. Eur J Radiol. 2013;82(2):258–63.
Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas. 2011;40(8):1271–5.
Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin NE, Eichler K, Zangos S, et al. Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: indications, outcomes and role in patient's management. Gynecol Oncol. 2012;124(2):225–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Auer, T.A., Collettini, F. (2023). Transarterial Chemoembolization with Degradable Starch Microspheres (DSM-TACE). In: Lucatelli, P. (eds) Transarterial Chemoembolization (TACE) . Springer, Cham. https://doi.org/10.1007/978-3-031-36261-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-36261-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36260-6
Online ISBN: 978-3-031-36261-3
eBook Packages: MedicineMedicine (R0)